Plant- and Microbial-Based Organic Disease Management for Grapevines: A Review
Abstract
1. Introduction
2. Materials and Methods
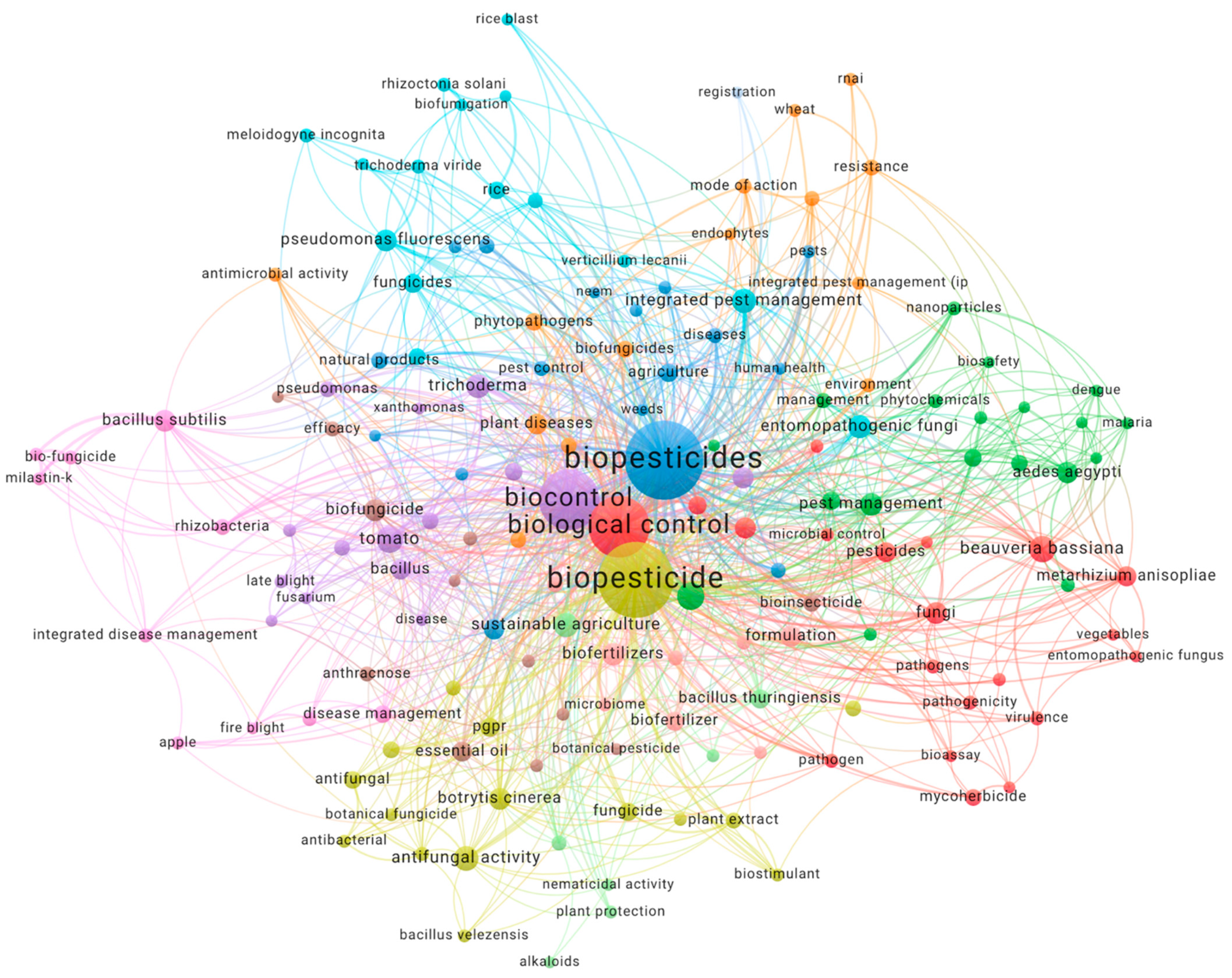
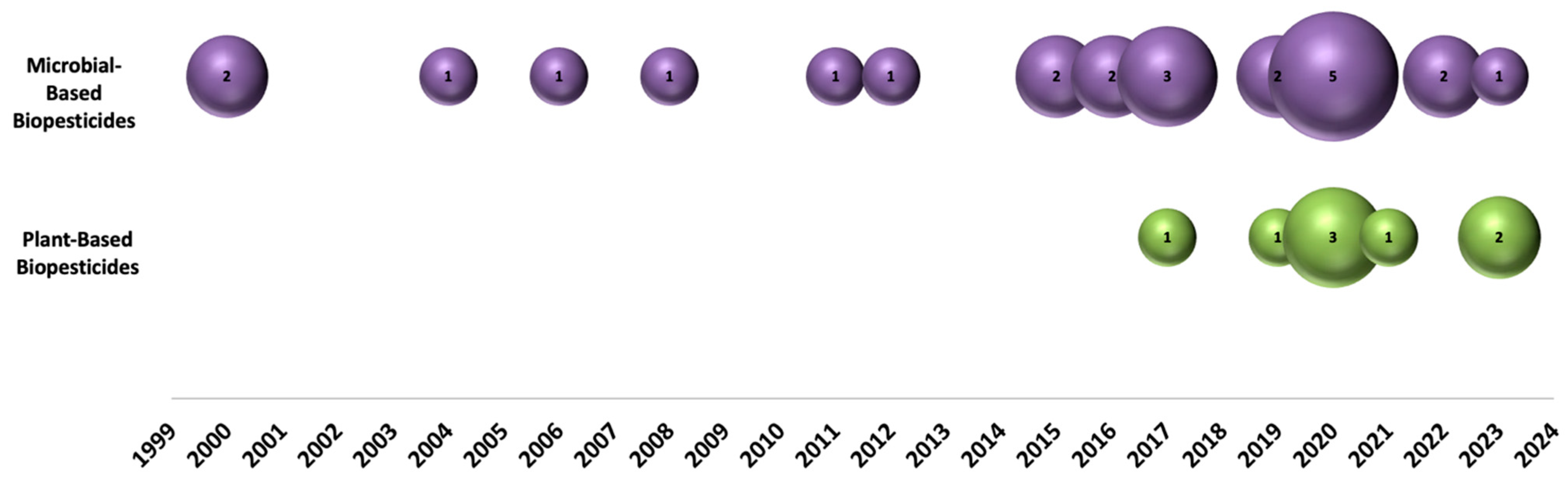
2.1. Bibliometric Analysis
2.2. Eligibility Criteria
2.3. Data Synthesis
3. Results and Discussion
3.1. Botanical Approaches to Organic Disease Management
3.1.1. Plant Extracts
3.1.2. Essential Oils
3.1.3. Compost
| Biopesticides | Pathogens | Bioactive Compounds | Effectiveness (in Lab, Greenhouse, and Field) | Ref. |
|---|---|---|---|---|
| Ligninsulfonate-based grape cane and apple extract | Plasmopara viticola | Vineatrol (36.6%) | GCE formulations alone reduced downy mildew disease severity in greenhouse trials by 29–69% in a dose-dependent manner, whereas a standard application of the copper-based agent alone reached 56%. When applied together, disease severity was diminished by 78–92%, revealing a synergistic effect that depended on the mixture ratio (lab and greenhouse) | [40] |
| Neem Ginger Garlic Eucalyptus Onion | Cladosporium cladosporioides | Hexaconazole (94.44%) Carbendazim (84.93%) propiconazole (81.53%) Difenconazole (75.97%) Thiophanate methyl (51.21%) | Cladosporium cladosporioides with 77% reduction in linear colony growth (lab) | [41] |
| Baccharis trimera and Baccharis dracunculifolia (essential oils) | Botrytis cinerea and Colletotrichum acutatum | Baccharis trimera: Carquejyl acetate (67.48%) Palustrol (3.12%) Globulol (2.41%) δ-cadinene (2.26%) Camphor (1.68%) Sabinene (1.45%) Baccharis dracunculifolia: Ledol (13.55%) Spathulenol (13.43%) Limonene (10.11%) Germacrene-δ-4-ol (5.39%) α-thujene (4.02%) | At concentrations of 50% and 100%, Baccharis trimera (BtEO) significantly inhibited Botrytis cinerea growth up to the 14th day For Colletotrichum acutatum, 12.5% and 25% inhibited growth until the 7th day, while 50% and 100% suppressed growth until the 14th day. Regarding Baccharis dracunculifolia (BdEO), 100% concentration of volatile compounds inhibited B. cinerea growth up to the 14th day, whereas for C. acutatum, all tested concentrations significantly suppressed growth until the 5th day, but the effect was less pronounced afterward at 200–600 ppm (lab) | [42] |
| Origanum vulgare essential oil vapor | Botrytis cinerea | p-Cymene (11.27%), Thymol (1.93%), Carvacrol (58.1%) | Oregano essential oil vapor achieved 100% inhibition of Botrytis cinerea growth in vitro. Chasselas berries resulted in 73% reduction in fungal growth (field) | [43] |
