Abstract
Natural plant- and algae-based extracts used in crop cultivation offer numerous advantages, including the potential to positively affect plant growth, exhibit hormonal activity, increase stress resistance, improve crop quality as environmentally benign alternatives to synthetic agrochemicals and help combat oxidative stress. The presented experiments aimed to compare the effectiveness of extracts from brown algae such as Ascophyllum nodosum and Fucus vesiculosus, as well as the plant Sideritis scardica, on the germination and initial growth of red kale (Brassica napus var. Pabularia) microgreens. Microgreens treated with aqueous extracts of A. nodosum, F. vesiculosus, as well as the control group, had the highest growth, whereas the lowest growth was observed in plants treated with water–ethanol extracts at the highest tested concentration (10%). The 10% water–ethanol extracts of brown algae reduced plant biomass, while aqueous extracts increased it. Applying water extracts of algae at concentrations (10, 1, 0.1%), as well as the water extract of S. scardica (10, 1%), led to an increase in the total phenolic content in the tested experimental groups. A significant influence on increasing total flavonoid content was noted for water extracts of F. vesiculosus at concentrations ranging from 0.1% to 10%. An opposite effect was observed for the water–ethanol extracts, where the lowest TFC was found in plants grown on mats soaked with 0.1% F. vesiculosus and 1% A. nodosum. All water–ethanol extracts tended to reduce the antioxidant activity of the tested red kale microgreens. In microgreens treated with water extracts of F. vesiculosus at concentrations of 1% and 10%, an increase in antioxidant activity was observed. Examining the impact of plant and algae extracts on kale germination and growth may provide valuable information on ways to improve the quality and health-promoting properties of kale microgreens.
1. Introduction
An increasing number of human health problems are associated with over- or under-consumption of specific food groups [1]. Inadequate fruit and vegetable consumption remains an ongoing global concern with economic, social, environmental and nutritional implications [2]. One solution to this problem may be the production of microgreens in a controlled environment, characterized by a high content of valuable secondary metabolites such as antioxidants and phenolic compounds [1]. The optimal protocols for growing microgreens with the highest valuable nutrient content are not well known due to high species variability and the need to maintain a trade-off between biomass production and secondary metabolite production. This phenomenon is known as the growth–defense trade-off hypothesis, which describes how plants can use the resources available to them by allocating them to growth or defense processes [1]. Significant differences can be observed between plants of the same species growing in different environments. Understanding the trade-off between plant growth, defense mechanisms and their phenotypic expression is one of the foundations for the development of sustainable agriculture [3]. Microgreens, vegetable or herb seedlings consumed at an early stage of growth are considered functional foods with high concentrations of minerals and beneficial bioactive compounds [4]. Compared to mature vegetables, microgreens contain higher levels of nutrients (minerals, vitamins, antioxidants and phenolic compounds) [5,6]. Vegetable producers are also interested in microgreens as a new specialty crop due to their high market value, consumer appeal, short production cycles, palatability and favorable phytochemical profile [4,7,8,9,10].
Microgreens are known for their high nutritional value and “nutrient density”; however, further research is needed to optimize the levels and ratios of the nutrients they contain. Understanding the impact of various growing conditions on the composition of microgreens will help to produce safe, easily available nutrient-rich crops [11]. Overall, addressing these challenges and focusing on future directions will contribute to the sustainable growth and widespread acceptance and recognition of microgreens as a nutritious and tasty food option [11]. Good handling practices for microgreens can improve product stability and extend storage time and shelf life of microgreens. Selection of light, temperature, nutrients, humidity and substrate is important [12]. There is a lack of research-based recommendations for specific crops, including fertilization, pre-sowing treatments to stimulate seed germination and their effect on the nutritional value of microgreens [4]. It has been suggested that more detailed standards should be established for species selection, growing media, lighting, temperature, humidity and nutrient management [11]. Microgreens can be produced year-round without fertilizers or pesticides [13]. Many commercial producers, as well as those producing microgreens for their own consumption, claim that they do not require fertilization, especially additional micronutrients, because most of the micronutrients necessary for proper seedling growth are already available in the seeds [14]. However, in some cases, especially in commercial microgreens production, additional agents (e.g., biostimulants) can be used to increase yield and quality, or to modify the chemical composition of the plants [15].
Treatment of plants with algae extracts contributes to the modification of their chemical composition through the expression of genes involved in the biosynthesis of various compounds, e.g., carbohydrates (starch, sucrose), amino acids (proline), sugar alcohols (inositol) and phenolic compounds [16]. It is also known that the application of aqueous extracts of Ascophyllum nodosum can increase production of low molecular weight antioxidants, including flavonoids and phenolic compounds. These are made during the biosynthesis process, which involves an enzyme called phenylalanine ammonia lyase (PAL). This enzyme catalyzes the initial step in the biosynthesis of phenolic compounds in plants and functions as a pivotal regulatory enzyme in their specialized metabolism [17,18].
PAL and polyphenol oxidase (PPO) are enzymes involved in the phenolic pathway, which plays a key role in plant defense and stress response [17,19].
The increase in PPO and PAL levels under the influence of the application of aqueous extracts of A. nodosum has been proven [17]. These enzymes affect the synthesis of phenolic compounds, including flavonoids in plants, particularly under abiotic stress conditions [20].
Plant and algal extracts could be a promising alternative to synthetic chemicals [17,21]. They are natural biostimulants that have the potential to support plant growth, improve their health, and increase resistance to abiotic and biotic stresses [15,21,22,23,24,25,26].
The effects of extracts and commercial preparations of Acophyllum nodosum are widely studied [27,28,29,30]. There are also publications in which the biostimulating effect of Fucus vesiculosus was tested [31].
