4.1. Chemical Composition of Almond Hulls
Optimizing both the quantity and quality of feed resources is crucial for identifying cost-effective alternatives and enhancing the economic efficiency of livestock operations. Among various possible alternatives, the inclusion of NaOH-treated AHs in sheep diets has been investigated in this study. Treatment of AHs with 4% NaOH altered the chemical composition of the parent material, potentially affecting their suitability as a feed resource. The NaOH treatment reduced OM, likely due to the addition of mineral matter with the NaOH, in agreement with the results reported by Bachmann et al. [
35] for wheat straw.
The NaOH treatment increased crude fat and protein and reduced fiber components, particularly NDF, ADF, and ADL. Uzatici et al. [
15] observed that treating common reed grass (
Phragmites australis) straw with NaOH increased the ash content and reduced OM, NDF, and ADF contents, without affecting CP content. This suggests that the effect of NaOH on CP may vary depending on the type of feed material. The reduction in NDF, ADF, and ADL observed in the present study may be attributed to the potential of NaOH to break ester bonds between cell wall components such as acetic acid, phenolic acids, cellulose, hemicellulose, and lignin. This process enhances nutrient availability for microorganisms, thereby improving digestibility [
5,
15]. Recently, Zoabi et al. [
36] reported that treating AHs with 4% NaOH for 40 days resulted in a reduction in CP, as well as structural and non-structural carbohydrate contents. Ibrahim et al. [
37] applied NaOH treatments at concentrations of 2%, 4%, 6%, and 8% to
Hedychium gardnerianum,
Pittosporum undulatum,
Arundo donax, and
Acacia melanoxylon. Ash content and dry matter digestibility were highest under the 8% NaOH treatment compared to the other concentrations.
NaOH treatment, if applied at high concentrations or without proper management, may pose environmental risks due to the formation of potentially toxic or polluting residues. To mitigate these risks, a low NaOH concentration (4%) was employed in our study to effectively disrupt the lignocellulosic matrix while preserving animal health and feed safety. By optimizing both the concentration of NaOH and the dietary inclusion rate, the use of treated AHs offers a sustainable strategy for enhancing ruminant productivity without exerting adverse effects on the environment.
4.2. In Vivo Digestibility in Rams
In our study, the inclusion of NaOH-treated AHs at either 20% or 40% in the diet of rams did not affect nutrient digestibility. This aligns with previous research suggesting that although NaOH can break down fibrous components such as cellulose and hemicellulose, its overall impact on digestibility may be limited. It was expected that NaOH would affect the digestibility of AH. Berger et al. [
38] observed that NaOH treatment of corn cobs at levels of 2.0%, 4.0%, 6.0%, and 8.0% linearly increased the rate of passage and decreased ruminal retention time with increasing levels of NaOH. Moreover, Jami et al. [
39] reported that including 5% NaOH-treated corn straw as a substitute for 15% wheat hay in the diets of lactating cows decreased voluntary feed intake without affecting in vivo DM and OM digestibility. However, it reduced CP digestibility and increased the digestibility of NDF, cellulose, and hemicellulose. Additionally, a reduction in rumination time was observed. Among the eight bacterial species quantified by real-time PCR, the NaOH-treated group showed a notable decrease in cellulolytic bacteria and an increase in lactic acid-utilizing bacteria.
It is well established that feeding NaOH-treated forages, particularly cereal straws, can lead to an increase in water intake by ruminants. This is largely attributed to the elevated sodium content and the osmolality of the treated feed, which stimulates water consumption to maintain the osmotic balance [
38]. Although water consumption was not recorded in the present study, no signs of excessive drinking behavior or dehydration were observed in Assaf sheep fed diets containing 20% or 40% NaOH-treated AH. This outcome may be due to the low NaOH concentration (4%) and the distinct structural and compositional characteristics of almond hulls compared to conventional cereal straws. Animals had free access to clean water throughout the duration of the experiment, and no metabolic disturbances related to fluid balance were noted. Although water intake was not quantitatively measured, our observations suggest that incorporating NaOH-treated AHs at the tested levels did not negatively impact animal hydration. Nonetheless, future studies could benefit from including precise measurements of water intake to confirm this assumption and fully assess the impact of alkali-treated AHs on water balance.
