1. Introduction
Hybridization is a strategy of genetic improvement, which allows the transfer of genes of interest among species (interspecific) or within the same species (intraspecific) in order to develop genetically superior genotypes. The knowledge of reproductive biology is based on the floral structure of a species and it is this which determines the nature of its reproductive process. The most important advances obtained in the genetic improvement of plants are associated with the knowledge of their reproductive system, through studies relating to the anthesis, the viability of the pollen and the receptivity of the stigma, among others [
1,
2,
3,
4]. A number of studies have been carried out on the floral biology, which include the morphological and reproductive characteristics of genotypes of Acerola (
Malpighia emarginata) revised by Gomes et al. [
3]. In
Withania ashwagandha sp.,
Jatropha curcas, and
Moringa oleifera Lam reproductive biology studies have been conducted [
4,
5,
6]. The viability of the pollen and cross-compatibility among various species of Capsicum has been examined [
7]. In the case of
Capsicum eximium, the interaction between the pollen and the pistil has been studied by Onus [
8] and ornamental peppers were studied by Crispin et al. [
9]. To our knowledge, reproductive biology studies of
Capsicum chinense Jacq. (
Habanero pepper) are limited. Mercado et al. [
10] commented that, in the case of chili, very little effort has been made to evaluate aspects of reproductive biology, even though these are useful in studies of pollen germination, pollen storage, and breeding programs.
Habanero pepper (
C. chínense) is a self-pollinated plant, hermaphrodite, with perfect, complete flowers, whose floral structure facilitates the emasculation and the pollination, in plant breeding programs of this species.
Habanero pepper is a traditional crop in Mexico, which is cultivated as a culinary product for exportation due to its taste and typical aroma, characterized by its high content of oleoresin and strong pungency, characteristics which have generated a significant demand in the national and international markets. Given the importance of the
Habanero pepper, a better knowledge of its floral biology would facilitate obtaining a greater fruit set, in both commercial cultivation and programs of genetic improvement by hybridization. The production of hybrid seed is expensive, very laborious and requires intense technical supervision, in some crops, the control of the pollination mechanisms can help to make the commercial production of hybrid seed more economical [
11,
12]. Given the importance of the hybridization as an improvement strategy and the lack of information regarding the floral biology of
C. chinense, this research aimed to study the reproductive biology of twelve genotypes of
C. chinense, specifically, the optimal moment to collect the pollen, appraise its viability and define the state of development of the flower bud in which the anthers are closed and stigma is receptive. The outcome of the study will help fill the knowledge gap regarding the reproductive biology of
C. chinense and will be useful for the plant breeder.
2. Materials and Methods
The investigation was conducted in a greenhouse, at 20–35 °C, 55–75% of relative humidity and cycles of natural light (approximately, 11 h of light, 13 h of darkness), located in the installations of the Center of Scientific Investigation of Yucatan, Merida, Yucatan, at 20°58′2.53″ Latitude North and 89°35′33.30″ Longitude West and at an altitude of 10 m above sea level [
13].
Twelve genotypes of
Habanero pepper were evaluated, of which five genotypes had red fruit, two genotypes with orange-colored fruit, three genotypes with yellow fruit and two genotypes with purple fruit (
Table 1). The experiments were conducted from August 2017 to May 2018, all the genotypes were studied at the same time. The seeds of each genotype were disinfected with commercial sodium hypochlorite (4%) for 10 min, after which they were planted in polystyrene trays with 200 receptacles. A mixed commercial substrate was used (Cosmopeat
® Cosmocel S.A., San Nicolás de los Garza, Nuevo León, México). The trays were kept covered with black plastic to maintain the temperature and humidity until germination. During the development of the plantlets, the commercial fertilizer Hakaphos
® was applied once a week 13-40-13 (1 g L
−1) (Compo Agro México S.A. de C.V., Jalisco, México) until they were transplanted to black bags [40 × 22 × 40 cm (0.035 cm
3)], containing a mixture of red soil and Cosmopeat
® in a proportion of 3:1 m/m. During the growth of the plants, the application of the Triple 18 fertilizer was carried out (1 g L
−1) (Royal Garden’s
®, Guadalajara, México).
