Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta
Abstract
:1. Introduction
2. Phytohormones
3. Compounds from Essential Oils
| Compounds | Producing Species | Host Plants | Reference | |
|---|---|---|---|---|
| Phenyl propanes | ||||
| Asarone | Muscodor tigerii | Cinnamomum camphora | [50] | |
| Eugenol | Annulohypoxylon stygium | not specified | [51] | |
| Alternaria sp. | Rosa damascaena | [52] | ||
| Monoterpenes | ||||
| Camphor | Nodulisporium sp. | Lagerstroemia loudoni | [47] | |
| Carene | Meliniomyces variabilis | Pinus sylvestris | [53] | |
| Nodulisporium sp. | L. loudoni | [47] | ||
| Cineole (eucalyptol) | Nodulisporium sp. | Persea indica | [54] | |
| L. loudoni | [47] | |||
| Cassia fistula | [55] | |||
| Limonene | unknown Sordariomycetes | Mentha piperita | [56] | |
| Wickerhamomyces anomalus | Lactuca sativa | [57] | ||
| Nodulisporium sp. | L. loudoni | [47] | ||
| Nigrograna mackinnonii | Guazuma ulmifolia | [58] | ||
| Myrcene | W. anomalus | L. sativa | [57] | |
| Nodulisporium sp. | L. loudoni | [47] | ||
| Ocimene | W. anomalus | L. sativa | [57] | |
| Nodulisporium sp. | L. loudoni | [47] | ||
| Phellandrene | Muscodor fengyangensis | Actinidia chinensis, Pseudotaxus chienii | [59] | |
| Muscodor yucatanensis | Bursera simaruba | [60] | ||
| unidentified strains | “weed grasses” | [61] | ||
| Muscodor sp. | O. granulata | [62] | ||
| Pinane | Nodulisporium sp. | L. loudoni | [47] | |
| Pinene | unknown Sordariomycetes | M. piperita | [56] | |
| M. variabilis, Phialocephala fortinii | P. sylvestris | [53] | ||
| W. anomalus | L. sativa | [57] | ||
| N. mackinnonii | G. ulmifolia | [58] | ||
| Sabinanes (thujanes) | Dihydroxysabinane | Phomopsis sp. | Camptotheca acuminata | [63] |
| Sabinene (thujene) | Phomopsis sp. | Odontoglossum sp. | [64] | |
| W. anomalus | L. sativa | [57] | ||
| Nodulisporium sp. | L. loudoni | [47] | ||
| Terpinene, terpineol | Nodulisporium sp. | L. loudoni | [47] | |
| N. mackinnonii | G. ulmifolia | [58] | ||
| Diterpenes | ||||
| Abietadiene | Xylaria sp. | Cupressus lusitanica | [65] | |
| Totarol | Xylaria sp. | C. lusitanica | [65] | |
| Sesquiterpenes | ||||
| Acoradiene | Phomopsis sp. | Odontoglossum sp. | [64] | |
| Aristolene | M. yucatanensis | B. simaruba | [60] | |
| Aromadendrene, isoledene | M. yucatanensis | B. simaruba | [60] | |
| Phoma sp. | Larrea tridentata | [66] | ||
| Xylaria sp. | C. lusitanica | [65] | ||
| Bisabolene | M. fengyangensis | A. chinensis, P. chienii | [59] | |
| Xylaria sp. | C. lusitanica | [65] | ||
| Bisabolol | Muscodor kashayum | Aegle marmelos | [67] | |
| Cadinanes | Cadalenes, calamenenes | Phomopsis cassiae | Cassia spectabilis | [68] |
| Bombamalone D, calamenenes, dysodensiol D, indicumolide C | Phomopsis sp. | Pleioblastus amarus | [69] | |
| Cadinene, amorphene, muurolene | Phoma sp. | L. tridentata | [66] | |
| Xylaria sp. | C. lusitanica | [65] | ||
| Cubenol | Xylaria sp. | C. lusitanica | [65] | |
| Carotol | M. tigerii | C. camphora | [50] | |
| Caryophyllane | Xylaria sp. | C. lusitanica | [65] | |
| Caryophyllene (humulene) | Muscodor albus | Cinnamomum zeylanicum | [45] | |
| G. ulmifolia | [70] | |||
| M. fengyangensis | A. chinensis, P. chienii | [59] | ||
| M. yucatanensis | B. simaruba | [60] | ||
| M. variabilis, P. fortinii | P. sylvestris | [53] | ||
| Phoma sp. | L. tridentata | [66] | ||
| Isocaryophyllene | Muscodor sutura | Prestonia trifidi | [71] | |
| Presilphiperfolanes | Xylaria sp. | Piper aduncum | [72] | |
| Cedranes | Cedrene, cedrol | Xylaria sp. | C. lusitanica | [65] |
| Diepicedrene-1-oxide | M. fengyangensis | A. chinensis, P. chienii | [59] | |
| Chamigrene | Phoma sp. | L. tridentata | [66] | |
| M. sutura | P. trifidi | [71] | ||
| Cuparene | unknown Sordariomycetes | M. piperita | [56] | |
| Drimanes | Albicanol | Perenniporia tephropora | Taxus chinensis var. mairei | [73] |
| Hydroxyconfertifolin | Phomopsis sp. | Rhizophora stylosa | [74] | |
| β-Elemene | Nodulisporium sp. | Cinnamomum loureirii | [46] | |
| M. yucatanensis | B. simaruba | [60] | ||
| Penicillium baarnense, Penicillium frequentans | Curcuma zedoaria | [75] | ||
| Eremophilanes | Eremophilanolides | Xylaria sp. | Licuala spinosa | [76] |
| Isopetasols | unidentified strain | Picea rubens | [77] | |
| Mairetolide A | Xylaria sp. | C. lusitanica | [78] | |
| Mairetolide F | Xylaria sp. | L. spinosa | [76] | |
| Valencene | M. albus | C. zeylanicum | [45] | |
| unidentified vine plant | [79] | |||
| Oryza granulata | [62] | |||
| Xylaria sp. | C. lusitanica | [78] | ||
| Eudesmanes, eudesmenes (selinenes) | Eutypella sp. | Etlingera littoralis | [80] | |
| Nodulisporium sp. | C. loureirii | [46] | ||
| Phoma sp. | L. tridentata | [66] | ||
| Xylaria sp. | C. lusitanica | [65] | ||
| Arundinols, arundinones | Microsphaeropsis arundinis | Ulmus macrocarpa | [81] | |
| Capitulatin B, hydroxycapitulatin B | Nigrospora oryzae | Aquilaria sinensis | [82] | |
| Farnesene | Xylaria sp. | C. lusitanica | [65] | |
| Guaianes: guaiene, guaiol (champacol), aciphyllene, bulnesene, gurjunene | M. albus | unidentified vine plant | [79] | |
| C. zeylanicum | [45] | |||
| Myristica fragrans | [83] | |||
| G. ulmifolia | [70] | |||
| Phoma sp. | L. tridentata | [66] | ||
| Xylaria sp. | C. lusitanica | [65] | ||
| Himachalene | Phoma sp. | L. tridentata | [66] | |
| Irones | Rhizopus oryzae | Iris germanica | [84] | |
| Longicyclene | M. variabilis, P. fortinii | P. sylvestris | [53] | |
| Longifolenes | M. yucatanensis | B. simaruba | [60] | |
| P. fortinii | P. sylvestris | [53] | ||
| Xylaria sp. | C. lusitanica | [65] | ||
| Longipinene | Phoma sp. | L. tridentata | [66] | |
| Occidentalol | Xylaria sp. | C. lusitanica | [65] | |
| Thujopsanol, thujopsene | Xylaria sp. | C. lusitanica | [65] | |
| M. sutura | P. trifidi | [71] | ||
| Ylangene | Phoma sp. | L. tridentata | [66] | |
| Zingiberene | Xylaria sp. | C. lusitanica | [65] | |
| Compounds | Producing Species | Host Plants | Reference |
|---|---|---|---|
| Botryosphaeridione | Phoma sp. | Melia azedarach | [85] |
| Cupressolides A-B | Xylaria sp. | Cupressus lusitanica | [78] |
| Dihydroberkleasmin A | Pestalotiopsis photiniae | Podocarpus macrophyllus | [86] |
| Eremoxylarins A-B | xylariaceous fungus | not specified | [87] |
| MBJ-0011-13 | Apiognomonia sp. | not specified | [88] |
| Periconianone B | Periconia sp. | Annona muricata | [89] |
| Pestalotiopsin A-B | P. photiniae | P. macrophyllus | [90] |
| Phomadecalins | Microdiplodia sp. | Pinus sp. | [91] |
| unnamed compounds | Xylaria sp. | Licuala spinosa | [76] |
| Xylaria sp. | mangrove plant | [92] | |
| Xylarenones | Xylaria sp. | Torreya jackii | [93] |
| Camarops sp | Alibertia macrophylla | [94,95] |
4. Other Terpenoids
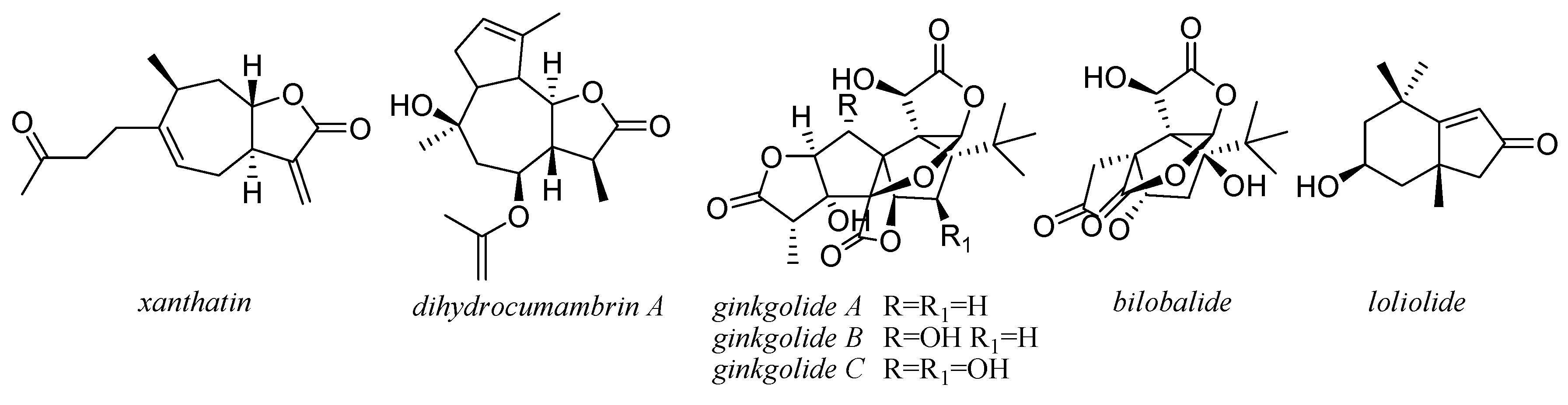
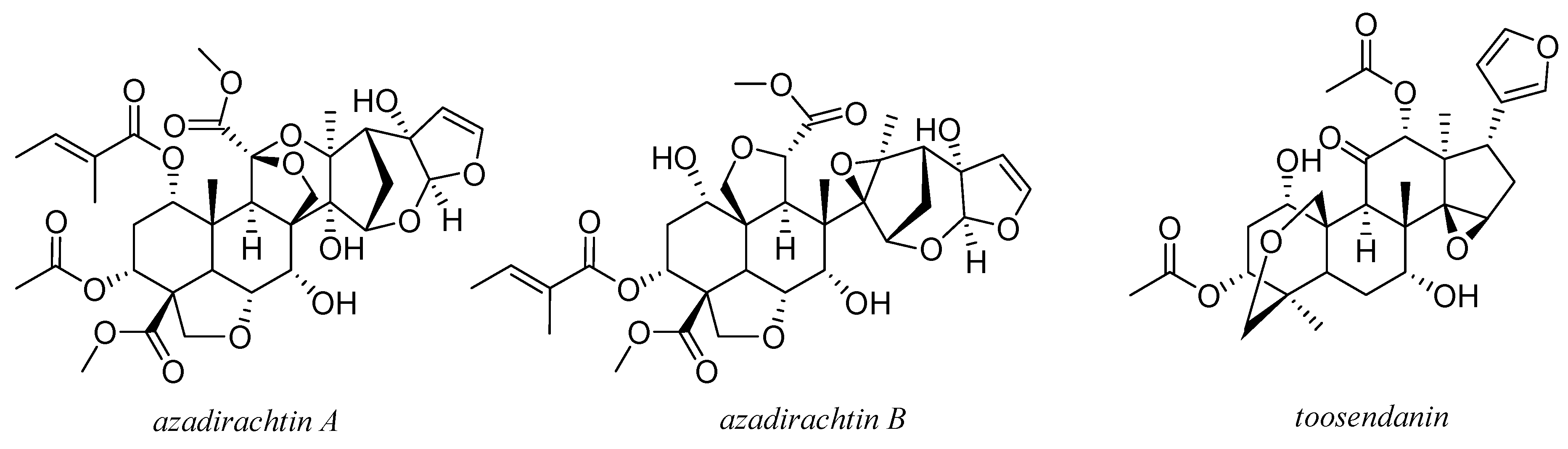
5. Coumarins
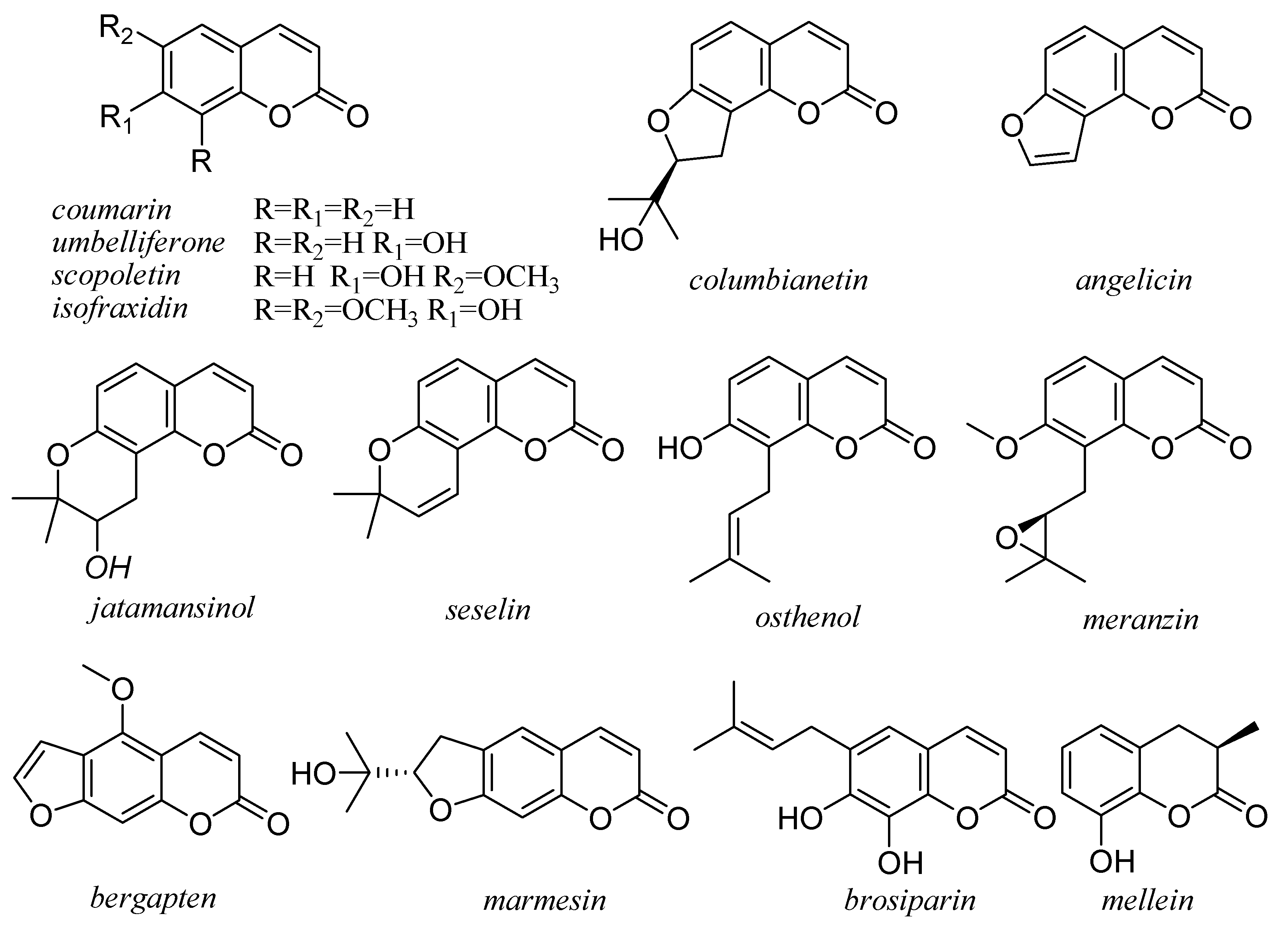
6. Flavonoids
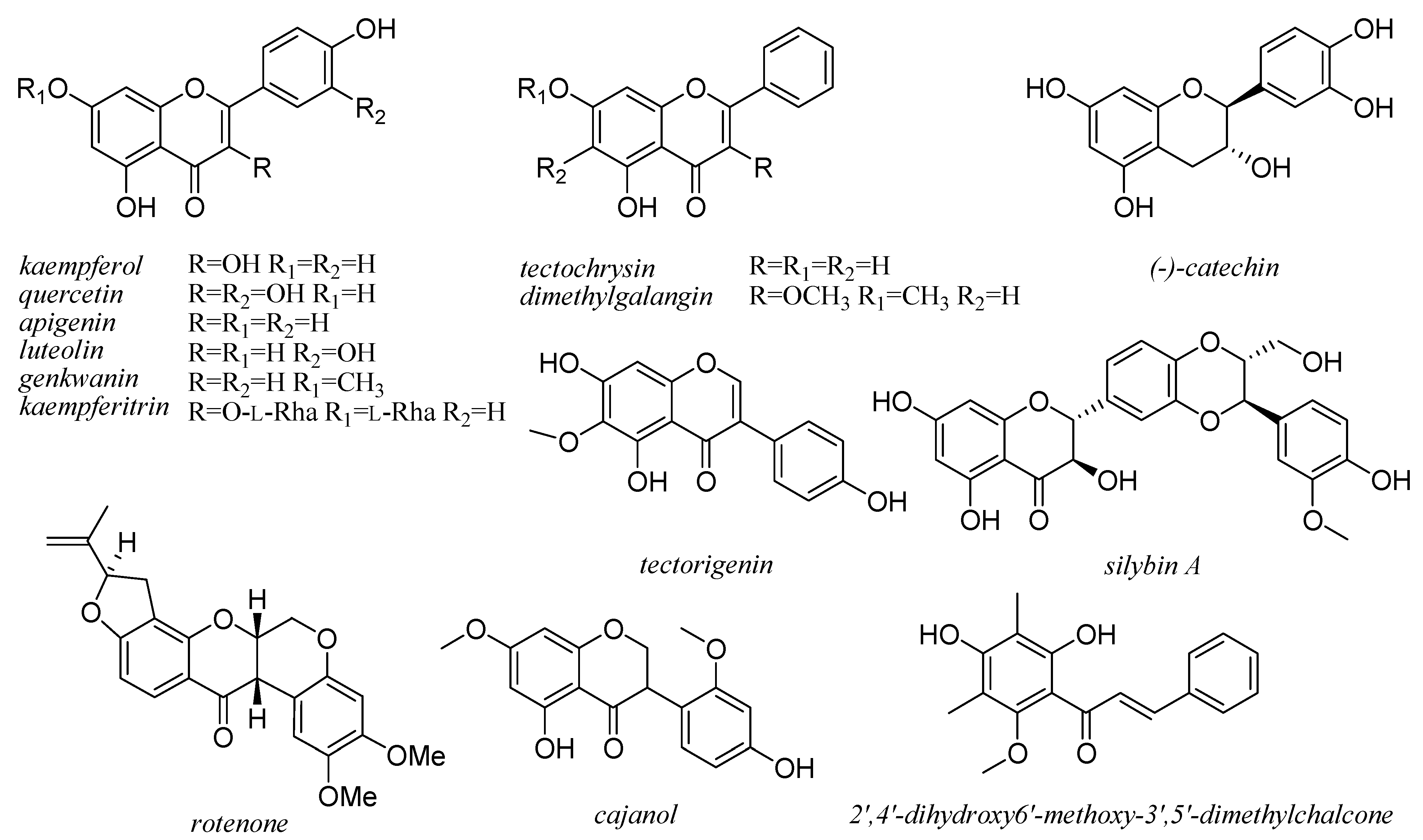
7. Xanthones and Quinones
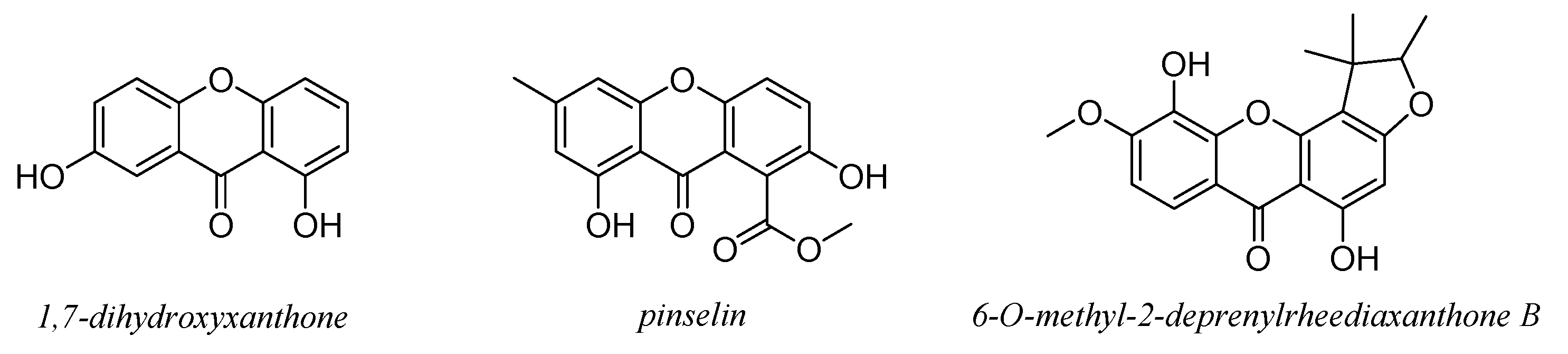
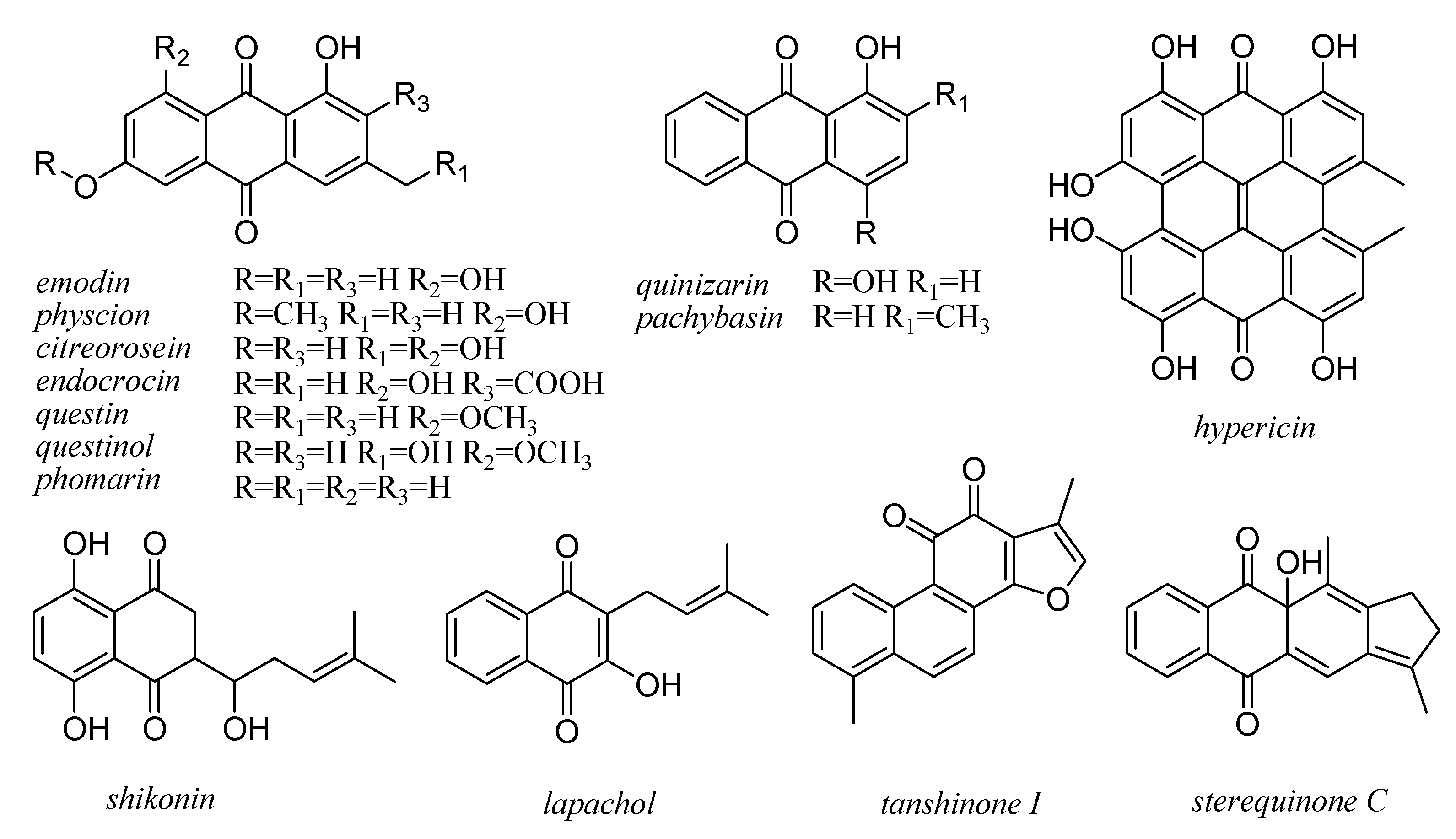
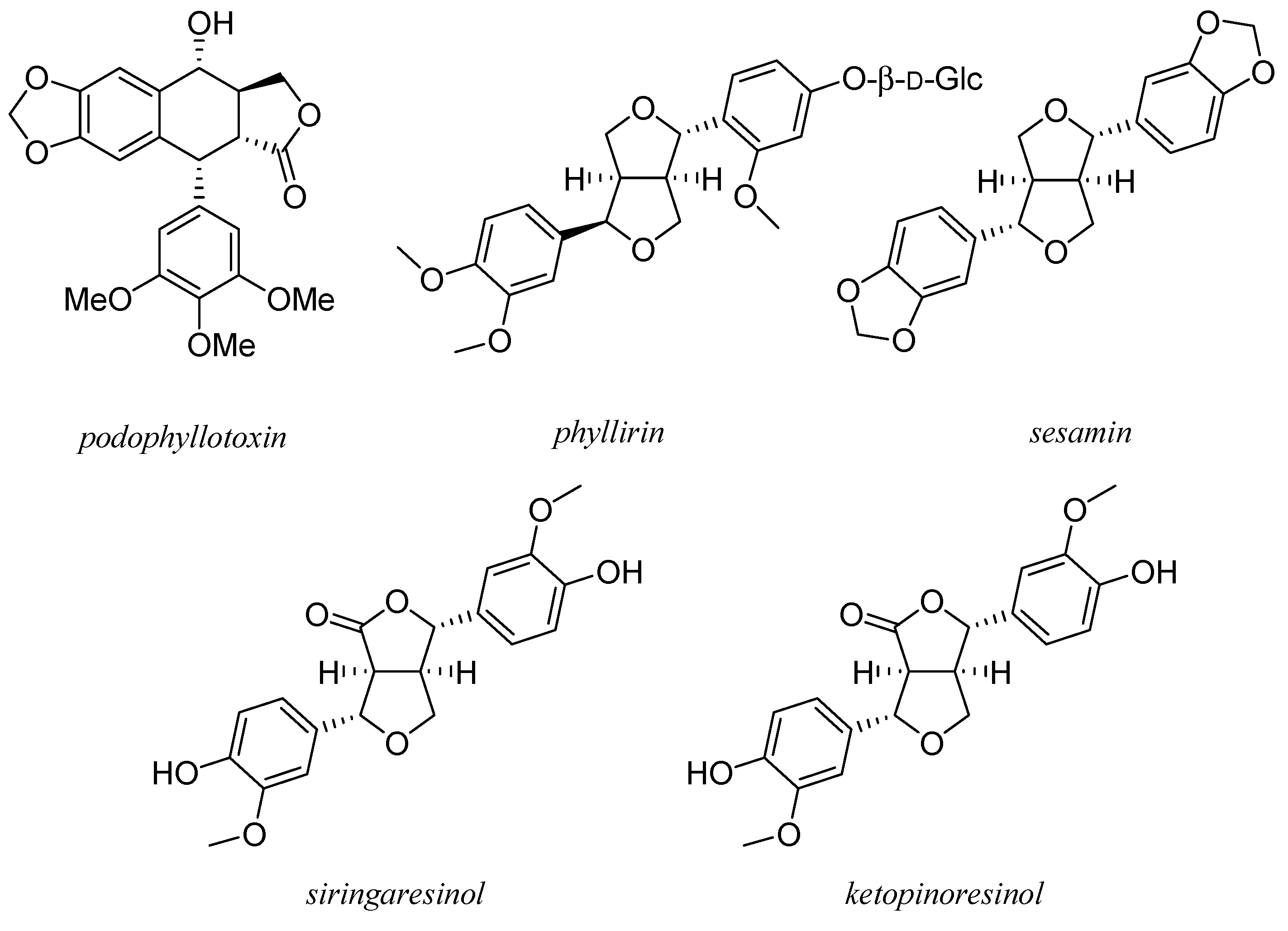
8. Lignans
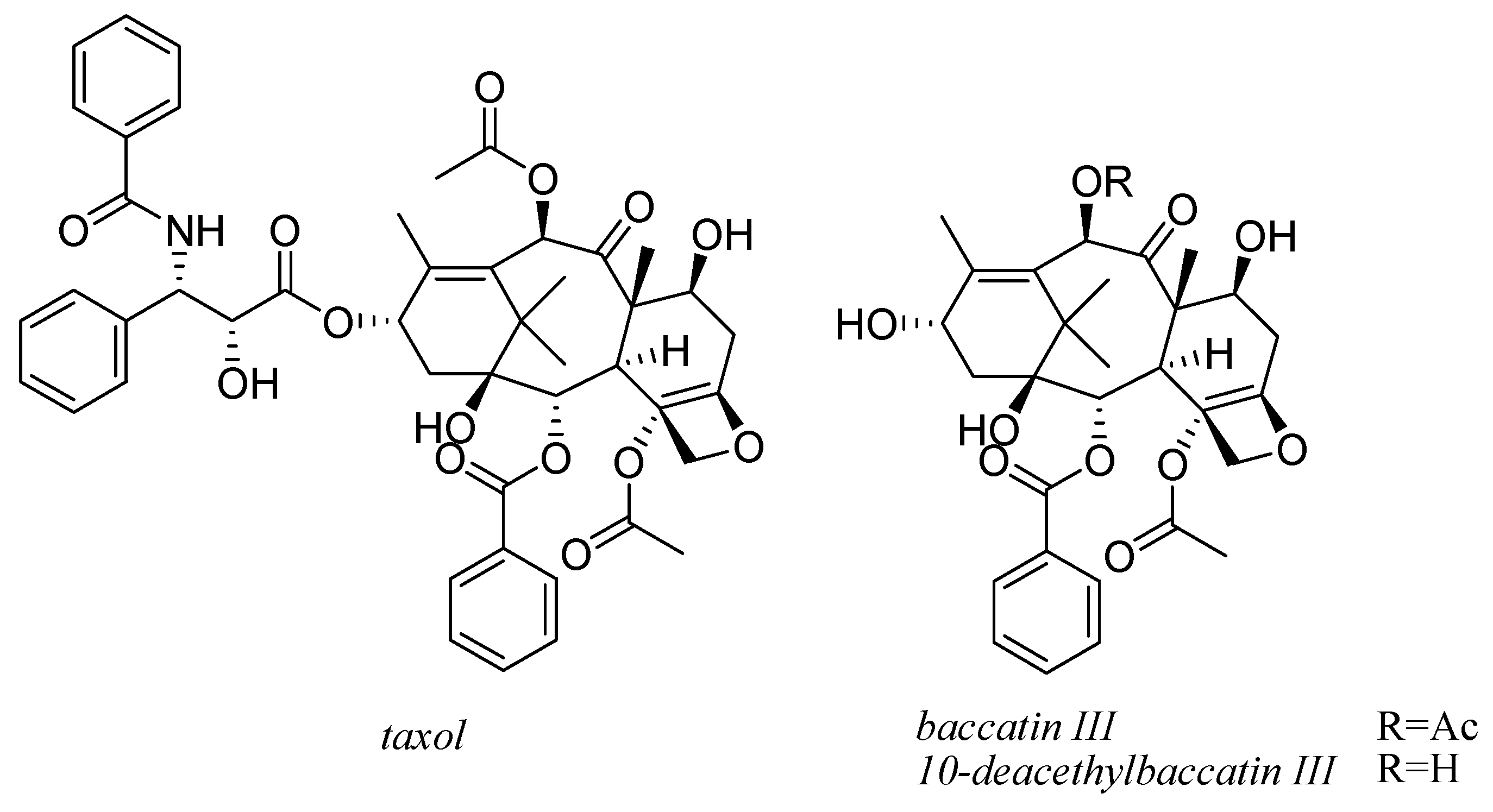
9. Taxol and Taxanes
| Species | Host plants | Reference |
|---|---|---|
| Acremonium sp. | Taxus globosa | [216] |
| Alternaria sp. | Taxus cuspidata Ginkgo biloba Corylus avellana | [217] [218] [219] |
| Aspergillus candidus | Taxus × media | [220] |
| Aspergillus fumigatus | Podocarpus sp. | [221] |
| Aspergillus niger var. taxi | T. cuspidata | [222] |
| Aspergillus sp. | Taxus chinensis | [223] |
| Bartalinia robillardoides | Aegle marmelos | [224] |
| Botryosphaeria sp. | T. globosa | [216] |
| Botrytis sp. | T. chinensis var. mairei T. cuspidata | [225] [226] |
| Ceratobasidium sp. | T. chinensis | [223] |
| Chaetomella raphigera | Terminalia arjuna | [227] |
| Cladosporium cladosporioides | T. media | [228] |
| Cladosporium oxysporum | Moringa oleifera | [229] |
| Cladosporium tenuissimum | T. chinensis | [223] |
| Colletotrichum gloeosporioides | Justicia gendarussa Plumeria acutifolia T. media Tectona grandis M. oleifera | [230] [231] [232] [233] [234] |
| Colletotrichum sp. | Maguireothamnus speciosus | [235] |
| Coniothyrium diplodiella | T. chinensis | [223] |
| Didymostilbe sp. | T. chinensis var. mairei | [236] |
| Ectostroma sp. | T. chinensis var. mairei | [225] |
| Epacris sp. | T. chinensis | [223] |
| Fusarium arthrosporioides | T. cuspidata | [237] |
| Fusarium lateritium | Taxus baccata | [217] |
| Fusarium mairei | T. chinensis var. mairei | [238] |
| Fusarium oxysporum | Rhizophora annamalayana | [239] |
| Fusarium proliferatum | T. media | [232] |
| Fusarium redolens | Taxus wallichiana | [240] |
| Fusarium solani | Taxus celebica T. chinensis | [241] [242] |
| Fusarium sp. | T. wallichiana T. globosa | [243] [216] |
| Gliocladium sp. | T. baccata | [244] |
| Guignardia mangiferae | T. media | [232] |
| Gyromitra sp. | T. globosa | [216] |
| Lasiodiplodia theobromae | Morinda citrifolia T. baccata | [245] [246] |
| Metarhizium anisopliae | T. chinensis | [223] |
| Monochaetia sp. | T. baccata | [217] |
| Mucor rouxianus | T. chinensis | [247] |
| Mucor sp. | T. chinensis var. mairei | [248] |
| Nigrospora sp. | T. globosa | [216] |
| Nodulisporium sylviforme | T. cuspidata | [249] |
| Ozonium sp. | T. chinensis var. mairei | [250] |
| Paraconiothyrium brasiliense | T. chinensis | [223] |
| Paraconiothyrium sp. | T. media | [251] |
| Penicillium aurantiogriseum | C. avellana | [252] |
| Penicillium raistrickii | Taxus brevifolia | [253] |
| Penicillium sp. | Taxus yunnanensis (=T. wallichiana) T. globosa T. chinensis | [254] [216] [255] |
| Periconia sp. | Torreya grandifolia | [256] |
| Pestalotia bicilia | T. baccata | [217] |
| Pestalotia pauciseta | Cardiospermum helicacabum Tabebuia pentaphylla | [257] [258] |
| Pestalotiopsis guepinii | Wollemia nobilis | [259] |
| Pestalotiopsis microspora | T. cuspidata, T. wallichiana Taxodium distichum | [260] [261] |
| Pestalotiopsis neglecta | T. cuspidata | [262] |
| Pestalotiopsis sp. | W. nobilis Catharanthus roseus | [259] [263] |
| Pestalotiopsis terminaliae | T. arjuna | [264] |
| Pestalotiopsis versicolor | T. cuspidata | [262] |
| Pezicula sp. | T. chinensis | [223] |
| Phoma betae | G. biloba | [265] |
| Phomopsis sp. | G. biloba, Larix leptolepis, T. cuspidata T. chinensis | [266] [223] |
| Phyllosticta melochiae | Melochia corchorifolia | [267] |
| Phyllosticta sp. | Ocimum basilicum | [268] |
| Phyllosticta spinarum | Cupressus sp. | [269] |
| Pithomyces sp. | Taxus sumatrana | [216] |
| Rhizopus sp. | T. media | [270] |
| Seimatoantlerium nepalense | T. wallichiana | [271] |
| Sordaria sp. | T. chinensis | [223] |
| Sporormia minima | T. wallichiana | [272] |
| Stegolerium kukenani | Stegolepisguianensis | [273] |
| Stemphylium sedicola | T. baccata | [274] |
| Taxomyces andreanae | T. brevifolia | [214] |
| Taxomyces sp. | Taxus sp. | [275] |
| Trichoderma sp. | T. chinensis | [223] |
| Trichothecium sp. | T. wallichiana | [272] |
| Tubercularia sp. | T. mairei | [276] |
| Xylaria sp. | M. speciosus T. chinensis | [235] [223] |
10. Quinoline Alkaloids
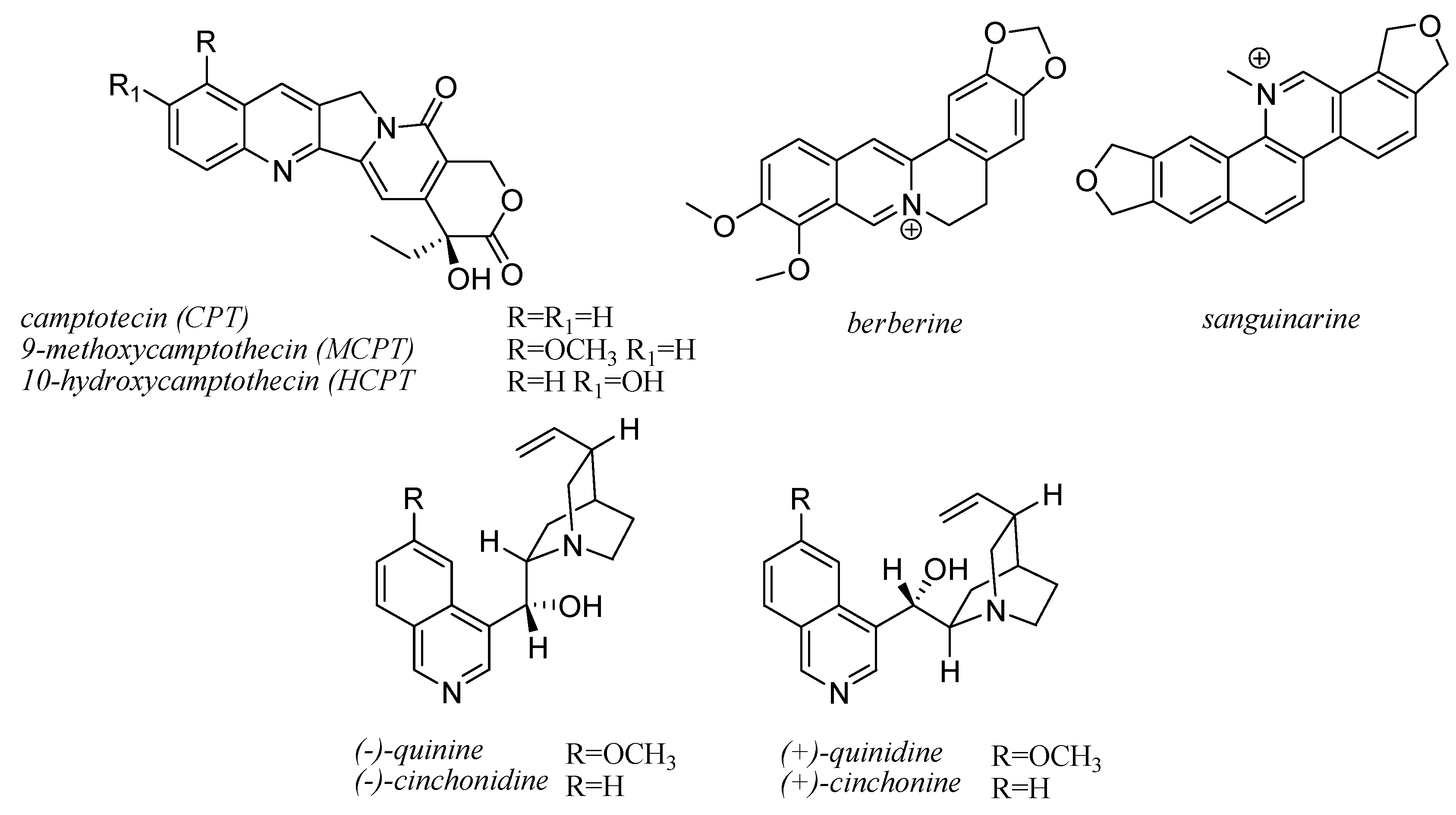
| Compounds | Fungal Species | Host Plants | References |
|---|---|---|---|
| CPT | Entrophospora infrequens | Nothapodytes foetida | [288,289] |
