Environmental Stability of Elevated α-Linolenic Acid Derived from a Wild Soybean in Three Asian Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Soybean Genotype
2.2. Growth Condition
2.3. Phenotype Determination by Gas Chromatography
2.4. Data Analysis
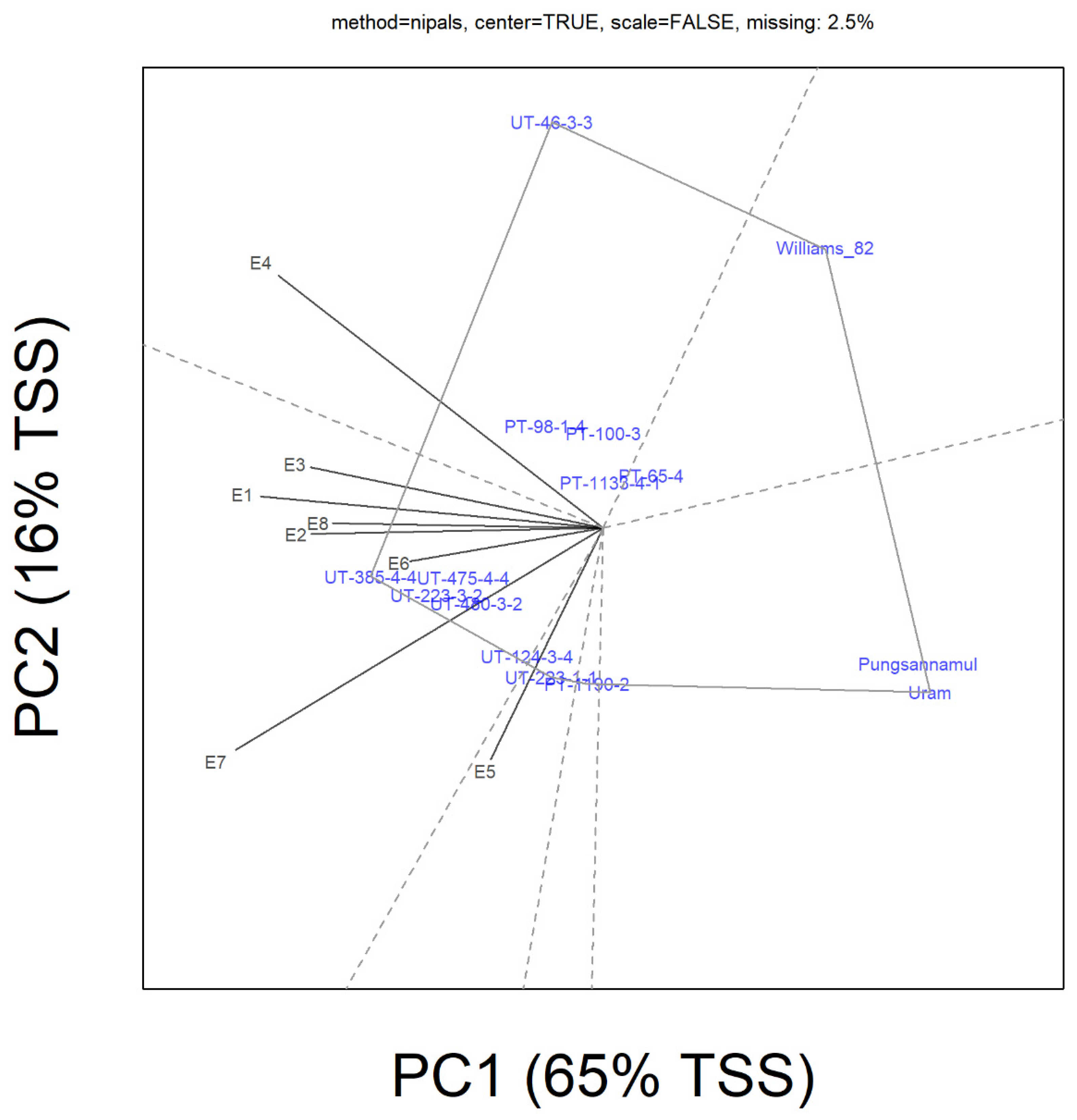
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- SoyStats. 2019. Available online: http://www.soystats.com (accessed on 1 December 2019).
