The Influence of Seed Viability on the Germination and In Vitro Multiple Shoot Regeneration of Soybean (Glycine max L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Effect of Seed Storage on Moisture and Standard Germination Test
2.3. Effect of Seed Storage on In Vitro Germination and Seedling Development for Shoot Induction
2.4. Explant Preparation and In Vitro Shoot Induction
2.5. Shoot Elongation and Rooting
2.6. Ex Vitro Plant Establishment
2.7. Experimental Design and Statistical Analysis
3. Results
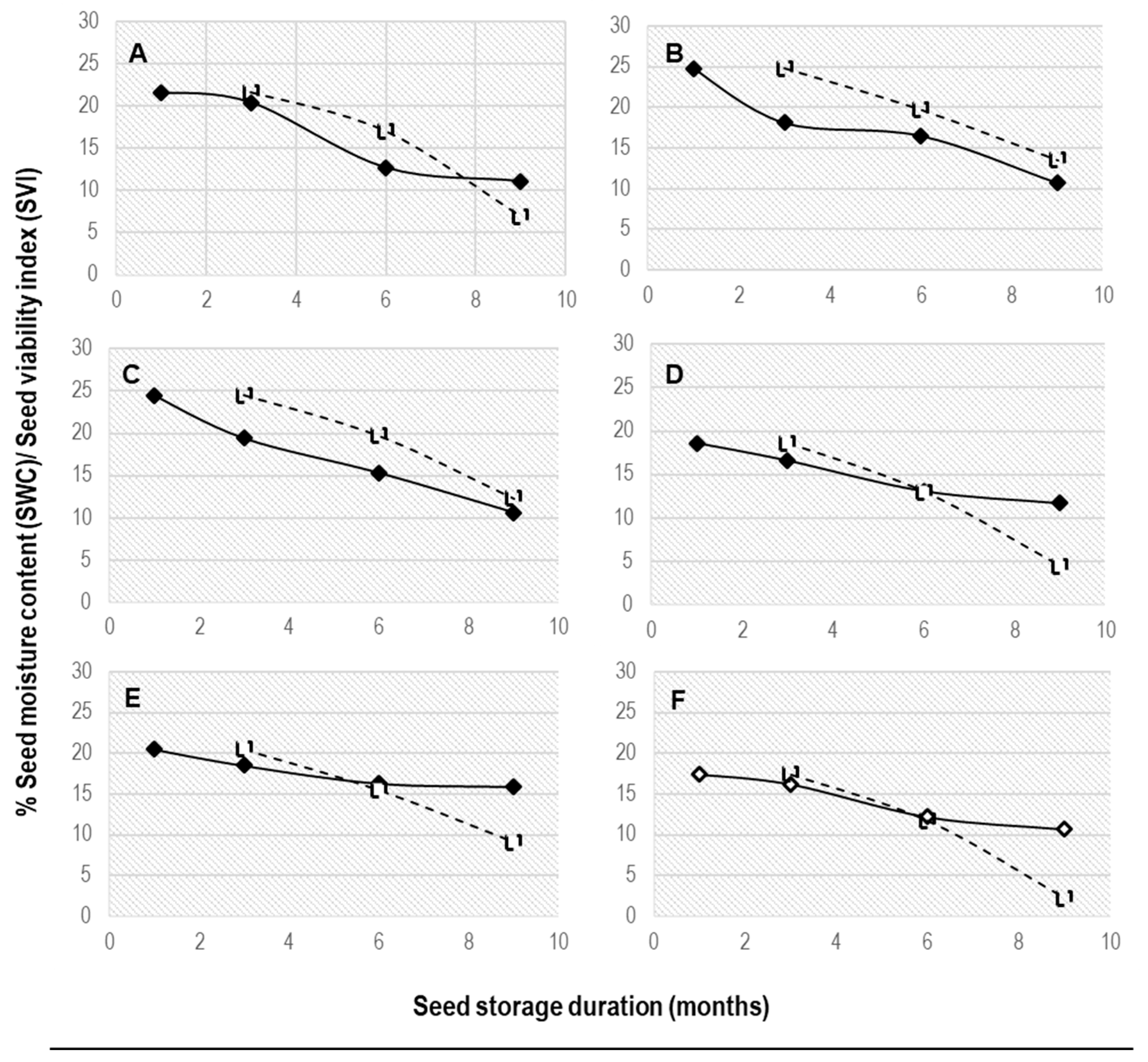
3.1. Effect of Seed Moisture Loss on Standard Germination Test
3.2. Effect of Seed Storage on In Vitro Seed Germination
3.3. Effect of Seed Storage Duration on In Vitro Shoot Induction
3.4. In Vitro Shoot Elongation and Rooting
3.5. Acclimatisation of Regenerated Plantlets
3.6. Flowering and Pod Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barwale, U.B.; Kerns, H.R.; Widholm, J.M. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta 1986, 167, 473–481. [Google Scholar] [CrossRef] [PubMed]
- De-Carvalho, M.H.C.; Van-Le, B.; Zuily-Fodil, Y.; Thi, T.T.P.; Van, K.T.T. Efficient whole plant regeneration of common bean (Phaseolus vulgaris L.) using thin-cell-layer culture and silver nitrate. Plant Sci. 2000, 157, 223–232. [Google Scholar]
- Franklin, G.; Capenter, L.; Davis, E.; Reddy, C.V.; Al-Abed, D.; Abou-Alaiwi, W.; Parani, M.; Smith, B.; Goldman, S.L.; Sairam, R.V. Factors influencing regeneration of soybean from mature and immature cotyledons. Plant Cell Tissue Organ Cult. 2004, 43, 73–79. [Google Scholar] [CrossRef]
- Mangena, P.; Mokwala, P.W.; Nikolova, R.V. In vitro multiple shoot induction in soybean. Int. J. Agric. Biol. 2015, 17, 838–842. [Google Scholar] [CrossRef]
- El-Shaieny, A.A.H. Seed germination percentage and early seedling establishment of five (Vigna unguiculata L. (Walp) genotypes under salt stress. Eur. J. Exp. Biol. 2015, 5, 22–32. [Google Scholar]
- Keneda, Y.; Tebei, Y.; Nishimura, S.; Harada, K.; Akihama, T.; Kitamura, K. Combination of thidiazuron and basal media with low salt concentrations increases the frequency of shoot organogenesis in soybeans [Glycine max (L.) Merr.]. Plant Cell Rep. 1997, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary-node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Shelar, V.K.; Shaikh, R.S.; Nikam, A.S. Soybean seed quality during storage: A review. Agric. Rev. 2008, 29, 121–131. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Hartmann and Kester’s Plant Propagation: Principles and Practice, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2011; pp. 670–679. [Google Scholar]