| CPT | Neurospora sp. | N. foetida | [290] |
| CPT | Nodulisporium sp. | N. foetida | [291] |
| CPT, HCPT, MCPT | Fusarium solani | Camptotheca acuminata | [292] |
| CPT, HCPT, MCPT | F. solani | Apodytes dimidiata | [293] |
| CPT | Botryosphaeria parva | Nothapodytes nimmoniana | [294] |
| CPT | Diaporthe conorum | N. nimmoniana | [294] |
| CPT | Fusarium oxysporum | N. nimmoniana | [294] |
| CPT | Fusarium sacchari | N. nimmoniana | [294] |
| CPT | F. solani | N. nimmoniana | [294] |
| CPT | Fusarium sp. | N. nimmoniana | [294] |
| CPT | Fusarium subglutinans | N. nimmoniana | [294] |
| CPT | Fusarium verticillioides | N. nimmoniana | [294] |
| CPT | Galactomyces sp. | N. nimmoniana | [294] |
| CPT | Irpex lacteus | N. nimmoniana | [294] |
| CPT | Phomopsis sp. | N. nimmoniana | [294] |
| CPT | Unidentified strains | N. nimmoniana | [294] |
| HCPT | Xylaria sp. | C. acuminata | [286] |
| HCPT | Valsa mali | C. acuminata | [295] |
| CPT | Aspergillus spp. | C. acuminata | [283] |
| CPT | Trichoderma atroviride | C. acuminata | [283] |
| CPT, HCPT, MCPT | Alternaria alternata | Miquelia dentata | [296] |
| CPT, HCPT, MCPT | Fomitopsis sp. | M. dentata | [296] |
| CPT, HCPT, MCPT | Phomopsis sp. | M. dentata | [296] |
| CPT | F. oxysporum | N. foetida | [297] |
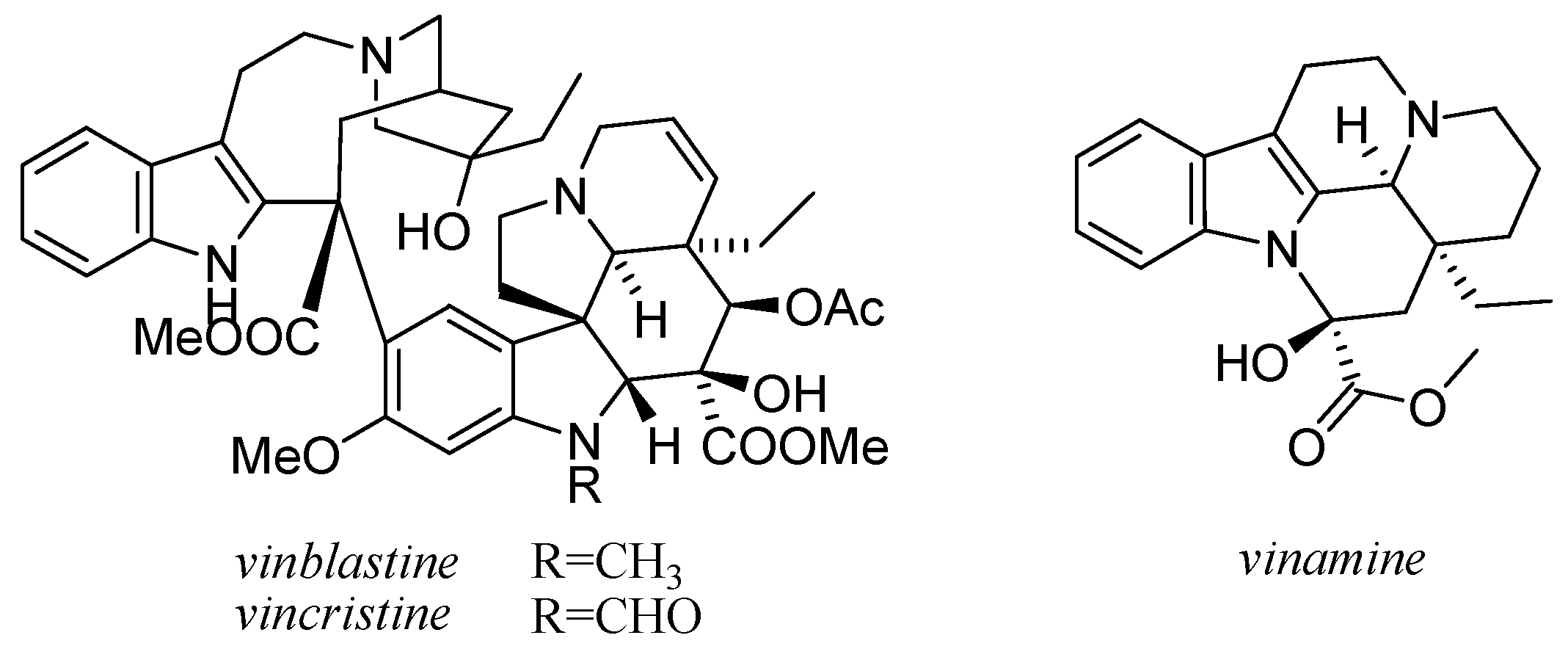
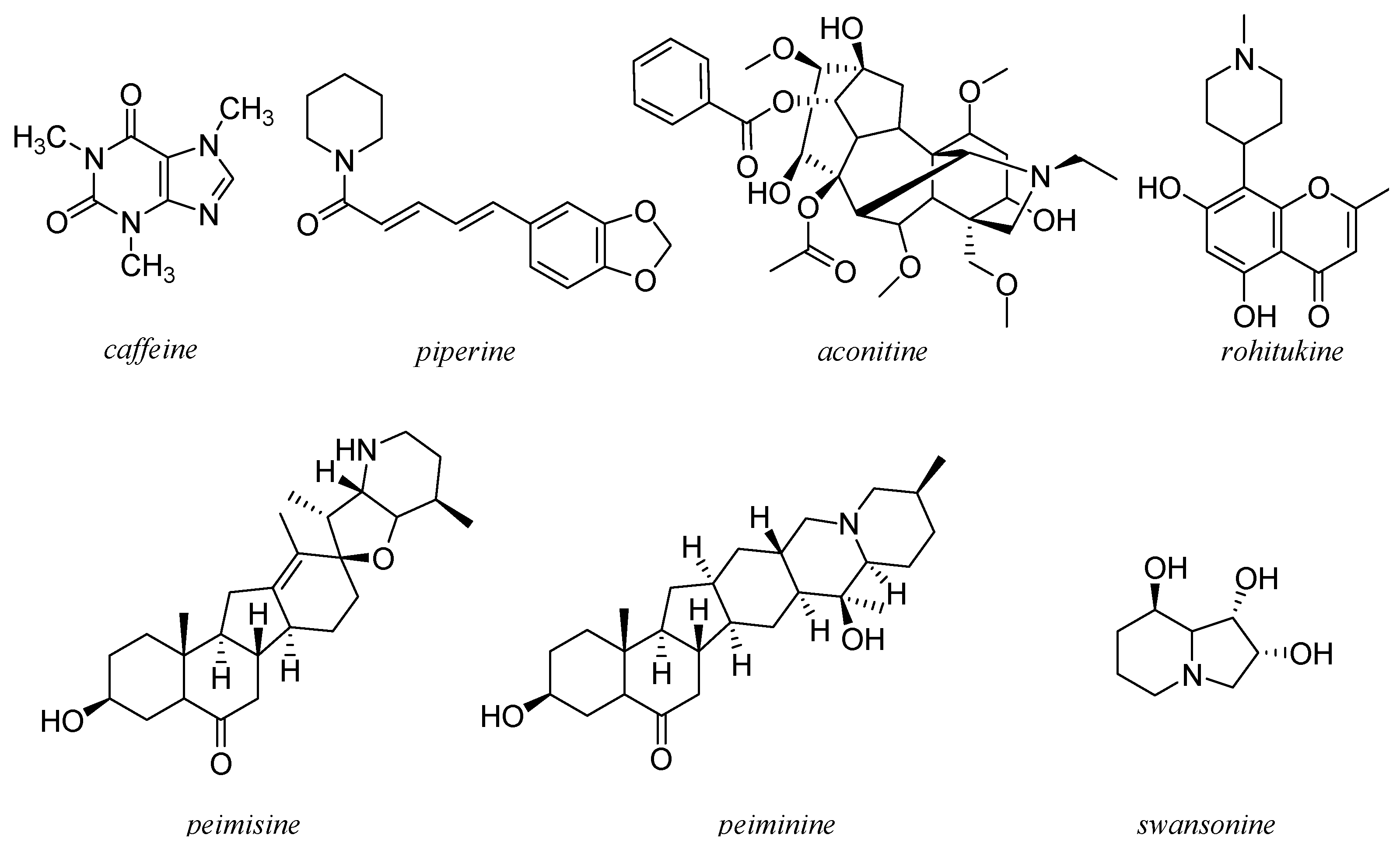
11. Other Alkaloids
12. 3-Nitropropionic Acid
13. Saponines
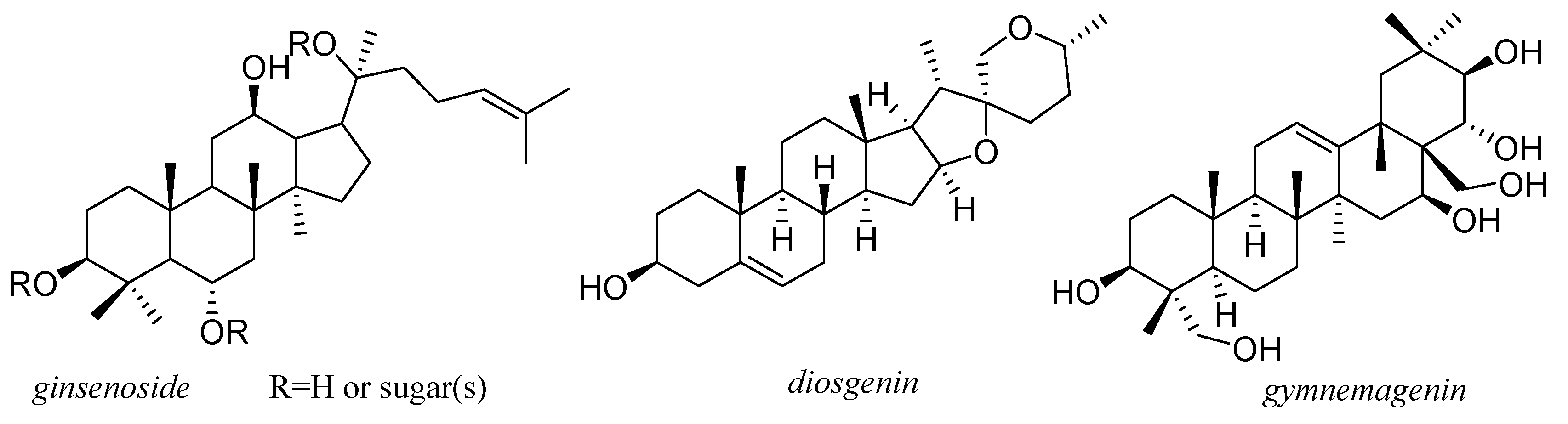
14. Miscellaneous Compounds
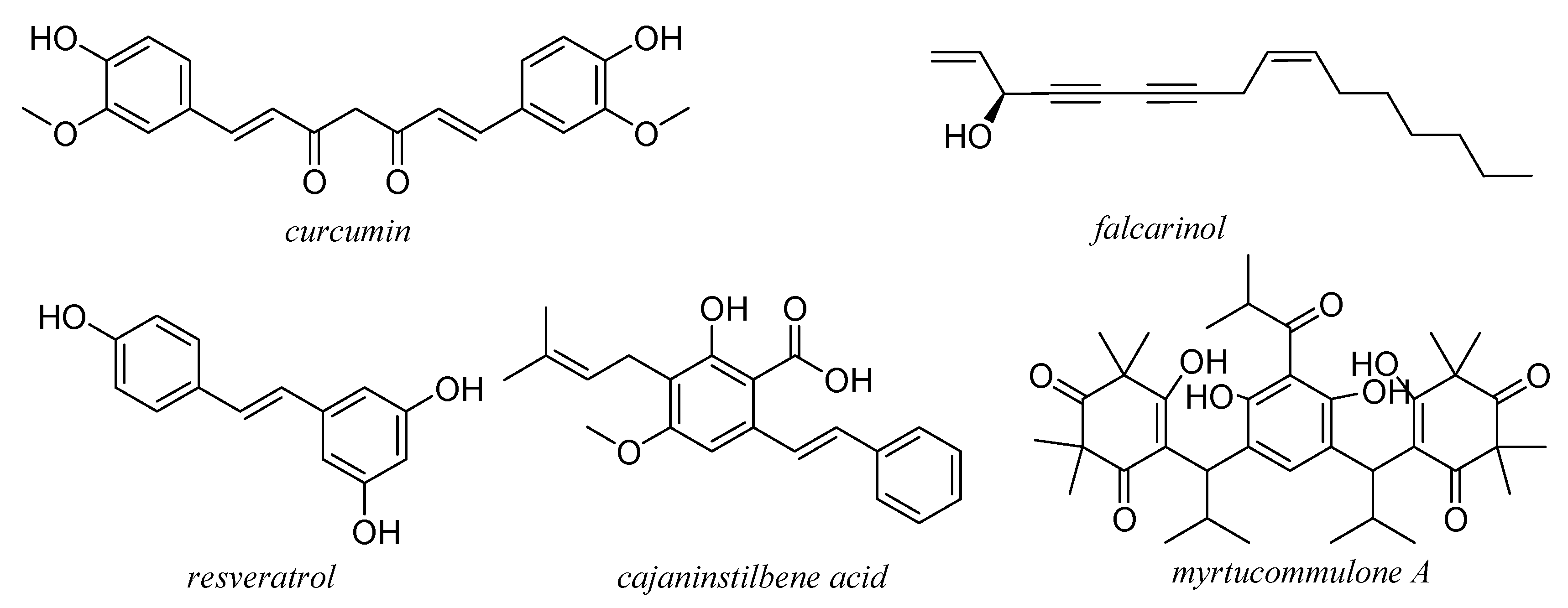
15. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kusari, S.; Spiteller, M. Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes? Nat. Prod. Rep. 2011, 28, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Mazzola, M. Auxin-mediated relationships between apple plants and root inhabiting fungi: Impact on root pathogens and potentialities of growth-promoting populations. Plant Pathol. 2015, 64, 843–851. [Google Scholar] [CrossRef]
- Kawaide, H. Biochemical and molecular analyses of gibberellin biosynthesis in fungi. Biosci. Biotechnol. Biochem. 2006, 70, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Bömke, C.; Tudzynski, B. Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry 2009, 70, 1876–1893. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, T.; Hayashi, T. Biochemical studies on Bakanae fungus of rice. Part 3. Physiological action of gibberellin on plants. J. Agric. Chem. Soc. Jpn. 1939, 15, 403–413. [Google Scholar]
- Porter, J.K.; Bacon, C.W.; Cutler, H.G.; Arrendale, R.F.; Robbins, J.D. In vitro auxin production by Balansia epichloe. Phytochemistry 1985, 24, 1429–1431. [Google Scholar] [CrossRef]
- De Battista, J.P.; Bacon, C.W.; Severson, R.; Plattner, R.D.; Bouton, J.H. Indole acetic acid production by the fungal endophyte of tall fescue. Agron. J. 1990, 82, 878–880. [Google Scholar] [CrossRef]
- Yue, Q.; Miller, C.J.; White, J.F.; Richardson, M.D. Isolation and characterization of fungal inhibitors from Epichloë festucae. J. Agric. Food Chem. 2000, 48, 4687–4692. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Belesky, D.P. Ecological importance of Neotyphodium spp. grass endophytes in agroecosystems. Grassland Sci. 2006, 52, 1–14. [Google Scholar] [CrossRef]
- Schardl, C.L. Fungal endophytes in Lolium and Festuca species. In Molecular Breeding of Forage and Turf; Yamada, T., Spangenberg, G., Eds.; Springer: New York, NY, USA, 2009; pp. 285–298. [Google Scholar]
- Di Menna, M.E.; Finch, S.C.; Popay, A.J.; Smith, B.L. A review of the Neotyphodium lolii/Lolium perenne symbiosis and its associated effects on animal and plant health, with particular emphasis on ryegrass staggers. N. Z. Vet. J. 2012, 60, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Ljungquist, P.O.; Stenström, E. Indole-3-acetic acid production by mycorrhizal fungi determined by gas chromatography-mass spectrometry. New Phytol. 1983, 94, 401–407. [Google Scholar] [CrossRef]
- Barroso, J.; Chaves Neves, H.; Pais, M.S. Production of indole-3-ethanol and indole-3-acetic acid by the mycorrhizal fungus of Ophrys lutea (Orchidaceae). New Phytol. 1986, 103, 745–749. [Google Scholar] [CrossRef]
- Rudawska, M.; Bernillon, J.; Gay, G. Indole compounds released by the ectendomycorrhizal fungal strain MrgX isolated from a pine nursery. Mycorrhiza 1992, 2, 17–23. [Google Scholar] [CrossRef]
- Lu, H.; Zou, W.X.; Meng, J.C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000, 151, 67–73. [Google Scholar] [CrossRef]
- Bhagobaty, R.K.; Joshi, S.R. Promotion of seed germination of green gram and chick pea by Penicillium verruculosum RS7PF, a root endophytic fungus of Potentilla fulgens L. Adv. Biotechnol. 2009, 8, 7–15. [Google Scholar]
- Hammerschmidt, L.; Wray, V.; Lin, W.; Kamilova, E.; Proksch, P.; Aly, A.H. New styrylpyrones from the fungal endophyte Penicillium glabrum isolated from Punica granatum. Phytochem. Lett. 2012, 5, 600–603. [Google Scholar] [CrossRef]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Sirrenberg, A.; Göbel, C.; Grond, S.; Czempinski, N.; Ratzinger, A.; Karlovsky, P.; Santos, P.; Feussner, I.; Pawlowski, K. Piriformospora indica affects plant growth by auxin production. Physiol. Plant. 2007, 131, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Varma, A.; Rexer, K.-H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Qiang, X.; Weiss, M.; Kogel, K.H.; Schäfer, P. Piriformospora indica—A mutualistic basidiomycete with an exceptionally large plant host range. Mol. Plant Pathol. 2012, 13, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Glawe, D.; Doty, S.L. Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol. Res. 2009, 113, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.C.; Yu, B.Y.; Li, X. Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. Afr. J. Biotechnol. 2008, 7, 3505–3510. [Google Scholar]
- Waqas, M.; Khan, A.L.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Phytohormone-producing fungal endophytes and hardwood-derived biochar interact to ameliorate heavy metal stress in soybeans. Biol. Fertil. Soils 2014, 50, 1155–1167. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Rim, S.O.; Lee, J.H.; Choi, W.Y.; Hwang, S.K.; Suh, S.J.; Lee, I.J.; Rhee, I.K.; Kim, J.G. Fusarium proliferatum KGL0401 as a new gibberellin-producing fungus. J. Microbiol. Biotechnol. 2005, 15, 809–814. [Google Scholar]
- Tsavkelova, E.A.; Bömke, C.; Netrusov, A.I.; Weiner, J.; Tudzynski, B. Production of gibberellic acids by an orchid-associated Fusarium proliferatum strain. Fungal Genet. Biol. 2008, 45, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Kim, H.-Y.; Chaudhary, M.F.; Hwang, Y.-H.; Shin, D.-H.; Kim, I.-K.; Lee, B.-H.; Lee, I.-J. Gibberellin production and plant growth enhancement by newly isolated strain of Scolecobasidium tshawytschae. J. Microbiol. Biotechnol. 2009, 19, 560–565. [Google Scholar] [PubMed]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Hamayun, M.; Kim, H.Y.; Yoon, H.J.; Seo, J.C.; Choo, Y.S.; Lee, I.J.; Kim, S.D.; Rhee, I.K.; Kim, J.G. A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellin production. Biotechnol. Lett. 2009, 31, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.S.; Kang, S.M.; Na, C.I.; Sohn, E.Y.; Hwang, Y.H.; Shin, D.H.; Lee, B.H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Khan, M.A.; Khan, A.L.; Kang, S.; Kim, S.K.; Joo, G.J.; Lee, I.L. Gibberellin production by pure cultures of a new strain of Aspergillus fumigatus. World J. Microbiol. Biotechnol. 2009, 25, 1785–1792. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kim, Y.H.; Kang, S.M.; Lee, I.J. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol. Biochem. 2011, 49, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kim, Y.H.; Kang, S.M.; Lee, J.H.; Lee, I.J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Proc. Biochem. 2011, 46, 440–447. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Iqbal, I.; Ahmad, B.; Lee, I.J. Isolation of a gibberellin-producing fungus (Penicillium sp. MH7) and growth promotion of crown daisy (Chrysanthemum coronarium). J. Microbiol. Biotechnol. 2010, 20, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qi, N.; Wang, S.; Gadhave, K.; Yang, S. Characterization of secondary metabolites of an endophytic fungus from Curcuma wenyujin. Curr. Microbiol. 2014, 69, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Crafts, C.B.; Miller, C.O. Detection and identification of cytokinins produced by mycorrhizal fungi. Plant Physiol. 1974, 54, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.J.; Tagu, D. The roles of auxins and cytokinins in mycorrhizal symbioses. J. Plant Growth Regul. 2000, 19, 144–154. [Google Scholar] [PubMed]
- Vadassery, J.; Ritter, C.; Venus, Y.; Camehl, I.; Varma, A.; Shahollari, B.; Novák, O.; Strnad, M.; Ludwig-Müller, J.; Oelmüller, R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol. Plant Microbe Interact. 2008, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Bhagobaty, R.K.; Joshi, S.R. Metabolite profiling of endophytic fungal isolates of five ethno-pharmacologically important plants of Meghalaya, India. J. Metabolomics Syst. Biol. 2011, 2, 20–31. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Dirkse, E.; Sears, J.; Markworth, C. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 2001, 147, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Ahn, J.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Potential of the volatile-producing fungus Nodulisporium sp. CF016 for the control of postharvest diseases of apple. Plant Pathol. J. 2010, 26, 253–259. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Nuangmek, W.; Matsui, K.; Lumyong, S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Prot. 2013, 45, 63–70. [Google Scholar] [CrossRef]
- Abrahão, M.R.; Molina, G.; Pastore, G.M. Endophytes: Recent developments in biotechnology and the potential for flavor production. Food Res. Int. 2013, 52, 367–372. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Meshram, V.; Kapoor, N. Muscodor tigerii sp. nov.—Volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann. Microbiol. 2015, 67, 47–57. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Chen, J.J.; Cheng, Y.C.; Hsieh, M.T.; Hsieh, S.Y.; Yuan, G.F.; Su, Y.S. Secondary metabolites from the endophytic fungus Annulohypoxylon stygium BCRC 34024. Chem. Nat. Compd. 2014, 50, 237–241. [Google Scholar] [CrossRef]
- Kaul, S.; Wani, M.; Dhar, K.L.; Dhar, M.K. Production and GC-MS trace analysis of methyl eugenol from endophytic isolate of Alternaria from rose. Ann. Microbiol. 2008, 58, 443–445. [Google Scholar] [CrossRef]
- Bäck, J.; Aaltonen, H.; Hellén, H.; Kajos, M.K.; Patokoski, J.; Taipale, R.; Pumpanen, J.; Heinonsalo, J. Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos. Environ. 2010, 44, 3651–3659. [Google Scholar] [CrossRef]