2.1. Anthesis
In each plant in the second bloom, the flowers in anthesis were counted (when the flower is open and the petals are separated from each other) (
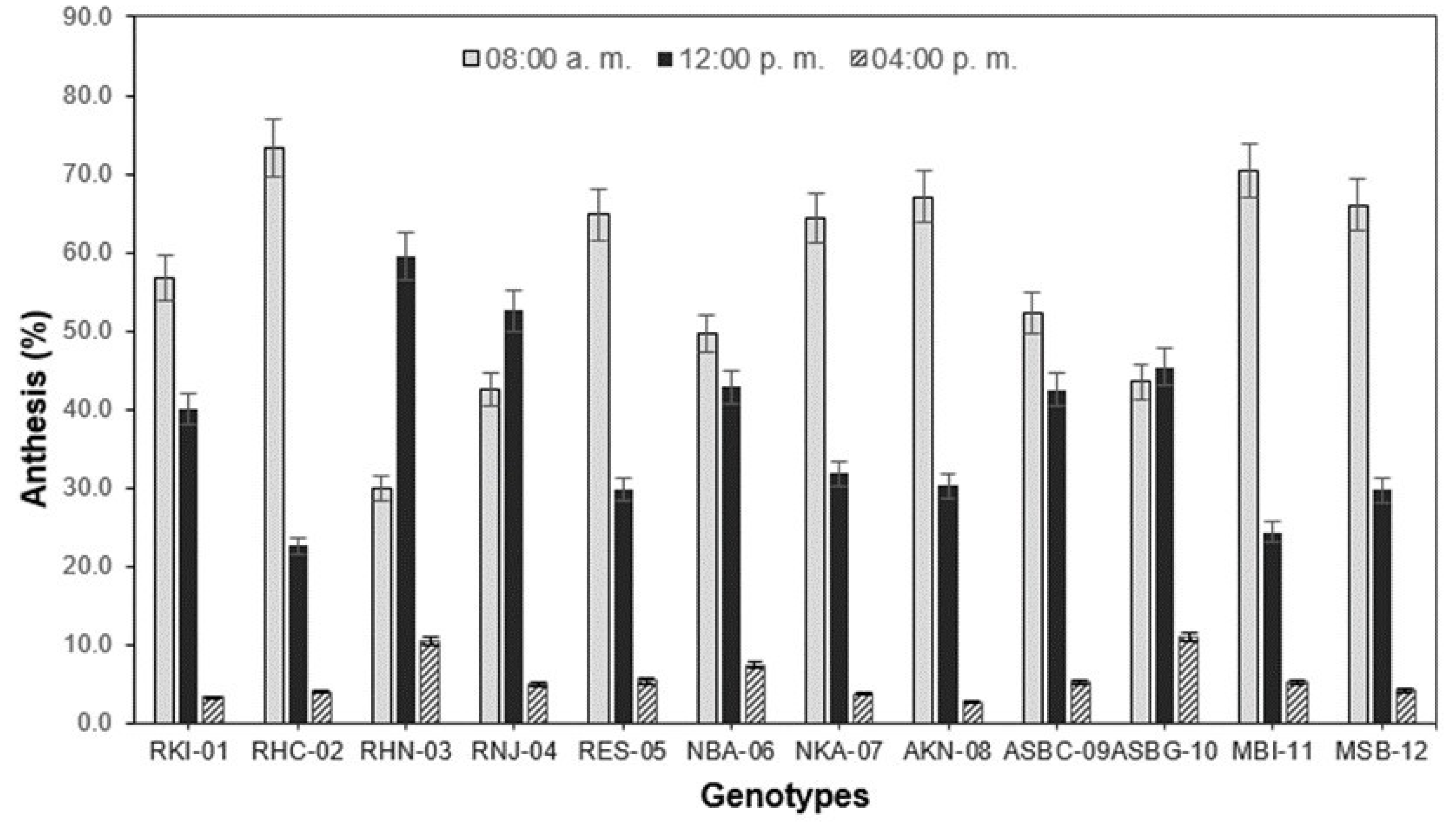
Figure 1). Once they were registered, they were subsequently eliminated from the plant in order to avoid re-counting. The counting was carried out daily in three timetables each day: 8:00 a.m., 12:00 p.m., and 4:00 p.m., over a period of 10 days. The anthesis was evaluated conducted with the aim of determining the moment of the day in which the largest number of flowers open is counted to carry out the collection of flowers and thus be able to extract the greatest amount of pollen, which is required during the crossing stage.
