Recent Advances in Nematicides and Their Modes of Action
Abstract
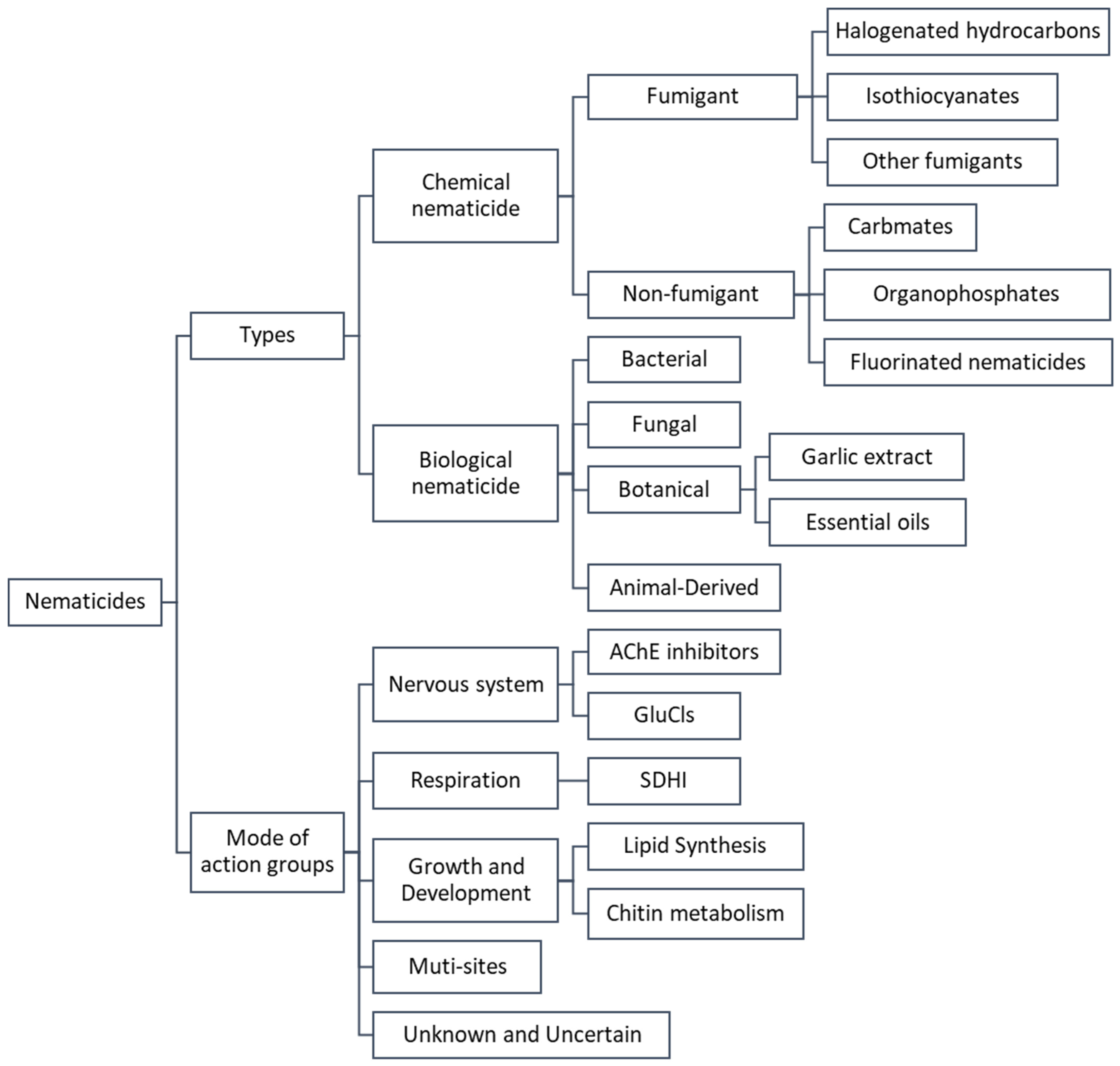
1. Overview
2. Methods
2.1. Study Selection
- (1)
- peer-reviewed original research or review articles;
- (2)
- clear description of nematicide categories, biochemical or physiological modes of action, field efficacy, or target specificity;
- (3)
- research addressing non-target toxicity, environmental behavior, or applications within integrated pest management (IPM).