- Tomsheck, A.R.; Strobel, G.A.; Booth, E.; Geary, B.; Spakowicz, D.; Knighton, B.; Floerchinger, C.; Sears, J.; Liarzi, O.; Ezra, D. Hypoxylon sp., an endophyte of Persea indica, producing 1,8-cineole and other bioactive volatiles with fuel potential. Microb. Ecol. 2010, 60, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Nigg, J.; Strobel, G.; Knighton, W.B.; Hilmer, J.; Geary, B.; Riyaz-Ul-Hassan, S.; Harper, J.K.; Valenti, D.; Wang, Y. Functionalized para-substituted benzenes as 1,8-cineole production modulators in an endophytic Nodulisporium species. Microbiology 2014, 160, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Mucciarelli, M.; Camusso, W.; Maffei, M.; Panicco, P.; Bicchi, C. Volatile terpenoids of endophyte-free and infected peppermint (Mentha piperita L.): Chemical partitioning of a symbiosis. Microb. Ecol. 2007, 54, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, A.Q.; Tang, X.K. Isolation, identification and volatile compound analysis of an aroma-producing endophytic yeast from romaine lettuce. Food Sci. 2011, 23, 33. [Google Scholar]
- Shaw, J.J.; Spakowicz, D.J.; Dalal, R.S.; Davis, J.H.; Lehr, N.A.; Dunican, B.F.; Orellana, E.A.; Narváez-Trujillo, A.; Strobel, S.A. Biosynthesis and genomic analysis of medium-chain hydrocarbon production by the endophytic fungal isolate Nigrograna mackinnonii E5202H. Appl. Microbiol. Biotechnol. 2015, 99, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Wang, G.P.; Mao, L.J.; Komon-Zelazowska, M.; Yuan, Z.L.; Lin, F.C.; Druzhinina, I.S.; Kubicek, C.P. Muscodor fengyangensis sp. nov. from southeast China: Morphology, physiology and production of volatile compounds. Fungal Biol. 2010, 114, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Oropeza, F.; Duarte, G.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Anaya, A.L. Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J. Chem. Ecol. 2010, 36, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.S.Y.; Mah, S.W.; Tee, C.S. Identification of volatile metabolites from fungal endophytes with biocontrol potential towards Fusarium oxysporum f. sp. cubense race 4. Am. J. Agric. Biol. Sci. 2010, 5, 177–182. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Su, Z.Z.; Mao, L.J.; Peng, Y.Q.; Yang, G.M.; Lin, F.C.; Zhang, C.L. Distinctive endophytic fungal assemblage in stems of wild rice (Oryza granulata) in China with special reference to two species of Muscodor (Xylariaceae). J. Microbiol. 2011, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lin, X.; Lu, C.; Hu, Z.; Huang, W.; Huang, Y.; Shen, Y. Secondary metabolites of Phomopsis sp. XZ-26, an endophytic fungus from Camptotheca acuminata. Eur. J. Org. Chem. 2009, 18, 2975–2982. [Google Scholar] [CrossRef]
- Singh, S.K.; Strobel, G.A.; Knighton, B.; Geary, B.; Sears, J.; Ezra, D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb. Ecol. 2011, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Santos Filho, F.C.; da Silva Amaral, L.; Rodrigues-Filho, E. Composition of essential oils from Cupressus lusitanica and a Xylariaceous fungus found on its leaves. Biochem. Syst. Ecol. 2011, 39, 485–490. [Google Scholar] [CrossRef]
- Strobel, G.; Singh, S.K.; Riyaz-Ul-Hassan, S.; Mitchell, A.M.; Geary, B.; Sears, J. An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett. 2011, 320, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Meshram, V.; Kapoor, N.; Saxena, S. Muscodor kashayum sp. nov.—A new volatile anti-microbial producing endophytic fungus. Mycology 2013, 4, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.H.; Teles, H.L.; Zanardi, L.M.; Marx Young, M.C.; Eberlin, M.N.; Hadad, R.; Pfenning, L.H.; Costa-Neto, C.M.; Castro-Gamboa, I.; da Silva Bolzani, V.; et al. Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae). Phytochemistry 2006, 67, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yang, S.X.; Liu, L.; Wang, Y. Chemical constituents and their toxic activity from the endophytic fungus Phomopsis sp. KY-12, isolated from Pleioblastus amarus. Nat. Prod. Res. Dev. 2014, 26, 1389–1392. [Google Scholar]
- Strobel, G.A.; Kluck, K.; Hess, W.M.; Sears, J.; Ezra, D.; Vargas, P.N. Muscodor albus E-6, an endophyte of Guazuma ulmifolia making volatile antibiotics: Isolation, characterization and experimental establishment in the host plant. Microbiology 2007, 153, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Kudalkar, P.; Strobel, G.; Riyaz-Ul-Hassan, S.; Geary, B.; Sears, J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 2012, 53, 319–325. [Google Scholar] [CrossRef]
- Silva, G.H.; de Oliveira, C.M.; Teles, H.L.; Pauletti, P.M.; Castro-Gamboa, I.; Silva, D.H.S.; da Silva Bolzani, V.; Young, M.C.M.; Costa-Neto, C.M.; Pfenning, L.H.; et al. Sesquiterpenes from Xylaria sp., an endophytic fungus associated with Piper aduncum (Piperaceae). Phytochem. Lett. 2010, 3, 164–167. [Google Scholar] [CrossRef]
- Wu, L.S.; Hu, C.L.; Han, T.; Zheng, C.J.; Ma, X.Q.; Rahman, K.; Qin, L.P. Cytotoxic metabolites from Perenniporia tephropora, an endophytic fungus from Taxus chinensis var. mairei. Appl. Microbiol. Biotechnol. 2013, 97, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Wei, W.; Guo, Y.; Wang, T.; Jiao, R.H.; Ng, S.W.; Tan, R.X.; Ge, H.M. Sesquiterpenoids from the mangrove-derived endophytic fungus Diaporthe sp. J. Nat. Prod. 2012, 75, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Q.; Zhang, L.Q.; Yang, J.; Li, Y.P. β-Elemene from Curcuma zedoaria endophytic fungus. Nat. Prod. Res. Dev. 2011, 23, 473–475. [Google Scholar]
- Isaka, M.; Chinthanom, P.; Boonruangprapa, T.; Rungjindamai, N.; Pinruan, U. Eremophilane-type sesquiterpenes from the fungus Xylaria sp. BCC 21097. J. Nat. Prod. 2010, 73, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Sumarah, M.W.; Puniani, E.; Sørensen, D.; Blackwell, B.A.; Miller, J.D. Secondary metabolites from anti-insect extracts of endophytic fungi isolated from Picea rubens. Phytochemistry 2010, 71, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.S.; Rodrigues-Filho, E. Two novel eremophilane sesquiterpenes from an endophytic xylariaceous fungus isolated from leaves of Cupressus lusitanica. J. Braz. Chem. Soc. 2010, 21, 1446–1450. [Google Scholar] [CrossRef]
- Atmosukarto, I.; Castillo, U.; Hess, W.M.; Sears, J.; Strobel, G. Isolation and characterization of Muscodor albus I-41.3 s, a volatile antibiotic producing fungus. Plant Sci. 2005, 169, 854–861. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Chanthaket, R.; Boonyuen, N.; Lumyong, S. γ-Lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 2009, 72, 1720–1722. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, X.; Li, E.; Guo, L.; Che, Y. Arundinols A-C and arundinones A and B from the plant endophytic fungus Microsphaeropsis arundinis. J. Nat. Prod. 2013, 76, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Y.; Pan, Q.; Tao, M.; Zhang, W. A new eudesmane sesquiterpene from Nigrospora oryzae, an endophytic fungus of Aquilaria sinensis. Rec. Nat. Prod. 2014, 8, 330–333. [Google Scholar]
- Sopalun, K.; Strobel, G.A.; Hess, W.M.; Worapong, J. A record of Muscodor albus, an endophyte from Myristica fragrans in Thailand. Mycotaxon 2003, 88, 239–247. [Google Scholar]
- Zhang, L.; Gu, S.; Shao, H.; Wei, R. Isolation, determination and aroma product characterization of fungus producing irone. Mycosystema 1999, 18, 49–54. [Google Scholar]
- Zhang, L.; Wang, S.Q.; Li, X.J.; Zhang, A.L.; Zhang, Q.; Gao, J.M. New insight into the stereochemistry of botryosphaeridione from a Phoma endophyte. J. Mol. Struct. 2012, 1016, 72–75. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, S.; Zhu, H.J.; Luo, D.Q. Dihydroberkleasmin A: A new eremophilane sesquiterpenoid from the fermentation broth of the plant endophytic fungus Pestalotiopsis photiniae. Molecules 2011, 16, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Murayama, T. New eremophilane-type sesquiterpenoids, eremoxylarins A and B from xylariaceous endophytic fungus YUA-026. Z. Naturforsch. B 2005, 60, 885–890. [Google Scholar] [CrossRef]
- Kawahara, T.; Itoh, M.; Izumikawa, M.; Sakata, N.; Tsuchida, T.; Shin-ya, K. Three eremophilane derivatives, MBJ-0011, MBJ-0012 and MBJ-0013, from an endophytic fungus Apiognomonia sp. f24023. J. Antibiot. 2013, 66, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ge, H.; Zou, J.H.; Tao, X.; Chen, R.; Dai, J. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014, 16, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhang, S.; Zhu, H.J.; Luo, D.Q.; Gao, X.Y. Eremophilane-type sesquiterpenoids from the fermentation broth of plant endophytic fungus Pestalotiopsis photiniae isolated from the Chinese Podocarpaceae plant Podocarpus macrophyllus. Helv. Chim. Acta 2011, 94, 1463–1469. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Koseki, T.; Murayama, T.; Shiono, Y. Eremophilane sesquiterpenes from the endophyte Microdiplodia sp. KS 75-1 and revision of the stereochemistries of phomadecalins C and D. Phytochem. Lett. 2010, 3, 148–151. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Huang, H.; Ma, L.; Wang, J.; Gu, Y.; Liu, L.; Lin, Y. Four eremophilane sesquiterpenes from the mangrove endophytic fungus Xylaria sp. BL321. Mar. Drugs 2012, 10, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Li, Y.Y.; Huang, Y.J.; Su, W.J.; Shen, Y.M. Three new sesquiterpenoids from Xylaria sp. NCY2. Helv. Chim. Acta 2008, 91, 46–52. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; Silva, G.H.; Regasini, L.O.; Flausino, O., Jr.; López, S.N.; Abissi, B.M.; Gomes de Souza Berlinck, R.; Durães Sette, L.; Costa Bonugli-Santos, R.; Rodrigues, A.; et al. Xylarenones C-E from an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2011, 74, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Gubiani, J.R.; Zeraik, M.L.; Oliveira, C.M.; Ximenes, V.F.; Nogueira, C.R.; Fonseca, L.M.; Sila, D.H.S.; da Silva Bolzani, V.; Araujo, A.R. Biologically active eremophilane-type sesquiterpenes from Camarops sp., an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2014, 77, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Pažoutová, S.; Follert, S.; Bitzer, J.; Keck, M.; Surup, F.; Šrůtka, P.; Holuša, J.; Stadler, M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013, 60, 107–123. [Google Scholar] [CrossRef]
- Vidal, S. Changes in suitability of tomato for whiteflies mediated by a non-pathogenic endophytic fungus. Entomol. Experim. Appl. 1996, 80, 272–274. [Google Scholar] [CrossRef]
- Jallow, M.F.; Dugassa-Gobena, D.; Vidal, S. Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod Plant Interact. 2008, 2, 53–62. [Google Scholar] [CrossRef]
- Hodges, R.; Porte, A.L. The structure of loliolide: A terpene from Lolium perenne. Tetrahedron 1964, 20, 1463–1467. [Google Scholar] [CrossRef]
- Wu, M.D.; Cheng, M.J.; Chen, I.S.; Su, Y.S.; Hsieh, S.Y.; Chang, H.S.; Chang, C.W.; Yuan, G.F. Phytochemical investigation of Annulohypoxylon ilanense, an endophytic fungus derived from Cinnamomum species. Chem. Biodivers. 2013, 10, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Ginesta-Peris, E.; Garcia-Breijo, F.J.; Primo-Yúfera, E. Antimicrobial activity of xanthatin from Xanthium spinosum L. Lett. Appl. Microbiol. 1994, 18, 206–208. [Google Scholar] [CrossRef]
- Nibret, E.; Youns, M.; Krauth-Siegel, R.L.; Wink, M. Biological activities of xanthatin from Xanthium strumarium leaves. Phytother. Res. 2011, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Han, T.; Wu, J.Z.; Zhang, Q.Y.; Zhang, H.; Huang, B.K.; Rahman, K.; Qin, L.P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine 2009, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.M.; Moharram, A.M.; Davis, R.; Panizzi, P.; Makboul, M.A.; Calderón, A.I. Characterisation of the metabolites of an antibacterial endophyte Botryodiplodia theobromae Pat. of Dracaena draco L. by LC–MS/MS. Nat. Prod. Res. 2015, 29. [Google Scholar] [CrossRef]