| Eucalyptus globulus Labill; Citrus limonum (L.) Burm; Cinnamomum zeylanicum Blume; Lavandula latifolia aspic; Rosmarinus officinalis L.; and Mentha spicata L. | Diplodia mutila, Neoscytalidium novaehollandia, Trichothecium roseum, and Neopestalotiopsis vitis | Eucalyptus globulus: Eucalyptol (63.99%) Benzene, 1-methyl-4-(1-methylethyl)- (11.20%) ɤ-Terpinene (8.39%) 2-Pinene (6.31%) Citrus limonum (L.) Burm: D-Limonene (46.82%) 1,7,7-trimethylbicyclo [2.2.1]Heptan-2-one (7.81%) Borneol (5.87%) Bicyclo [3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1 S)-(4.81%) Cinnamomum zeylanicum: cis-Cinnamaldehyde (87.53%) 2-Pinene (25.41%) Lavandula latifolia: Linalyl acetate (15.44%) Linalool (14.22%) 1,7,7-trimethylbicyclo [2.2.1]heptan-2-one (11.44%) Borneol (8.68%) Rosmarinus officinalis L.: 2-Oxabicyclo [2.2.2]octane, 1,3,3-trimethyl- (24.66%) m-Eugenol (12.47%) 1,7,7-trimethylbicyclo [2.2.1]heptan-2-one (22.52%) Bicyclo [2.2.1]heptane, 2,2-dimethyl-3-methylene-, (1 S) (7.64%) Mentha spicata L: 2-Oxabicyclo [2.2.2]octane, 1,3,3-trimethyl- (73.54%) o-Cymene (11.08%) ɤ-Terpinene (4.77%) | Trichothecium roseum, inhibited growth by 81.37%, Diplodia mutila by 47.45%. Neopestalotiopsis vitis reduced growth by 73.33%, Neoscytalidium novaehollandiae achieved 56.79% inhibition (field and lab) | [44] |
| 26 essential oils Cinnamomum zeylanicum and Cymbopogon citratus | Pseudocercopora vitis and Sphaceloma ampelinum | Cinnamomum zeylanicum: (E)-cinnamaldehyde (92.36%) (E)-cinnamyl acetate (1.48%) 1,8-cineole (1.05%) Cymbopogon citratus Geranial (58.89%) Neral (38.50%) | Cinnamomum zeylanicum (cinnamon) and Cymbopogon citratus (lemongrass) exhibited 100% inhibition of Sphaceloma ampelinum and Pseudocercospora vitis spore germination in both vapor and liquid phases (field and lab) | [45] |
| Lavandula × hybrida (lavender) Rosmarinus officinalis (rosemary), Mentha piperita (peppermint), and Thymus vulgaris (thyme) | Botrytis cinerea | Lavandula × hybrida: Linalool (34.4%) Linalyl acetate (25.8%) 1,8-Cineol (6.88%) Camphor (6.28%); Rosmarinus officinalis: 1,8-Cineol (48.24%) a-Pinene (10.26%) b-Pinene (8.55%) Camphor (8.66%); Mentha piperita: Menthol (34.71%) Menthone (27.44%) Menthyl acetate (4.92%); Thymus vulgaris: Thymol (51.83%) Cymene (20.26%) γ-Terpinen (6.91%) Linalool (4.45%) | Rosemary oil reduced the incidence by 70% and 66% (7 day), peppermint oil reductions of 53% and 60% (5 day) (lab) | [46] |
| Lantana | Escherichia coli, Salmonella, Xanthomonas citrus, Xanthomonas campestris, Erwinia carotovora, and Pseudomonas aerogenosa | Hexadecane (3.93%), tetradecane (3.05), heptacosane (5.49), heptadecane (3.05%), and heptacosane 1-chloro- (5.49%) | Lantana compost extract showed 1.08-fold inhibition of Pseudomonas aeruginosa, 0.79–1.08-fold inhibition of Erwinia carotovora, 0.88–0.96-fold inhibition of Xanthomonas campestris, and 0.88–1.08-fold inhibition of Xanthomonas citrus (field and lab) | [47] |
3.2. Microbial Organic Disease Management
3.2.1. Bacteria-Based Biopesticides
3.2.2. Fungi-Based Biopesticides
3.2.3. Yeast-Based Biopesticides
3.2.4. Commercial Biopesticides Based on Microorganisms
| Biopesticides | Pathogens | Effectiveness (in Lab, Greenhouse and Field) | Ref. |
|---|---|---|---|
| Pseudomonas sp. I2R21 and Pseudomonas sp. W1R33 | Neofusicoccum luteum and N. parvum | The endophytic bacteria reduced lesion length by 32–52% (field) | [37] |