2.2. Position of the Pistil with Regard to the Anthers
The position of the pistil was determined with respect to the anthers in order to determine the probability of the occurrence of cross-pollination in each genotype evaluated. For this, 60 flowers in anthesis, between 8:00–10:00 a.m. were randomly selected and considered in order to evaluate how many of them presented: inserted pistil (−1), pistil at the same level as the anthers (0), pistil slightly exserted (pistil up to 1 mm above the anthers) (2) and pistil exserted (pistil > 1 mm with respect to the anthers (3) (
Figure 2).
2.3. Dehiscence of the Anthers
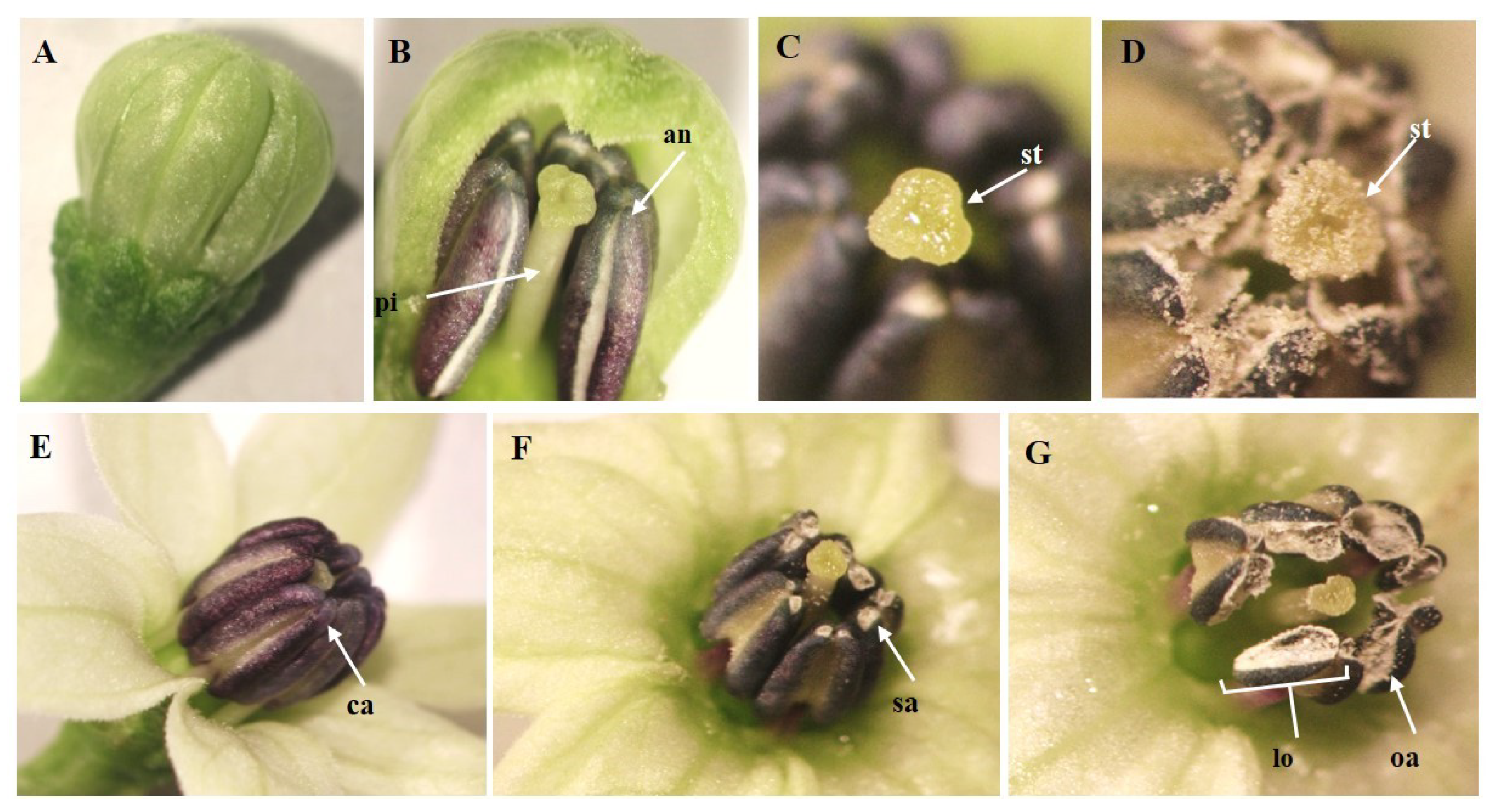
In order to determine the dehiscence of the anthers, the observations were conducted randomly on 60 flower buds (one day before the anthesis) and on 60 flowers the day of the anthesis in three timetables: 8:00 a.m., 10:00 a.m., and 12:00 p.m., for each time and each genotype. The dehiscence of the anthers was classified as: closed anthers (ca), semi-open anthers (sa) and open anthers (oa). In addition, four open flowers were marked in each plant in order to monitor the time of dehiscence of the anthers, and the observation was conducted every two hours, after which they were eliminated from the plant. The total closed anthers (ca) semi-open anthers (sa) and open anthers (oa) evaluated was expressed in percentage (%).