- Cui, Y.; Yi, D.; Bai, X.; Sun, B.; Zhao, Y.; Zhang, Y. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 2012, 83, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Carman, R.M.; Marty, R.A. Diterpenoids. IX. Agathis microstachya oleoresin. Aust. J. Chem. 1966, 19, 2403–2406. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga V.F., Jr.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhao, P.J.; Ma, J.; Lu, C.H.; Shen, Y.M. Labdane and tetranorlabdane diterpenoids from Botryosphaeria sp. MHF, an endophytic fungus of Maytenus hookeri. Helv. Chim. Acta 2009, 92, 1118–1125. [Google Scholar] [CrossRef]
- Gardner, D.R.; Panter, K.E.; Stegelmeier, B.L. Implication of agathic acid from Utah juniper bark as an abortifacient compound in cattle. J. Appl. Toxicol. 2010, 30, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Verma, V.C.; Lamshoeft, M.; Spiteller, M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J. Microbiol. Biotechnol. 2012, 28, 1287–1294. [Google Scholar] [CrossRef]
- Dos Santos, R.M.G.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar]
- Wang, Q.; Fu, Y.; Gao, J.; Wang, Y.; Li, X.; Zhang, A. Preliminary isolation and screening of the endophytic fungi from Melia azedarach L. Acta Agric. Boreali-Occident. Sin. 2007, 16, 224–227. [Google Scholar]
- Man, S.; Gao, W.; Wei, C.; Liu, C. Anticancer drugs from traditional toxic Chinese medicines. Phytother. Res. 2012, 26, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chatare, V.; Pal, M. Isocoumarin and its derivatives: An overview on their synthesis and applications. Curr. Org. Chem. 2011, 15, 782–800. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, T.; Govindappa, M.; Ramachandra, Y.L. In vitro antioxidant and antimicrobial activity of partially purified coumarins from fungal endophytes of Crotalaria pallida. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 58–72. [Google Scholar]
- Gnonlonfin, G.B.; Sanni, A.; Brimer, L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; Cai, X.; She, Z.; Lin, Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp. (ZH16). Nat. Prod. Res. 2012, 26, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Wu, M.D.; Chen, J.J.; Hsieh, S.Y.; Yuan, G.F.; Chen, I.S.; Chang, C.W. Secondary metabolites from the endophytic fungus of Annulohypoxylon ilanense. Chem. Nat. Compd. 2013, 49, 523–525. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; She, Z.; Lin, Y. Isoflavones from the mangrove endophytic fungus Fusarium sp. (ZZF41). Nat. Prod. Commun. 2010, 5, 1771–1773. [Google Scholar] [PubMed]
- Nishikawa, E. Biochemisty of filamentous fungi. II. A metabolic product of Aspergillus melleus Yukawa. Part I. Bull. Agric. Chem. Soc. Jpn. 1933, 9, 107–109. [Google Scholar] [CrossRef]
- Krohn, K.; Bahramsari, R.; Flörke, U.; Ludewig, K.; Kliche-Spory, C.; Michel, A.; Aust, H.-J.; Draeger, S.; Schulz, B.; Antus, S. Dihydroisocoumarins from fungi: Isolation, structure elucidation, circular dichroism and biological activity. Phytochemistry 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Das, A.J. Moringa oleifera (Lamm.): A plant with immense importance. J. Biol. Act. Prod. Nat. 2012, 2, 307–315. [Google Scholar] [CrossRef]
- Chacón-Morales, P.; Amaro-Luis, J.M.; Bahsas, A. Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus. Avan. Quim. 2013, 8, 145–151. [Google Scholar]
- Oliveira, C.M.; Regasini, L.O.; Silva, G.H.; Pfenning, L.H.; Young, M.C.M.; Berlinck, R.G.S.; Bolzani, V.S.; Araujo, A.R. Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp., endophytic fungi associated with Piper aduncum and Alibertia macrophylla. Phytochem. Lett. 2011, 4, 93–96. [Google Scholar] [CrossRef]
- Fan, N.W.; Chang, H.S.; Cheng, M.J.; Hsieh, S.Y.; Liu, T.W.; Yuan, G.F.; Chen, I.S. Secondary metabolites from the endophytic fungus Xylaria cubensis. Helv. Chim. Acta 2014, 97, 1689–1699. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, Y.L.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.S.; Murgu, M.; Rodrigues-Fo, E.; de Souza, A.Q.; de Moura Sarquis, M.I. A saponin tolerant and glycoside producer xylariaceous fungus isolated from fruits of Sapindus saponaria. World J. Microbiol. Biotechnol. 2008, 24, 1341–1348. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Yuan, G.F.; Chen, Y.L.; Su, Y.S.; Hsieh, M.T.; Chen, I.S. Secondary metabolites and cytotoxic activities from the endophytic fungus Annulohypoxylon squamulosum. Phytochem. Lett. 2012, 5, 219–223. [Google Scholar] [CrossRef]
- Cheng, M.J.; Wu, M.D.; Hsieh, S.Y.; Hsieh, M.T.; Chen, I.S.; Yuan, G.F. Constituents of the endophytic fungus Annulohypoxylon boveri var. microspora BCRC 34012. Helv. Chim. Acta 2011, 94, 1108–1114. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Hyde, K.D.; Corke, H.; Sun, M. Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers. 2008, 33, 61–75. [Google Scholar]
- Qiu, M.; Xie, R.S.; Shi, Y.; Zhang, H.; Chen, H.M. Isolation and identification of two flavonoid-producing endophytic fungi from Ginkgo biloba L. Ann. Microbiol. 2010, 60, 143–150. [Google Scholar] [CrossRef]
- Huang, J.X.; Zhang, J.; Zhang, X.R.; Zhang, K.; Zhang, X.; He, X.R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014, 52, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Gajbhiye, S.; Roy, S.; Dudhale, R.; Chowdhary, A. Determination of kaempferol in extracts of Fusarium chlamydosporum, an endophytic fungi of Tylophora indica (Asclepeadaceae) and its anti-microbial activity. J. Pharm. Biol. Sci. 2014, 9, 51–55. [Google Scholar]
- Cheng, M.J.; Wu, M.D.; Hsieh, S.Y.; Su, Y.S.; Chen, I.S.; Yuan, G.F. Secondary metabolites from the endophytic fungus Annulohypoxylon boveri var. microspora BCRC 34012. Chem. Nat. Compd. 2011, 47, 536–540. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, D.; Luo, M.; Wang, W.; Zhao, C.; Zu, Y.; Fu, Y.; Wink, M. In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int. 2014, 56, 243–251. [Google Scholar] [CrossRef]
- Luo, M.; Liu, X.; Zu, Y.; Fu, Y.; Zhang, S.; Yao, L.; Efferth, T. Cajanol, a novel anticancer agent from pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2010, 188, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, C.; Wang, W.; Zhao, C.; Luo, M.; Mu, F.; Fu, Y.; Zu, Y.; Yao, M. Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (Cajanus cajan [L.] Millsp.). J. Appl. Microbiol. 2013, 115, 102–113. [Google Scholar] [PubMed]
- Gao, Y.; Zhao, J.; Zu, Y.; Fu, Y.; Liang, L.; Luo, M.; Wang, W.; Efferth, T. Antioxidant properties, superoxide dismutase and glutathione reductase activities in HepG2 cells with a fungal endophyte producing apigenin from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int. 2012, 49, 147–152. [Google Scholar] [CrossRef]
- Zhou, S.L.; Wang, M.X.; Chen, S.L. Two compounds from the endophytic Colletotrichum sp. of Ginkgo biloba. Nat. Prod. Commun. 2011, 6, 1131–1132. [Google Scholar]
- Tian, Y.; Amand, S.; Buisson, D.; Kunz, C.; Hachette, F.; Dupont, J.; Nay, B.; Prado, S. The fungal leaf endophyte Paraconiothyrium variabile specifically metabolizes the host-plant metabolome for its own benefit. Phytochemistry 2014, 108, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.L.; Goodhue, L.D.; Jones, H.A. The constituents of Derris and other rotenone-bearing plants. Chem. Rev. 1942, 30, 33–48. [Google Scholar] [CrossRef]
- Hu, M.Y.; Zhong, G.H.; Sun, Z.T.; Sh, G.; Liu, H.M.; Liu, X.Q. Insecticidal activities of secondary metabolites of endophytic Pencillium sp. in Derris elliptica Benth. J. Appl. Entomol. 2005, 129, 413–417. [Google Scholar] [CrossRef]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.-C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Agrawal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 4448–4457. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Deb, U.; Bhaskar, A.S.; Prasad, G.B.; Rao, P.V. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice. Environ. Toxicol. 2007, 22, 472–479. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Raja, H.A.; Graf, T.N.; Faeth, S.H.; Cech, N.B.; Oberlies, N.H. Flavonolignans from Aspergillus iizukae, a fungal endophyte of milk thistle (Silybum marianum). J. Nat. Prod. 2014, 77, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.B.; Mahajan, S.K.; Katti, S.A. Chalcone: A versatile molecule. J. Pharm. Sci. Res. 2009, 1, 11–22. [Google Scholar]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, L.Y.; Lu, Y.H. Ceriporia lacerata DMC1106, a new endophytic fungus: Isolation, identification, and optimal medium for 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone production. Biotechnol. Bioprocess Eng. 2013, 18, 669–678. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally occurring xanthones: Chemistry and biology. J. Appl. Chem. 2013. [Google Scholar] [CrossRef]
- Nagem, T.J.; de Oliveira, F.F. Xanthones and other constituents of Vismia parviflora. J. Braz. Chem. Soc. 1997, 8, 505–508. [Google Scholar] [CrossRef]
- Kato, L.; Alves de Oliveira, C.M.; Vencato, I.; Lauriucci, C. Crystal structure of 1,7-dihydroxyxanthone from Weddellina squamulosa Tul. J. Chem. Crystall. 2005, 35, 23–26. [Google Scholar] [CrossRef]
- Law, K.K.; Chan, T.L.; Tam, S.W.; Shatin, N.T. Synthesis of pinselic acid and pinselin. J. Org. Chem. 1979, 44, 4452–4453. [Google Scholar] [CrossRef]
- Yang, H.Y.; Gao, Y.H.; Niu, D.Y.; Yang, L.Y.; Gao, X.M.; Du, G.; Hu, Q.F. Xanthone derivatives from the fermentation products of an endophytic fungus Phomopsis sp. Fitoterapia 2013, 91, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.E.; Aumond, M.C.; Mallet, S.; Dumontet, V.; Litaudon, M.; Rondeau, D.; Richomme, P. Antioxidant xanthones from Garcinia vieillardii. J. Nat. Prod. 2004, 67, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Danielsen, K.; Aksnes, D.W.; Francis, G.W. NMR study of some anthraquinones from rhubarb. Magn. Reson. Chem. 1992, 30, 359–360. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Res. 2012, 3, 291–319. [Google Scholar]
- Jayasuriya, H.; Koonchanok, N.M.; Geahlen, R.L.; McLaughlin, J.L.; Chang, C.J. Emodin, a protein tyrosine kinase inhibitor from Polygonum cuspidatum. J. Nat. Prod. 1992, 55, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Hwang, T.L.; Hu, J.W.; Fang, J.Y. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother. Res. 2008, 22, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.M.R.; Rodrigues-Filho, E.; Moitinho, M.L.R.; Santos, L.S. Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia azedarach. J. Braz. Chem. Soc. 2005, 16, 280–283. [Google Scholar] [CrossRef]
- Wang, F.W.; Hou, Z.M.; Wang, C.R.; Li, P.; Shi, D.H. Bioactive metabolites from Penicillium sp., an endophytic fungus residing in Hopea hainanensis. World J. Microbiol. Biotechnol. 2008, 24, 2143–2147. [Google Scholar] [CrossRef]
- Marinho, A.M.R.; Marinho, P.S.B.; Santos, L.S.; Rodrigues Filho, E.; Ferreira, I.C.P. Active polyketides isolated from Penicillium herquei. Anais Acad. Bras. Ciências 2013, 85, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cai, X.L.; Yang, H.; Xia, X.K.; Guo, Z.Y.; Yuan, J.; Li, M.F.; She, Z.G.; Lin, Y.C. The bioactive metabolites of the mangrove endophytic fungus Talaromyces sp. ZH-154 isolated from Kandelia candel (L.) Druce. Planta Med. 2010, 76, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Lin, Y.C.; Zhou, S.N. Anthraquinone derivatives isolated from marine fungus #2526 from the South China Sea. Chin. J. Org. Chem. 2004, 24, 1114–1117. [Google Scholar]