- Raza, G.; Singh, M.B.; Bhalla, P.L. In vitro plant regeneration from commercial cultivars of soybean. BioMed Res. Int. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Govender, V.; Aveling, T.A.S.; Kritzinger, Q. The effect of traditional storage methods on germination and vigour of maize (Zea mays L.) from northern KwaZulu-Natal and southern Mozambique. SA J. Bot. 2008, 74, 190–196. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Badola, H.K. Effect of storage conditions and storage period on seed germination in eleven populations of Swertia chirayita: A critically endangered medicinal herb in Himalaya. Sci. World J. 2012, 2012, 128105. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.H.; Florentine, S.K.; Chauhan, B.S.; McLaren, D.A.; Palmer, G.C.; Wright, W. Influence of various environmental factors on seed germination and seedling emergence of noxious environmental weed: Green Galenia (Galenia pubescens). Weed Sci. 2016, 64, 486–494. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). Handbook for Seedling Evaluation; International Seed Testing Association: Bassersdorf, Switzerland, 2003. [Google Scholar]
- Paz, M.M.; Shou, H.X.; Guo, Z.B.; Zhang, Z.Y.; Banerjee, A.K.; Wang, K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 2004, 136, 167–179. [Google Scholar] [CrossRef]
- Pierik, R.L.M. In Vitro Culture of Higher Plants; Kluwer Academic Publishers: Alphen aan den Rijn, Netherlands, 1997; pp. 45–82. [Google Scholar]
- Malik, C.P.; Yoti, J. Seed deterioration: A review. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 374–385. [Google Scholar]
- Rosenberg, L.A.; Rinne, R.W. Moisture loss of a prerequisite for seedling growth in soybean seeds (Glycine max L. Merr.). J. Exp. Bot. 1986, 37, 1663–1674. [Google Scholar] [CrossRef]
- Dadlani, M.; Kuchlan, M.K.; Samuel, S.D.K. Seed coat properties and longevity of soybean seeds. J. New Seeds 2010, 11, 239–249. [Google Scholar]
- Shaban, M. Study on some aspects of seed viability and vigor. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1692–1697. [Google Scholar]
- Jepleting, C.G. Seed Quality of Soybean (Glycine max L. Merrill) Genotypes under Varying Storage and Priming Methods, Mother Plant Nutrient Profile and Agro-Ecologies in Kenya. Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2015. [Google Scholar]
- Pinthus, M.J.; Kimel, U. Speed of germination as a criterion of seed vigour in soybeans. Crop. Sci. 1978, 19, 291–292. [Google Scholar] [CrossRef]
- Gleekia-kerkula, L.M. Germination responses as influenced by varying oil and protein contents of nine soybean (Glycine max (L.) Merr.) genotypes stored under two storage environments. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Accra, Ghana, 2012. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Ann. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Olhoft, P.M.; Somers, D.A. L-Cysteine increase Agrobacterium-mediated T-DNA delivery into soybean cotyledonary-node cells. Plant Cell Rep. 2001, 20, 706–711. [Google Scholar]
- Edelstein, M.F.; Cordineau, F.; Kigel, J.; Herson, H. Seed coat structure and oxygen availability control low temperature germination of melon (Cucumis melo) seeds. Physiol. Plant. 1995, 93, 451–456. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoeseyou, O.; Altman, A. Biotechnology of plant osmotic stress tolerance: Physiological and molecular considerations. Acta Hortic. 2001, 560, 285–292. [Google Scholar] [CrossRef]