Uzatici et al. [
15] observed only marginal improvements in the digestibility of common reed straw following NaOH treatment. Similarly, Reed and Brown [
11] reported that increasing the inclusion level of untreated AHs in the diet to 25% and 35% reduced the digestibility of DM, OM, and fiber. However, Zoabi et al. [
36] stated that treating AHs with 4% NaOH for 40 days increased in vitro DM digestibility without affecting the digestibility of fibers. Ibrahim et al. [
37] reported that NaOH treatment of different plant byproducts did not affect the invitro OM digestibility; however, a 2% NaOH concentration positively influenced the digestibility of both ADF and NDF. Untreated AHs have a high fiber content and, when fed in excess, can limit the digestive utilization of other dietary components and reduce overall feed intake [
40]. Our results suggest that NaOH treatment can improve the digestibility of AH. However, several factors may influence the effectiveness of this treatment, including animal physiology, the specific AH variety, processing methods, digestive capacity, and the adaptations of rumen microbes in sheep consuming AH. Yalchi [
10] found that AHs are richer in sugars and more digestible than alfalfa hay. Swanson et al. [
41] observed that pure AHs are more digestible than raw AHs containing debris. Additionally, natural compounds in AHs, such as tannins, can reduce protein digestibility [
5,
42]. According to Kahlaoui et al. [
7], tannin content in AHs ranges from 70 to 120 mg/g, which has been associated with reduced protein digestibility. Despite the limited benefits of NaOH treatment, AHs can still be effectively incorporated into livestock diets without adversely affecting total tract digestibility.
4.3. Reproductive Performance
There is a lack of specific studies directly examining the effects of AH inclusion in the diets of rams or ewes on their reproductive performance. However, studies on other species have shown positive or neutral outcomes. For instance, almond-supplemented diets have been reported to improve reproductive function in male rats [
43]. The inclusion of up to 15% AHs in a laying hen diet had no adverse effect on egg production or egg quality [
44].
The significant effects observed in rams fed AH-supplemented diets, particularly the AH40 diet, suggest enhanced reproductive performance and improved semen quality. Rams fed the AH40 diet showed the best outcomes in terms of libido score, testicular circumference, and semen volume. Improvements in sperm concentration, total motility, progressive motility, and fast motility indicate enhanced sperm vigor and functionality. Additionally, spermatozoa from AH40 rams showed increased curvilinear and straight-line velocities, supporting enhanced sperm movement and fertilization potential. Reductions in abnormal motility patterns, such as circular, local, and immotile sperm, as well as fewer morphological defects, including mid-piece abnormalities, spiraled tail, separated tail, and curved tail, further indicate improved sperm integrity and quality, particularly with the AH40 diet [
45]. These enhancements in both sperm motility and morphology seem to demonstrate the response to dietary interventions.
Almond hulls are rich in fiber, fatty acids, and antioxidants [
7,
46,
47,
48,
49], which may affect rumen fermentation and nutrient utilization, subsequently supporting overall reproductive function [
50]. The antioxidant properties of AHs may contribute to reducing sperm defects [
51], while maintaining a balanced pH contributes to a healthier semen environment. These findings underscore the pivotal role of nutrition in optimizing overall reproductive health and highlight the potential of dietary interventions to improve both physiological and reproductive performance in livestock [
52,
53].
Incorporating AHs into ewe diets had a sizeable impact on reproductive performance, as evidenced by increased key traits such as fertility, prolificacy, fecundity, numeric productivity (NP), and weight productivity (WP), along with decreased abortion rate and lamb mortality. Notably, AH40 treatment resulted in the highest fertility, prolificacy, and fecundity, indicating optimal reproductive performance. The AH20 treatment showed intermediate values for these indicators, while the control treatment had the lowest. This analysis highlights the beneficial effects of AHs on reproductive outcomes in ewes [
13]. Higher fertility rates for ewes supplemented with AH20 and AH40, as compared with those of the control group, suggest that AHs improved conception rates in ewes, while increased prolificacy and fecundity indicate a greater number of lambs born and fewer losses. It can be suggested that improving the body conditions of ewes through flushing before and during mating, combined with increasing the inclusion rates of AH, was an effective strategy to enhance fertility, which is known to have low heritability [
54]. Acock et al. [
55] reported that feeding gestating beef cows wheat straw treated with NaOH did not affect calf birth weight, incidence of calving difficulty, or the subsequent reproductive performance of the cows.
The increase in the number of weaned lambs produced per ewe could be attributed to increased colostrum intake, leading to better health and reduced lamb mortality in the offspring of ewes fed the AH40 diet [
56]. The higher litter weight at weaning in the AH40 group would also reflect improved lamb survival and greater growth rate in milk-fed lambs, resulting in heavier lambs at weaning. Based on these findings, the addition of AHs up to 40% to sheep diets (both ewes and rams) before and during mating seems to be a worthwhile feeding practice to improve animal performance, and a cost-effective strategy to improve sheep breeder income.