- Liu, J.Y.; Song, Y.C.; Zhang, Z.; Wang, L.; Guo, Z.J.; Zou, W.X.; Tan, R.X. Aspergillus fumigatus CY018, an endophytic fungus in Cynodon dactylon as a versatile producer of new and bioactive metabolites. J. Biotechnol. 2004, 114, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chen, Y.W.; Qin, S.; Huang, R. A new spiroketal from Aspergillus terreus, an endophytic fungus in Opuntia ficusindica Mill. J. Basic Microbiol. 2008, 48, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Derksen, G.C.H.; Van Beek, T.A. Rubia tinctorum L. Stud. Nat. Prod. Chem. 2002, 26, 629–684. [Google Scholar]
- Gautam, R.; Karkhile, K.V.; Bhutani, K.K.; Jachak, S.M. Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumex nepalensis. Planta Med. 2010, 76, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Awakawa, T.; Wakimoto, T.; Abe, I. Induced production of novel prenyldepside and coumarins in endophytic fungi Pestalotiopsis acaciae. Tetrahedron Lett. 2013, 54, 5814–5817. [Google Scholar] [CrossRef]
- Ogwuru, N.; Adamczeski, M. Bioactive natural products derived from Polygonum species of plants: Their structures and mechanisms of action. Stud. Nat. Prod. Chem. 2000, 22, 607–642. [Google Scholar]
- Li, D.L.; Li, X.M.; Li, T.G.; Dang, H.Y.; Proksch, P.; Wang, B.G. Benzaldehyde derivatives from Eurotium rubrum, an endophytic fungus derived from the mangrove plant Hibiscus tiliaceus. Chem. Pharm. Bull. 2008, 56, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Metabolites from the endophytic mitosporic Dothideomycete sp. LRUB20. Phytochemistry 2009, 70, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Borges, W.D.S.; Pupo, M.T. Novel anthraquinone derivatives produced by Phoma sorghina, an endophyte found in association with the medicinal plant Tithonia diversifolia (Asteraceae). J. Braz. Chem. Soc. 2006, 17, 929–934. [Google Scholar] [CrossRef]
- Imre, S.; Sar, S.; Thomson, R.H. Anthraquinones in Digitalis species. Phytochemistry 1976, 15, 317–320. [Google Scholar] [CrossRef]
- Arrebola, M.L.; Ringbom, T.; Verpoorte, R. Anthraquinones from Isoplexis isabelliana cell suspension cultures. Phytochemistry 1999, 52, 1283–1286. [Google Scholar] [CrossRef]
- Imre, S. Flavone and anthraquinone dyes from Digitalis species. I. isolation of a new anthraquinone dye from Digitalis viridiflora leaves. Phytochemistry 1969, 8, 315. [Google Scholar] [CrossRef]
- Sun, P.; Huo, J.; Kurtan, T.; Mandi, A.; Antus, S.; Tang, H.; Draeger, S.; Schulz, B.; Hussain, H.; Krohn, K.; et al. Structural and stereochemical studies of hydroxyanthraquinone derivatives from the endophytic fungus Coniothyrium sp. Chirality 2013, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, D.; Jamal, Y.; Agusta, A. Pachybasin, a major metabolite from culture broth of endophytic Coelomyceteous AFKR-18 fungus isolated from a yellow moonsheed plant, Arcangelisia flava (L.) Merr. Hayati J. Biosci. 2014, 21, 95–100. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshöft, M.; Zühlke, S.; Spiteller, M. An endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 2008, 71, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Zühlke, S.; Kosuth, J.; Čellárová, E.; Spiteller, M. Light-independent metabolomics of endophytic Thielavia subthermophila provides insight into microbial hypericin biosynthesis. J. Nat. Prod. 2009, 72, 1825–1835. [Google Scholar] [CrossRef]
- Kumar, U.S.; Aparna, P.; Rao, R.J.; Rao, T.P.; Rao, J.M. 1-Methyl anthraquinones and their biogenetic precursors from Stereospermum personatum. Phytochemistry 2003, 63, 925–929. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.H.; Jiang, C.; Lü, J. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, D.; Wei, X. Mutation breeding of Emericella foeniculicola TR21 for improved production of tanshinone IIA. Proc. Biochem. 2011, 46, 2059–2063. [Google Scholar] [CrossRef]
- Ming, Q.L.; Han, T.; Li, W.; Zhang, Q.Y.; Zhang, H.; Zheng, C.J.; Huang, F.; Rahman, K.; Qin, L.P. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Su, C.; Zheng, C.; Jia, M.; Zhang, Q.; Zhang, H.; Rahman, K.; Han, T.; Qin, L. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Zhang, N.; Turpin, J.A.; Buckheit, R.W.; Osterling, C.; Oppenheim, J.J.; Zack Howard, O.M. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2003, 47, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Movahhedin, N.; Albadry, M.; Hamann, T. Isolation and characterization of cytotoxic compounds from endophytes of an endangered American cactus, Mammillaria hahniana. Planta Med. 2014, 80, PD136. [Google Scholar] [CrossRef]
- Epifano, F.; Genovese, S.; Fiorito, S.; Mathieu, V.; Kiss, R. Lapachol and its congeners as anticancer agents: A review. Phytochem. Rev. 2014, 13, 37–49. [Google Scholar] [CrossRef]
- Sadananda, T.S.; Nirupama, R.; Chaithra, K.; Govindappa, M.; Chandrappa, C.P.; Vinay Raghavendra, B. Antimicrobial and antioxidant activities of endophytes from Tabebuia argentea and identification of anticancer agent (lapachol). J. Med. Plants Res. 2011, 5, 3643–3652. [Google Scholar]
- Channabasava, R.; Govindappa, M. First report of anticancer agent, lapachol producing endophyte, Aspergillus niger of Tabebuia argentea and its in vitro cytotoxicity assays. Bangladesh J. Pharmacol. 2014, 9, 129–139. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcı́a, P.A.; Miguel del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Inamori, Y.; Kubo, M.; Tsujibo, H.; Ogawa, M.; Baba, K.; Kozawa, M.; Fujita, E. The biological activities of podophyllotoxin compounds. Chem. Pharm. Bull. 1986, 34, 3928–3932. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.; Di, X.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina L, and related lignans against larvae of Pieris rapae L. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- You, Y. Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. Curr. Pharm. Design 2005, 11, 1695–1717. [Google Scholar] [CrossRef]
- Yang, X.; Guo, S.; Zhang, L.; Shao, H. Select of producing podophyllotoxin endophytic fungi from podophyllin plant. Nat. Prod. Res. Dev. 2003, 15, 419–422. [Google Scholar]
- Lu, L.; He, J.; Yu, X.; Li, G.; Zhang, X. Studies on isolation and identification of endophytic fungi strain SC13 from harmaceutical plant Sabina vulgaris Ant. and metabolites. Acta Agric. Boreali-Occident. Sin. 2006, 15, 85–89. [Google Scholar]
- Eyberger, A.L.; Dondapati, R.; Porter, J.R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Ram, M.; Alam, P.; Ahmad, M.M.; Mohammad, A.; Al-Qurainy, F.; Khan, S.; Abdin, M.Z. Fusarium solani, P1, a new endophytic podophyllotoxin-producing fungus from roots of Podophyllum hexandrum. Afr. J. Microbiol. Res. 2012, 6, 2493–2499. [Google Scholar]
- Kour, A.; Shawl, A.S.; Rehman, S.; Sultan, P.; Qazi, P.H.; Suden, P.; Khajuria, R.K.; Verma, V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J. Microbiol. Biotechnol. 2008, 24, 1115–1121. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshöft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhang, L.; Guo, Y.; Lu, Y.; Lin, D. Phillyrin, a natural lignan, attenuates tumor necrosis factor α-mediated insulin resistance and lipolytic acceleration in 3T3-L1 adipocytes. Planta Med. 2014, 80, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wei, X.; Wang, J. Phillyrin produced by Colletotrichum gloeosporioides, an endophytic fungus isolated from Forsythia suspensa. Fitoterapia 2012, 83, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [PubMed]
- Vidensek, N.; Lim, P.; Campbell, A.; Carlson, C. Taxol content in bark, wood, root, leaf, twig and seedling from several Taxus species. J. Nat. Prod. 1990, 53, 1609–1610. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014, 32, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Soca-Chafre, G.; Rivera-Orduña, F.N.; Hidalgo-Lara, M.E.; Hernandez-Rodriguez, C.; Marsch, R.; Flores-Cotera, L.B. Molecular phylogeny and paclitaxel screening of fungal endophytes from Taxus globosa. Fungal Biol. 2011, 115, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Hess, W.M.; Ford, E.; Sidhu, R.S.; Yang, X. Taxol from fungal endophytes and the issue of biodiversity. J. Ind. Microbiol. 1996, 17, 417–423. [Google Scholar] [CrossRef]
- Kim, S.U.; Strobel, G.A.; Ford, E. Screening of taxol-producing endophytic fungi from Ginkgo biloba and Taxus cuspidata in Korea. Agric. Chem. Biotechnol. 1999, 42, 97–99. [Google Scholar]
- Michalczyk, A.; Cieniecka-Rosłonkiewicz, A.; Cholewińska, M. Plant endophytic fungi as a source of paclitaxel. Herba Pol. 2014, 60, 22–33. [Google Scholar]
- Zhang, P.; Zhou, P.-P.; Yu, L.-J. An endophytic taxol-producing fungus from Taxus x media, Aspergillus candidus MD3. FEMS Microbiol. Lett. 2009, 293, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ran, X.; Wang, J. Isolation and identification of a taxol-producing endophytic fungus from Podocarpus. Acta Microbiol. Sin. 2008, 48, 589–595. [Google Scholar]
- Zhao, K.; Ping, W.; Li, Q.; Hao, S.; Zhao, L.; Gao, T.; Zhou, D. Aspergillus niger var. taxi, a new species variant of taxol-producing fungus isolated from Taxus cuspidata in China. J. Appl. Microbiol. 2009, 107, 1202–1207. [Google Scholar]
- Liu, K.; Ding, X.; Deng, B.; Chen, W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J. Ind. Microbiol. Biotechnol. 2009, 36, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, V.; Muthumary, J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J. Microbiol. Biotechnol. 2008, 24, 717–724. [Google Scholar] [CrossRef]
- Hu, K.; Tan, F.; Tang, K.; Zhu, S.; Wang, W. Isolation and screening of endophytic fungi synthesizing taxol from Taxus chinensis var. mairei. J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2006, 31, 134–137. [Google Scholar]
- Zhao, K.; Zhao, L.; Jin, Y.; Wei, H.; Ping, W.; Zhou, D. Isolation of a taxol-producing endophytic fungus and inhibiting effect of the fungus metabolites on HeLa cell. Mycosystema 2008, 27, 735–744. [Google Scholar]
- Gangadevi, V.; Muthumary, J. A novel endophytic taxol-producing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl. Biochem. Biotechnol. 2009, 158, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhou, P.P.; Yu, L.J. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr. Microbiol. 2009, 59, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.G.; Manikandan, R.; Arulvasu, C.; Pandi, M. Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 667–674. [Google Scholar]
- Gangadevi, V.; Muthumary, J. Isolation of Colletotrichum gloeosporioides, a novel endophytic taxol-producing fungus from the leaves of a medicinal plant, Justicia gendarussa. Mycol. Balc. 2008, 5, 1–4. [Google Scholar]
- Nithya, K.; Muthumary, J. Growth studies of Colletotrichum gloeosporioides (Penz.) Sacc.—A taxol producing endophytic fungus from Plumeria acutifolia. Indian J. Sci. Technol. 2009, 2, 14–19. [Google Scholar]
- Xiong, Z.Q.; Yang, Y.Y.; Zhao, N.; Wang, Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Murugesan, S.; Mohan, V.; Muthumary, J. Taxol producing fungal endophyte, Colletotrichum gleospoiroides (Penz.) from Tectona grandis L. Curr. Biotica 2013, 7, 8–15. [Google Scholar]