2.2. Data Extraction and Synthesis
3. Mode of Action of the Main Nematicide Groups
3.1. Disruption of Nematode Nervous System
3.1.1. Acetylcholinesterase Inhibitors (Organophosphates and Carbamates)
3.1.2. Allosteric Modulators of Glutamate-Gated Chloride Channels (Avermectins)
3.2. Inhibition of Nematode Respiration
Succinate Dehydrogenase Inhibitors (Fluopyram, Cyclobutrifluram)
3.3. Growth and Development Regulation
3.3.1. Lipid Biosynthesis and Acetyl-CoA Carboxylase Inhibitors (Spirotetramat)
3.3.2. Chitin Biosynthesis and Degradation
3.4. Multi-Site Activity—Fumigants
3.4.1. Halogenated Hydrocarbons
3.4.2. Isothiocyanates
3.4.3. Other Fumigants
3.5. Unknown or Uncertain Mode of Action
3.5.1. Fluensulfone
3.5.2. Tioxazafen
3.5.3. Fluazaindolizine
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaxter, M.L. Nematoda: Genes, genomes and the evolution of parasitism. Adv. Parasitol. 2003, 54, 101–195. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A Threat to Sustainability of Agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Jones, J.; Gheysen, G.; Fenoll, C. Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bell, C.H. Fumigation in the 21st century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Nicol, J.; Turner, S.; Coyne, D.L.; Nijs, L.d.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Grewal, P.S.; Ehlers, R.-U.; Shapiro-Ilan, D.I. Nematodes as Biocontrol Agents; CABI: Atlanta, GA, USA, 2005. [Google Scholar]
- Ciancio, A.; Mukerji, K.G. Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; Volume 2. [Google Scholar]
- Oliveira, C.M.G.D.; Monteiro, A.R.; Blok, V.C. Morphological and molecular diagnostics for plant-parasitic nematodes: Working together to get the identification done. Tropical Plant Pathology 2011, 36, 65–73. [Google Scholar]
- Bridge, J.; Starr, J.L. Plant Nematodes of Agricultural Importance: A Color Handbook; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- You, L.; Wu, D.; Zhang, R.; Wang, D.; Fu, Z.Q. Bioactivated and selective: A promising new family of nematicides with a novel mode of action. Mol. Plant 2023, 16, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Lahm, G.P.; Desaeger, J.; Smith, B.K.; Pahutski, T.F.; Rivera, M.A.; Meloro, T.; Kucharczyk, R.; Lett, R.M.; Daly, A.; Smith, B.T. The discovery of fluazaindolizine: A new product for the control of plant parasitic nematodes. Bioorg. Med. Chem. Lett. 2017, 27, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cai, S.; Deng, Y.; Cao, S.; Yang, X.; Lu, Y.; Li, W.; Chen, H. Efficacy of cyclobutrifluram in controlling Fusarium crown rot of wheat and resistance risk of three Fusarium species to cyclobutrifluram. Pestic. Biochem. Physiol. 2024, 198, 105723. [Google Scholar] [CrossRef]
- Seong, J.; Shin, J.; Kim, K.; Cho, B.-K. Microbial production of nematicidal agents for controlling plant-parasitic nematodes. Process Biochem. 2021, 108, 69–79. [Google Scholar] [CrossRef]
- Bargmann, C.I. Neurobiology of the Caenorhabditis elegans genome. Science 1998, 282, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Combes, D.; Fedon, Y.; Toutant, J.-P.; Arpagaus, M. Acetylcholinesterase genes in the nematode Caenorhabditis elegans. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2001; Volume 209, pp. 207–239. [Google Scholar]