2.4. Receptivity of the Stigma
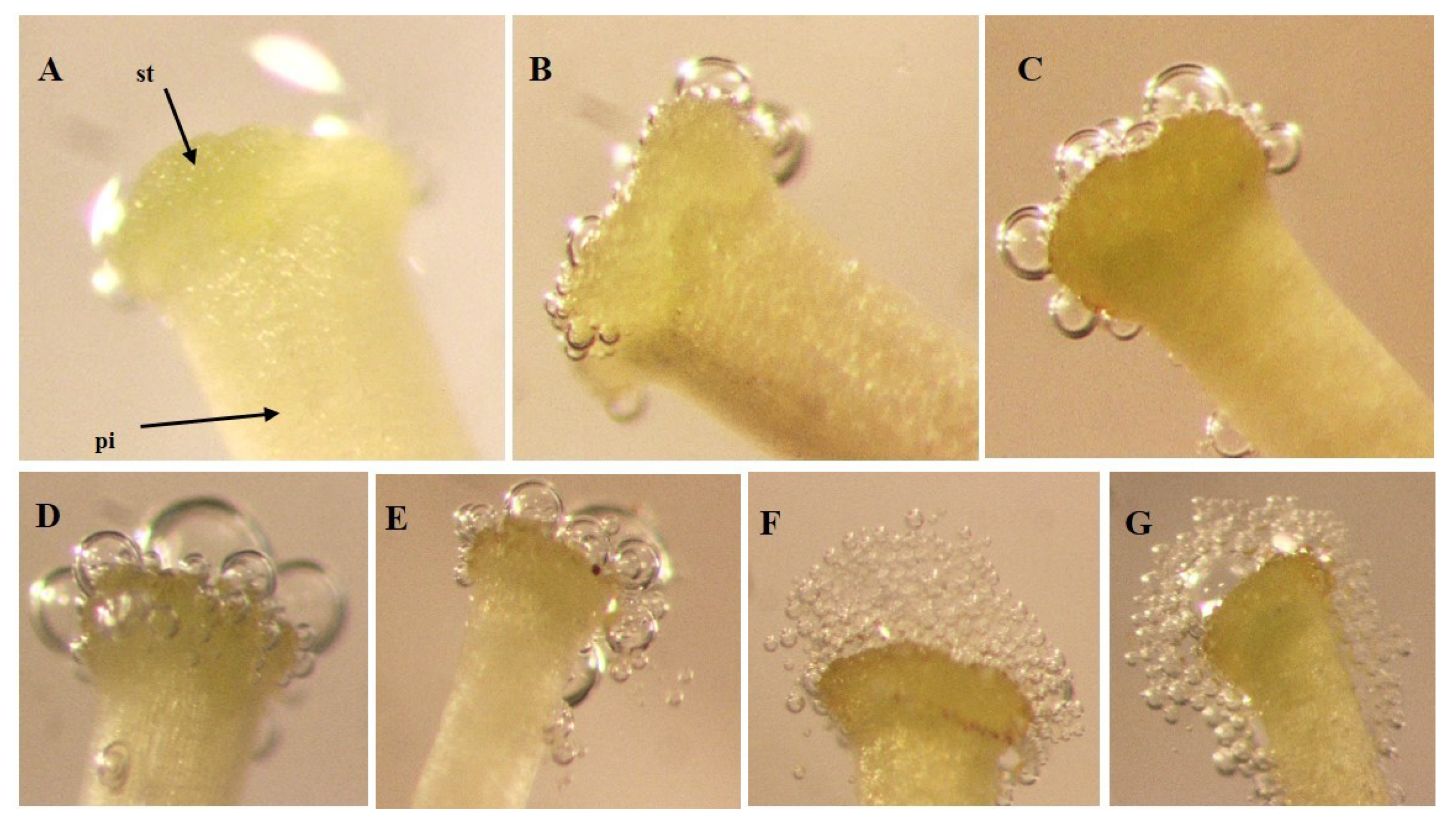
Receptivity of the stigma was evaluated using the rapid drop hydrogen peroxide test (H
2O
2) at 3% [
14]. To achieve this, 10 μL of H
2O
2 was applied on the stigma with a micropipette (Micropipette 1–10 μL), after which the observations were carried out with the aid of a stereoscopic microscope (Nikon SMZ800 (Nikon INC., Japan) with Canon DS126311 camera (Canon INC., Taiwan)). Twenty stigmas of each genotype were evaluated by floral stage: (a) flower buds before anthesis (before anthesis), (b) flowers in day of anthesis (during anthesis) and (c) flowers 24 h after the day of anthesis (after anthesis). In all cases, stigmas of recently collected flowers were used.