- Raj, K.G.; Rajapriya, P.; Muthumary, J.; Pandi, M. Molecular identification and characterization of the taxol-producing Colletotrichum gloeosporioides from Moringa oleifera Linn. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R., Dubey, N., Raghuwanshi, R., Eds.; Springer India: New Delhi, India, 2014; pp. 111–120. [Google Scholar]
- Strobel, G.A.; Ford, E.; Li, J.-Y.; Sears, J.; Sidhu, R.S.; Hess, W.M. Seimatoantlerium tepuiense gen. nov., a unique epiphytic fungus producing taxol from the Venezuelan Guyana. Syst. Appl. Microbiol. 1999, 22, 426–433. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, K. A new endophytic taxol- and baccatin III-producing fungus isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2011, 10, 16379–16386. [Google Scholar]
- Li, C.-T.; Li, Y.; Wang, Q.-J.; Sung, C.-K. Taxol production by Fusarium arthrosporioides isolated from yew, Taxus cuspidata. J. Med. Biochem. 2008, 27, 454–458. [Google Scholar] [CrossRef]
- Xu, F.; Tao, W.; Chang, L.; Guo, L. Strain improvement and optimization of the media of taxol-producing fungus Fusarium mairei. Biochem. Eng. J. 2006, 31, 67–73. [Google Scholar] [CrossRef]
- Elavarasi, A.; Rathna, G.S.; Kalaiselvam, M. Taxol producing mangrove endophytic fungi Fusarium oxysporum from Rhizophora annamalayana. Asian Pac. J. Trop. Biomed. 2012, 2, S1081–S1085. [Google Scholar] [CrossRef]
- Garyali, S.; Reddy, M.S. Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J. Microbiol. Biotechnol. 2013, 23, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, B.V.S.K.; Das, P.; Surendranath, K.; Karande, A.A.; Jayabaskaran, C. Production of paclitaxel by Fusarium solani isolated from Taxus celebica. J. Biosci. 2008, 33, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.W.; Liu, K.H.; Chen, W.Q.; Ding, X.W.; Xie, X.C. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J. Microbiol. Biotechnol. 2009, 25, 139–143. [Google Scholar] [CrossRef]
- Gogoi, D.K.; Deka Boruah, H.P.; Saikia, R.; Bora, T.C. Optimization of process parameters for improved production of bioactive metabolite by a novel endophytic fungus Fusarium sp. DF2 isolated from Taxus wallichiana of North East India. World J. Microbiol. Biotechnol. 2008, 24, 79–87. [Google Scholar] [CrossRef]
- Sreekanth, D.; Syed, A.; Sarkar, S.; Sarkar, D.; Santhakumari, B.; Ahmad, A.; Khan, I. Production, purification and characterization of taxol and 10DAB III from a new endophytic fungus Gliocladium sp. isolated from the Indian yew tree, Taxus baccata. J. Microbiol. Biotechnol. 2009, 19, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Pandi, M.; Kumaran, R.S.; Choi, Y.-K.; Kim, H.J.; Muthumary, J. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodia theobromae, an endophytic fungus of the medicinal plant Morinda citrifolia. Afr. J. Biotechnol. 2011, 10, 1428–1435. [Google Scholar]
- Venkatachalam, R.; Subban, K.; Paul, M.J. Taxol from Botryodiplodia theobromae (BT 115)-an endophytic fungus of Taxus baccata. J. Biotechnol. 2008, 136, S189–S190. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Y.; Yu, X.; Guo, B.; Tang, K. A new endophytic taxane production fungus from Taxus chinensis. Appl. Biochem. Microbiol. 2009, 45, 81–86. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Zhu, H.; Tang, K. Identification of a taxol-producing endophytic fungus EFY-36. Afr. J. Biotechnol. 2009, 8, 2623–2625. [Google Scholar]
- Zhao, K.; Zhou, D.P.; Ping, W.X.; Ge, J. Study on the preparation and regeneration of protoplast from taxol-producing fungus Nodulisporium sylviforme. Nat. Sci. 2004, 2, 52–59. [Google Scholar]
- Guo, B.H.; Wang, Y.C.; Zhou, X.W.; Hu, K.; Tan, F.; Miao, Z.Q.; Tang, K.X. An endophytic taxol-producing fungus BT2 isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2006, 5, 875–877. [Google Scholar]
- Soliman, S.S.M.; Tsao, R.; Raizada, M.N. Chemical inhibitors suggest endophytic fungal paclitaxel is derived from both mevalonate and non-mevalonate-like pathways. J. Nat. Prod. 2011, 74, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, H.; Barrero, R.A.; Zhang, B.; Sun, G.; Wilson, I.W.; Xie, F.; Walker, K.D.; Parks, J.W.; Bruce, R.; et al. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genomics 2014, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Stierle, D.; Stierle, S. Bioactive compounds from four endophytic Penicillium sp. of a northwest pacific yew tree. Nat. Prod. Chem. 2000, 24, 933–977. [Google Scholar]
- Liu, J.J.; Gong, H.X.; Yang, D.L.; Chen, S.J.; Yang, L. Study on endophytic fungi producing taxol isolated from Taxus yunnanensis. Prog. Mod. Biomed. 2006, 6, 53–55. [Google Scholar]
- Wang, Y.; Ma, Z.; Hu, F.; Fan, M.; Li, Z. Isolation and screening of endophytic fungi producing taxol from Taxus chinensis of Huangshan. Nat. Prod. Res. Dev. 2014, 26, 1624–1627. [Google Scholar]
- Li, J.Y.; Sidhu, R.S.; Ford, E.J.; Long, D.M.; Hess, W.M.; Strobel, G.A. The induction of taxol production in the endophytic fungus—Periconia sp. from Torreya grandifolia. J. Ind. Microbiol. Biotechnol. 1998, 20, 259–264. [Google Scholar] [CrossRef]
- Gangadevi, V.; Murugan, M.; Muthumary, J. Taxol determination from Pestalotiopsis pauciseta, a fungal endophyte of a medicinal plant. Chin. J. Biotechnol. 2008, 24, 1433–1438. [Google Scholar] [CrossRef]
- Vennila, R.; Thirunavukkarasu, S.V.; Muthumary, J. In-vivo studies on anticancer activity of taxol isolated from an endophytic fungus Pestalotiopsis pauciseta Sacc. VM1. Asian J. Pharm. Clin. Res. 2010, 3, 30–34. [Google Scholar]
- Strobel, G.A.; Hess, W.M.; Li, J.-Y.; Ford, E.; Sears, J.; Sidhu, R.S.; Summerell, B. Pestalotiopsis guepinii, a taxol-producing endophyte of the Wollemi pine, Wollemia nobilis. Aust. J. Bot. 1997, 45, 1073–1082. [Google Scholar] [CrossRef]
- Strobel, G.; Yang, X.; Sears, J.; Kramer, R.; Sidhu, R.S.; Hess, W.M. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 1996, 142, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Strobel, G.; Sidhu, R.; Hess, W.M.; Ford, E.J. Endophytic taxol producing fungi from bald cypress Taxodium distichum. Microbiology 1996, 142, 2223–2226. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Kim, H.J.; Hur, B.-K. Taxol promising fungal endophyte, Pestalotiopsis species isolated from Taxus cuspidata. J. Biosci. Bioeng. 2010, 110, 541–546. [Google Scholar]
- Srinivasan, K.; Muthumary, J. Taxol production from Pestalotiopsis sp. an endophytic fungus isolated from Catharanthus roseus. J. Ecobiotechnol. 2009, 1, 28–31. [Google Scholar]
- Gangadevi, V.; Muthumary, J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun tree). Biotechnol. Appl. Biochem. 2009, 52, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Choi, Y.K.; Lee, S.; Jeon, H.J.; Jung, H.; Kim, H.J. Isolation of taxol, an anticancer drug produced by the endophytic fungus, Phoma betae. Afr. J. Biotechnol. 2014, 11, 950–960. [Google Scholar]
- Kumaran, R.S.; Hur, B.-K. Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol. Biotechnol. Appl. Biochem. 2009, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Muthumary, J.; Hur, B.-K. Isolation and identification of taxol, an anticancer drug from Phyllosticta melochiae Yates, an endophytic fungus of Melochia corchorifolia L. Food Sci. Biotechnol. 2008, 17, 1246–1253. [Google Scholar]
- Gangadevi, V.; Muthumary, J. Endophytic fungal diversity from young, mature and senescent leaves of Ocimum basilicum L. with special reference to taxol production. Indian J. Sci. Technol. 2007, 1, 1–12. [Google Scholar]
- Kumaran, R.S.; Muthumary, J.; Hur, B.-K. Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp. Eng. Life Sci. 2008, 8, 438–446. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Zhang, P.; Wang, C.; Liu, B.; Liu, T.; Fu, C.; Yu, L. Isolation and identification of a taxol-producing endophytic fungus identified from Taxus media. Agric. Sci. Technol. Hunan 2010, 11, 38–40. [Google Scholar]
- Bashyal, B.; Li, J.Y.; Strobel, G.; Hess, W.M.; Sidhu, R. Seimatoantlerium nepalense, an endophytic taxol producing coelomycete from Himalayan yew (Taxus wallachiana). Mycotaxon 1999, 72, 33–42. [Google Scholar]
- Shrestha, K.; Strobel, G.A.; Shrivastava, S.P.; Gewali, M.B. Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med. 2001, 67, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Hess, W.M.; Baird, G.; Ford, E.; Li, J.Y.; Sidhu, R.S. Stegolerium kukenani gen. et sp. nov. an endophytic, taxol producing fungus from the Roraima and Kukenan tepuis of Venezuela. Mycotaxon 2001, 78, 353–361. [Google Scholar]
- Mirjalili, M.H.; Farzaneh, M.; Bonfill, M.; Rezadoost, H.; Ghassempour, A. Isolation and characterization of Stemphylium sedicola SBU-16 as a new endophytic taxol-producing fungus from Taxus baccata grown in Iran. FEMS Microbiol. Lett. 2012, 328, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Li, A.M.; Wang, X.L. Separation of a fungus producing taxol. Sci. China Ser. C. 2001, 44, 156–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Li, G.; Lu, H.; Zheng, Z.; Huang, Y.; Su, W. Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol. Lett. 2000, 193, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G. The phytochemistry of the yew tree. Nat. Prod. Rep. 1995, 12, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zaiyou, J.; Li, M.; Guifang, X.; Xiuren, Z. Isolation of an endophytic fungus producing baccatin III from Taxus wallichiana var. mairei. J. Ind. Microbiol. Biotechnol. 2013, 40, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Zhou, X.; Zhao, W.; Jian, Z. Isolation and identification of a 10-deacetyl baccatin-III-producing endophyte from Taxus wallichiana. Appl. Biochem. Biotechnol. 2015, 175, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Colombo, A.L.; Fedeli, L.; Pavesi, A.; Quaroni, S.; Saracchi, M.; Ventrella, G. Isolation of endophytic fungi and actinomycetes taxane producers. Ann. Microbiol. 2000, 50, 3–14. [Google Scholar]
- Staniek, A.; Woerdenbag, H.J.; Kayser, O. Screening the endophytic flora of Wollemia nobilis for alternative paclitaxel sources. J. Plant Interact. 2010, 5, 189–195. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on camptothecin biosynthesis. J. Nat. Prod. 2011, 74, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Qu, X.; Chen, F.; Bao, J.; Zhang, G.; Luo, Y. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 2013, 97, 9365–9375. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Reinscheid, U.M. Camptothecin-resistant fungal endophytes of Camptotheca acuminata. Mycol. Prog. 2004, 3, 189–192. [Google Scholar] [CrossRef]
- Kusari, S.; Košuth, J.; Čellárová, E.; Spiteller, M. Survival-strategies of endophytic Fusarium solani against indigenous camptothecin biosynthesis. Fungal Ecol. 2011, 4, 219–223. [Google Scholar] [CrossRef]
- Liu, K.; Ding, X.; Deng, B.; Chen, W. 10-Hydroxycamptothecin produced by a new endophytic Xylaria sp., M20, from Camptotheca acuminata. Biotechnol. Lett. 2010, 32, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, A.; Srivastava, S. Enhanced camptothecin production by ethanol addition in the suspension culture of the endophyte, Fusarium solani. Bioresour. Technol. 2015, 188, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.C.; Verma, V.; Amna, T.; Qazi, G.N.; Spiteller, M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J. Nat. Prod. 2005, 68, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Puri, S.C.; Verma, V.; Sharma, J.P.; Khajuria, R.K.; Musarrat, J.; Spiteller, M.; Qazi, G.N. Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Can. J. Microbiol. 2006, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Shawl, A.S.; Kour, A.; Andrabi, R.; Sudan, P.; Sultan, P.; Verma, V.; Qazi, G.N. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl. Biochem. Microbiol. 2008, 44, 203–209. [Google Scholar] [CrossRef]