| B. subtilis | E. necator | Powdery mildew severity reduced by 96% (greenhouse) | [48] |
| Lactiplantibacillus plantarum | Pseudomonas syringae pv. syringae and B. cinerea | Lactiplantibacillus plantarum Q4 reduced Pseudomonas syringae pv. syringae infection by 45% and significantly decreased Botrytis cinereal induced fruit rot, proving its biocontrol potential (lab) | [68] |
| Bacillus thuringiensis | Oenococcus oeni | Bacillus thuringiensis reduced Oenococcus oeni by 70–80% without affecting fermentation (field) | [50] |
| Bacillus velezensis | B. cinerea, Monilinia fructicola, M. laxa, Penicillium digitatum, P. expansum, and P. italicum | B. velezensis inhibited Monilinia fructicola (66%), M. laxa (72%), Penicillium italicum (80%), and completely suppressed B. cinerea (100%). In vivo, strain I3 reduced gray mold by 50%, and BUZ-14 lowered brown rot severity from 60 mm to 4 mm. VOCs like diacetyl eliminated gray mold (100%) and reduced blue mold by 60% (field) | [49] |
| Trichoderma harzianum M10 and T22 and Trichoderma atroviride P1. | Uncinula necator | Trichoderma harzianum M10 and T. atroviride P1 reduced powdery mildew by 60%, increased grape yield by 63–97%, and boosted antioxidant activity by 48.7%, proving their efficacy in disease control and fruit quality enhancement (field) | [51] |
| Aspergillus carbonarius | Penicillium adametzioides | P. adametzioides reduced OTA in grape juice by 80–90%, showing high efficiency (field) | [52] |
| Trichoderma | Plasmopara viticola | Trichoderma harzianum with potassium tartrate reduced grape downy mildew by 78.9% (year 1) and 81.8% (year 2) (field) | [53] |
| Neurospora sp. Arthrinium sp. Pestalotiopsis sp. Hypocrea lixii Fusarium sp. | Cladosporium cladosporioides | Neurospora sp.: 3.7 mm → 95.9% inhibition Arthrinium sp.: 7.5 mm → 91.7% inhibition Pestalotiopsis sp.: 9 mm → 90.0% inhibition Hypocrea lixii: 9 mm → 90.0% inhibition Fusarium sp.: 9.5 mm → 89.4% inhibition (lab) | [41] |
| Trichoderma atroviride, A. pullulans, and B. subtilis | B. cinerea | Pathogen suppression efficiency was 72–85% (field) | [54] |
| A. pullulans and Potassium Bicarbonate | B. cinerea | Potassium bicarbonate: 20%, Aureobasidium pullulans: 13% efficacy at 3% severity (field) | [56] |
| Candida guilliermondii, strain A42 and Acremonium cephalosporium, strain B11 | B. cinerea, Aspergillus niger, and Rhizopus stolonifera | A42 reduced grape rot to 8–22% (lab) and B11 to 16–82%, confirming A42’s superior efficacy (lab), A42 reduced grape rot to 22–30% (field) and B11 to 30–48% (field), confirming B11’s superior efficacy | [36] |
| Aureobasidium pullulans | Aspergillus carbonarius | A. pullulans (isolate Y-1) effectively inhibited the growth of A. carbonarius, reducing it by 14–92% compared to untreated berries. The fungicide reduced OTA levels by 97%–99% and A. pullulans isolate Y-1—by 99% (field) | [57] |