- Piotte, C.; Arthaud, L.; Abad, P.; Rosso, M.-N. Molecular cloning of an acetylcholinesterase gene from the plant parasitic nematodes, Meloidogyne incognita and Meloidogyne javanica. Mol. Biochem. Parasitol. 1999, 99, 247–256. [Google Scholar] [CrossRef]
- Fanelli, E.; Vovlas, A.; D’Addabbo, T.; De Luca, F. Molecular mechanism of Cinnamomum zeylanicum and Citrus aurantium essential oils against the root-knot nematode, Meloidogyne incognita. Sci. Rep. 2025, 15, 6077. [Google Scholar] [CrossRef]
- Zhong, D.; De-liang, P.; Bida, G.; Qi, H. Molecular cloning and characterization of a new acetylcholinesterase gene Dd-ace-1 from Ditylenchus destructor. J. Hunan Agric. Univ. 2010, 36, 437–441. [Google Scholar]
- Cui, R.; Zhang, L.; Chen, Y.; Huang, W.; Fan, C.; Wu, Q.; Peng, D.; da Silva, W.; Sun, X. Expression and evolutionary analyses of three acetylcholinesterase genes (Mi-ace-1, Mi-ace-2, Mi-ace-3) in the root-knot nematode Meloidogyne incognita. Exp. Parasitol. 2017, 176, 75–81. [Google Scholar] [CrossRef]
- Costa, J.C.; Lilley, C.J.; Atkinson, H.J.; Urwin, P.E. Functional characterisation of a cyst nematode acetylcholinesterase gene using Caenorhabditis elegans as a heterologous system. Int. J. Parasitol. 2009, 39, 849–858. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, D.-W.; Choi, J.Y.; Je, Y.H.; Koh, Y.H.; Lee, S.H. Three acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus: Insights into distinct physiological functions. Mol. Biochem. Parasitol. 2011, 175, 154–161. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, D.-W.; Koh, Y.H.; Lee, S.H. A Soluble Acetylcholinesterase Provides Chemical Defense against Xenobiotics in the Pinewood Nematode. PLoS ONE 2011, 6, e19063. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.H.; Kwon, D.H.; Cha, D.J.; Kim, J.H. Mutation and duplication of arthropod acetylcholinesterase: Implications for pesticide resistance and tolerance. Pestic. Biochem. Physiol. 2015, 120, 118–124. [Google Scholar] [CrossRef]
- Opperman, C.H.; Chang, S. Nematode acetylcholinesterases: Molecular forms and their potential role in nematode behavior. Parasitol. Today 1992, 8, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.A. RNA Interference as a Tool to Control Plant Parasitic Nematode Infestation in Key Plant Crops. Ph.D. Thesis, Queen’s University Belfast, Belfast, UK, 2015. [Google Scholar]
- Pree, D.J.; Townshend, J.L.; Cole, K.J. Inhibition of Acetylcholinesterases from Aphelenchus avenae by Carbofuran and Fenamiphos. J. Nematol. 1990, 22, 182–186. [Google Scholar]
- Le Patourel, G.N.J.; Wright, D.J. Some factors affecting the susceptibility of two nematode species to phorate. Pestic. Biochem. Physiol. 1976, 6, 296–305. [Google Scholar] [CrossRef]
- Cleland, T.A. Inhibitory glutamate receptor channels. Mol. Neurobiol. 1996, 13, 97–136. [Google Scholar] [CrossRef]
- Yates, D.M.; Portillo, V.; Wolstenholme, A.J. The avermectin receptors of Haemonchus contortus and Caenorhabditis elegans. Int. J. Parasitol. 2003, 33, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Shoop, W.L.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Crump, A.; Ōmura, S. Ivermectin, ‘wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 13–28. [Google Scholar] [CrossRef]
- Cully, D.F.; Vassilatis, D.K.; Liu, K.K.; Paress, P.S.; Van der Ploeg, L.H.T.; Schaeffer, J.M.; Arena, J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 1994, 371, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.A.; Smith, M.M.; Vassilatis, D.K.; Avery, L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2000, 97, 2674–2679. [Google Scholar] [CrossRef]