- Ensminger, M.E.; Ensminger, A.H. Foods & Nutrition Encyclopedia, Two Volume Set; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Fehr, W.R. Breeding for modified fatty acid composition in soybean. Crop Sci. 2007, 47, S-72–S-87. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Rosner, B.A.; Hennekens, C.H.; Willett, W.C. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997, 337, 1491–1499. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, 3978. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Final determination regarding partially hydrogenated oils. Fed. Regist. 2015, 80, 116. [Google Scholar]
- Bilyeu, K.; Gillman, J.D.; LeRoy, A.R. Novel FAD3 mutant allele combinations produce soybeans containing 1% linolenic acid in the seed oil. Crop Sci. 2011, 51, 259–264. [Google Scholar] [CrossRef]
- Fehr, W.R.; Welke, G.A.; Hammond, E.G.; Duvick, D.N.; Cianzio, S.R. Inheritance of reduced linolenic acid concentration in soybean genotypes A16 and A17. Crop Sci. 1992, 32, 903–906. [Google Scholar] [CrossRef]
- Kim, M.; Song, J.T.; Bilyeu, K.D.; Lee, J.D. A new low linolenic acid allele of GmFAD3A gene in soybean PE1690. Mol. Breed. 2015, 35, 155. [Google Scholar] [CrossRef]
- Pham, A.T.; Shannon, J.G.; Bilyeu, K.D. Combinations of mutant FAD2 and FAD3 genes to produce high oleic acid and low linolenic acid soybean oil. Theor. Appl. Genet. 2012, 125, 503–515. [Google Scholar] [CrossRef]
- Wilson, R.F.; Burton, J.W.; Brim, C.A. Progress in the Selection for Altered Fatty Acid Composition in Soybeans 1. Crop Sci. 1981, 21, 788–791. [Google Scholar] [CrossRef]
- Liu, K. Chemistry and nutritional value of soybean components. In Soybeans; Springer: Boston, MA, USA, 1997; pp. 25–113. [Google Scholar]
- Watanabe, T. Science of Tofu, Chapter 2, Manufacture of Tofu; Food Journal Co. Ltd.: Kyoto, Japan, 1997. [Google Scholar]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; De Kock, M.; Smuts, C.M.; De Villiers, C.; Swanevelder, S.; Gelderblom, W.C.A. Dietary modulation of fatty acid profiles and oxidative status of rat hepatocyte nodules: Effect of different n−6/n−3 fatty acid ratios. Lipids 2004, 39, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Weber, P.C. Are we what we eat? Fatty acids in nutrition and in cell membranes: Cell functions and disorders induced by dietary conditions. In Fish Fats and Your Health; Svanoy Foundation: Svanoybukt, Norway, 1989; Volume 9, p. 18. [Google Scholar]
- Asekova, S.; Chae, J.H.; Ha, B.K.; Dhakal, K.H.; Chung, G.; Shannon, J.G.; Lee, J.D. Stability of elevated α-linolenic acid derived from wild soybean (Glycine soja Sieb. & Zucc.) across environments. Euphytica 2014, 195, 409–418. [Google Scholar]