Incorporating AHs into ewe diets led to significant improvements in milk composition, with higher levels of fat, protein, lactose, total solids, and solids-not-fat. These effects became more pronounced as the amount of AHs in the diet increased, following a linear trend that suggests a consistent increase in these nutritional components in milk with greater AH inclusion. The increase in fat content is attributed to the addition of a high-fiber feedstuff in the ration, a relationship that has been extensively studied [
57,
58]. Jami et al. [
39] reported that including 5% NaOH-treated corn straw in the diet of lactating cows increased both milk fat and milk protein contents. Although we did not measure the profile of ruminal volatile fatty acids, Zoabi et al. [
36] reported that treating AHs with 4% NaOH increased the production of ruminal acetate, which is highly correlated with milk fat concentration [
59]. Swanson et al. [
13] support our findings that dietary AHs can be associated with increased milk fat content, although a potential decrease in milk protein was observed in this study, in contrast with our results showing an increase in protein content. Similarly, Williams et al. [
14] reported reductions in both milk production and protein yield when AHs were included in the diet. This discrepancy suggests that the effect of AHs on milk protein may vary depending on breed differences and genetic factors influencing milk production and lactation [
60,
61,
62], and on the composition of the diet in which AHs is incorporated. Despite the increase in protein levels observed in our study, the levels remained within the typical range for the Assaf breed, known for its relatively high protein content [
63]. The elevated protein levels in Assaf sheep milk are particularly important for high-quality cheese production, as proteins are critical for curd formation during cheese-making. Higher protein concentrations contribute to increased cheese yield, improved texture, and a more concentrated flavor [
64]. Even moderate increases in protein and total solids in milk can enhance cheese production [
65,
66], further underscoring Assaf sheep’s potential to produce protein-rich milk for premium dairy production. The increase in the milk lactose concentration with the AH40 diet highlights the effectiveness of NaOH treatment in enhancing fiber breakdown [
67,
68], which may increase the availability of carbohydrates necessary for lactose synthesis. Earlier studies by Can et al. [
69] reported that goats fed a diet containing 400 g of untreated AHs and shells per kg of diet DM had greater DM intake (3% of BW) compared to those fed 400 g of wheat straw per kg of diet DM (2.37% of BW). The AHs and shells used in this study were soft, easily breakable by hand, and more palatable than straw, leading to greater consumption rates among the goats. Although not recorded in our study, any increase in feed intake in ewes receiving AHs diets would raise energy supply through the entire reproduction cycle from flushing to steaming and the post-lambing period.
4.4. Growth Performance of Lambs
Feeding AHs to Sarda lambs did not adversely affect growth performance, feed conversion ratio, or carcass weight, and improved the oxidative stability of meat when refrigerated [