The effect of Sideritis scardica on human health has been widely studied, but there is no proven effect on plants. The rich composition of bioactive compounds suggests that this plant may have biostimulating properties. S. scardica extracts exhibit high levels of phenolics compounds and significant antioxidant activity, pointing to their potential application as antioxidant sources [32]. A large number of chemical compounds have also been detected, more than 100 metabolites, including five sugar acids and saccharides, 21 carboxylic, hydroxybenzoic, hydroxycinnamic acids, and derivatives, 15 acylquinic acids, 10 phenylpropanoid glycosides, four iridoid glycosides, 28 flavonoids, seven fatty acids, and four organosulfur compounds [33].
Microgreens, when cultivated in a controlled environment, have been found to exhibit rich phytochemical profiles. It has also been suggested that extracts with potential biostimulant effects can be used to improve microgreens’ phytochemical characteristics, with natural algal and plant extracts being a possible option. Red kale, which is rich in vitamins, minerals and bioactive compounds, and is, therefore, a valuable ingredient in the human diet, could be a suitable model for research on the effect of natural biostimulants on the growth and composition of young seedlings [34,35,36,37].
In this study, the influence of plant- and algae-based extracts on the germination process and growth of young red kale plants was analyzed. The aim of the work was to assess the effect of selected natural extracts on plants at the initial stage of growth and to assess how they can be naturally used in the cultivation of microgreens. It was hypothesized that natural extracts would enhance plant growth parameters and increase antioxidant activity. The hypothesis was to be verified by the following indicators, among others: germination energy and capacity, Maguire and Pieper index, plant growth and weight, TPC and TFC content and antioxidant activity.
The research results provide valuable information on the potential applications of plant and algae extracts in agriculture, especially in the context of improving the germination and growth parameters of crops grown as microgreens, as illustrated by the example of kale.
2. Materials and Methods
The experiments were conducted in two stages. In the first stage, the impact of the extracts on seed germination energy and germination capacity was evaluated by germinating seeds on Petri dishes. In the second stage, the influence of the tested extracts on the initial growth and chemical composition of plants grown on linen mats was assessed.
2.1. Preparation of Extracts
The extracts were obtained from the brown algae Ascophyllum nodosum (A. nodosum, ASC) and Fucus vesiculosus (F. vesiculosus, FUC) and from the plant Sideritis scardica (S. scardica, SID). The aqueous extracts were prepared according to the procedure of Drygaś et al. [17,21]. The water–ethanol extracts were prepared as follows:
Twenty-five grams (25 g) of crushed plant and algae material were weighed using a RADWAG (Radom, Poland) scale and subsequently extracted with a 1:1 v/v mixture of H2O (DI water 0.05 µS, Hydrolab) and EtOH (analytical grade PA-01-0300-W, PAE001, Pol-Aura) in an ultrasonic bath for two intervals of 30 min at 40 °C, utilizing separate portions of the pure extraction mixture. The resulting extracts were filtered and combined, and the final volume was adjusted to 500 mL in a volumetric flask with the extraction mixture.
Ethanol extracts were prepared in the same way as water–ethanol extracts, using only ethanol as a solvent. The resulting extract was then referred to as 100% extract (undiluted). Subsequent dilutions (10%, 1%, 0.1%, 0.01%) were prepared with distilled water.
2.2. Assay of Extracts (Screening)
The methods and techniques for assays of extracts are described in detail in the publication of Drygaś et al. [17]. The names of the tests performed and the expected results are summarized in Table 1.

Table 1.
Phytochemical tests used to determine the composition of the extracts obtained.
2.3. Germination Test
The research material consisted of red kale (Brassica napus var. Pabularia) of the Red Russian variety (TORAF, Maciejów, Poland, lot number 98451CIN0DS). The first experiment was carried out in a growth chamber (producer, city) at a temperature of 20 °C without access to light for a period of 30 d. Germinating seeds were counted daily at equal time intervals. The germination experiment was set up on 5.5 cm diameter Petri dishes lined with six layers of medium porosity filter paper, in three replicates for each object. Each dish of filter paper was soaked with 3 mL of the appropriate extract, and 50 kale seeds were sown. The extracts were applied to the paper once. The variants used are listed in Table 2.

Table 2.
Variants of extracts used in the experiment according to source (plant, algae), concentration (dilution), solvents used.
2.4. Pot Experiment
The pot experiment was conducted in a completely randomized design with 3 replications for each object. The substrate for the production of microgreens in the experiment was linen mats with an area of 200 cm2 and a thickness of 5 mm, made of flax fiber (T-shop, Wroclaw, Poland). Each mat was soaked with the corresponding extracts in the amount of 50 mL, and seeds were sown in the amount of 3 g. The experiment was conducted in a growth chamber at a temperature of 20 °C without access to light until the seeds germinated. After germination, the containers were exposed to light. The plant growing chamber emitted photosynthetically active radiation within the range of approximately 400 to 700 nm, with an intensity of 800 µmol m−2/s−1. The photoperiod was 10 h of light and 14 h of dark. On the third day of the experiment, the germinating seeds were again watered with extracts at a rate of 25 mL per mat. In total, 75 mL of extract was used per mat. Further watering was carried out with distilled water. On the twelfth day, the plants were removed simultaneously from all objects, cut off just above the surface of the mat and subjected to chemical composition analyses. Plants at harvest had fully developed cotyledons and the first true leaves appearing. Biometric measurements were carried out on 20 plants from each container. The height and fresh weight of the microgreens (per plant) were determined.
2.5. Assays for Antioxidant Activity
The sample for the determination of antioxidant activity, total polyphenol content (TPC) and flavonoid content (TFC), was prepared by homogenizing 100 mg of ground and lyophilized tissue (pre-freezing at −64 °C; shelf temperature: 28 °C; pressure 24 Pa) with 4 mL of 50% methanol and centrifuging the homogenate at 10,000× g for 30 min. The obtained supernatant was used for analysis.