- Dhakal, K.H.; Lee, J.D.; Jeong, Y.S.; Kim, H.S.; Shannon, J.G.; Hwang, Y.H. Stability of linolenic acid in seed oil of soybean accessions with elevated linolenic acid concentration. J. Food Agric. Environ. 2013, 11, 80–85. [Google Scholar]
- Chae, J.H.; Ha, B.K.; Chung, G.; Park, J.E.; Park, E.; Ko, J.M.; Shannon, J.G.; Song, J.T.; Lee, J.D. Identification of environmentally stable wild soybean genotypes with high alpha-linolenic acid concentration. Crop Sci. 2015, 55, 1629–1636. [Google Scholar] [CrossRef]
- Ha, B.K.; Kim, H.J.; Velusamy, V.; Vuong, T.D.; Nguyen, H.T.; Shannon, J.G.; Lee, J.D. Identification of quantitative trait loci controlling linolenic acid concentration in PI483463 (Glycine soja). Theor. Appl. Genet. 2014, 127, 1501–1512. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Kim, M.; Song, J.T.; Bilyeu, K.D.; Lee, J.D. Genetic improvement of the fatty acid biosynthesis system to alter the ω-6/ω-3 ratio in the soybean seed. J. Am. Oil Chem. Soc. 2017, 94, 1403–1410. [Google Scholar] [CrossRef]
- Pantalone, V.R.; Rebetzke, G.J.; Burton, J.W.; Wilson, R.F. Genetic regulation of linolenic acid concentration in wild soybean Glycine soja accessions. J. Am. Oil Chem. Soc. 1997, 74, 159–163. [Google Scholar] [CrossRef]
- Lee, J.D.; Bilyeu, K.D.; Shannon, J.G. Genetics and breeding for modified fatty acid profile in soybean seed oil. J. Crop Sci. Biotech. 2007, 10, 201–210. [Google Scholar]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil concentrations and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Hou, G.; Ablett, G.R.; Pauls, K.P.; Rajcan, I. Environmental effects on fatty acid levels in soybean seed oil. J. Am. Oil Chem. Soc. 2006, 83, 759–763. [Google Scholar] [CrossRef]
- Howell, R.W.; Collins, F.I. Factors Affecting Linolenic and Linoleic Acid Concentration of Soybean Oil1. Agron. J. 1957, 49, 593–597. [Google Scholar] [CrossRef]
- Rennie, B.D.; Tanner, J.W. Fatty acid composition of oil from soybean seeds grown at extreme temperatures. J. Am. Oil Chem. Soc. 1989, 66, 1622–1624. [Google Scholar] [CrossRef]
- Wilson, R.F. Seed Composition. In Soybeans: Improvement, Production, and Uses; American Society of Agronomy, Inc.: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar]
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acids and sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Oliva, M.L.; Shannon, J.G.; Sleper, D.A.; Ellersieck, M.R.; Cardinal, A.J.; Paris, R.L.; Lee, J.D. Stability of fatty acid profile in soybean genotypes with modified seed oil composition. Crop Sci. 2006, 46, 2069–2075. [Google Scholar] [CrossRef]