8,
40,
70,
71]. Our study involved suckling lambs (not fattening lambs), and AHs were included in the diets of the nursing ewes. Incorporating AHs into the diet of ewes improved the growth performance of their lambs, particularly those born from ewes fed the AH40 diet. A meta-analysis by Roca Fraga et al. [
72] highlighted the critical importance of managing ewe nutrition during late pregnancy to meet the basic nutritional requirements for optimal weight, vitality, and survival of newborn lambs. In our study, lamb weight at birth was slightly reduced by the inclusion of AHs in the ewe diet. In comparison, lower lamb birth weights were reported by Bekkouche et al. [
73]. These variations could be attributed to differences in breed, ewe nutrition during pre- and post-partum periods, litter size, and lamb sex. It is noteworthy that ewes fed the AH20 and AH40 diets showed greater litter sizes (prolificacy) than the control group, which likely contributed to the smaller individual lamb size at birth. A reduction of 500 g in birth weight is considered more detrimental to the potential performance of a twin lamb compared to a typically heavier singleton [
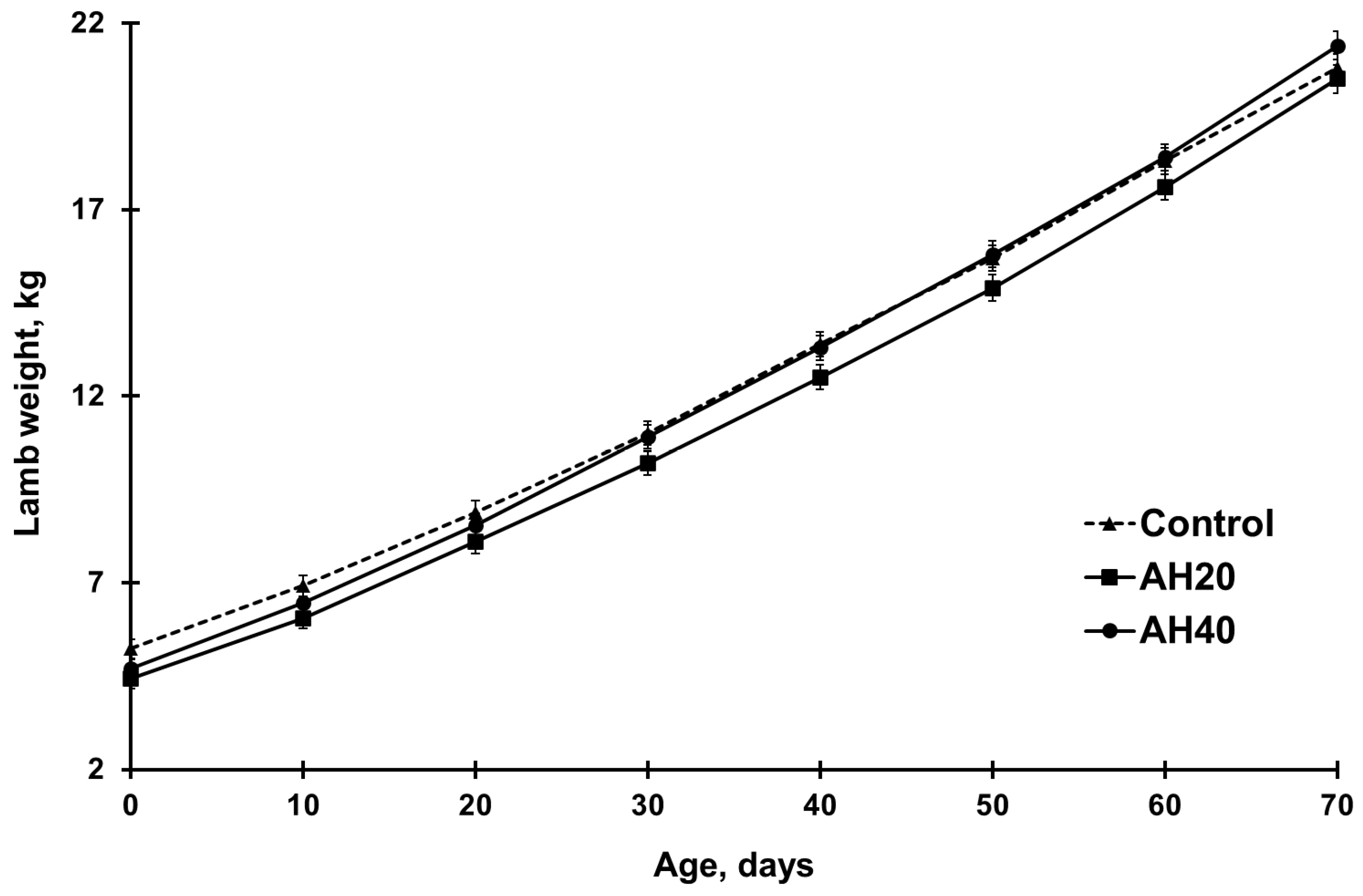
72]. This emphasizes the need to identify singleton- and multiple-bearing ewes during pregnancy and to adjust their feeding accordingly to meet pregnancy-specific nutritional requirements. As the trial progressed, lambs nursed by dams fed the AH40 diet exhibited a faster growth rate, particularly between 30 and 70 days of age. This resulted in greater weaning weights and higher average daily gains across the intervals of 0–30, 10–30, and 30–70 days. This enhanced growth rate can be attributed to a higher consumption of milk from their mothers, which also had superior nutritional content, as milk from ewes fed the AH40 diet contained more fat, lactose, protein, and total solids than milk from control ewes. Consequently, milk-fed lambs from AH40 ewes received more nutrients during suckling. This suggests that higher levels of AHs may enhance lamb growth, likely due to improved milk yield. Significant linear and quadratic trends also indicate a relationship between AH inclusion and lamb growth, with optimal effects becoming more apparent as the lambs were heavier. It can be suggested that during times of high feed prices, AHs are a potential cost-controlling feed source in growing lamb diets.
Although this study primarily focused on the biological responses to dietary inclusion of NaOH-treated AHs, it is worth noting that AHs are an abundant, low-cost agro-industrial byproduct. The use of treated AHs in sheep diets could significantly reduce feed costs. Preliminary economic observations indicate a potential reduction in feed expenses due to partial replacement of conventional ingredients with AHs, particularly at the 40% inclusion level, which showed enhanced productive and reproductive performance. However, a comprehensive cost–benefit analysis considering treatment costs, labor, and market prices is necessary to fully validate its economic viability. This will be the focus of future work to better support the adoption of AHs as a cost-effective feed resource under commercial farming conditions.