Antioxidant activity was measured against DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals, and results were expressed as Trolox equivalents (mg TE g−1 d.m.). Briefly, 2 µL of the supernatant was mixed with 198 µL of DPPH methanol solution. The initial concentration was 1 mmol⋅L−1, which was then diluted to an absorbance of A515 = 1.0 ± 0.3 before analysis. The absorbance of the reaction mixture was measured at 515 nm using a microplate reader (Biotek, Winooski, VT, USA) after 30 min of incubation in darkness [38].
For TPC, 10 µL of the supernatant was pipetted into a plate well containing 90 µL of distilled water, 20 µL of Follin–Ciocalteu reagent (diluted 1:1 with distilled water) and 30 µL of 20% sodium carbonate. After 30 min incubation in the dark, the absorbance of the solution was measured at 700 nm. The results were expressed as gallic acid equivalents (mg GAE g−1 d.m.).
TFC was analyzed by a colorimetric method based on the formation of flavonoid complexes with aluminum chloride in an alkaline medium. Briefly, 20 µL of the extract was mixed with 80 µL of methanol, 10 µL of 5% sodium nitrite, 10 µL of 10% aluminum chloride, 100 µL of sodium hydroxide and 30 µL of distilled water. After incubation for 15 min, the absorbance was monitored at 430 nm. The results were expressed as quercetin equivalents (mg QE g−1).
3. Results
3.1. Chemical Content of the Extracts
Table 3 presents the results of the phytochemical screening for the aqueous, hydroethanolic and ethanolic extracts of Sideritis scardica, as well as for the hydroethanolic and ethanolic extracts of Ascophyllum nodosum and Fucus vesiculosus. The results of the analyses of the aqueous extracts from A. nodosum and F. vesiculosus are provided, respectively, in subsequent publications [17,21].

Table 3.
Qualitative analysis (phytochemical screening) of the extracts. ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract.
In the water–ethanol extract of Fucus vesiculosus, the test suggested the presence of tannins, whereas the test result for the aqueous extract was inconclusive. In the Liebermann–Burchard test (for cardiac glycosides, steroids, and terpenoids), the water–ethanol extracts produced negative results, while the aqueous solutions tested positive. Meanwhile, in the water–ethanol extract of F. vesiculosus, the presence of steroids and terpenoids was confirmed by the Salkowski test. For A. nodosum extracts, significant difference in composition between the water and water–ethanol versions was shown by the lead acetate test. The water–ethanol extract was found to contain tannins and no flavonoids, whereas the opposite result was observed for the aqueous extract. The greatest differences between the aqueous and aqueous–ethanolic extracts of S. scardica were found in the Libermann–Burchard test for cardiac glycosides and steroids. The aqueous extract gave a positive result, whereas the aqueous–ethanol extract produced a negative result. Three tests for alkaloids (Mayer, Wagner, Draggendorf) were either negative or inconclusive, but the results of the aqueous and aqueous–ethanol extracts tested by this method did not overlap, suggesting a change in the phytochemical profile of extracts, depending on the composition of the extraction solvent.
3.2. Germination Indexes
In the Petri dishes experiment, selected parameters and indicators related to the germination of red kale seeds were tested. Germination energy and germination capacity, Maguire’s index and Pieper’s index were calculated after 10 and 25 d, and the percentage of healthy, non-germinating and damaged seeds was also calculated. The results of these calculations are given in Table 4. Germination energy for the control was over 83%. This parameter was not significantly reduced by the use of water extracts of A. nodosum (except the most concentrated) and water extract of F. vesiculosus at a concentration of 0.1%. All water–ethanol and ethanol extracts used caused reduction in the germination energy of kale seeds. Very similar results were obtained for germination capacity and Maguire’s index (calculated both after 10 d and after 25 d). Pieper’s index was increased by treating seeds with most of the water–ethanol and ethanol extracts. All water–ethanol extracts also increased the percentage of healthy non-germinating seeds, which was not the case with water extracts (except for S. scardica plant at a concentration of 0.01%). The use of some water–ethanol extracts of F. vesiculosus resulted in an increase in the percentage of rotten and damaged seeds.

Table 4.
Parameters describing kale germination under the influence of the applied extracts. ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
3.3. Linen Mat Experiment
The experiment determined the height and fresh weight of kale microgreens, as well as the content of TPC, TFC and antioxidant potential measured by activity against the DPPH radical. The average height of the kale plants in this experiment was 4.2 cm. In general, the microgreens treated with water extracts of A. nodosum, F. vesiculosus and the control group were the tallest (Figure 1). The water–ethanol extract of S. scardica also had a positive effect on plant growth. The lowest growth of the tested plants (2.0–2.3 cm) was found in the plots treated with water–ethanol extracts at the highest concentration tested (10%). All water–ethanol extracts (except 0.1% of S. scardica extract) significantly reduced plant growth.
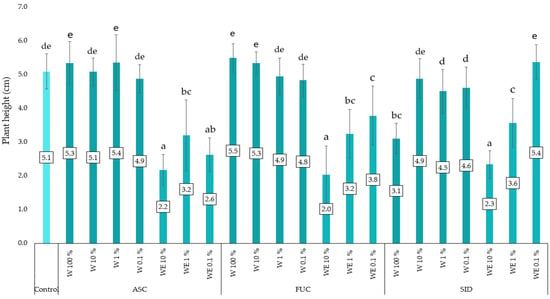
Figure 1.
Height of the young kale plants as a function of the type and dose of the extract applied to the mats (n = 60). ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
Figure 2 shows the fresh mass of kale microgreens as a function of the type of extract with which the substrate (flax mats) was soaked. Water–ethanol extracts of brown algae at the highest concentration tested (10%) significantly reduced the collected plant biomass. A positive effect on the fresh mass was observed for objects treated with the aqueous extract of F. vesiculosus and A. nodosum at the highest concentration tested.