| Candida sake | B. cinerea | C. sake reduced B. cinerea incidence by up to 80%, depending on the formulation used (lab) | [58] |
| Kluyveromyces thermotolerans | Aspergillus carbonarius and A. niger | The growth rate of fungi was reduced by 11% to 82.5% (field) | [59] |
| L. thermotolerans strains (RCKT4 and RCKT5) | Aspergillus Nigri | Achieved 27–100% reduction in OTA accumulation (greenhouse and field) | [60] |
| H. uvarum | Aspergillus tubingensis | H. uvarum with trehalose reduced grape rot by 70% and boosted defense enzyme activity, with CAT up 23-fold and PPO up 9.5-fold, enhancing resistance to Aspergillus tubingensis (lab) | [61] |
| Yarrowia lipolytica | Penicillium rubens | Yarrowia lipolytica reduced grape decay to 12.45% (from 79.15%), inhibited spore germination (7.22%), and lowered OTA from 74.61 to 0.32 ng/grape. It boosted defense enzymes, with catalase increasing 8.33× (lab) | [62] |
| K. thermotolerans, P. guillermondii, H. uvarum, Z. fermentati, C. flavus, and C. valdiviana | Aspergillus carbonarius and A. niger | Metschnikowia pulcherrima (77–100%) and Issatchenkia orientalis (100%) inhibited A.carbonarius and A. niger, reducing fungal colonization in grapes and preventing OTA contamination (lab) | [63] |
| Metschnikowia pulcherrima LS16, A. pullulans LS30, and A. pullulans AU34-2 | A. carbonarius | Metschnikowia pulcherrima LS16 and A. pullulans (LS30 and AU34-2) reduced Aspergillus carbonarius infection by 69–99% and lowered (OTA) contamination by up to 93.5%, with effectiveness varying by humidity levels (lab) | [64] |
| Aureobasidium pullulans, Cryptococcus magnus, and Candida sake | Aspergillus tubingensis | A. pullulans reduced A. tubingensis by 17.1–95.7% (lab) | [65] |
| Rhodotorula LS15 | Penicillium digitatum, Rhizopus stolonifera, and A. niger | Preharvest LS15 reduced gray mold on grapes by 28.3–38.2% (lab) | [66] |
| Eco-pesticide® (Trichoderma asperellum), Bio-Pulse® (Trichoderma asperellum and Bacillus amyloliquefaciens), and Bio-Care 24® (Bacillus amyloliquefaciens) | E. necator | PDI reduced to 22.37 (Eco-Pesticide®), 22.62 (Bio-Pulse®), and 24.62 (Bio-Care 24®) on leaves, and to 24.71, 24.94, and 26.77 on bunches (field) | [67] |
| Biopesticide Serenade Max (B. subtilis) | B. cinerea | Serenade Max reduced fruit rot by approximately 52.4–71.1% (field) | [69] |
3.3. Mechanisms and Applications of Organic Disease Management in Grapevines
4. Challenges in the Development and Application of Plant-Based and Microbial Agents for Organic Disease Management
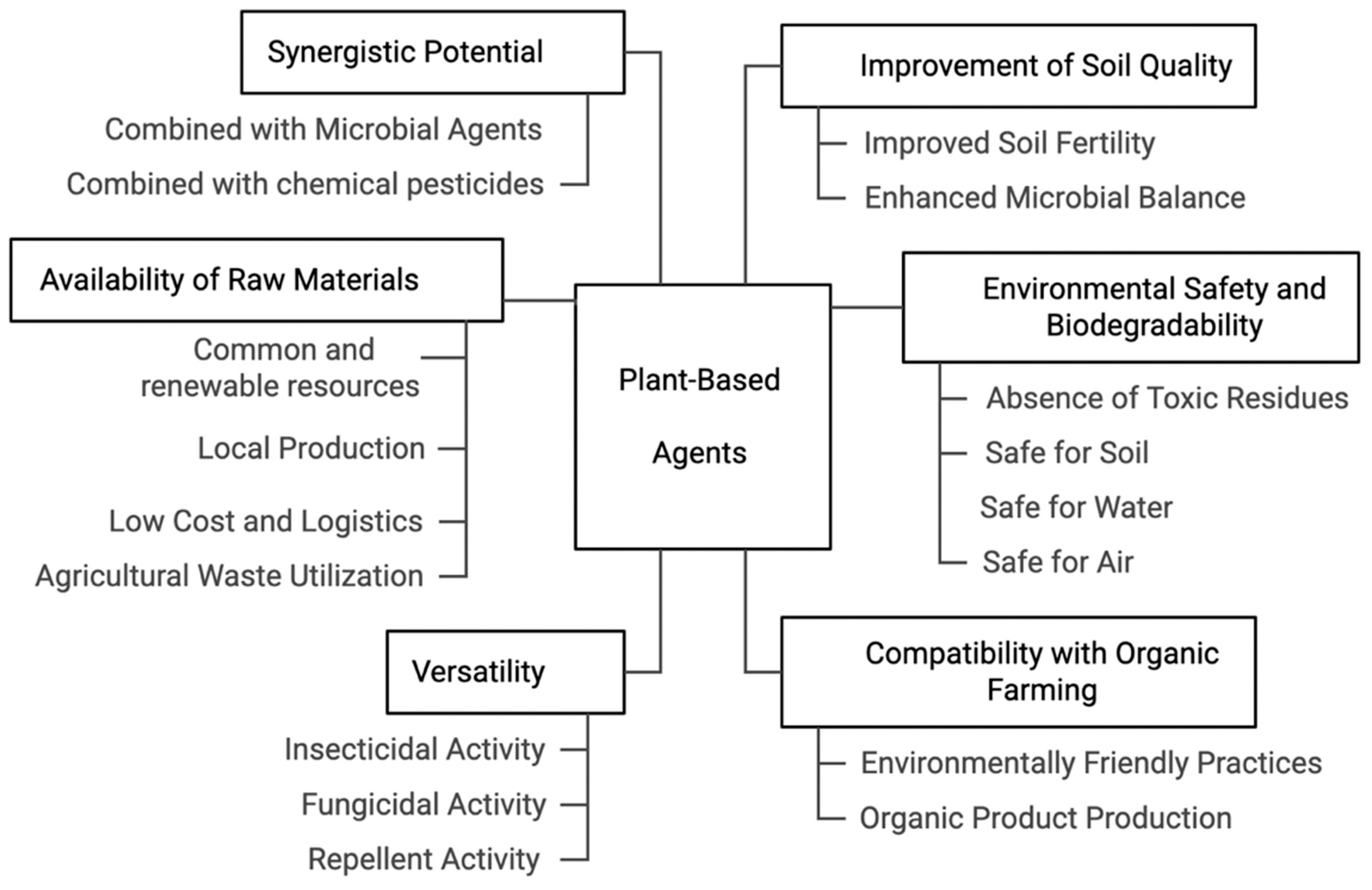
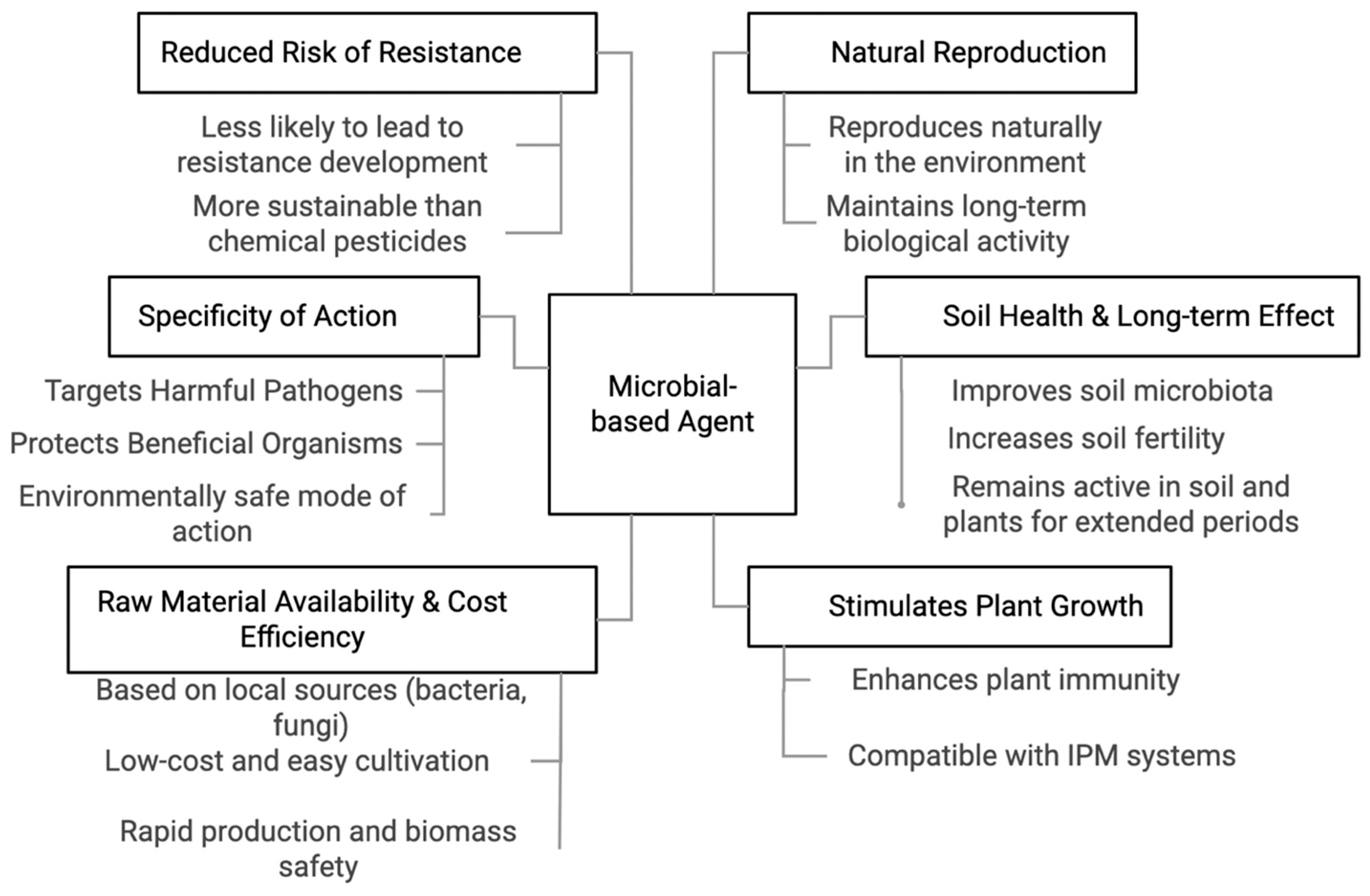
5. Benefits of Plant- and Microbial-Based Organic Disease Management in Sustainable Agriculture
5.1. Plant-Based Organic Disease Management
5.2. Microbial-Based Organic Disease Management
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine. Available online: https://www.oiv.int/ (accessed on 18 September 2023).