- Feng, X.P.; Hayashi, J.; Beech, R.N.; Prichard, R.K. Study of the nematode putative GABA type-A receptor subunits: Evidence for modulation by ivermectin. J. Neurochem. 2002, 83, 870–878. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 2005, 131, S85–S95. [Google Scholar] [CrossRef] [PubMed]
- Laughton, D.L.; Lunt, G.G.; Wolstenholme, A.J. Reporter gene constructs suggest that the Caenorhabditis elegans avermectin receptor beta-subunit is expressed solely in the pharynx. J. Exp. Biol. 1997, 200, 1509–1514. [Google Scholar] [CrossRef]
- Kondrashov, F.A.; Koonin, E.V.; Morgunov, I.G.; Finogenova, T.V.; Kondrashova, M.N. Evolution of glyoxylate cycle enzymes in Metazoa: Evidence of multiple horizontal transfer events and pseudogene formation. Biol. Direct 2006, 1, 31. [Google Scholar] [CrossRef]
- Veloukas, T.; Karaoglanidis, G.S. Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Manag. Sci. 2012, 68, 858–864. [Google Scholar] [CrossRef]
- Foote, E.; Jordan, D.; Gorny, A.; Dunne, J.; Lux, L.; Shew, B.; Ye, W. Previous Cropping Sequence Affects Plant-Parasitic Nematodes and Yield of Peanut and Cotton More than Continuous Use of Fluopyram. Crops 2025, 5, 12. [Google Scholar] [CrossRef]
- Faske, T.R.; Hurd, K. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to Fluopyram. J. Nematol. 2015, 47, 316–321. [Google Scholar]
- Oka, Y.; Saroya, Y. Effect of fluensulfone and fluopyram on the mobility and infection of second-stage juveniles of Meloidogyne incognita and. Pest Manag. Sci. 2019, 75, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Wang, Y.; Liu, H.; Zhang, S.; Ji, X.; Qiao, K. Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 2022, 286, 131830. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Wang, Q. Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation. Chin. Chem. Lett. 2022, 33, 626–642. [Google Scholar] [CrossRef]
- Heydari, F.; Rodriguez-Crespo, D.; Wicky, C. The New Nematicide Cyclobutrifluram Targets the Mitochondrial Succinate Dehydrogenase Complex in Caenorhabditis elegans. J. Dev. Biol. 2023, 11, 39. [Google Scholar] [CrossRef]
- Liu, W.; Shao, H.; Qi, D.; Huang, X.; Chen, J.; Zhou, L.; Guo, K. The New Nematicide Cyclobutrifluram Targets the Mitochondrial Succinate Dehydrogenase Complex in Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2024, 25, 6914. [Google Scholar] [CrossRef] [PubMed]
- Lümmen, P.; Khajehali, J.; Luther, K.; Van Leeuwen, T. The cyclic keto-enol insecticide spirotetramat inhibits insect and spider mite acetyl-CoA carboxylases by interfering with the carboxyltransferase partial reaction. Insect Biochem. Mol. Biol. 2014, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vang, L.E.; Opperman, C.H.; Schwarz, M.R.; Davis, E.L. Spirotetramat causes an arrest of nematode juvenile development. Nematology 2016, 18, 121–131. [Google Scholar] [CrossRef]
- Fahs, H.Z.; Refai, F.S.; Gopinadhan, S.; Moussa, Y.; Gan, H.H.; Hunashal, Y. A new class of natural anthelmintics targeting lipid metabolism. Nat. Commun. 2025, 16, 305. [Google Scholar] [CrossRef]
- Guest, M.; Kriek, N.; Flemming, A.J. Studies of an insecticidal inhibitor of acetyl-CoA carboxylase in the nematode C. elegans. Pestic. Biochem. Physiol. 2020, 169, 104604. [Google Scholar] [CrossRef]