The genotype ASBG-10 was discarded in the evaluation of receptivity of the stigma and the viability of pollen, due to the fact that it presented a high incidence of floral abortion: and thus, there were insufficient flowers for the evaluations.
2.5. Viability of the Pollen
In order to determine the viability of the pollen, 50–60 flowers in anthesis were randomly collected from each genotype and the pollen was extracted with the aid of tweezers. Two methods were used to evaluate the viability: staining with acetocarmine at 1% [
2,
15] and by manual pollination. In the case of the method with staining, first 0.001 g of pollen was weighed and added to 50 µL of distilled water; subsequently, 50 µL of a solution of acetocarmine at 1% was added, this was agitated for one minute and allowed to incubate at 4 °C for 30 min in darkness. The pollen grains, which take the color are viable or fertile, while those that remain without color are non-viable or sterile. The grains of pollen were counted with a Neubauer camera, where 10 µL of the dilution of the coloring containing pollen was placed and the counting was carried out with the aid of an optical microscope (ZEISS, Axioplan, Carl Zeiss AG, Germany), in order to determine the percentage of viability (%V).
In order to evaluate the viability of the pollen by means of the manual pollination method; 10 flower buds were pollinated for each genotype per treatment (0 h, 24 h and 48 h of refrigeration at 4 °C). The recently collected pollen was designated as zero hour (0 h), the pollen collected from the other treatments was placed in sealed vials which were stored in refrigeration at 4 °C for 24 h and for 48 h for their posterior evaluation. Subsequently, the flower buds were pollinated manually and were covered with small bags of glassine until the fruit ripened. The following aspects were evaluated: the number of fruits set (FS) fruit length (cm), fruit width (cm), fruit weight (g) and number of seeds per fruit (NSF).
2.6. Data Analysis
The experimental design randomized complete block design and was used with two repetitions and ten plants as the experimental unit. Anthesis was analyzed based on an analysis with factorial arrangement A × B (Factor A: genotypes, Factor B: timetables). The averages of each timetable were calculated during the ten days, an analysis of the variance was carried out (ANOVA) and the
t Tests LSD (Least significant difference) were applied with
p < 0.05 in order to determine the significance of the differences among variables, using the SAS program version 9.1 for Windows [
16]. Chi-square test (
Χ2) was used to analyze the position of the pistil and the dehiscence of the anthers. The receptivity of the stigma was analyzed by means of a binomial test, in which the number (1) was assigned when the presence of bubbles was observed in the stigma (receptive stigma) and (0) when the presence of bubbles was not observed in the stigma (non-receptive stigma). Data were analyzed with the IBM statistical program PSS Statistics version 22 [
17] with
p-value < 0.05 used as a significant difference level.
4. Discussion
In general, for most of the genotypes, the highest number of flowers in anthesis was registered at 8:00 a.m. (
Figure 3), with the exception of the genotypes RHN-03 and RNJ-04, in which the aperture of the flowers occurred with greater frequency after 12:00 p.m. With these results it is possible to establish that this is the moment of the day in which their pollen should be collected, when these genotypes are used as masculine progenitors, within a cross-breeding program. In a similar study by Aleemullah et al. [
18] which analyzed the phenology of
Capsicum annuum flowering, it was observed that the anthesis was presented with greater frequency during the morning. In a study of the reproductive biology of
Jatropha curcas, Rincón-Rabanales et al. [
6] found that the flowers, both masculine and feminine, begin to open at 8:00 h, and that the maximum aperture of the flowers is registered at 9:00 h, while Mir et al. [
5], studying the reproductive biology of
Withania ashwagandha sp. Novo (Solanaceae), they observed that the anthesis occurred in an interval between 8:00–11:00 h. These results allow the inference that the more usual anthesis in several species including a
Capsicum chinense is that flowers open in the morning.