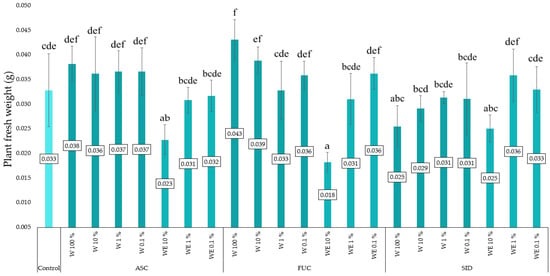
Figure 2.
Fresh mass of young kale (per plant) depending on the type and dose of extract applied to the linen mats (n = 60). ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
Application of water extracts of both brown seaweeds in concentrations of 10, 1 and 0.1% resulted in an increase in TPC in the experimental objects tested (Figure 3). Application of aqueous extracts at concentrations of 10% and 1% resulted in a significant increase in the content of total phenolic compounds and a significant increase in their content under the influence of 0.1% concentration. The applied water–ethanol extracts had no significant effect on this parameter in kale microgreens.
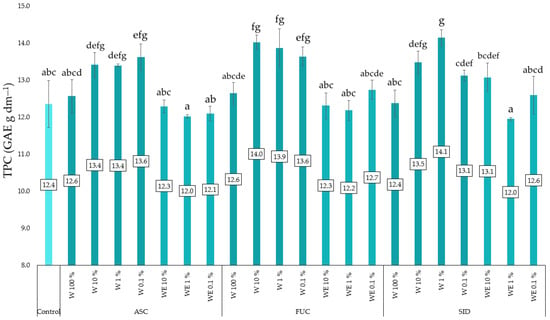
Figure 3.
Content of total phenolic compounds (TPC) in kale microgreens as a function of the type and dose of the extract. ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
The studies conducted show that water extracts of F. vesiculosus in concentrations ranging from 0.1 to 10% had a significant effect on increasing the total flavonoid content in young red kale plants (Figure 4). The opposite effect can be observed in the case of water–ethanol extracts, where the lowest TFC content was found in plants growing on mats soaked with 0.1% F. vesiculosus and 1% A. nodosum.
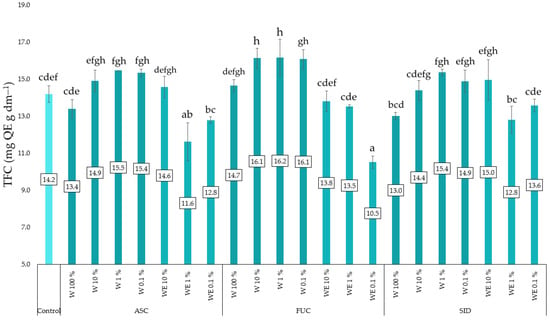
Figure 4.
The content of total flavonoids (TFC) in kale microgreens is dependent on the type and dose of the extract. ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
All water–ethanol extracts caused a tendency to reduce the antioxidant activity of the red kale microgreens tested. The lowest and statistically significant activity against DPPH was found in objects treated with the water–ethanol extract of A. nodosum at a concentration of 1%. All aqueous extracts (except the most concentrated ones) tended to increase antioxidant activity. However, 100% aqueous extracts tended to slightly decrease this activity. In microgreens treated with aqueous extracts of F. vesiculosus at concentrations of 1% and 10%, a tendency to increase antioxidant activity was observed. The results are presented in Figure 5.
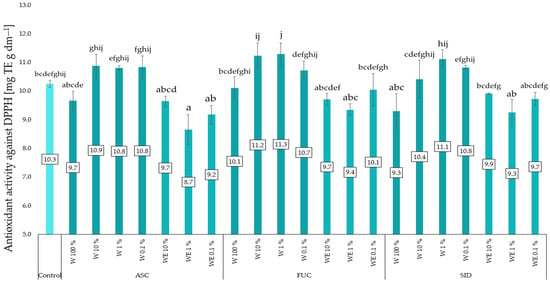
Figure 5.
Antioxidant activity (anti-DPPH) in kale microgreens depending on type and dose of extract. ASC—Ascophyllum nodosum; FUC—Fucus vesiculosus; SID—Sideritis scardica; W—water (aqueous) extract; WE—water–ethanol extract; E—ethanol extract. Differences between results are indicated by lower case letters; significance level was defined as p < 0.05.
4. Discussion
Germination-related indices, such as germination energy, germination rate or seed vigor, indicate the ability of seeds to germinate quickly and effectively and determine their ability to survive and develop under different environmental conditions. As part of the experiment, selected parameters and indices related to the germination of red kale seeds in the presence of selected plant and algal extracts were calculated. It was shown that germination energy was not reduced after the use of water extracts of A. nodosum (except for the highest concentration and water extract of F. vesiculosus at a concentration of 0.1%). All applied water–ethanol and ethanol extracts caused a reduction in the germination energy of curly kale seeds. Very similar results were obtained for germination capacity and the Maguire index, which describes the speed of seed germination. It was, therefore, concluded that aqueous extracts of brown algae at appropriate concentrations should not have a negative effect on the germination energy and capacity of kale seeds.
Silva et al. [39] tested the effect of water and water–ethanol preparations of the brown algae A. nodosum and Sargassum muticum on rice and lettuce plants at concentrations of 0, 25, 75 and 100%. Seeds of two lettuce varieties (green and purple) germinated at different concentrations, with the highest percentage of germinated seeds for extracts at 25% concentration and the lowest for 100% concentration. The values obtained at the lower concentration (25%) were similar to those of the control. The Maguire index changed in a similar way; the highest values were observed in treatments with a concentration of 25% and the lowest in treatments with a concentration of 100% [39]. These results are consistent with our observations, where the use of algal water extracts at low concentrations did not worsen the germination parameters of kale plants.