- Mubeen, I.; Fawzi Bani Mfarrej, M.; Razaq, Z.; Iqbal, S.; Naqvi, S.A.H.; Hakim, F.; Mosa, W.F.A.; Moustafa, M.; Fang, Y.; Li, B. Nanopesticides in Comparison with Agrochemicals: Outlook and Future Prospects for Sustainable Agriculture. Plant Physiol. Biochem. 2023, 198, 107670. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Syrgabek, Y.; Alimzhanova, M. Modern Analytical Methods for the Analysis of Pesticides in Grapes: A Review. Foods 2022, 11, 1623. [Google Scholar] [CrossRef] [PubMed]
- Syrgabek, Y.; Alimzhanova, M.; García-encina, P.A.; Jose, J.; Rebeca, L. Greenness Evaluation of Sample Preparation Methods by GAPI for the Determination of Pesticides in Grape: A Review. Trends Environ. Anal. Chem. 2023, 39, e00206. [Google Scholar] [CrossRef]
- Tukenova, Z.; Mustafayev, M.; Alimzhanova, M.; Akylbekova, T.; Ashimuly, K. Influence of Pesticides on the Biological Activity of Light Chestnut Soils in South-East Kazakhstan. J. Water Land Dev. 2021, 48, 141–147. [Google Scholar] [CrossRef]
- Han, C.; Hu, B.; Liu, B.; Jin, J.; Ye, M.; Fu, C.; Shen, Y. Determination of Four Amide Fungicides in Grape Wine by Gas Chromatography Coupled with Tandem Mass Spectrometry. Food Anal. Methods 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Sadanov, A.; Alimzhanova, M.; Ismailova, E.; Shemshura, O.; Ashimuly, K.; Molzhigitova, A.; Daugaliyeva, S. Antagonistic and Protective Activity of Lactobacillus Plantarum Strain 17 M against E. Amylovora. World J. Microbiol. Biotechnol. 2023, 39, 314. [Google Scholar] [CrossRef]
- Billiard, K.M.; Dershem, A.R.; Gionfriddo, E. Solid-Phase Microextraction: A Review. Molecules 2020, 25, 5297. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhao, H.-Y.; Shen, Q.; Qi, P.-P.; Wang, X.-Q.; Xu, H.; Di, S.-S.; Wang, Z.-W. High-Throughput Determination of Fungicides in Grapes Using Thin-Film Microextraction Coupled with Liquid Chromatography–Tandem Mass Spectrometry. J. Sep. Sci. 2020, 43, 1558–1565. [Google Scholar] [CrossRef]
- Pang, G.; Chang, Q.; Bai, R.; Fan, C.; Zhang, Z.; Yan, H.; Wu, X. Simultaneous Screening of 733 Pesticide Residues in Fruits and Vegetables by a GC/LC-Q-TOFMS Combination Technique. Engineering 2020, 6, 432–441. [Google Scholar] [CrossRef]
- Jia, M.; Zhongbo, E.; Zhai, F.; Bing, X. Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules 2020, 25, 3590. [Google Scholar] [CrossRef] [PubMed]
- Collimore, W.A.; Bent, G.A. A Newly Modified QuEChERS Method for the Analysis of Organochlorine and Organophosphate Pesticide Residues in Fruits and Vegetables. Environ. Monit. Assess. 2020, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- İçli, N.; Tahmas Kahyaoğlu, D. Investigation of Pesticide Residues in Fresh Sultani Grapes and Antioxidant Properties of Fresh/Sun-Dried/Oven-Dried Grapes. Turk. J. Agric. For. 2020, 44, 350–360. [Google Scholar] [CrossRef]
- Tleubayeva, M.I.; Datkhayev, U.M.; Alimzhanova, M.; Ishmuratova, M.Y.; Korotetskaya, N.V.; Abdullabekova, R.M.; Flisyuk, E.V.; Gemejiyeva, N.G. Component Composition and Antimicrobial Activity of CO2 Extract of Portulaca oleracea, Growing in the Territory of Kazakhstan. Sci. World J. 2021, 2021, 5434525. [Google Scholar] [CrossRef]
- Ciasca, B.; Pecorelli, I.; Lepore, L.; Paoloni, A.; Catucci, L.; Pascale, M.; Lattanzio, V.M.T. Rapid and Reliable Detection of Glyphosate in Pome Fruits, Berries, Pulses and Cereals by Flow Injection—Mass Spectrometry. Food Chem. 2020, 310, 125813. [Google Scholar] [CrossRef]
- Pérez-Mayán, L.; Ramil, M.; Cela, R.; Rodríguez, I. Multiresidue Procedure to Assess the Occurrence and Dissipation of Fungicides and Insecticides in Vineyard Soils from Northwest Spain. Chemosphere 2020, 261, 127696. [Google Scholar] [CrossRef]
- Valera-Tarifa, N.M.; Santiago-Valverde, R.; Hernández-Torres, E.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Development and Full Validation of a Multiresidue Method for the Analysis of a Wide Range of Pesticides in Processed Fruit by UHPLC-MS/MS. Food Chem. 2020, 315, 126304. [Google Scholar] [CrossRef] [PubMed]
- Syrgabek, Y.; Alimzhanova, M.; Yegemova, S.; Batyrbekova, S. Vacuum-Assisted Headspace-Solid Phase Microextraction of Pesticides in Grape Samples. Adv. Sample Prep. 2024, 11, 1–15. [Google Scholar] [CrossRef]
- Aitzhanova, A.; Oleinikova, Y.; Mounier, J.; Hymery, N.; Leyva Salas, M.; Amangeldi, A.; Saubenova, M.; Alimzhanova, M.; Ashimuly, K.; Sadanov, A. Dairy Associations for the Targeted Control of Opportunistic Candida. World J. Microbiol. Biotechnol. 2021, 37, 143. [Google Scholar] [CrossRef]
- Koilybayeva, M.; Shynykul, Z.; Ustenova, G.; Abzaliyeva, S.; Alimzhanova, M.; Amirkhanova, A.; Turgumbayeva, A.; Mustafina, K.; Yeleken, G.; Raganina, K.; et al. Molecular Characterization of Some Bacillus Species from Vegetables and Evaluation of Their Antimicrobial and Antibiotic Potency. Molecules 2023, 28, 3210. [Google Scholar] [CrossRef] [PubMed]
- Tleubayeva, M.I.; Abdullabekova, R.M.; Datkhayev, U.; Ishmuratova, M.Y.; Alimzhanova, M.B.; Kozhanova, K.K.; Seitaliyeva, A.M.; Zhakipbekov, K.S.; Iskakova, Z.B.; Serikbayeva, E.A.; et al. Investigation of CO2 Extract of Portulaca oleracea for Antioxidant Activity from Raw Material Cultivated in Kazakhstan. Int. J. Biomater. 2022, 2022, 6478977. [Google Scholar] [CrossRef]