With the analysis of the pistil position, with respect to the anthers in the genotypes studied (
Table 4), it was possible to appreciate that only AKI-08 presented pistil exserted in all its flowers evaluated (100%), while in the genotypes RKI-01, NBA-06, and NKA-07, the pistil at the level of the anthers predominated in the majority of their flowers (70% and 78%). Most of the genotypes presented the pistil slightly exserted with a percentage in a range of 50–67%. These results indicate that, at least under the edaphoclimatic conditions of the Peninsula of Yucatan,
Capsicum chinense behaves in the same way as a
facultative allogamous species. This classification of
Habanero pepper has been repeatedly mentioned, in previous studies [
19,
20,
21,
22], which report out-crossing rates of 7% to 91%. With these results, it is possible to confirm that an important level of allogamy exists in the species, which can vary among genotypes and must be taken into account in the genetic improvement programs and those of
Habanero pepper seed production. Opedal [
23] mention that the herkogamy or the spatial separation of anthers and stigmas inside the flowers is a trait that promotes cross-fertilization or avoids autogamy. Larrinaga et al. [
24], studying the floral morphology of
Narcissus cyclamineus (Amaryllidaceae), observed that the relative position of the stigmas and anthers has a significant effect on the success of female reproduction. Similarly, information has been reported for tomato by Pan et al. [
25], indicating that autogamy is usually associated with the position of the anther in relation to the stigma. In cross-pollination, the stigmatic surface receives the pollen of the neighboring flowers, while the self-pollinated flowers are typically characterized by a stigmatic surface that is imbedded inside their own anther.
According to the observations carried out with respect to the dehiscence of the anthers one day before the anthesis (flower bud), 100% of the anthers are closed (
Table 6). The dehiscence of the anthers occurred longitudinally, with the observation of pollen grains one hour after aperture of the flower. Similar results were reported by Aleemullah et al. [
18] who worked with
Capsicum annuum and observed that the dehiscence of the anthers was longitudinal and the liberation of the pollen grains initiated one hour after the aperture of the flower. In other species; Khanduri et al. [
26] reported that in
Cornus capitata, the aperture of the anther also occurred from the apex to the base, which was also reported by Douglas and Freyre et al. [