Karbarz et al. [40] tested the effect of Planktochlorella nurekis microalgae clones on the germination of four plant species, including Brassica oleracea L. variety Cezar broccoli. In the case of broccoli, there were statistically significant differences in germination energy compared to the control sample when a 1% extract concentration was used. Interestingly, the broccoli seed germination test showed that treatment with a 1% concentration of extracts reduced seed germination energy in two clones of these microalgae, while the remaining clones did not significantly affect this germination parameter [40]. The greatest variation in the effect of extracts was observed for the germination energy of lettuce seeds, where six algal clones reduced germination energy. Changes in the germination energy of broccoli seeds in the cited work, using seaweed extracts at medium (5%) and highest (10%) concentrations, did not reach a statistically significant level [40]. In our experiment, the Pieper index value increased as a result of treating seeds with most water–ethanol and ethanol extracts. All water–ethanol extracts also increased the percentage of healthy, non-germinating seeds, which did not happen in the case of water extracts (except for S. scardica plant at a concentration of 0.01%). Some water–ethanol extracts of F. vesiculosus also increased the percentage of infected, rotten seeds. The lower the Pieper index, the higher the seed vigor and the faster (less “stretched” in time) emergence. This means that only water extracts did not worsen the parameters characterizing seed vigor.
In an experiment, Salehi et al. [41] tested the effect of ethanol on the germination of three turf grass species. The experimental results showed that the germination percentage increased with increasing ethanol concentration to 0.5% and 0.3% for Lolium perenne L. and Festuca arundinacea Schreb, respectively. However, in both genera, the germination percentage decreased when the ethanol concentration was increased to 3%. In Cynodon dactylon L., the germination percentage increased when the ethanol concentration was increased to 3%. Soaking the seeds in 10% ethanol resulted in inhibition of germination. Miyoshi and Sato [30] studied the effect of ethanol on the germination of Japanese and Indian rice (Oryza sativa L.) seeds under anaerobic and aerobic conditions. In dehulled Japonica rice seeds, ethanol at a concentration of 0.5–5% overcame the inhibition of germination caused by dehulling. Maximum germination (often 100%) was achieved at concentrations of 3–5% (30 days after anthesis) or 1–4.5% (60 days after anthesis). Higher ethanol concentrations inhibited germination. In intact seeds, ethanol at the concentrations tested caused inhibition, no effect or minimal stimulation of germination. Under anaerobic conditions, ethanol inhibited the germination process stimulated by the absence of oxygen. In the case of Indian rice, 0.5 and 1% ethanol slightly inhibited germination, while 2% stimulated germination as the seeds matured. The best results were obtained at 3% ethanol, and at 5–6% germination dropped to 0%. As with Japonica rice, ethanol caused inhibition, no effect or minimal stimulation of germination in unhulled seeds. Ethanol had little or no effect on germination of hulled seeds under anaerobic conditions [42]. This study shows that the effect of ethanol on germination can vary even in different varieties of the same plant.
In our experiment, utilization of any of the ethanol-based extracts resulted in a deterioration of the germination parameters. In the experiment, conducted on linen mats, the effect of the tested extracts on the height, fresh mass and selected antioxidant properties of the microgreens produced was determined. The growth (height) of microgreens can be an indicator of the health/condition and growth potential of the plant. The fresh mass of the plant is an indicator of its overall health/condition, and a higher biomass is the result of a better use of available resources (water, light, nutrients). In the experiment conducted, the highest kale microgreens were obtained under the influence of the use of aqueous extracts of A. nodosum and F. vesiculosus in different concentrations and as a control, while the highest concentration of water–ethanol extracts (10%) significantly reduced plant growth. The use of extracts with high ethanol concentrations showed a decrease in plant growth, which was most likely related to the toxic effect of ethyl alcohol on plants. Alcohol in low concentrations has a stimulating effect on plants, while an increase in concentration causes a toxic effect. This is related to oxidative stress and the production of reactive oxygen species, which damage cells. However, the use of water-based extracts causes less stress on plants, which translates into greater growth. High concentrations of ethanol can lead to osmotic stress in plants. This stress disrupts the water balance within plant cells, causing dehydration and impaired cellular functions. Osmotic stress can result in reduced cell turgor pressure, which is essential for maintaining cell structure and growth.
In the experiment by Karbarz et al. [40], Planktochlorella nurekis algal clones’ extracts used at a concentration of 1% did not change the length of broccoli sprouts. The application of algae extract at a concentration of 5% caused a slight increase in the length of broccoli sprouts treated with an extract from one of the algae clones. The length of radish sprouts (also belonging to the Brassicaceae family) was reduced by the influence of three of the clones’ extracts used. The length of broccoli sprout leaves was not changed by the application of the 10% concentration. The 10% extract concentration caused a significant reduction in the length of radish sprouts (Raphanis sativus L., variety Mino Early) for all algae clones.
In the experiment by Park et al. [43], biomass, filtrate and suspension of the microalga Chlorella vulgaris were tested as a biostimulator for the growth and secondary metabolite content of “Red Russian” kale (Brassica napus var. Pabularia). There was no significant difference in plant growth parameters between C. vulgaris suspension and biomass treatments, while the filtered supernatant had a negative effect on kale growth, reducing dry mass by 37% [43]. The experiment by Karbarz et al. [40] investigated the effect of selected clones of P. nurekis algae on the fresh mass of plants. In the case of broccoli sprouts, the use of a concentration of 1% did not lead to significant changes in fresh mass (for any of the clones). Under the influence of microalgae extracts at a concentration of 5%, the mass of the sprouts of the plants tested either decreased or increased (depending on the clone used). Algae extracts at a concentration of 10% did not cause any changes in the weight of broccoli sprouts compared to the control. In the case of radish, however, the use of extracts from several algal clones at concentrations of 5% and 10% caused a significant decrease in the fresh weight of the plants compared to the control (Karbarz et al. [40]).
In our experiment, the use of water–ethanol extracts of brown algae at the highest concentration (10%) caused a decrease in kale biomass, while the water extract of F. vesiculosus at the same concentration had the most beneficial effect on the fresh weight of plants.
Different extraction methods can also lead to differences in the composition of the plant or algal extracts produced [44,45,46,47].