- Rehman, S.; Shawl, A.S.; Kour, A.; Sultan, P.; Ahmad, K.; Khajuria, R.; Qazi, G.N. Comparative studies and identification of camptothecin produced by an endophyte at shake flask and bioreactor. Nat. Prod. Res. 2009, 23, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Zühlke, S.; Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 2009, 72, 2–7. [Google Scholar] [CrossRef]
- Shweta, S.; Zuehlke, S.; Ramesha, B.T.; Priti, V.; Kumar, P.M.; Ravikanth, G.; Spiteller, M.; Vasudeva, R.; Shaanker, R.U. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 2010, 71, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Gurudatt, P.S.; Priti, V.; Shweta, S.; Ramesha, B.T.; Ravikanth, G.; Vasudeva, R.; Amna, T.; Deepika, S.; Ganeshaiah, K.N.; Uma Shaanker, R.; et al. Attenuation of camptothecin production and negative relation between hyphal biomass and camptothecin content in endophytic fungal strains isolated from Nothapodytes nimmoniana Grahm (Icacinaceae). Curr. Sci. 2010, 98, 1006–1010. [Google Scholar]
- Min, C.; Wang, X. Isolation and identification of the 10-hydroxycamptothecin-producing endophytic fungi from Camptotheca acuminata Decne. Acta Bot. Boreal.-Occident. Sin. 2009, 29, 614–617. [Google Scholar]
- Shweta, S.; Gurumurthy, B.R.; Ravikanth, G.; Ramanan, U.S.; Shivanna, M.B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 2013, 20, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Musavi, S.F.; Dhavale, A.; Balakrishnan, R.M. Optimization and kinetic modeling of cell-associated camptothecin production from an endophytic Fusarium oxysporum NFX06. Prep. Biochem. Biotechnol. 2015, 45, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Neto, V.F.; Brandão, M.G.L.; Stehmann, J.R.; Oliveira, L.A.; Krettli, A.U. Antimalarial activity of Cinchona-like plants used to treat fever and malaria in Brazil. J. Ethnopharmacol. 2003, 87, 253–256. [Google Scholar] [CrossRef]
- Maehara, S.; Simanjuntak, P.; Kitamura, C.; Ohashi, K.; Shibuya, H. Cinchona alkaloids are also produced by an endophytic filamentous fungus living in Cinchona plant. Chem. Pharm. Bull. 2011, 59, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Simanjuntak, P.; Kitamura, C.; Ohashi, K.; Shibuya, H. Bioproduction of Cinchona alkaloids by the endophytic fungus Diaporthe sp. associated with Cinchona ledgeriana. Chem. Pharm. Bull. 2012, 60, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Simanjuntak, P.; Maetani, Y.; Kitamura, C.; Ohashi, K.; Shibuya, H. Ability of endophytic filamentous fungi associated with Cinchona ledgeriana to produce Cinchona alkaloids. J. Nat. Med. 2013, 67, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, D.; Agastian, P. Berberine production by endophytic fungus Fusarium solani from Coscinium fenestratum. Int. J. Biol. Pharm. Res. 2013, 4, 1239–1245. [Google Scholar]
- Grenby, T.H. The use of sanguinarine in mouthwashes and toothpaste compared with some other antimicrobial agents. Br. Dent. J. 1995, 178, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Min, C.; Ge, M.; Zuo, R. An endophytic sanguinarine-producing fungus from Macleaya cordata, Fusarium proliferatum BLH51. Curr. Microbiol. 2014, 68, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.A.; Ducki, S.; Hirst, N.; McGown, A.T. Tubulin and microtubules as targets for anticancer drugs. Prog. Cell Cycle Res. 2002, 5, 309–325. [Google Scholar]
- Guo, B.; Li, H.; Zhang, L. Isolation of a fungus producting vinblastine. J. Yunnan Univ. (Nat. Sci.) 1997, 20, 214–215. [Google Scholar]
- Zhang, L.; Guo, B.; Li, H.; Zeng, S.; Shao, H.; Gu, S.; Wei, R. Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin. Tradit. Herb. Drugs 1999, 31, 805–807. [Google Scholar]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ahmad, A. Biotransformation of vinblastine to vincristine by the endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. Biocatal. Biotransform. 2013, 31, 89–93. [Google Scholar] [CrossRef]
- Yin, H.; Sun, Y.H. Vincamine-producing endophytic fungus isolated from Vinca minor. Phytomedicine 2011, 18, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Sunila, E.S.; Kuttan, G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Ethnopharmacol. 2004, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Norman, S.; Krongkarn, C. Piperine is anti-hyperlipidemic and improves endothelium-dependent vasorelaxation in rats on a high cholesterol diet. J. Physiol. Biomed. Sci. 2012, 25, 27–30. [Google Scholar]
- Huan, Q.U.; Min, L.V.; Hui, X.U. Piperine: Bioactivities and structural modifications. Mini Rev. Med. Chem. 2015, 15, 145–156. [Google Scholar]
- Verma, V.C.; Lobkovsky, E.; Gange, A.C.; Singh, S.K.; Prakash, S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J. Antibiot. 2011, 64, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Chithra, S.; Jasim, B.; Anisha, C.; Mathew, J.; Radhakrishnan, E.K. LC-MS/MS based identification of piperine production by endophytic Mycosphaerella sp. PF13 from Piper nigrum. Appl. Biochem. Biotechnol. 2014, 173, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Chithra, S.; Jasim, B.; Sachidanandan, P.; Jyothis, M.; Radhakrishnan, E.K. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine 2014, 21, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liang, J.; Li, Q.; Kong, X.; Chen, R.; Jin, Y. Cladosporium cladosporioides XJ-AC03, an aconitine-producing endophytic fungus isolated from Aconitum leucostomum. World J. Microbiol. Biotechnol. 2013, 29, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2012, 17, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Kumara, P.M.; Soujanya, K.N.; Ravikanth, G.; Vasudeva, R.; Ganeshaiah, K.N.; Shaanker, R.U. Rohitukine, a chromone alkaloid and a precursor of flavopiridol, is produced by endophytic fungi isolated from Dysoxylum binectariferum Hook.f and Amoora rohituka (Roxb). Wight & Arn. Phytomedicine 2014, 21, 541–546. [Google Scholar] [PubMed]
- Kumara, P.M.; Zuehlke, S.; Priti, V.; Ramesha, B.T.; Shweta, S.; Ravikanth, G.; Vasudeva, R.; Santhoshkumar, T.R.; Spiteller, M.; Uma Shaanker, R. Fusarium proliferatum, an endophytic fungus from Dysoxylum binectariferum Hook.f, produces rohitukine, a chromane alkaloid possessing anti-cancer activity. Antonie Van Leeuwenhoek 2012, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Hou, K.; Gao, F.; Hu, B.; Chen, Q.; Wu, W. Peimisine and peiminine production by endophytic fungus Fusarium sp. isolated from Fritillaria unibracteata var. wabensis. Phytomedicine 2014, 21, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Gardner, D.R.; Pfister, J.A. Swainsonine-containing plants and their relationship to endophytic fungi. J. Agric. Food Chem. 2014, 62, 7326–7334. [Google Scholar] [CrossRef] [PubMed]
- Pryor, B.M.; Creamer, R.; Shoemaker, R.A.; McLain-Romero, J.; Hambleton, S. Undifilum, a new genus for endophytic Embellisia oxytropis and parasitic Helminthosporium bornmuelleri on legumes. Botany 2009, 87, 178–194. [Google Scholar] [CrossRef]
- Cook, D.; Gardner, D.R.; Grum, D.; Pfister, J.A.; Ralphs, M.H.; Welch, K.D.; Green, B.T. Swainsonine and endophyte relationships in Astragalus mollissimus and Astragalus lentiginosus. J. Agric. Food Chem. 2011, 59, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Romero, J.; Liddell, C.; Creamer, R. Production of swainsonine by fungal endophytes of locoweed. Mycol. Res. 2003, 107, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Ralphs, M.H.; Creamer, R.; Baucom, D.; Gardner, D.R.; Welsh, S.L.; Graham, J.D.; Hart, C.; Cook, D.; Stegelmeier, B.L. Relationship between the endophyte Embellisia spp. and the toxic alkaloid swainsonine in major locoweed species (Astragalus and Oxytropis). J. Chem. Ecol. 2008, 34, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Baucom, D.L.; Romero, M.; Belfon, R.; Creamer, R. Two new species of Undifilum, fungal endophytes of Astragalus (locoweeds) in the United States. Botany 2012, 90, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Grum, D.S.; Cook, D.; Baucom, D.; Mott, I.W.; Gardner, D.R.; Creamer, R.; Allen, J.G. Production of the alkaloid swainsonine by a fungal endophyte in the host Swainsona canescens. J. Nat. Prod. 2013, 76, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, J.; Lu, W.; Ma, Y.; Zhao, B.; Wang, J. Isolation and identification of swainsonine-producing fungi found in locoweeds and their rhizosphere soil. Afr. J. Microbiol. Res. 2012, 6, 4959–4969. [Google Scholar]
- Chen, S.; Zhao, B.; Gao, R.; Li, R. The progress in research of swainsonine immunogenicity and anticancer-activity. Nat. Prod. Res. Dev. 2003, 16, 66–70. [Google Scholar]
- Fiaux, H.; Kuntz, D.A.; Hoffman, D.; Janzer, R.C.; Gerber-Lemaire, S.; Rose, D.R.; Juillerat-Jeanneret, L. Functionalized pyrrolidine inhibitors of human type II α-mannosidases as anti-cancer agents: Optimizing the fit to the active site. Bioorg. Med. Chem. 2008, 16, 7337–7346. [Google Scholar] [CrossRef] [PubMed]
- Oldrup, E.; McLain-Romero, J.; Padilla, A.; Moya, A.; Gardner, D.; Creamer, R. Localization of endophytic Undifilum fungi in locoweed seed and influence of environmental parameters on a locoweed in vitro culture system. Botany 2010, 88, 512–521. [Google Scholar] [CrossRef]
- Hipkin, C.R.; Simpson, D.J.; Wainwright, S.J.; Salem, M.A. Nitrification by plants that also fix nitrogen. Nature 2004, 430, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Isarangkul, D.; Kittakoop, P. 3-Nitropropionic acid (3-NPA), a potent antimycobacterial agent from endophytic fungi: Is 3-NPA in some plants produced by endophytes? J. Nat. Prod. 2005, 68, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.T.; Touster, O.; Brockman, J.E. The production of beta-nitropropionic acid by a strain of Aspergillus flavus. J. Biol. Chem. 1951, 188, 685–693. [Google Scholar] [PubMed]
- Hershenhorn, J.; Vurro, M.; Zonn, M.C.; Stierle, A.; Strobel, G. Septoria cirsii, a potential biocontrol agent of Canada thistle and its phytotoxin—β-nitropropionic acid. Plant Sci. 1993, 94, 227–234. [Google Scholar] [CrossRef]
- Wei, D.-L.; Chang, S.-C.; Lin, S.-C.; Doong, M.-L.; Jong, S.-C. Production of 3-nitropropionic acid by Arthrinium species. Curr. Microbiol. 1994, 28, 1–5. [Google Scholar] [CrossRef]
- Schwarz, M.; Köpcke, B.; Weber, R.W.; Sterner, O.; Anke, H. 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry 2004, 65, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.C.; Pamphile, J.A.; Sarragiotto, M.H.; Clemente, E. Production of 3-nitropropionic acid by endophytic fungus Phomopsis longicolla isolated from Trichilia elegans A. JUSS ssp. elegans and evaluation of biological activity. World J. Microbiol. Biotechnol. 2013, 29, 923–932. [Google Scholar] [PubMed]
- Elsässer, B.; Krohn, K.; Flörke, U.; Root, N.; Aust, H.J.; Draeger, S.; Schulz, B.; Antus, S.; Kurtán, T. X-ray structure determination, absolute configuration and biological activity of phomoxanthone A. Eur. J. Org. Chem. 2005, 21, 4563–4570. [Google Scholar] [CrossRef]