27] in species of
Nolana (Solanaceae). Based on the results obtained from the evaluation of anther aperture, the recommendation is to perform the emasculation and pollination at the same time, in the stage flower bud (one day before the anthesis) in the in order to ensure that the anthers are closed without risk of self-pollination.
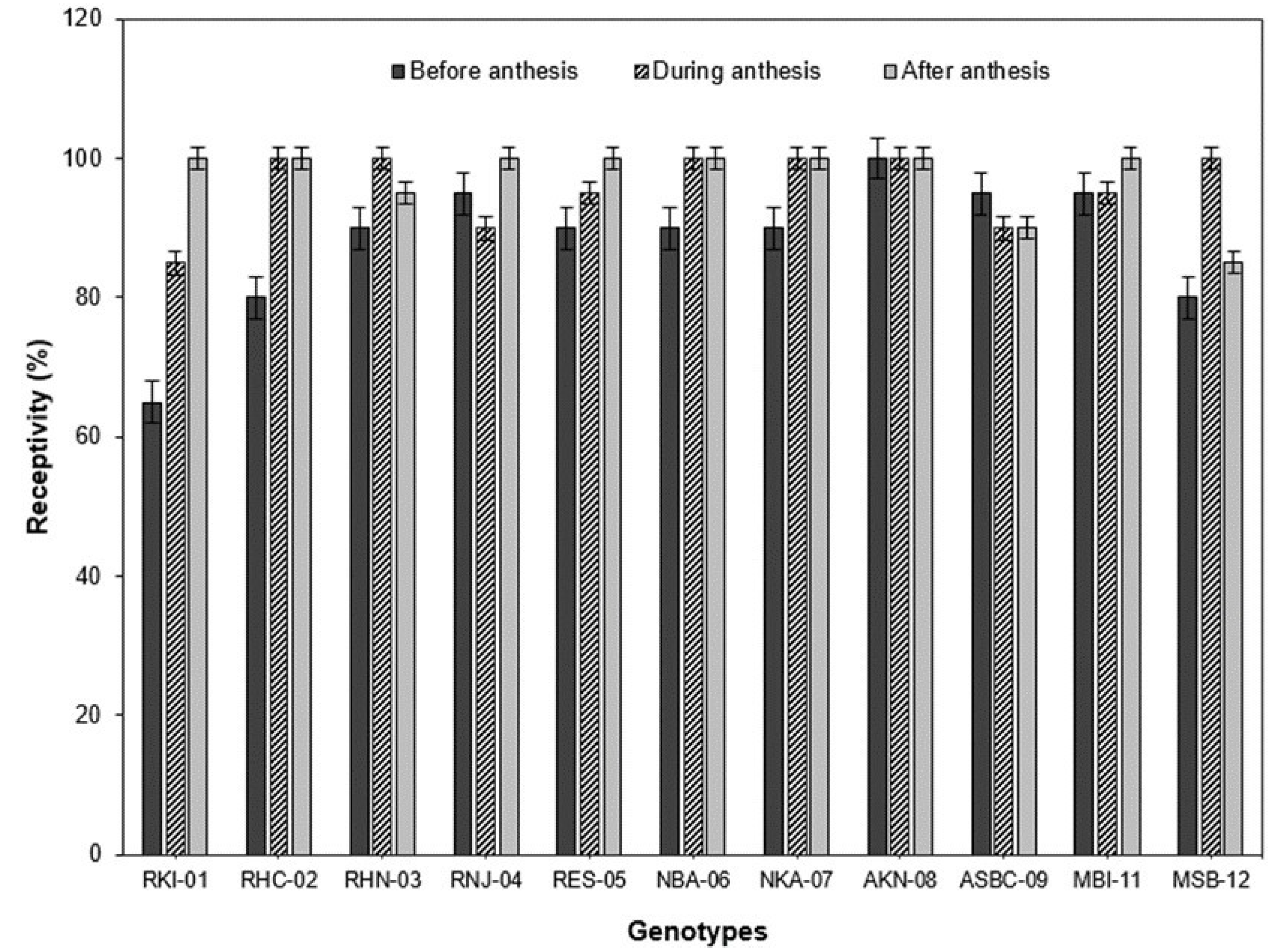
With the analysis of the behavior of the different genotypes regarding the receptivity of the stigma, in the genotypes RKI-01 and RES-05 it was possible to observe a gradual increase in the receptivity of the stigma from one day before the anthesis, to one day after the anthesis (
Figure 5). However, the genotypes RHN-03 and MSB-12 presented a different pattern in comparison with the rest of the genotypes in which the receptivity of the stigma was low before the anthesis, increased during the anthesis and diminished after the anthesis. In a similar study, Aleemullah et al. [
18] determined the period of receptivity of
C. annuum, indicating that the pistil was most receptive in −1 (one-day pre-anthesis), 0 (day of anthesis) and +1 (day post-anthesis). Recently, Crispim et al. [
9] observed that, although the flowers of the ornamental chili plant are receptive from the flower bud phase, the most significant receptivity was observed after the anthesis, Zhang et al. [
4], in a study of the floral biology and the receptivity of the
Moringa oleifera Lam. pistil, reported that the greatest receptivity of the stigma was registered on days 1 and 2 after the anthesis, with more than 93% of the stigmas receptive. In
Habanero pepper, the greatest receptivity was presented one day after the anthesis for the majority of the genotypes (
Figure 6). These results facilitate the efficient planning and design of a program of cross-breeding in order to obtain
Habanero pepper hybrids. According to Ofuso-Anim et al. [
28], the stigma of chili flowers (
Capsicum annuum) maintains its receptivity for three days (the day of the anthesis and the two days after the anthesis). In our study, the receptivity of the stigma was positive from one day before the anthesis to one day after the anthesis in all of the genotypes (
Table 8). It is also recommended to carry out the cross-breeding one day before the anthesis (floral bud), given that the anthers are completely closed (100%) in most of the genotypes (
Table 6). Gomes et al. [
3] who studied the reproductive biology of aceroleira (
Malpighia emarginata) genotypes, also indicate that the flower buds are suitable to emasculate.