- Bernard, R.L.; Cremeens, C.R. Registration of ‘Williams 82’ Soybean. Crop Sci. 1988, 28, 1027–1028. [Google Scholar] [CrossRef]
- Lee, C.; Choi, M.S.; Kim, H.T.; Yun, H.T.; Lee, B.; Chung, Y.S.; Kim, R.W.; Choi, H.K. Soybean [Glycine max (L.) Merrill]: Importance as a Crop and Pedigree Reconstruction of Korean Varieties. Plant Breed. Biotech. 2015, 3, 179–196. [Google Scholar] [CrossRef]
- Ko, J.M.; Han, W.Y.; Kim, H.T.; Lee, Y.H.; Choi, M.S.; Lee, B.W.; Shin, S.U.; Seo, J.H.; Oh, K.W.; Yun, H.T.; et al. Soybean Cultivar for Soy-paste, ‘Uram’ with Mechanization Harvesting, Large Seed, Disease Resistance and High Yield. Korean J. Breed. Sci. 2016, 48, 301–306. [Google Scholar] [CrossRef]
- Buss, G.R.; Camper, H.M., Jr.; Roane, C.W. Registration of ‘Hutcheson’ soybean. Crop Sci. 1988, 28, 1024–1025. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill 1. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Lee, J.D.; Bilyeu, K.D.; Pantalone, V.R.; Gillen, A.M.; So, Y.S.; Shannon, J.G. Environmental stability of oleic acid concentration in seed oil for soybean lines with FAD2-1A and FAD2-1B mutant genes. Crop Sci. 2012, 52, 1290–1297. [Google Scholar] [CrossRef]
- Eberhart, S.T.; Russell, W.A. Stability parameters for comparing varieties 1. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Yan, W. GGEbiplot—A Windows application for graphical analysis of multienvironment trial data and other types of two-way data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef]
- Bellaloui, N.; Smith, J.R.; Gillen, A.M.; Fisher, D.K.; Mengistu, A. Effect of shade on seed protein, oil, fatty acids, and minerals in soybean lines varying in seed germinability in the early soybean production system. Am. J. Plant Sci. 2012, 3, 84–95. [Google Scholar] [CrossRef]
- Primomo, V.S.; Falk, D.E.; Ablett, G.R.; Tanner, J.W.; Rajcan, I. Genotypex environment interactions, stability, and agronomic performance of soybean with altered fatty acid profiles. Crop Sci. 2002, 42, 37–44. [Google Scholar] [CrossRef]
- Wilcox, J.R.; Cavins, J.F. Normal and low linolenic acid soybean strains: Response to planting date. Crop Sci. 1992, 32, 1248–1251. [Google Scholar] [CrossRef]
- Hymowitz, T. The history of the soybean. In Soybeans; AOCS Press: Urbana, IL, USA, 2008; pp. 1–31. [Google Scholar]
| Name | Pedigree Information a) | Generation | Trait |
|---|---|---|---|