High levels of antioxidants in plants can indicate their ability to resist oxidative stress, which is important for their survival and nutritional quality. Analysis of antioxidant content can, therefore, indicate which extracts contribute to the production of microgreens, with a better ability to defend against oxidative stress and higher nutritional value [48,49,50].
Brassicaceae vegetables are rich in many health-promoting phytochemicals, but the exact metabolite composition is highly dependent on the cultivation conditions and environment [51]. For example, Liu et al. [51] presented the first report on the content of phenolic compounds, among others, in microgreens of kale and broccoli grown in growth chambers and on windowsills. The plants were harvested 10 days after sowing, and the results showed a clear variability in the qualitative composition of secondary metabolites between the growth conditions. However, no significant difference in total phenolic content was observed between the cultivation variants [51].
In an experiment investigating the effect of C. vulgaris on the phenolic compounds of “Red Russian” kale [43], the total phenolic and flavonoid content tended to increase after the use of filtered supernatant at 100x dilution. TFC and TPC increased in comparison to the control group when supernatant or 50x diluted suspension (fresh, living, unprocessed cultures) was used [43]. In the present experiment, aqueous extracts of seaweed at concentrations of 10, 1 and 0.1% increased the phenolic content of kale, whereas aqueous ethanol extracts had no such effect. In the experiment by Drygaś et al. [17] with arugula, aqueous extracts of A. nodosum increased the content of TPC, TFC and anti-DPPH activity, provided they were not used at high concentrations (10%).
High concentrations of ethanol can have toxic effects on plants, inhibiting their growth and the production of phenolic compounds. In the experiment by Shin et al. [52], it was found that in all three plants tested (Ocimum basilicum, Agastache rugosa, Artemisia annua), high concentrations of ethanol induced oxidative stress, which led to an increase in the total content of phenolic compounds as a defense mechanism in the plants. In the plants tested, DPPH radical scavenging activity increased in cultivation variants exposed to ethanol. The authors of the study believe that the exogenous concentration of ethanol did not affect the flavonoid pathway, and no specific trends in total flavonoid content were observed in their study. Therefore, it can be expected that low concentrations of ethanol will increase the dry weight of plant shoots, while high concentrations of ethanol are considered to induce severe oxidative stress in plants [52]. Based on our own research, it is concluded that water–ethanol extracts may cause greater oxidative stress in plants, which may negatively affect TPC production. The conducted studies showed that TPC in plants decreased with increasing alcohol concentrations in extracts. A similar relationship was also found in the studies of Stoica et al. [53], where TPC increased with increasing solvent polarity. This may be due to the different polarities of each group of antioxidant compounds present in algal biomass extracts.
However, the solubility and separation properties of polyphenols are influenced by their structural differences. For example, the structure of any compound has a significant influence on its polarity level, conjugation, and interaction with the sample matrix [54]. In addition, the lower TPC value caused by the application of an extract with a high ethanol concentration might cause cell damage, which ultimately reduced the production of phenolic compounds. Water-based extracts may be less stressful to plants, resulting in higher levels of these compounds. Phenolic compounds act as antioxidants to help plants cope with oxidative stress. However, excessive stress, particularly that caused by substances such as ethanol, can overwhelm the plant’s defenses and lead to cell damage, which can ultimately reduce the production of phenolics. In the case of water-based extracts of brown algae, plants may be able to cope better with stress and effectively increase the production of phenolic compounds. On the other hand, water–ethanol extracts may cause too much stress, exceeding the plants’ adaptive capacity, which may explain the lack of increase in phenolic content.
The use of pre-sowing seed soaking to accelerate seed germination should be considered in the context of its possible effects in terms of reduced microgreen yield and mineral content [4].
In our experiment with red kale, treatment with water extracts of F. vesiculosus at concentrations of 0.1, 1 and 10% led to increase of the flavonoid content in red kale, whereas water–ethanol extracts decreased this parameter.
Apart from the possible reasons for this effect mentioned above, possibilities such as differences in the composition of extracts made with different solvents cannot be excluded. The choice and composition of the solvent system (i.e., water, water–ethanol mix, or ethanol alone) play a pivotal role in determining the selectivity of extracted compounds, as illustrated by the differences observed in the presence or absence of tannins, flavonoids, steroids and terpenoids in the tested extracts. For instance, in Fucus vesiculosus, tannins were confirmed only in the water–ethanol extract, and the presence of steroids and terpenoids differed between the Liebermann–Burchard and Salkowski tests, depending on whether the extracts were purely aqueous or contained ethanol. Similarly, Ascophyllum nodosum extracts displayed a marked distinction: the water–ethanol extract contained tannins but no flavonoids, whereas the aqueous extract showed the reverse. In Sideritis scardica, the greatest discrepancy emerged in the Liebermann–Burchard test for cardiac glycosides and steroids, which were detected in the aqueous extract but not in the water–ethanol version.
These variations align with well-established principles that polar solvents (like water) better dissolve polar compounds (e.g., many phenolics and glycosides), while mixtures of water and ethanol or pure ethanol can extract moderate to less polar molecules (e.g., certain terpenoids and some phenolics). Tannins, for example, may exhibit variable solubility in water or ethanol–water mixtures, depending on their molecular weight and structural properties. The presence of hydroxyl groups, for example, in some flavonoids tends to increase their polarity, hence producing better extraction yields with polar solvents (like water) [55,56,57,58,59]. Collectively, these findings highlight the crucial role of solvent selection in optimizing the extraction of target compounds and phytochemical profiles of extracts in underscoring the importance of adjusting solvent polarity to achieve a desired biochemical profile in plant and algae extracts.
Water–ethanol extracts reduced the antioxidant activity of red kale, with the lowest anti-DPPH activity at 1% A. nodosum extract, whereas F. vesiculosus water extracts at concentrations of 1 and 10% increased this activity.
Similarly, aqueous extracts of F. vesiculosus increased the activity against DPPH in barley microgreens in the work of Drygaś et al. [21].
