Environmental and Colony-Related Factors Linked to Small Hive Beetle (Aethina tumida) Infestation in Apis mellifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
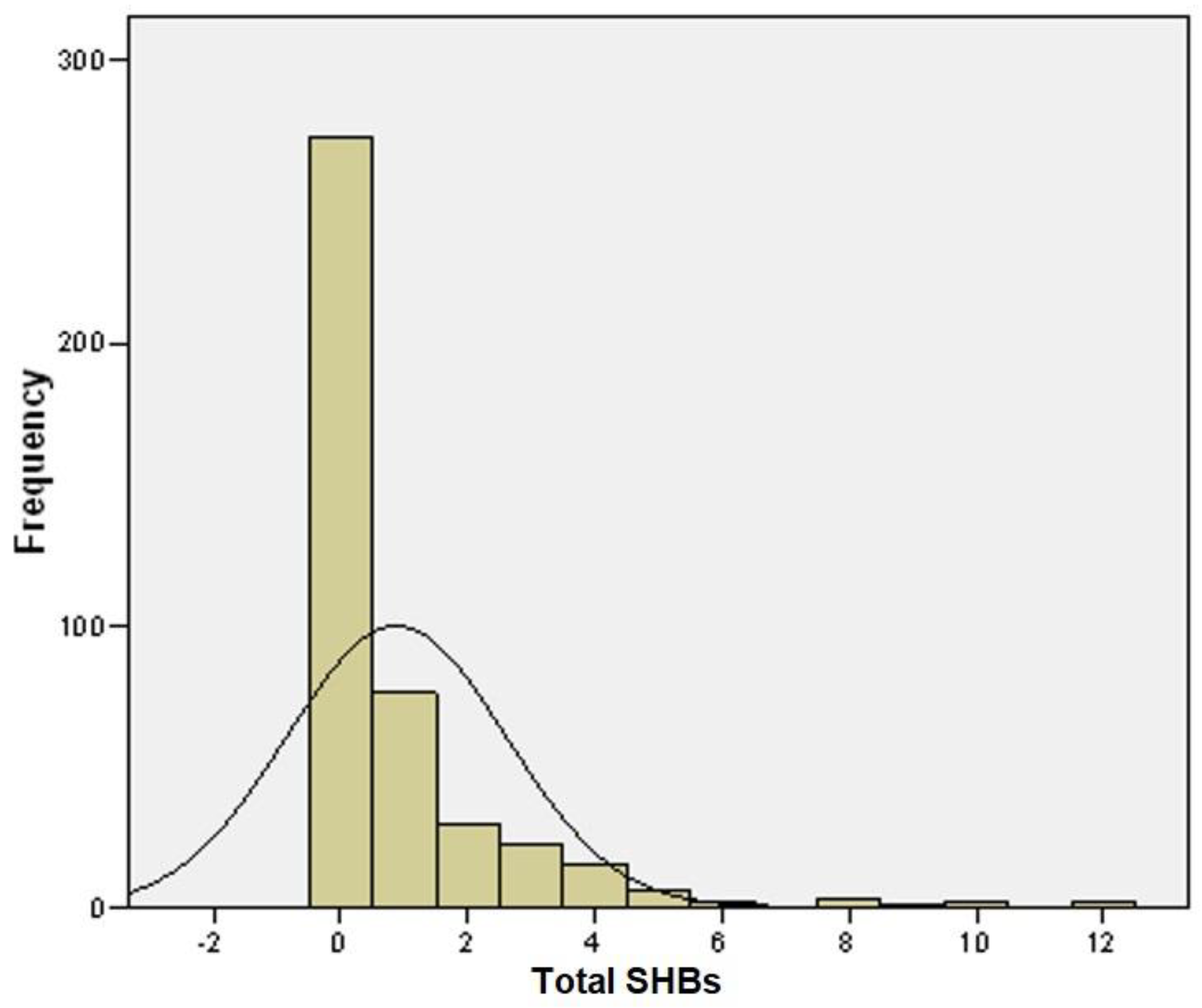
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, P.; Ellis, J.D. The Small Hive Beetle (Aethina tumida Murray, Coleoptera: Nitidulidae): Distribution, Biology and Control of an Invasive Species. J. Apic. Res. 2008, 47, 181–183. [Google Scholar] [CrossRef]
- World Organisation of Animal Health. Infestation with Aethina tumida (Small Hive Beetle). Terrestrial Animal Health Code, Chapter 9.4. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aethina_tumida.pdf (accessed on 10 March 2025).