| PT-65-4 | Pungsannamul x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| PT-98-1-4 | Pungsannamul x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| PT-100-3 | Pungsannamul x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| PT-1190-2 | Pungsannamul x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| PT-1133-4-1 | Pungsannamul x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-46-3-3 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-124-3-4 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-223-1-1 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-223-3-2 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-385-4-4 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-475-4-4 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| UT-480-3-2 | Uram x TR166-552 | F4:8 / F4:9 | Elevated 18:3 |
| Pungsannamul | Check | Normal 18:3 | |
| Uram | Check | Normal 18:3 | |
| Williams82 | Check | Normal 18:3 |
| Environments. | Location | Year | Latitude | Planting Date |
|---|---|---|---|---|
| E1 | Hanoi, Vietnam | 2018 | 21°7′ N | 20 Sep. 2018 |
| E2 | Hanoi, Vietnam | 2019 | 21°7′ N | 7 Feb. 2019 |
| E3 | Vientiane, Laos | 2018 | 18°8′ N | 2 Sep. 2018 |
| E4 | Vientiane, Laos | 2019 | 18°8′ N | 10 Aug. 2019 |
| E5 | Gunwi, Republic of Korea | 2018 | 36º11′ N | 22 May 2018 |
| E6 | Gunwi, Republic of Korea | 2018 | 36º11′ N | 19 Jun. 2018 |
| E7 | Gwangju, Republic of Korea | 2018 | 35º17′ N | 29 May 2018 |
| E8 | Gwangju, Republic of Korea | 2018 | 35º17′ N | 29 Jun. 2018 |
| Source. | df | Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | α-linolenic Acid |
|---|---|---|---|---|---|---|
| Genotype (G) | 14 | 3.15 *** | 0.45 *** | 223.84 *** | 103.83 *** | 29.11 *** |
| Environment (E) | 7 | 8.27 *** | 1.46 *** | 234.98 *** | 108.40 *** | 90.96 *** |
| Replication in E | 8 | 0.60 *** | 0.06 | 4.94 | 4.26 | 0.61 |
| G*E | 95 | 0.43 *** | 0.14 *** | 43.55 *** | 28.38 *** | 2.66 *** |
| Error | 98 | 0.12 | 0.05 | 4.56 | 3.01 | 0.63 |
| Genotype | Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid (ω-6) | α-linolenic Acid(ω-3) | Ratio of ω-6 and ω-3 |
|---|---|---|---|---|---|---|
| Mean (%) ± Standard Deviation | ||||||
| PT-65-4 | 11.7 ± 0.5 | 3.3 ± 0.3 | 17.4 ± 3.0 | 57.3 ± 2.7 | 10.3 ± 1.7 | 5.6 |
| PT-98-1-4 | 11.2 ± 0.3 | 3.2 ± 0.5 | 17.0 ± 2.4 | 57.0 ± 2.1 | 11.6 ± 1.6 | 4.9 |
| PT-100-3 | 11.6 ± 0.7 | 3.0 ± 0.4 | 17.4 ± 3.1 | 57.6 ± 2.6 | 10.5 ± 1.2 | 5.5 |
| PT-1190-2 | 12.0 ± 0.4 | 3.1 ± 0.3 | 17.9 ± 7.3 | 55.8 ± 5.1 | 11.3 ± 2.7 | 4.9 |
| PT-1133-4-1 | 12.0 ± 0.8 | 3.2 ± 0.4 | 17.0 ± 1.9 | 57.1 ± 1.5 | 10.7 ± 1.6 | 5.3 |
| UT-46-3-3 | 11.6 ± 0.8 | 3.6 ± 0.4 | 18.3 ± 4.1 | 55.3 ± 2.7 | 11.2 ± 2.1 | 4.9 |