- Lin, C.J.; Siddique, S. Parasitic nematodes: Dietary habits and their implications. Trends Parasitol. 2024, 40, 230–240. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and degradation as targets for pesticide action. Arch. Insect Biochem. Physiol. 1993, 22, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.D.; Spindler-Barth, M.; Londershausen, M. Chitin metabolism: A target for drugs against parasites. Parasitol. Res. 1990, 76, 283–288. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, D. Nematode chitin and application. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; pp. 209–219. [Google Scholar]
- Fanelli, E.; Di Vito, M.; Jones, J.T.; De Giorgi, C. Analysis of chitin synthase function in a plant parasitic nematode, Meloidogyne artiellia, using RNAi. Gene 2005, 349, 87–95. [Google Scholar] [CrossRef]
- Opperman, C.H.; Bird, D.M.; Williamson, V.M.; Rokhsar, D.S.; Burke, M.; Cohn, J.; Windham, E. Sequence and genetic map of Meloidogyne hapla: A compact nematode genome for plant parasitism. Proc. Natl. Acad. Sci. USA 2008, 105, 14802–14807. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li, J.; Zhang, K. Cloning of the gene Lecanicillium psalliotae chitinase Lpchi1 and identification of its potential role in the biocontrol of root-knot nematode Meloidogyne incognita. Appl. Microbiol. Biotechnol. 2007, 76, 1309–1317. [Google Scholar] [CrossRef]
- Tachu, B.; Pillai, S.; Lucius, R.; Pogonka, T. Essential role of chitinase in the development of the filarial nematode Acanthocheilonema viteae. Infect. Immun. 2008, 76, 221–228. [Google Scholar] [CrossRef]
- Maeda, I.; Kohara, Y.; Yamamoto, M.; Sugimoto, A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 2001, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, X.; Guan, T.; Peng, D.; Li, H. Versatile glycoside hydrolase family 18 chitinases for fungi ingestion and reproduction in the pinewood nematode Bursaphelenchus xylophilus. Int. J. Parasitol. 2016, 46, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kiewnick, S.; Sikora, R.A. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol. Control 2006, 38, 179–187. [Google Scholar] [CrossRef]
- Poelarends, G.J.; Wilkens, M.; Larkin, M.J.; Elsas, J.D.v.; Janssen, D.B. Degradation of 1,3-Dichloropropene by Pseudomonas cichorii 170. Appl. Environ. Microbiol. 1998, 64, 2931–2936. [Google Scholar] [CrossRef]
- Price, N.R. The mode of action of fumigants. J. Stored Prod. Res. 1985, 21, 157–164. [Google Scholar] [CrossRef]
- Lin, C.-M.; Preston, J.F., III; Wei, C.-I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157: H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Pearson, P.G.; Slatter, J.G.; Rashed, M.S.; Han, D.H.; Baillie, T.A. Carbamoylation of peptides and proteins in vitro by S-(N-methylcarbamoyl)glutathione and S-(N-methylcarbamoyl)cysteine, two electrophilic S-linked conjugates of methyl isocyanate. Chem. Res. Toxicol. 1991, 4, 436–444. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.; Zhao, Y.; Ma, Z.; Zhang, X. Comparative studies on mitochondrial electron transport chain complexes of Sitophilus zeamais treated with Allyl isothiocyanate and Calcium phosphide. Pestic. Biochem. Physiol. 2016, 126, 70–75. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Z.; Zhang, X.; Wu, H. Transcriptomic alterations in Sitophilus zeamais in response to allyl isothiocyanate fumigation. Pestic. Biochem. Physiol. 2017, 137, 62–70. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, Y.; Wu, H.; Xu, N.; Ma, Z.; Zhang, C. Function of four mitochondrial genes in fumigation lethal mechanisms of Allyl Isothiocyanate against Sitophilus zeamais adults. Pestic. Biochem. Physiol. 2021, 179, 104947. [Google Scholar] [CrossRef]