Water extracts may better support plant metabolism, leading to increased synthesis of antioxidant compounds. In addition, ethanol itself may have a negative effect on this parameter. The components present in water extracts may also act synergistically to increase the antioxidant activity in the microgreens studied [60]. Water–ethanol extracts may not have the same synergistic effects, which may explain the lower antioxidant activity of microgreens cultivated utilizing such extracts.
5. Conclusions
The study investigated the effect of selected extracts on selected parameters of germination, initial growth and antioxidant activity using the red kale as an example. Aqueous extracts of brown algae (Ascophyllum nodosum, Fucus vesiculosus) and Sideritis scardica plants stimulated plant growth (height, weight) and increased the content of beneficial nutrients (total phenolic compounds, flavonoids). On the other hand, water–ethanol extracts in a certain concentration range had a negative effect, e.g., plants treated with 10% water–ethanol extracts showed reduced biomass. Generally, aqueous extracts in higher concentrations significantly increased TPC. Water–ethanol extracts generally reduced TFC and antioxidant activity. Further research is recommended to determine the potential of natural plant extracts and algae in the production of microgreens. This research provides valuable information for improving the quality and health benefits of kale microgreens through the strategic use of these extracts. The results of this study can be used by farmers and gardeners to improve their growing practices, while scientists can use these results as a basis for further research in this area.
Author Contributions
Conceptualization, B.D. and E.S.-K.; methodology, T.P., B.D., E.S.-K. and J.K.; formal analysis, B.D, E.S.-K. and C.P; investigation, B.D., E.S.-K., J.K., T.P. and M.J.-P., data curation, B.D.; validation, T.P., visualization, B.D. and E.S.-K., writing—original draft preparation, B.D.; writing—review and editing, B.D. and M.J.-P.; supervision, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
Author Joanna Kreczko was CEO/shareholder of the company Urtica Technologies Sp. z o. o. (Gdańsk, Poland). The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TPC | Total Phenolic Compound |
| TFC | Total Flavonoids |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| ASC | Ascophyllum nodosum |
| FUC | Fucus vesiculosus |
| SID | Sideritis scardica |
| W | Water (aqueous) extract |
| WE | Water–ethanol extract |
| E | Ethanol extract |
| TE | Trolox equivalent |
| GAE | Gallic acid equivalent |
| QE | Gallic acid equivalent |
References
- Cowden, R.J.; Ghaley, B.B.; Henriksen, C.B. Analysis of light recipe, seeding density, and fertilization effects on secondary metabolite accumulation and growth-defense responses in Brassicaceae microgreens. Food Biosci. 2024, 59, 104071. [Google Scholar] [CrossRef]
- Goryńska-Goldmann, E.; Murawska, A.; Balcerowska-Czerniak, G. Consumer Profiles of Sustainable Fruit and Vegetable Consumption in the European Union. Sustainability 2023, 15, 15512. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Li, T.; Lalk, G.T.; Bi, G. Fertilization and Pre-Sowing Seed Soaking Affect Yield and Mineral Nutrients of Ten Microgreen Species. Horticulturae 2021, 7, 14. [Google Scholar] [CrossRef]
- Mallor, C.; Bertolín, J.R.; Paracuellos, P.; Juan, T. Nutraceutical Potential of Leafy Vegetables Landraces at Microgreen, Baby, and Adult Stages of Development. Foods 2023, 12, 3173. [Google Scholar] [CrossRef]
- Gunjal, M.; Singh, J.; Kaur, J.; Kaur, S.; Nanda, V.; Sharma, A.; Rasane, P. Microgreens: Cultivation practices, bioactive potential, health benefits, and opportunities for its utilization as value-added food. Food Biosci. 2024, 62, 105133. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G.; De Pascale, S. Sprouts, Microgreens and Edible Flowers as Novel Functional Foods. Agronomy 2021, 11, 2568. [Google Scholar] [CrossRef]
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Partap, M.; Sharma, D.; Deekshith, H.N.; Thakur, M.; Verma, V.; Bhargava, U.B. Microgreen: A tiny plant with superfood potential. J. Funct. Foods 2023, 107, 105697. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, T.; Singh, S.P.; Bhardwaj, A.; Srivastava, D.; Kumar, R. Prospects of microgreens as budding living functional food: Breeding and biofortification through OMICS and other approaches for nutritional security. Front. Genet. 2023, 14, 1053810. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Patanè, C. Effect of Application of Biostimulants on the Biomass, Nitrate, Pigments, and Antioxidants Content in Radish and Turnip Microgreens. Agronomy 2023, 13, 145. [Google Scholar] [CrossRef]
- Di Gioia, F.; Hong, J.C.; Pisani, C.; Petropoulos, S.A.; Bai, J.; Rosskopf, E.N. Yield performance, mineral profile, and nitrate content in a selection of seventeen microgreen species. Front. Plant Sci. 2023, 14, 1220691. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing Sustainability by Improving Plant Salt Tolerance through Macro- and Micro-Algal Biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Shukla, P.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological And Transcriptomics Analysis reveal that asco phyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 2018, 38, 463–478. [Google Scholar] [CrossRef]
- Drygaś, B.; Piechowiak, T.; Balawejder, M.; Matłok, N.; Kreczko, J.; Puchalski, C. The Eliciting Effect of Aqueous Extracts from Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens. Sustainability 2024, 16, 7436. [Google Scholar] [CrossRef]
- Marchica, A.; Cotrozzi, L.; Detti, R.; Lorenzini, G.; Pellegrini, E.; Petersen, M.; Nali, C. The Biosynthesis of Phenolic Compounds Is an Integrated Defence Mechanism to Prevent Ozone Injury in Salvia officinalis. Antioxidants 2020, 9, 1274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive Roles of Polyphenol Oxidase in Plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: New York, NY, USA, 2008; pp. 253–270. [Google Scholar]