- Sheidaei, S.; Abad, H.S.S.; Hamidi, A.; Mohammadi, G.H.; Moghaddam, A. Relationship between laboratory indices of soybean seed vigour with field emergence and yield. Int. J. Biosci. 2014, 5, 281–287. [Google Scholar]


| Seed Mass (g) | |||||
|---|---|---|---|---|---|
| Cultivars | 0 Months | 3 Months | 6 Months | 9 Months | Seed Moisture (%) |
| Dundee | 21.603 b | 20.404 a | 13.072 c | 11.709 b | 22.7 d |
| LS 677 | 24.842 a | 18.099 c | 16.493 a | 10.741 d | 29.4 b |
| LS 678 | 24.524 a | 19.439 b | 15.252 b | 10.638 d | 27.9 a |
| Peking | 18.613 d | 16.643 e | 12.744 d | 11.149 c | 20.5 e |
| TGx 1835-10E | 20.522 c | 18.523 d | 16.255 a | 15.854 a | 13.3 f |
| TGx 1740-2F | 17.333 e | 12.177 f | 11.167 e | 10.669 d | 25.9 c |
| Soybean Genotypes | 0 Months | 3 Months | 6 Months | 9 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SVI | MSN ± SD | RE (%) | SVI | MSN ± SD | RE (%) | SVI | MSN ± SD | RE (%) | SVI | MSN ± SD | RE (%) | |
| Dundee | 9.6 d | 10.9 ± 1.41 e | 90.2 e | 9.3 c | 9.0 ± 3.14 c | 89.2 c | 9.3 b | 8.9 ± 3.49 b | 79.4 c | 6.7 b | 7.4 ± 4.63 b | 52.5 d |
| LS 677 | 12.5 a | 13.5 ± 0.35 a | 96.5 a | 9.8 a | 13.0 ± 1.56 b | 96.0 a | 9.6 a | 9.4 ± 3.43 a | 83.6 b | 8.9 a | 8.0 ± 4.79 a | 70.9 b |
| LS 678 | 12.7 a | 11.0 ± 0.71 c | 94.5 c | 9.8 b | 10.7 ± 3.04 c | 92.3 b | 8.9 c | 8.4 ± 3.06 b | 86.3 a | 6.2 b | 6.8 ± 4.34 c | 64.0 a |
| Peking | 11.3 b | 13.7 ± 1.41 b | 95.8 b | 9.1 f | 12.0 ± 3.33 a | 95.7 a | 7.6 e | 9.1 ± 3.51 a | 78.6 c | 7.7 c | 7.8 ± 4.63 a | 59.5 c |
| TGx 1835-10E | 10.3 c | 10.3 ± 3.04 d | 92.8 d | 8.9 d | 8.5 ± 0.34 c | 80.6 e | 8.6 c | 7.6 ± 2.52 c | 63.2 e | 5.8 c | 5.5 ± 3.86 d | 42.8 e |
| TGx 1740-2F | 8.8 e | 8.5 ± 0.35 f | 90.9 e | 7.6 e | 5.9 ± 2.34 d | 88.5 d | 8.0 d | 2.2 ± 1.83 d | 72.1 d | 6.6 d | 5.3 ± 3.22 d | 40.4 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangena, P.; Mokwala, P.W. The Influence of Seed Viability on the Germination and In Vitro Multiple Shoot Regeneration of Soybean (Glycine max L.). Agriculture 2019, 9, 35. https://doi.org/10.3390/agriculture9020035
Mangena P, Mokwala PW. The Influence of Seed Viability on the Germination and In Vitro Multiple Shoot Regeneration of Soybean (Glycine max L.). Agriculture. 2019; 9(2):35. https://doi.org/10.3390/agriculture9020035
Chicago/Turabian StyleMangena, Phetole, and Phatlane William Mokwala. 2019. "The Influence of Seed Viability on the Germination and In Vitro Multiple Shoot Regeneration of Soybean (Glycine max L.)" Agriculture 9, no. 2: 35. https://doi.org/10.3390/agriculture9020035
APA StyleMangena, P., & Mokwala, P. W. (2019). The Influence of Seed Viability on the Germination and In Vitro Multiple Shoot Regeneration of Soybean (Glycine max L.). Agriculture, 9(2), 35. https://doi.org/10.3390/agriculture9020035