- Suazo, A.; Torto, B.; Teal, P.; Tumlinson, J. Response of the Small Hive Beetle (Aethina tumida) to Honey Bee (Apis mellifera) and Beehive-Produced Volatiles. Apidologie 2003, 34, 525–533. [Google Scholar] [CrossRef]
- Torto, B.; Suazo, A.; Alborn, H.; Tumlinson, J.H.; Teal, P.E.A. Response of the Small Hive Beetle (Aethina tumida) to a Blend of Chemicals Identified from Honeybee (Apis mellifera) Volatiles. Apidologie 2005, 36, 523–532. [Google Scholar] [CrossRef]
- Roth, M.A.; Gross, A.D.; Wilson, J.M. Small Hive Beetle. Available online: https://www.pubs.ext.vt.edu/ENTO/ENTO-338/ENTO-338.html (accessed on 10 March 2025).
- Araneda, X.; Aldea, P.; Freire, X. Small Hive Beetle (Aethina tumida Murray), A potential thret to beekeeping in Chile. Chil. J. Agric. Anim. Sci. 2021, 37, 3–10. [Google Scholar] [CrossRef]
- Kulishenko, O.; Davydenko, P.; Borovyk, I.; Radzykhovskyi, M.; Gutyj, B. Small Hive Beetle (Aethina tumida) Threat on the Horizon. Ukr. J. Vet. Agric. Sci. 2023, 6, 72–77. [Google Scholar] [CrossRef]
- Palmeri, V.; Scirtò, G.; Malacrinò, A.; Laudani, F.; Campolo, O. A Scientific Note on a New Pest for European Honeybees: First Report of Small Hive Beetle Aethina tumida, (Coleoptera: Nitidulidae) in Italy. Apidologie 2014, 46, 527–529. [Google Scholar] [CrossRef]
- Mutinelli, F.; Montarsi, F.; Federico, G.; Granato, A.; Ponti, A.M.; Grandinetti, G.; Ferre, N.; Franco, S.; Duquesne, V.; Rivière, M.P.; et al. Detection of Aethina tumida Murray (Coleoptera: Nitidulidae.) in Italy: Outbreaks and Early Reaction Measures. J. Apic. Res. 2014, 53, 569–575. [Google Scholar] [CrossRef]
- Granato, A.; Zecchin, B.; Baratto, C.; Duquesne, V.; Negrisolo, E.; Chauzat, M.P.; Ribière-Chabert, M.; Cattoli, G.; Mutinelli, F. Introduction of Aethina tumida (Coleoptera: Nitidulidae) in the Regions of Calabria and Sicily (Southern Italy). Apidologie 2016, 48, 194–203. [Google Scholar] [CrossRef]
- Cini, A.; Santosuosso, U.; Papini, A. Uncovering the Spatial Pattern of Invasion of the Honeybee Pest Small Hive Beetle, Aethina tumida, in Italy. Rev. Bras. Entomol. 2019, 63, 12–17. [Google Scholar] [CrossRef]
- Istituto Zooprofilattico Sperimentale Delle Venezie Centro DI Referenza Nazionale per L’Apicoltura. Casi Confermati Di Aethina tumida, Situazione Aggiornata Al 9 October 2024. Available online: https://www.izsvenezie.it/documenti/temi/api/aethina-thumida/2024/tabella-1-casi-confermati.pdf (accessed on 10 March 2025).
- Salvioni, C.; Cerroni, S. Eliciting Beekeepers’ Preferences for the Small Hive Beetle Control Policy in Italy: A Contingent Valuation Survey Approach. Agric. Food Econ. 2023, 11, 29. [Google Scholar] [CrossRef]
- Rivera-Gomis, J.; Bubnic, J.; Ribarits, A.; Moosbeckhofer, R.; Alber, O.; Kozmus, P.; Jannoni-Sebastianini, R.; Haefeker, W.; Köglberger, H.; Smodis Skerl, M.M.I.; et al. Good Farming Practices in Apiculture. Rev. Sci. Tech. L’OIE 2020, 38, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.; der Steen, J.; Guzman, E. Standard Methods for Estimating Strength Parameters of Apis mellifera Colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Kullenberg, C.; Kasperowski, D. What Is Citizen Science?—A Scientometric Meta-Analysis. PLoS ONE 2016, 11, e0147152. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gomis, J.; Gregorc, A.; Maroni Ponti, A.; Artese, F.; Zowitsky, G.; Formato, G. Monitoring of Small Hive Beetle (Aethina tumida Murray) in Calabria (Italy) from 2014 to 2016: Practical Identification Methods. J. Apic. Sci. 2017, 61, 257. [Google Scholar] [CrossRef]
- Uzunov, A.; Ralph, B.; Costa, C.; Mondet, F.; Bienkowska, M.; Hatjina, F.; Meixner, M.; Andonov, S.; Kovačić, M.; Dall’Olio, R.; et al. Book of Methods Performance Testers; EurBeST Project (AGRI-2017-0346); Bee Institute: Kirchhain, Germany, 2021. (In English) [Google Scholar]