- Drygaś, B.; Piechowiak, T.; Kreczko, J.; Matłok, N.; Saletnik, B.; Balawejder, M. The Utilisation of Fucus vesiculosus Algae Extracts in the Production of Microgreens Hordeum vulgare L. with an Increased Content of Selected Bioactive Compounds. Plants 2024, 13, 2871. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ahmad, A.; Blasco, B.; Martos, V. Combating Salinity Through Natural Plant Extracts Based Biostimulants: A Review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef]
- Zulfigar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Thangavel, K.; Nalliappan, S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. [Google Scholar] [CrossRef]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Vicente, O.; Verdeguer, M. Effect of Two Biostimulants, Based on Ascophyllum nodosum Extracts, on Strawberry Performance under Mild Drought Stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Krautforst, K.; Szymczycha-Madeja, A.; Wełna, M.; Michalak, I. Brown seaweed: Fucus vesiculosus as a feedstock for agriculture and environment protection. Sci. Rep. 2023, 13, 10065. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Papliński, R.; Mysiak, B.; Nurzyńska-Wierdak, R. Phenolic Profile and Antioxidant Activity of Extracts from Aerial Parts of Thymus vulgaris L. and Sideritis scardica Griseb. Appl. Sci. 2025, 15, 3842. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Voynikov, Y.; Gevrenova, R.; Balabanova, V. A Comprehensive Phytochemical Analysis of Sideritis scardica Infusion Using Orbitrap UHPLC-HRMS. Molecules 2024, 29, 204. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Jacobo-Velázquez, D.A. Improving the Health-Benefits of Kales (Brassica oleracea L. var. acephala DC) through the Application of Controlled Abiotic Stresses: A Review. Plants 2021, 10, 2629. [Google Scholar] [CrossRef]
- Satheesh, N.; Workneh Fanta, S.; Yildiz, F. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Khalid, W.; Iqra; Afzal, F.; Rahim, M.A.; Rehman, A.A.; Rasul, H.F.U.; Arshad, M.S.; Ambreen, S.; Zubair, M.; Safdar, S.; et al. Industrial applications of kale (Brassica oleracea var. sabellica) as a functional ingredient: A review. Int. J. Food Prop. 2023, 26, 489–501. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. 2018, 59, 2411–2422. [Google Scholar] [CrossRef]
- Piechowiak, T.; Sowa-Borowiec, P. Changes in redox status in raspberry (Rubus idaeus L.) fruit during ripening. Biocatal. Agric. Biotechnol. 2024, 61, 103380. [Google Scholar] [CrossRef]
- Silva, L.D.; Bahcevandziev, K.; Pereira, L. Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Ocean. Limnol. 2019, 37, 918–927. [Google Scholar] [CrossRef]
- Karbarz, M.; Piziak, M.; Żuczek, J.; Duda, M. Influence of Microalgae Planktochlorella nurekis Clones on Seed Germination. Agronomy 2023, 13, 9. [Google Scholar] [CrossRef]
- Salehi, M.R.; Ashiri, F.; Salehi, H. Effect of Different Ethanol Concentrations on Seed Germination of Three Turfgrass Genera. Adv. Nat. Appl. Sci. 2008, 2, 6–9. [Google Scholar]
- Miyoshi, K.; Sato, T. The Effects of Ethanol on the Germination of Seeds of Japonica and Indica Rice (Oryza sativa L.) under Anaerobic and Aerobic Conditions. Ann. Bot. 1997, 79, 391–395. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, J.-E.; Truong, T.Q.; Koo, S.Y.; Choi, J.-H.; Kim, S.M. Effect of Chlorella vulgaris on the Growth and Phytochemical Contents of “Red Russian” Kale (Brassica napus var. Pabularia). Agronomy 2022, 12, 2138. [Google Scholar] [CrossRef]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro- and microalgae extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. Pharmacogn. J. 2017, 6, 32–36. Available online: https://www.phytojournal.com/archives/2017/vol6issue1/PartA/6-1-23-924.pdf (accessed on 15 April 2025).
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction Processes with Several Solvents on Total Bioactive Compounds in Different Organs of Three Medicinal Plants. Molecules 2020, 25, 4672. [Google Scholar] [CrossRef] [PubMed]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; Oulad El Majdoub, Y.; Kounnoun, A.; Miceli, N.; Fernanda Taviano, M.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.Y.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022, 2, 101–113. Available online: https://pubs.acs.org/doi/pdf/10.1021/acsfoodscitech.1c00355 (accessed on 15 April 2025). [CrossRef]
- Shin, J.; Lee, Y.; Hahm, S.; Lee, K.; Park, J. Effects of Exogenous Ethanol Treatment in Nutrient Solution on Growth and Secondary Metabolite Contents of Three Herb Species in an Indoor Vertical Farming System. Plants 2023, 12, 3842. [Google Scholar] [CrossRef] [PubMed]
- Stoica, R.; Velea, S.; Ilie, L.; Calugareanu, M.; Ghimis, S.B.; Ion, R.-M. The Influence of Ethanol Concentration on the Total Phenolics and Antioxidant Activity of Scenedesmus Opoliensis Algal Biomass Extracts. Rev. Chim. 2013, 64, 304–306. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–2014. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 28, 1118761. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, S.B.; Wulandari, S.; Nurfitriani, R.A.; Awaludin, A. The potential solvent for tannin extraction as a feed additive made of coffee husk (Coffea canephora) using Soxhlet Method. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012024. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Fereira, O.; Pinho, S.P. Solubility of Flavonoids in Pure Solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Xia, B.-H.; Yu, Z.-L.; Lu, Y.-A.; Liu, S.-J.; Li, Y.-M.; Xie, M.-X.; Lin, L.-M. Green and Efficient Extraction of Phenolic Components from Plants with Supramolecular Solvents: Experimental and Theoretical Studies. Molecules 2024, 29, 2067. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).