- Cao, A.; Guo, M.; Yan, D.; Mao, L.; Wang, Q.; Li, Y.; Duan, X.; Wang, P. Evaluation of sulfuryl fluoride as a soil fumigant in China. Pest Manag. Sci. 2013, 70, 219–227. [Google Scholar] [CrossRef]
- Bonifácio, L.F.; Sousa, E.; Naves, P.; Inácio, M.L.; Henriques, J.; Mota, M.; Barbosa, P.; Drinkall, M.J.; Buckley, S. Efficacy of sulfuryl fluoride against the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchidae), in Pinus pinaster boards. Pest Manag. Sci. 2014, 70, 6–13. [Google Scholar] [CrossRef]
- Meikle, R.W.; Stewart, D.; Globus, O.A. Fumigant Mode of Action, Drywood Termite Metabolism of Vikane Fumigant as Shown by Labeled Pool Technique. J. Agric. Food Chem. 1963, 11, 226–230. [Google Scholar] [CrossRef]
- Yan, D.; Cao, A.; Wang, Q.; Li, Y.; Canbin, O.; Guo, M.; Guo, X. Dimethyl disulfide (DMDS) as an effective soil fumigant against nematodes in China. PLoS ONE 2019, 14, e0224456. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Tenorio, M.A.; Tello, J.C.; Zanón, M.J.; de Cara, M. Soil disinfestation with dimethyl disulfide (DMDS) to control Meloidogyne and Fusarium oxysporum f. sp. radicis-lycopersici in a tomato greenhouse. Crop Prot. 2018, 112, 133–140. [Google Scholar] [CrossRef]
- Dugravot, S.; Grolleau, F.; Macherel, D.; Rochetaing, A.; Hue, B.; Stankiewicz, M.; Huignard, J.; Lapied, B. Dimethyl Disulfide Exerts Insecticidal Neurotoxicity Through Mitochondrial Dysfunction and Activation of Insect KATP Channels. J. Neurophysiol. 2003, 90, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Zhang, D.; Fang, W.; Li, Y.; Cao, A.; Wang, Q.; Yan, D. Transcriptome reveals the toxicity difference of dimethyl disulfide by contact and fumigation on Meloidogyne incognita through calcium channel-mediated oxidative phosphorylation. J. Hazard. Mater. 2023, 460, 132268. [Google Scholar] [CrossRef]
- Kearn, J.; Ludlow, E.; Dillon, J.; O’Connor, V.; Holden-Dye, L. Fluensulfone is a nematicide with a mode of action distinct from anticholinesterases and macrocyclic lactones. Pestic. Biochem. Physiol. 2014, 109, 44–57. [Google Scholar] [CrossRef]
- Kearn, J.; Lilley, C.; Urwin, P.; O’Connor, V.; Holden-Dye, L. Progressive metabolic impairment underlies the novel nematicidal action of fluensulfone on the potato cyst nematode Globodera pallida. Pestic. Biochem. Physiol. 2017, 142, 83–90. [Google Scholar] [CrossRef]
- Hada, A.; Singh, D.; Venkata Satyanarayana, K.K.V.; Chatterjee, M.; Phani, V.; Rao, U. Effect of fluensulfone on different functional genes of root-knot nematode Meloidogyne incognita. J. Nematol. 2021, 53, e2021-73. [Google Scholar] [CrossRef]
- Wram, C.L.; Hesse, C.N.; Zasada, I.A. Transcriptional changes of biochemical pathways in Meloidogyne incognita in response to non-fumigant nematicides. Sci. Rep. 2022, 12, 9875. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.X.; Song, B. Chemical Nematicides: Recent Research Progress and Outlook. J. Agric. Food Chem. 2020, 68, 12175–12188. [Google Scholar] [CrossRef] [PubMed]