| UT-124-3-4 | 11.5 ± 0.3 | 3.4 ± 0.5 | 16.7 ± 4.0 | 56.8 ± 2.6 | 11.5 ± 2.4 | 4.9 |
| UT-223-1-1 | 11.3 ± 1.1 | 3.3 ± 0.4 | 19.8 ± 5.2 | 54.3 ± 3.9 | 11.3 ± 2.6 | 4.8 |
| UT-223-3-2 | 10.9 ± 0.7 | 3.1 ± 0.3 | 17.1 ± 1.4 | 56.7 ± 1.5 | 12.2 ± 1.9 | 4.6 |
| UT-385-4-4 | 11.6 ± 0.4 | 3.1 ± 0.3 | 16.1 ± 1.9 | 56.3 ± 1.1 | 12.9 ± 1.7 | 4.4 |
| UT-475-4-4 | 11.5 ± 0.8 | 3.3 ± 0.3 | 17.8 ± 3.2 | 55.4 ± 2.8 | 12.1 ± 2.0 | 4.6 |
| UT-480-3-2 | 10.7 ± 0.9 | 3.4 ± 0.3 | 17.4 ± 2.1 | 56.5 ± 1.7 | 11.9 ± 2.1 | 4.7 |
| Mean | 11.5 | 3.3 | 17.5 | 56.3 | 11.5 | 4.9 |
| Pungsannamul | 11.3 ± 0.8 | 3.0 ± 0.2 | 27.7 ± 10.4 | 49.9 ± 8.2 | 8.1 ± 2.8 | 6.2 |
| Uram | 10.6 ± 0.7 | 3.5 ± 0.5 | 30.9 ± 13.2 | 47.2 ± 10.5 | 7.9 ± 3.0 | 6.0 |
| Williams 82 | 10.5 ± 1.1 | 3.5 ± 0.4 | 21.0 ± 3.7 | 56.1 ± 2.9 | 8.9 ± 2.3 | 6.3 |
| Mean | 10.8 | 3.3 | 26.5 | 51.0 | 8.3 | 6.2 |
| Overall mean | 11.3 | 3.3 | 19.3 | 55.3 | 10.8 | 5.1 |
| LSD (5%) a) | 0.3 | 0.2 | 1.6 | 1.3 | 0.6 | |
| Genotype | α-linolenic Acid (%) | Mean ± SE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 a) | E2 | E3 | E4 | E5 | E6 | E7 | E8 | ||
| PT-65-4 | 10.7 | 9.9 | 8.7 | 7.8 | . | 12.6 | 10.4 | 11.7 | 10.3 ± 3.9 |
| PT-98-1-4 | 11.5 | 9.3 | 10.1 | 10.1 | 12.4 | 14.6 | 11.7 | 11.7 | 11.4 ± 4.0 |
| PT-100-3 | 11.7 | 10.1 | 9.5 | 8.7 | . | 12.2 | 11.0 | 11.2 | 10.6 ± 4.0 |
| PT-1190-2 | 12.1 | 9.9 | 10.6 | 5.4 | 13.5 | 13.7 | 11.7 | 12.5 | 11.2 ± 3.9 |
| PT-1133-4-1 | 11.9 | 9.9 | 8.9 | 8.1 | 11.9 | 11.8 | 11.4 | 12.0 | 10.7 ± 3.8 |
| UT-46-3-3 | 14.2 | 10.2 | 11.2 | 9.8 | 9.5 | 11.4 | 8.3 | 14.0 | 11.1 ± 3.9 |
| UT-124-3-4 | 11.5 | 10.1 | 8.9 | 8.0 | 13.5 | 14.3 | 13.4 | 13.5 | 11.6 ± 4.1 |
| UT-223-1-1 | 13.2 | 10.8 | 9.1 | 6.0 | 12.7 | 13.1 | 12.9 | 12.8 | 11.3 ± 4.0 |
| UT-223-3-2 | 12.5 | 10.8 | 10.5 | 9.8 | . | 14.5 | 14.5 | 12.8 | 12.2 ± 4.6 |
| UT-385-4-4 | 13.5 | 11.2 | 10.9 | 10.6 | 14.1 | 13.8 | 14.8 | 14.3 | 12.9 ± 4.6 |
| UT-475-4-4 | 13.2 | 9.8 | 9.4 | 10.0 | 14.1 | 13.6 | 13.7 | 13.3 | 12.1 ± 4.3 |
| UT-480-3-2 | 13.3 | 10.4 | 10.4 | 8.2 | 13.5 | 14.0 | 13.1 | 13.4 | 12.0 ± 4.3 |
| Pungsannamul | 8.2 | 8.2 | 5.9 | 3.4 | 12.5 | 11.6 | 8.3 | 8.7 | 8.3 ± 2.9 |
| Uram | 8.5 | 4.7 | 6.2 | 3.6 | 12.9 | 9.6 | 9.3 | 10.6 | 8.2 ± 2.9 |
| Williams 82 | 8.2 | 6.6 | 6.8 | 9.2 | 9.8 | 13.1 | 7.9 | 9.9 | 8.9 ± 3.2 |
| Mean | 11.6 | 9.5 | 9.1 | 7.9 | 12.5 | 12.9 | 11.5 | 12.2 | 10.9 |
| LSD (5%) b) | 1.9 | 2.0 | 0.7 | 0.5 | 2.5 | 2.6 | 2.2 | 1.6 | |
| Temperature (°C) c) | 27.5 | 30.2 | 32.6 | 33.7 | 25.3 | 23.0 | 25.7 | 22.6 | |