- Pu, C.H.; Lin, S.K.; Chuang, W.C.; Shyu, T.H. Modified QuEChERS Method for 24 Plant Growth Regulators in Grapes Using LC-MS/MS. J. Food Drug Anal. 2018, 26, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Abdulra’uf, L.B.; Tan, G.H. Chemometric Approach to the Optimization of HS-SPME/GC-MS for the Determination of Multiclass Pesticide Residues in Fruits and Vegetables. Food Chem. 2015, 177, 267–273. [Google Scholar] [CrossRef]
- Moinfar, S.; Jamil, L.A.; Sami, H.Z.; Ataei, S. An Innovative Continuous Sample Drop Flow Microextraction for GC–MS Determination of Pesticides in Grape Juice and Water Samples. J. Food Compos. Anal. 2021, 95, 103695. [Google Scholar] [CrossRef]
- López-Zapata, S.P.; Castaño-Zapata, J. In Vitro Effect of Four Fungicides on Colletotrichum Gloeosporioides Causing Anthracnosis on the Red Globe Grape Variety. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2020, 44, 747–758. [Google Scholar] [CrossRef]
- Sanghavi, K.; Sanghavi, M.; Rajurkar, A.M. Early Stage Detection of Downey and Powdery Mildew Grape Disease Using Atmospheric Parameters through Sensor Nodes. Artif. Intell. Agric. 2021, 5, 223–232. [Google Scholar] [CrossRef]
- Liang, Z.; Mahmoud Abdelshafy, A.; Luo, Z.; Belwal, T.; Lin, X.; Xu, Y.; Wang, L.; Yang, M.; Qi, M.; Dong, Y.; et al. Occurrence, Detection, and Dissipation of Pesticide Residue in Plant-Derived Foodstuff: A State-of-the-Art Review. Food Chem. 2022, 384, 132494. [Google Scholar] [CrossRef] [PubMed]
- Shemshura, O.; Shemsheyeva, Z.; Alimzhanova, M.; Sadanov, A.; Bozena, L.; Khalima, K. Antifungal activity of trichoderma asperellum and a profile of its volatile organic compounds. Pak. J. Bot. 2024, 56, 1187–1191. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Val, J.; Ballestero, D.; Navarro, E.; Sánchez, E.; González-Coloma, A.; Mainar, A.M. Ecotoxicity of a New Biopesticide Produced by Lavandula Luisieri on Non-Target Soil Organisms from Different Trophic Levels. Sci. Total Environ. 2019, 671, 83–93. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- López-Serna, R.; Ernst, F.; Wu, L. Analysis of Cinnamaldehyde and Diallyl Disulfide as Eco-Pesticides in Soils of Different Textures—A Laboratory-Scale Mobility Study. J. Soils Sediments 2016, 16, 566–580. [Google Scholar] [CrossRef]
- Enarevba, D.R.; Haapala, K.R. The Emerging Hemp Industry: A Review of Industrial Hemp Materials and Product Manufacturing. AgriEngineering 2024, 6, 2891–2925. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Eirian Jones, E.; Monk, J.; Ridgway, H.J. Using Bacterial Endophytes from a New Zealand Native Medicinal Plant for Control of Grapevine Trunk Diseases. Biol. Control 2017, 114, 65–72. [Google Scholar] [CrossRef]
- Naves, V.M.L.; dos Santos, M.H.; Ribeiro, I.S.; da Silva, C.A.; Silva, N.C.; da Silva, M.A.; da Silva, G.A.; Dias, A.L.T.; Ionta, M.; Dias, D.F. Antimicrobial and Antioxidant Activity of Garcinia Brasiliensis Extracts. S. Afr. J. Bot. 2019, 124, 244–250. [Google Scholar] [CrossRef]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Besrukow, P.; Will, F.; Dussling, S.; Berkelmann-Löhnertz, B.; Schweiggert, R. Additive and Synergistic Antifungal Effects of Copper and Phenolic Extracts from Grape Cane and Apples. Pest Manag. Sci. 2023, 79, 3334–3341. [Google Scholar] [CrossRef]
- Mengal, H.S.; Ali Abro, M.; Jatoi, G.H.; Nawab, L.; Poussio, G.B.; Ahmed, N.; Zehri, A.Q.; Ali, A. Efficacy of Different Fungicides, Botanical Extracts and Bio-Control Agents against Cladosporium Cladosporioides, the Causal Agent of Cladosporium Rot in Grapes. Acta Ecol. Sin. 2020, 40, 300–305. [Google Scholar] [CrossRef]
- Pedrotti, C.; da Silva Ribeiro, R.T.; Schwambach, J. Control of Postharvest Fungal Rots in Grapes through the Use of Baccharis Trimera and Baccharis Dracunculifolia Essential Oils. Crop Prot. 2019, 125, 1–7. [Google Scholar] [CrossRef]
- Burggraf, A.; Rienth, M. Origanum Vulgare Essential Oil Vapour Impedes Botrytis Cinerea Development on Grapevine (Vitis vinifera) Fruit. Phytopathol. Mediterr. 2020, 59, 331–344. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Lahlali, R.; Laasli, S.; Goura, K.; Fardi, M. The Potency and Effectiveness of Six Essential Oils in Controlling Grapevine Trunk Diseases in Morocco. J. Nat. Pestic. Res. 2023, 6, 100053. [Google Scholar] [CrossRef]
- Rozwalka, L.C.; Moreira, R.R.; Ballesteros Garcia, M.J.; Marques, F.A.; May De Mio, L.L. Chemical Components of Essential Oils as a Base to Control Two Grape Pathogens: Sphaceloma Ampelinum and Pseudocercopora Vitis. J. Phytopathol. 2020, 168, 342–352. [Google Scholar] [CrossRef]
- Servili, A.; Feliziani, E.; Romanazzi, G. Exposure to Volatiles of Essential Oils Alone or under Hypobaric Treatment to Control Postharvest Gray Mold of Table Grapes. Postharvest Biol. Technol. 2017, 133, 36–40. [Google Scholar] [CrossRef]
- Rai, R.; Singh, R.K.; Suthar, S. Production of Compost with Biopesticide Property from Toxic Weed Lantana: Quantification of Alkaloids in Compost and Bacterial Pathogen Suppression. J. Hazard. Mater. 2021, 401, 123332. [Google Scholar] [CrossRef] [PubMed]