- Slomczynska, U.; South, M.S.; Bunkers, G.J.; Edgecomb, D.; Wyse-Pester, D.; Selness, S.; Ding, Y.; Christiansen, J.; Ediger, K.; Miller, W.; et al. Tioxazafen: A New Broad-Spectrum Seed Treatment Nematicide. In Discovery and Synthesis of Crop Protection Products; American Chemical Society: Washington, DC, USA, 2015; Volume 1204, pp. 129–147. [Google Scholar]
- Faske, T.R.; Brown, K.; Kelly, J. Toxicity of Tioxazafen to Meloidogyne Incognita and Rotylenchulus Reniformis. J. Nematol. 2022, 54, 20220007. [Google Scholar] [CrossRef]
- Tzortzakakis, E.A.; Thoden, T.C.; Chatzaki, A. Investigation of fluazaindolizine as a potential novel tool to manage Xiphinema index. Crop Prot. 2024, 180, 106636. [Google Scholar] [CrossRef]
- Qiao, K.; Liu, Q.; Zhang, S. Evaluation of fluazaindolizine, a new nematicide for management of Meloidogyne incognita in squash in calcareous soils. Crop Prot. 2021, 143, 105469. [Google Scholar] [CrossRef]
| Registration Year | Active Ingredient | Company | Chemical Group | Notes |
|---|---|---|---|---|
| 2024 | Trifluenfuronate | Shandong Zhongnong United Industry | Trifluorobutene compound | May act on mitochondrial fatty acid β-oxidation inhibitor; |
| 2022 | Cyclobutrifluram | Syngenta | Novel nicotinamide compound | Fluorinated nematicide |
| 2021 | Fluazaindolizine | Corteva Agriscience | Novel sulfonamide compound | First nematicide product from Corteva |
| 2017 | Tioxazafen | Monsanto | Oxadiazole compound | May cause allergic skin reactions. |
| 2014 | Fluensulfone | ADAMA | Heterocyclic fluorosulfonyl compound | Fluorinated nematicide |
| 2010 | Fluopyram | Bayer | Pyridine ethyl benzamide compound | Fluorinated nematicide; First nematicide acts on succinate dehydrogenase |
| Mode of Action (MoA) | Representative Nematicides |
|---|---|
| Nerve action: Acetylcholinesterase (AChE) inhibitors | Carbamates: Aldicarb, Carbofuran, Oxamyl, etc. Organophosphates: Fosthiazate, Phorate, etc. |
| Nerve action: Glutamate-gated chloride channel (GluCl) allosteric modulators | Abamectin |
| Respiration: Mitochondrial complex II, electron transport inhibitors (Succinate-coenzyme Q reductase inhibitors) | Fluopyram, Cyclobutrifluram |
| Lipid synthesis and growth regulation: Acetyl-CoA carboxylase (ACCase) inhibitors | Spirotetramat |
| Compounds with unknown or uncertain mode of action | Fluensulfone, Tioxazafen, Triflumezopyrim, Furfural, Iprodione |
| Compounds with unknown or uncertain mode of action: Assumed multi-site inhibitors | Fumigants: Halogenated hydrocarbons, Methyl isothiocyanates (MITCs), etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yan, D.; Ghaderi, R.; He, J.; Cao, A.; Wang, Q. Recent Advances in Nematicides and Their Modes of Action. Agriculture 2026, 16, 21. https://doi.org/10.3390/agriculture16010021
Yan D, Ghaderi R, He J, Cao A, Wang Q. Recent Advances in Nematicides and Their Modes of Action. Agriculture. 2026; 16(1):21. https://doi.org/10.3390/agriculture16010021
Chicago/Turabian StyleYan, Dongdong, Reza Ghaderi, Jizheng He, Aocheng Cao, and Qiuxia Wang. 2026. "Recent Advances in Nematicides and Their Modes of Action" Agriculture 16, no. 1: 21. https://doi.org/10.3390/agriculture16010021
APA StyleYan, D., Ghaderi, R., He, J., Cao, A., & Wang, Q. (2026). Recent Advances in Nematicides and Their Modes of Action. Agriculture, 16(1), 21. https://doi.org/10.3390/agriculture16010021