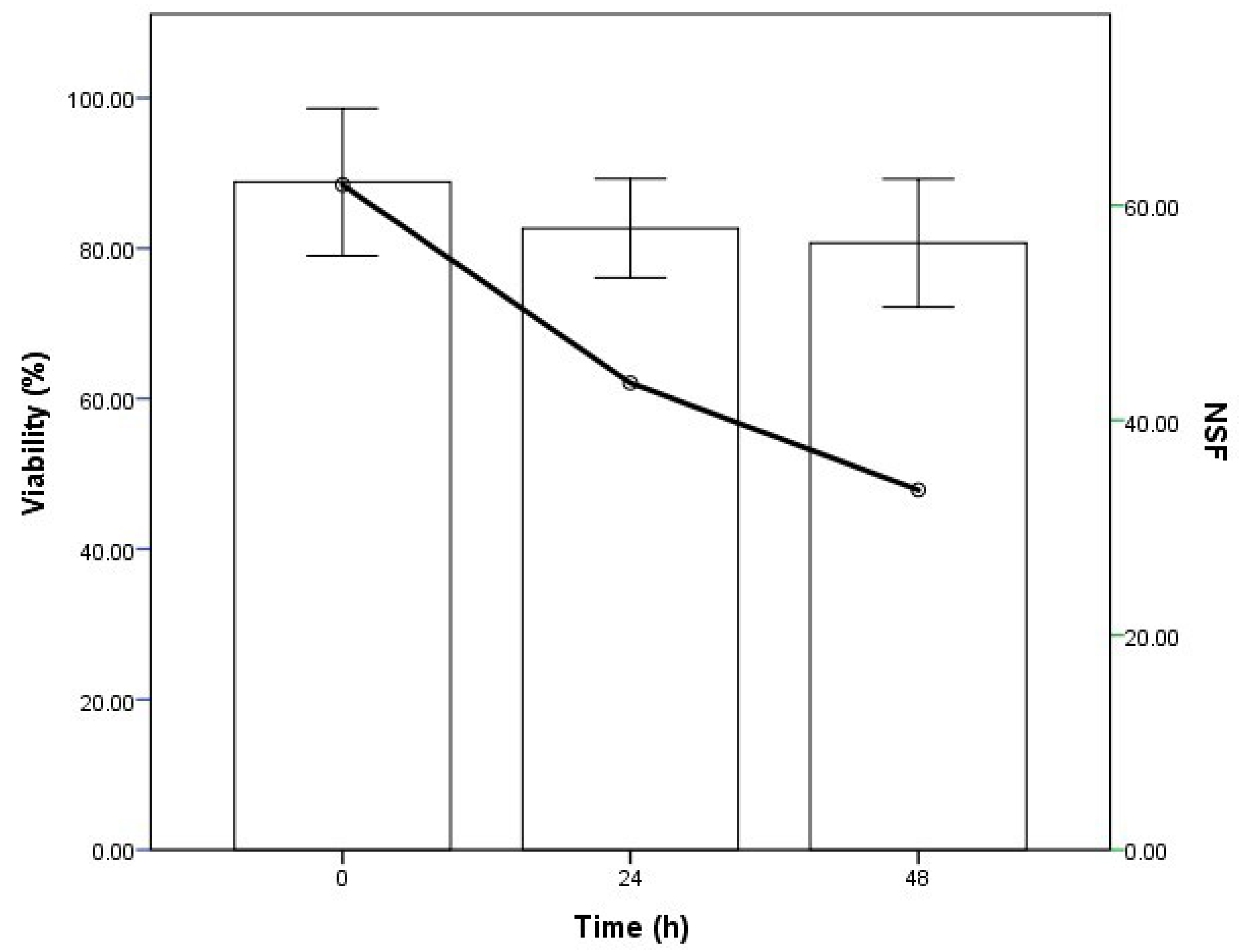
The viability of the pollen varied, depending on the time of conservation (in refrigeration) after collection. Of the three times evaluated (0, 24 and 48 h), the best time for use was the time of harvesting (0 h), given that the pollen presented a greater percentage of viability (88%) and a greater number of seeds per fruit (NSF), although the percentage of fruit set (%FS) was low (
Table 9 and
Figure 7). Mir et al. [
5] concluded that low fructification in cross-pollination after emasculation is an indicator of partial fixation of autogamy in the
Withania ashwagandha sp. Novo (Solanaceae). In general, the plants with flowers possess a wide range of morphological and physiological mechanisms that influence their patterns of reproduction and in particular in the degree of self-fertilization. As for the NSF Garcia et al. [
29], evaluating the characteristics of the fruit of wild pepper (
Capsicum flexuosum), observed that when manual pollination was carried out, the number of seeds per fruit increased with the size of the fruit. Thus, being able to know when the greater viability and germination of the pollen occur would result in greater fruit formation and high seed yield.