- Netala, V.R.; Ghosh, S.B.; Bobbu, P.; Anitha, D.; Tartte, V. Triterpenoid saponins: A review on biosynthesis, applications and mechanism of their action. Int. J. Pharm. Pharm. Sci. 2014, 7, 24–28. [Google Scholar]
- Osbourn, A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996, 1, 4–9. [Google Scholar] [CrossRef]
- Dhankhar, S.; Kumar, S.; Dhankhar, S.; Yadav, J.P. Antioxidant activity of fungal endophytes isolated from Salvadora oleoides Decne. Int. J. Pharm. Pharm. Sci. 2012, 4, 381–385. [Google Scholar]
- Prabavathy, D.; Nachiyar, C. Cytotoxic potential and phytochemical analysis of Justicia beddomei and its endophytic Aspergillus sp. Asian J. Pharm. Clin. Res. 2013, 6, 159–161. [Google Scholar]
- Nath, A.; Joshi, S.R. Bioactivity assessment of endophytic fungi associated with the ethnomedicinal plant Potentilla fulgens. World J. Pharm. Res. 2013, 2, 2596–2607. [Google Scholar]
- Govindappa, M.; Bharath, N.; Shruthi, H.B.; Santoyo, G. In vitro antioxidant activity and phytochemical screening of endophytic extracts of Crotalaria pallida. Free Radic. Antioxid. 2011, 1, 79–86. [Google Scholar] [CrossRef]
- Govindappa, M.; Channabasava, R.; Sowmya, D.V.; Meenakshi, J.; Shreevidya, M.R.; Lavanya, A.; Santoyo, G.; Sadananda, T.S. Phytochemical screening, antimicrobial and in vitro anti-inflammatory activity of endophytic extracts from Loranthus sp. Pharmacognosy J. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Pragathi, D.; Vijaya, T.; Mouli, K.C.; Anitha, D. Diversity of fungal endophytes and their bioactive metabolites from endemic plants of Tirumala hills-Seshachalam biosphere reserve. Afr. J. Biotechnol. 2013, 12, 4317–4323. [Google Scholar]
- Saraswaty, V.; Srikandace, Y.; Simbiyani, N.A.; Setiyanto, H.; Udin, Z. Antioxidant activity and total phenolic content of endophytic fungus Fennellia nivea NRRL 5504. Pakistan J. Biol. Sci. 2013, 16, 1574–1578. [Google Scholar] [CrossRef]
- Karunai Selvi, B.; Balagengatharathilagam, P. Isolation and screening of endophytic fungi from medicinal plants of Virudhunagar district for antimicrobial activity. Int. J. Sci. Nat. 2014, 5, 147–155. [Google Scholar]
- Yadav, M.; Yadav, A.; Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, S256–S261. [Google Scholar] [CrossRef]
- Nath, A.; Chattopadhyay, A.; Joshi, S.R. Biological activity of endophytic fungi of Rauwolfia serpentina Benth: An ethnomedicinal plant used in folk medicines in Northeast India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 233–240. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.; You, X.; Li, Y. Isolation and characterization of saponin-producing fungal endophytes from Aralia elata in Northeast China. Int. J. Mol. Sci. 2012, 13, 16255–16266. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, H.; You, X.; Li, Y. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus 2013, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; Dang, L.Z.; Li, J.F.; Zou, C.G.; Zhang, K.Q.; Li, G.H. Biotransformation of saponins by endophytes isolated from Panax notoginseng. Chem. Biodivers. 2013, 10, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.H.; Du, X.W.; Xu, Y.; Xu, X.M.; Mou, J.C.; Yu, D.; Wu, J.K.; Meng, F.J.; Liu, Y.; Wang, W.L.; et al. Screening for differentially expressed genes in endophytic fungus strain 39 during co-culture with herbal extract of its host Dioscorea nipponica Makino. Curr. Microbiol. 2014, 69, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosgenin: A concise report. Nat. Prod. Bioprospect. 2012, 2, 46–52. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, X.; Yang, C.; Wu, X.; Zhang, L. Endophytic fungi of Paris polyphylla var. yunnanensis and steroid analysis in the fungi. Nat. Prod. Res. Dev. 2004, 16, 198–200. [Google Scholar]
- Cao, X.; Li, J.; Zhou, L.; Xu, L.; Li, J.; Zhao, J. Determination of diosgenin content of the endophytic fungi from Paris polyphylla var. yunnanensis by using an optimum ELISA. Nat. Prod. Res. Dev. 2007, 19, 1020–1023. [Google Scholar]
- Zhang, R.; Li, P.; Xu, L.; Chen, Y.; Sui, P.; Zhou, L.; Li, J. Enhancement of diosgenin production in Dioscorea zingiberensis cell culture by oligosaccharide elicitor from its endophytic fungus Fusarium oxysporum Dzf17. Nat. Prod. Commun. 2009, 4, 1459–1462. [Google Scholar] [PubMed]
- Li, P.; Mou, Y.; Shan, T.; Xu, J.; Li, Y.; Lu, S.; Zhou, L. Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules 2011, 16, 9003–9016. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, P.; Li, H.; Xu, L.; Zhao, J.; Shan, T.; Zhou, L.G. Enhancement of diosgenin production in Dioscorea zingiberensis seedling and cell cultures by beauvericin from the endophytic fungus Fusarium redolens Dzf2. J. Med. Plants Res. 2011, 5, 6550–6554. [Google Scholar]
- Parthasarathy, R.; Sathiyabama, M. Gymnemagenin-producing endophytic fungus isolated from a medicinal plant Gymnema sylvestre R. Br. Appl. Biochem. Biotechnol. 2014, 172, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, Q.; Liu, Y.; Pan, Z. Alternaria sp. MG1, a resveratrol-producing fungus: Isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 2012, 95, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, Y.; Luo, M.; Zu, Y.; Wang, W.; Zhao, C.; Gu, C. Endophytic fungi from pigeon pea (Cajanus cajan (L.) Millsp.) produce antioxidant cajaninstilbene acid. J. Agric. Food Chem. 2012, 60, 4314–4319. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Nicoletti, R.; Ferranti, P.; Caira, S.; Misso, G.; Castellano, M.; Di Lorenzo, G.; Caraglia, M. Myrtucommulone production by a strain of Neofusicoccum australe endophytic in myrtle (Myrtus communis). World J. Microbiol. Biotechnol. 2014, 30, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Budhiraja, A.; Nepali, K.; Sapra, S.; Gupta, S.; Kumar, S.; Dhar, K.L. Bioactive metabolites from an endophytic fungus of Aspergillus species isolated from seeds of Gloriosa superba Linn. Med. Chem. Res. 2013, 22, 323–329. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Q.; Sun, F.M.; An, T. Gentiopicrin-producing endophytic fungus isolated from Gentiana macrophylla. Phytomedicine 2009, 16, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Ahmed, M.; Zargar, K.; Sharma, P.; Dhar, M.K. Prospecting endophytic fungal assemblage of Digitalis lanata Ehrh. (foxglove) as a novel source of digoxin: A cardiac glycoside. 3 Biotech 2013, 3, 335–340. [Google Scholar] [CrossRef]
- Wu, S.B.; Long, C.; Kennelly, E.J. Structural diversity and bioactivities of natural benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.W.; Ye, Y.H.; Ding, H.; Chen, Y.X.; Tan, R.X.; Song, Y.C. Benzophenones from Guignardia sp. IFB-E028, an endophyte on Hopea hainanensis. Chem. Biodivers. 2010, 7, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Otsuki, S.; Sakurai, H.; Yamashita, K.; Ozeki, T.; Oshima, Y. Benzophenones from an endophytic fungus, Graphiopsis chlorocephala, from Paeonia lactiflora cultivated in the presence of an NAD+-dependent HDAC inhibitor. Org. Lett. 2013, 15, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, X.M.; Li, C.S.; Wang, B.G. Diphenyl ether and benzophenone derivatives from the marine mangrove-derived fungus Penicillium sp. MA-37. Phytochem. Lett. 2014, 9, 22–25. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Houbraken, J.; Popma, S.; Samson, R.A. Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum, producing the anticancer compound asperphenamate. FEMS Microbiol. Lett. 2013, 339, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.; Totzke, F.; Zirrgiebel, U.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Onocha, P.A.; Okorie, D.A.; Connolly, J.D.; Roycroft, D.S. Monoterpene diol, iridoid glucoside and dibenzo-α-pyrone from Anthocleista djalonensis. Phytochemistry 1995, 40, 1183–1189. [Google Scholar] [CrossRef]
- Shibuya, H.; Kitamura, C.; Maehara, S.; Nagahata, M.; Winarno, H.; Simanjuntak, P.; Kim, H.-S.; Wataya, Y.; Ohashi, K. Transformation of Cinchona alkaloids into 1-N-oxide derivatives by endophytic Xylaria sp. isolated from Cinchona pubescens. Chem. Pharm. Bull. 2003, 51, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Agusta, A.; Maehara, S.; Ohashi, K.; Simanjuntak, P.; Shibuya, H. Stereoselective oxidation at C-4 of flavans by the endophytic fungus Diaporthe sp. isolated from a tea plant. Chem. Pharm. Bull. 2005, 53, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Agusta, A.; Ohashi, K.; Maehara, S.; Simanjuntak, P. Biooxidation of (+)-catechin and (−)-epicatechin into 3, 4-dihydroxyflavan derivatives by the endophytic fungus Diaporthe sp. isolated from a tea plant. Chem. Pharm. Bull. 2005, 53, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Ikeda, M.; Haraguchi, H.; Kitamura, C.; Nagoe, T.; Ohashi, K.; Shibuya, H. Microbial conversion of curcumin into colorless hydroderivatives by the endophytic fungus Diaporthe sp. associated with Curcuma longa. Chem. Pharm. Bull. 2011, 59, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Agusta, A.; Wulansari, D.; Nurkanto, A.; Fathoni, A. Biotransformation of protoberberine alkaloids by the endophytic fungus Coelomycetes AFKR-3 isolated from yellow moonsheed plant (Archangelisia flava (L.) Merr.). Proc. Chem. 2014, 13, 38–43. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhang, W.; Su, C.L.; Zhu, H.; Dai, C.C. Biodegradation of the phytoestrogen luteolin by the endophytic fungus Phomopsis liquidambari. Biodegradation 2015, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.W.; Li, L.; Dai, C.C. The potential application of the endophyte Phomopsis liquidambari to the ecological remediation of long-term cropping soil. Appl. Soil Ecol. 2013, 67, 20–26. [Google Scholar] [CrossRef]
- Heinig, U.; Scholz, S.; Jennewein, S. Getting to the bottom of taxol biosynthesis by fungi. Fungal Divers. 2013, 60, 161–170. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Hertweck, C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 2005, 437, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Partida-Martinez, L.P.; Groth, I.; Schmitt, I.; Richter, W.; Roth, M.; Hertweck, C. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus. Int. J. Syst. Evol. Microbiol. 2007, 57, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.T.; Arnold, A.E. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl. Environ. Microbiol. 2010, 76, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, H.; Shimaji, M.; Sakai, A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. Nature 1987, 328, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.; Raizada, M.N. Interactions between co-habitating fungi elicit synthesis of taxol from an endophytic fungus in host Taxus plants. Front. Microbiol. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, R.; Fiorentino, A. Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta. Agriculture 2015, 5, 918-970. https://doi.org/10.3390/agriculture5040918
Nicoletti R, Fiorentino A. Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta. Agriculture. 2015; 5(4):918-970. https://doi.org/10.3390/agriculture5040918
Chicago/Turabian StyleNicoletti, Rosario, and Antonio Fiorentino. 2015. "Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta" Agriculture 5, no. 4: 918-970. https://doi.org/10.3390/agriculture5040918
APA StyleNicoletti, R., & Fiorentino, A. (2015). Plant Bioactive Metabolites and Drugs Produced by Endophytic Fungi of Spermatophyta. Agriculture, 5(4), 918-970. https://doi.org/10.3390/agriculture5040918