| Genotype | Mean of α-linolenic Acid | Range of α-linolenic Acid | CV | Stability Coefficients (bE) a) | Mean Rank b) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Rank | % | Rank | % | Rank | bE | Rank | P | r2 | ||
| PT-65-4 | 10.3 | 12 | 5.4 | 5 | 16.1 | 6 | −0.9 | 8 | 0.001 | 0.9 | 6 |
| PT-98-1-4 | 11.6 | 5 | 5.3 | 4 | 13.5 | 3 | −0.8 | 4 | 0.007 | 0.7 | 3 |
| PT-100-3 | 10.5 | 11 | 3.5 | 1 | 11.7 | 1 | −0.7 | 2 | <0.001 | 0.9 | 1 |
| PT-1190-2 | 11.3 | 7 | 9.3 | 14 | 24.0 | 12 | −1.4 | 13 | 0.001 | 0.9 | 13 |
| PT-1133-4-1 | 10.7 | 10 | 4.2 | 2 | 14.7 | 4 | −0.8 | 5 | <0.001 | 0.9 | 4 |
| UT-46-3-3 | 11.2 | 9 | 6.4 | 9 | 18.5 | 9 | −0.3 | 1 | 0.511 | 0.1 | 9 |
| UT-124-3-4 | 11.5 | 6 | 7.0 | 10 | 20.5 | 10 | −1.3 | 11 | <0.001 | 0.9 | 10 |
| UT-223-1-1 | 11.3 | 8 | 8.3 | 12 | 22.7 | 11 | −1.3 | 12 | 0.001 | 0.8 | 11 |
| UT-223-3-2 | 12.2 | 2 | 5.7 | 6 | 15.4 | 5 | −0.9 | 7 | 0.004 | 0.8 | 5 |
| UT-385-4-4 | 12.9 | 1 | 5.2 | 3 | 13.3 | 2 | −0.9 | 6 | 0.002 | 0.8 | 2 |
| UT-475-4-4 | 12.1 | 3 | 5.9 | 7 | 16.4 | 7 | −1.0 | 9 | 0.001 | 0.9 | 7 |
| UT-480-3-2 | 11.9 | 4 | 6.2 | 8 | 17.7 | 8 | −1.1 | 10 | <0.001 | 1.0 | 8 |
| Pungsannamul | 8.1 | 14 | 9.2 | 13 | 35.4 | 14 | −1.4 | 14 | 0.003 | 0.8 | 14 |
| Uram | 7.9 | 15 | 9.5 | 15 | 37.7 | 15 | −1.6 | 15 | 0.001 | 0.8 | 15 |
| Williams 82 | 8.9 | 13 | 7.8 | 11 | 25.4 | 13 | −0.7 | 3 | 0.106 | 0.4 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Kim, M.; Ali, L.; Tayade, R.; Jo, D.; Le, D.T.; Phommalth, S.; Ha, B.-K.; Kang, S.; Song, J.T.; et al. Environmental Stability of Elevated α-Linolenic Acid Derived from a Wild Soybean in Three Asian Countries. Agriculture 2020, 10, 70. https://doi.org/10.3390/agriculture10030070
Jo H, Kim M, Ali L, Tayade R, Jo D, Le DT, Phommalth S, Ha B-K, Kang S, Song JT, et al. Environmental Stability of Elevated α-Linolenic Acid Derived from a Wild Soybean in Three Asian Countries. Agriculture. 2020; 10(3):70. https://doi.org/10.3390/agriculture10030070
Chicago/Turabian StyleJo, Hyun, Minsu Kim, Liakat Ali, Rupesh Tayade, Danim Jo, Duc Thao Le, Siviengkhek Phommalth, Bo-Keun Ha, Sungtaeg Kang, Jong Tae Song, and et al. 2020. "Environmental Stability of Elevated α-Linolenic Acid Derived from a Wild Soybean in Three Asian Countries" Agriculture 10, no. 3: 70. https://doi.org/10.3390/agriculture10030070
APA StyleJo, H., Kim, M., Ali, L., Tayade, R., Jo, D., Le, D. T., Phommalth, S., Ha, B.-K., Kang, S., Song, J. T., & Lee, J.-D. (2020). Environmental Stability of Elevated α-Linolenic Acid Derived from a Wild Soybean in Three Asian Countries. Agriculture, 10(3), 70. https://doi.org/10.3390/agriculture10030070