- Crisp, P.; Wicks, T.J.; Lorimer, M.; Scott, E.S. An Evaluation of Biological and Abiotic Controls for Grapevine Powdery Mildew, 1. Greenhouse Studies. Aust. J. Grape Wine Res. 2006, 12, 192–202. [Google Scholar] [CrossRef]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal Activity of the Volatile Organic Compounds Produced by Bacillus Velezensis Strains against Postharvest Fungal Pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Bae, S.; Fleet, G.H.; Heard, G.M. Occurrence and Significance of Bacillus Thuringiensis on Wine Grapes. Int. J. Food Microbiol. 2004, 94, 301–312. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and Its Secondary Metabolites Improve Yield and Quality of Grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Ahmed, H.; Strub, C.; Hilaire, F.; Schorr-Galindo, S. First Report: Penicillium Adametzioides, a Potential Biocontrol Agent for Ochratoxin-Producing Fungus in Grapes, Resulting from Natural Product Pre-Harvest Treatment. Food Control 2015, 51, 23–30. [Google Scholar] [CrossRef]
- El-Sharkawy, H.H.A.; Abo-El-Wafa, T.S.A.; Mostafa, N.A.; Yousef, S.A.M. Boosting Biopesticide Potential of Trichoderma Harzianum for Controlling the Downy Mildew and Improving the Growth and the Productivity of King Ruby Seedless Grape. Egypt. J. Biol. Pest Control 2023, 33, 61. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining Biocontrol Agents with Different Mechanisms of Action in a Strategy to Control Botrytis Cinerea on Grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Zahavi, T.; Cohen, L.; Weiss, B.; Schena, L.; Daus, A.; Kaplunov, T.; Zutkhi, J.; Ben-Arie, R.; Droby, S. Biological Control of Botrytis, Aspergillus and Rhizopus Rots on Table and Wine Grapes in Israel. Postharvest Biol. Technol. 2000, 20, 115–124. [Google Scholar] [CrossRef]
- Laurent, A.; Makowski, D.; Aveline, N.; Dupin, S.; Miguez, F.E. On-Farm Trials Reveal Significant but Uncertain Control of Botrytis Cinerea by Aureobasidium Pullulans and Potassium Bicarbonate in Organic Grapevines. Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Dimakopoulou, M.; Tjamos, S.E.; Antoniou, P.P.; Pietri, A.; Battilani, P.; Avramidis, N.; Markakis, E.A.; Tjamos, E.C. Phyllosphere Grapevine Yeast Aureobasidium Pullulans Reduces Aspergillus Carbonarius (Sour Rot) Incidence in Wine-Producing Vineyards in Greece. Biol. Control 2008, 46, 158–165. [Google Scholar] [CrossRef]
- Marín, A.; Cháfer, M.; Atarés, L.; Chiralt, A.; Torres, R.; Usall, J.; Teixidó, N. Effect of Different Coating-Forming Agents on the Efficacy of the Biocontrol Agent. Biol. Control 2016, 96, 108–119. [Google Scholar] [CrossRef]
- Ponsone, M.L.; Chiotta, M.L.; Combina, M.; Dalcero, A.; Chulze, S. Biocontrol as a Strategy to Reduce the Impact of Ochratoxin A and Aspergillus Section Nigri in Grapes. Int. J. Food Microbiol. 2011, 151, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ponsone, M.L.; Nally, M.C.; Chiotta, M.L.; Combina, M.; Köhl, J.; Chulze, S.N. Evaluation of the Effectiveness of Potential Biocontrol Yeasts against Black Sur Rot and Ochratoxin A Occurring under Greenhouse and Field Grape Production Conditions. Biol. Control 2016, 103, 78–85. [Google Scholar] [CrossRef]
- Apaliya, M.T.; Zhang, H.; Yang, Q.; Zheng, X.; Zhao, L.; Kwaw, E.; Mahunu, G.K. Hanseniaspora Uvarum Enhanced with Trehalose Induced Defense-Related Enzyme Activities and Relative Genes Expression Levels against Aspergillus Tubingensis in Table Grapes. Postharvest Biol. Technol. 2017, 132, 162–170. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, L.; Zhang, X.; Dhanasekaran, S.; Abdelhai, M.H.; Yang, Q.; Jiang, Z.; Zhang, H. Study on Biocontrol of Postharvest Decay of Table Grapes Caused by Penicillium Rubens and the Possible Resistance Mechanisms by Yarrowia Lipolytica. Biol. Control 2019, 130, 110–117. [Google Scholar] [CrossRef]
- Bleve, G.; Grieco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of Epiphytic Yeasts with Potential for Biocontrol of Aspergillus Carbonarius and A. Niger on Grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef]
- De Curtis, F.; de Felice, D.V.; Ianiri, G.; De Cicco, V.; Castoria, R. Environmental Factors Affect the Activity of Biocontrol Agents against Ochratoxigenic Aspergillus Carbonarius on Wine Grape. Int. J. Food Microbiol. 2012, 159, 17–24. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Christou, O.; Tsolakidou, M.D.; Tsaltas, D.; Ioannou, N. Isolation, Identification and in Vitro Screening of Grapevine Yeasts for the Control of Black Aspergilli on Grapes. Biol. Control 2015, 88, 46–53. [Google Scholar] [CrossRef]
- Schena, L.; Ippolito, A.; Zahavi, T.; Cohen, L.; Droby, S. Molecular Approaches to Assist the Screening and Monitoring of Postharvest Biocontrol Yeasts. Eur. J. Plant Pathol. 2000, 106, 681–691. [Google Scholar] [CrossRef]
- Malviya, D.; Thosar, R.; Kokare, N.; Pawar, S.; Singh, U.B.; Saha, S.; Rai, J.P.; Singh, H.V.; Somkuwar, R.G.; Saxena, A.K. A Comparative Analysis of Microbe-Based Technologies Developed at ICAR-NBAIM Against Erysiphe Necator Causing Powdery Mildew Disease in Grapes (Vitis vinifera L.). Front. Microbiol. 2022, 13, 871901. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus plantarum Strains from Sourdoughs for Biosuppression of Pseudomonas syringae pv. syringae and Botrytis cinerea in Table Grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef]
- Thomidis, T.; Pantazis, S.; Konstantinoudis, K. Evaluation of Serenade Max to Control Fruit Rot of Grapes. J. Agric. Sci. 2016, 8, 212. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; DiVenere, D.; Salerno, M. Effects of Pre- and Postharvest Chitosan Treatments to Control Storage Grey Mold of Table Grapes. J. Food Sci. 2002, 67, 1862–1867. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Godena, S. Potential of Plant-Based Agents as Next-Generation Plant Growth-Promotors and Green Bactericides Against Pseudomonas savastanoi pv. savastanoi. Agronomy 2025, 15, 819. [Google Scholar] [CrossRef]