- Spiewok, S.; Pettis, J.; Duncan, M.; Spooner-Hart, R.; Westervelt, D.; Neumann, P. Small Hive Beetle, Aethina tumida, Populations I: Infestation Levels of Honeybee Colonies, Apiaries and Regions. Apidologie 2007, 38, 595–605. [Google Scholar] [CrossRef]
- Ellis, J.; Delaplane, K. Small hive beetle (Aethina tumida) oviposition behaviour in sealed brood cells with notes on the removal of the cell contents by European honey bees (Apis mellifera). J. Apic. Res. 2008, 47, 210–215. [Google Scholar] [CrossRef]
- Noor-ul-Ane, M.; Jung, C. Temperature-Dependent Development and Survival of Small Hive Beetle, Aethina tumida (Coleoptera: Nitidulidae). J. Apic. Res. 2020, 59, 807–816. [Google Scholar] [CrossRef]
- Mekonnen, E.; Gela, A.; Bezzabih, A. On-Farm Evaluation and Demonstration of Small Hive Beetle Trapping Technology. Int. J. Energy Environ. Sci. 2021, 6, 158–162. [Google Scholar] [CrossRef]
- Torto, B.; Arbogast, R.; Alborn, H.; Suazo, A.; VanEngelsdorp, D.; Boucias, D.; Tumlinson, J.; Teal, P. Composition of Volatiles from Fermenting Pollen Dough and Attractiveness to the Small Hive Beetle Aethina tumida, a Parasite of the Honeybee Apis mellifera. Apidologie 2007, 38, 380–389. [Google Scholar] [CrossRef]
- Nolan, M.P. Hood WM Comparison of Two Attractants to Small Hive Beetles, Aethina tumida, in Honey Bee Colonies. J. Apic. Res. 2008, 47, 229–233. [Google Scholar] [CrossRef]
- Benda, N.; Boucias, D.; Torto, B.; Teal, P. Detection and Characterization of Kodamaea ohmeri Associated with Small Hive Beetle Aethina tumida Infesting Honey Bee Hives. J. Apic. Res. 2008, 47, 194–201. [Google Scholar] [CrossRef]
- Leemon, D. In-Hive Fungal Biocontrol of Small Hive Beetle; Rural Industries Research and Development Corporation: Canberra, Australia, 2012; pp. 1–69. [Google Scholar]
- Montgomery, M.E.; Wargo, P.M. Ethanol and Other Host-Derived Volatiles as Attractants to Beetles That Bore into Hardwoods. J. Chem. Ecol. 1983, 9, 181–190. [Google Scholar] [CrossRef]
- Hayes, R.; Rice, S.; Amos, B.; Leemon, D. Increased Attractiveness of Honeybee Hive Product Volatiles to Adult Small Hive Beetle, Aethina tumida, Resulting from Small Hive Beetle Larval Infestation. Entomol. Exp. Appl. 2015, 155, 240–248. [Google Scholar] [CrossRef]
- Lundie, A.E. The Small Hive Beetle, A. tumida; Scientific Bulletin 220; Department of Agriculture and Forestry, Government Printer: Pretoria, South Africa, 1940. [Google Scholar]
- Bestmann, H.J.; Vostrowsky, O. Pheromones of the Coleoptera. In Handbook of Natural Pesticides; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, pp. 95–183. [Google Scholar]
- Cuthbertson, A.; Wakefield, M.; Powell, M.; Marris, G.; Anderson, H.; Budge, G.; Mathers, J.; Blackburn, L.; Brown, M. The Small Hive Beetle Aethina tumida: A Review of Its Biology and Control Measures. Curr. Zool. 2013, 59, 644–653. [Google Scholar] [CrossRef]
- Dekebo, A.; Seokmin, H.; Jung, C. Attractiveness of the Small Hive Beetle (Aethina tumida) to Volatiles from Honey Bee (Apis mellifera) and Beehive Materials. J. Apic. 2017, 32, 315–326. [Google Scholar] [CrossRef]
- Breed, M.D.; Guzmán-Novoa, E.; Hunt, G.J. Organization, Genetics, and Comparisons with Other Bees. Annu. Rev. Entomol. 2004, 49, 271–298. [Google Scholar] [CrossRef]
- Kayode, A.; Neumann, P. Small Hive Beetle Infestation Levels of Honey Bee Colonies Correlate with Precipitation and Forest Cover. Apidologie 2018, 49, 517–525. [Google Scholar]
- Nacko, S.; Hall, M.; Duncan, M.; Cook, J.; Riegler, M.; Spooner-Hart, R. Scientific Note on Small Hive Beetle Infestation of Stingless Bee (Tetragonula carbonaria) Colony Following a Heat Wave. Apidologie 2020, 51, 199–201. [Google Scholar] [CrossRef]
| Apiary | Season | Number of Hive Inspections/Apiary During the First Control | Number of Hive Inspections/Apiary During the Second Control | Number of Hive Inspections/Apiary During the Third Control | Total Number of Hive Inspections/Apiary | Total Number of SHBs Detected in the Whole Apiary | Average Number (SHBs/Hive Inspection) |
|---|---|---|---|---|---|---|---|
| Apiary 1 | Late winter | 8 | 8 | 8 | 24 | 11 | 0.46 |
| Autumn | NA | NA | NA | NA | NA | NA | |
| Apiary 2 | Late winter | 26 | 26 | 26 | 78 | 18 | 0.23 |
| Autumn | 36 | 36 | 33 | 105 | 245 | 2.33 | |
| Apiary 3 | Late winter | 10 | 8 | 8 | 26 | 16 | 0.62 |
| Autumn | 10 | 10 | 10 | 30 | 3 | 0.10 | |
| Apiary 4 | Late winter | 7 | 7 | 7 | 21 | 20 | 0.95 |
| Autumn | 19 | 17 | 16 | 52 | 8 | 0.15 | |
| Apiary 5 | Late winter | 6 | 6 | 6 | 18 | 3 | 0.17 |
| Autumn | 8 | 8 | 7 | 23 | 29 | 1.26 | |
| Apiary 6 | Late winter | 10 | 10 | 10 | 30 | 13 | 0.43 |
| Autumn | 8 | 8 | 8 | 24 | 13 | 0.54 | |
| Total Apiaries | Both seasons | 148 | 144 | 139 | 431 | 379 | 0.83 |
| Minimum in Each Colony | Maximum in Each Colony | Average (M) | Standard Deviation (SD) | Coefficient of Variation (CV = SD/M) | |
|---|---|---|---|---|---|
| Total number of SHBs | 0 | 12 | 0.88 | 1.73 | 1.96 |
| Total number of combs | 2 | 10 | 5.74 | 1.72 | 0.30 |
| Number of combs covered by adult bees | 1 | 10 | 5.27 | 1.66 | 0.31 |
| Number of combs containing brood | 0 | 7 | 2.93 | 1.30 | 0.44 |
| Number of storage combs | 0 | 10 | 2.32 | 1.22 | 0.53 |
| Exposure (0 = Shadow; 1 = Sun) | 0 | 1 | 0.74 | 0.44 | 0.60 |
| Queen (0 = Orphan; 1 = Queen right) | 0 | 1 | 0.95 | 0.21 | 0.22 |
| Calendar Months | Late Winter | Autumn | |||||
|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Sep | Oct | Nov | Average | |
| Average number of SHBs/hive | 0.46 | 0.28 | 0.49 | 1.80 | 1.16 | 0.81 | 0.83 |
| 0.41 | 1.26 | ||||||
| Factors to Be Compared with the SHB Infestation Level | ∆-GLM Extremes Impact |
|---|---|
| SHB infestation level in the previous month | ±1.192 |
| Seasons | ±0.258 |
| Total number of combs | ±1.877 |
| Number of combs covered by adult bees | ±2.543 |
| Combs surveillance | ±0.935 |
| Exposure to sunlight | ±0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Ruggiero, C.; Gyorffy, A.; Artese, F.; De Carolis, A.; De Simone, A.; Pietropaoli, M.; Pedrelli, C.; Formato, G. Environmental and Colony-Related Factors Linked to Small Hive Beetle (Aethina tumida) Infestation in Apis mellifera. Agriculture 2025, 15, 962. https://doi.org/10.3390/agriculture15090962
Di Ruggiero C, Gyorffy A, Artese F, De Carolis A, De Simone A, Pietropaoli M, Pedrelli C, Formato G. Environmental and Colony-Related Factors Linked to Small Hive Beetle (Aethina tumida) Infestation in Apis mellifera. Agriculture. 2025; 15(9):962. https://doi.org/10.3390/agriculture15090962
Chicago/Turabian StyleDi Ruggiero, Camilla, Andrea Gyorffy, Francesco Artese, Alessandra De Carolis, Angelo De Simone, Marco Pietropaoli, Camilla Pedrelli, and Giovanni Formato. 2025. "Environmental and Colony-Related Factors Linked to Small Hive Beetle (Aethina tumida) Infestation in Apis mellifera" Agriculture 15, no. 9: 962. https://doi.org/10.3390/agriculture15090962
APA StyleDi Ruggiero, C., Gyorffy, A., Artese, F., De Carolis, A., De Simone, A., Pietropaoli, M., Pedrelli, C., & Formato, G. (2025). Environmental and Colony-Related Factors Linked to Small Hive Beetle (Aethina tumida) Infestation in Apis mellifera. Agriculture, 15(9), 962. https://doi.org/10.3390/agriculture15090962