- Burman, E.; Bengtsson-Palme, J. Microbial Community Interactions Are Sensitive to Small Changes in Temperature. Front. Microbiol. 2021, 12, 672910. [Google Scholar] [CrossRef]
- Wend, K.; Zorrilla, L.; Freimoser, F.M.; Gallet, A. Microbial pesticides–challenges and future perspectives for testing and safety assessment with respect to human health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Furuya, S.; Mochizuki, M.; Aoki, Y.; Kobayashi, H.; Takayanagi, T.; Shimizu, M.; Suzuki, S. Isolation and Characterization of Bacillus Subtilis KS1 for the Biocontrol of Grapevine Fungal Diseases. Biocontrol Sci. Technol. 2011, 21, 705–720. [Google Scholar] [CrossRef]
- Ren, C.; Mohamed, M.S.M.; Aini, N.; Kuang, Y.; Liang, Z. CRISPR/Cas in Grapevine Genome Editing: The Best is Yet to Come. Horticulturae 2024, 10, 965. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial Biofungicides as a Substitute for Chemical Fungicides in the Control of Phytopathogens: Current Perspectives and Research Directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef]
- Lemos, W.J.; Bovo, B.; Nadai, C.; Crosato, G.; Carlot, M.; Favaron, F.; Giacomini, A.; Corich, V. Biocontrol Ability and Action Mechanism of Starmerella Bacillaris (Synonym Candida Zemplinina) Isolated from Wine Musts against Gray Mold Disease Agent Botrytis Cinerea on Grape and Their Effects on Alcoholic Fermentation. Front. Microbiol. 2016, 7, 1249. [Google Scholar] [CrossRef]

| Mechanism | Description | Organic Agent | Mechanism of Action |
|---|---|---|---|
| Resource competition | Compete with pathogens for nutrients and colonization sites | Aureobasidium pullulans | Inhibits Botrytis cinerea by occupying infection sites and limiting resource availability |
| Biosynthesis of antimicrobial compounds | Produce lipopeptides or secondary metabolites that inhibit pathogen growth | Bacillus subtilis and Leptospermum scoparium | Disrupt fungal membranes and suppress germination |
| Physical barrier formation and immune induction | Stimulate host defense enzymes and form protective films on plant surfaces | Chitosan | Activates peroxidase and phenylalanine ammonia-lyase activity; forms a protective layer on grapevine tissues |
| Direct pathogen disruption | Destroy fungal spores or membranes through biochemical interactions | Essential oils (eucalyptus, rosemary, and cinnamon) | Disrupt spore structure and inhibit fungal development |
| Soil microbiome enhancement | Improve beneficial soil microbiota and suppress harmful pathogens | Lantana camara compost | Releases bioactive compounds that reduce pathogen levels and enhance soil health |
| Agent Type | Category | Challenge | Solution | Mechanism of Action |
|---|---|---|---|---|
| Plant-based | Stability | Degradation under light and temperature | Microencapsulation and controlled storage | Creates a protective barrier to prevent degradation by light and heat |
| Standardization | Variable chemical composition | Standardized extraction and quality control | Ensures consistent bioactivity through quantitative analysis | |
| Narrow spectrum | Limited activity against specific pathogens | Combination with other agents and IPM | Broadens activity through synergistic effects | |
| Environmental sensitivity | UV-induced degradation | Optimized application timing and UV protectants | Minimizes photodegradation by application under low-light conditions with UV blockers | |
| Microbial | Stability | Loss of viability during storage and transport | Lyophilization and cold chain | Preserves cell structure and viability under dry and cold conditions |
| Standardization | Inconsistent formulation with adjuvants | Encapsulation, stabilization | Improves stability and compatibility under various conditions | |
| Narrow spectrum | Limited efficacy against diverse pathogens | Microbial consortia | Combines multiple mechanisms of action (e.g., antibiotics and competition) | |
| Environmental sensitivity | Sensitivity to temperature, humidity, and UV | Optimized application and UV blockers | Enhances survival under stress via timing and protective additives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimzhanova, M.; Meirbekov, N.; Syrgabek, Y.; López-Serna, R.; Yegemova, S. Plant- and Microbial-Based Organic Disease Management for Grapevines: A Review. Agriculture 2025, 15, 963. https://doi.org/10.3390/agriculture15090963
Alimzhanova M, Meirbekov N, Syrgabek Y, López-Serna R, Yegemova S. Plant- and Microbial-Based Organic Disease Management for Grapevines: A Review. Agriculture. 2025; 15(9):963. https://doi.org/10.3390/agriculture15090963
Chicago/Turabian StyleAlimzhanova, Mereke, Nurkanat Meirbekov, Yerkanat Syrgabek, Rebeca López-Serna, and Saltanat Yegemova. 2025. "Plant- and Microbial-Based Organic Disease Management for Grapevines: A Review" Agriculture 15, no. 9: 963. https://doi.org/10.3390/agriculture15090963
APA StyleAlimzhanova, M., Meirbekov, N., Syrgabek, Y., López-Serna, R., & Yegemova, S. (2025). Plant- and Microbial-Based Organic Disease Management for Grapevines: A Review. Agriculture, 15(9), 963. https://doi.org/10.3390/agriculture15090963







