Abstract
China has approximately 3.43 million hectares of tea plantations, which offer significant potential for carbon sequestration and the reduction of CO2 emissions. However, the mechanisms underlying the stability and mineralization of soil organic carbon (SOC) in different tea plantations remain unclear. This study aimed to comprehensively evaluate the effects of chemical, physical, and microbial factors on SOC mineralization in tea plantations with different methods of forest conversion to tea plantations and different ages of tea plants. Our findings indicate that forest conversion to tea plantation methods and tea planting age significantly influence SOC mineralization. Specifically, the SOC mineralization in tea plantations reclaimed by clear-cutting and burning (FMT4) was lower than in those reclaimed by partial cutting (MT3, MT30, and MT150). This variation is attributed to differences in the chemical structure of SOC, which showed higher proportions of aromatic C (33.4%) and carbonyl/carboxyl C (7.8%), alongside lower proportions of O-alkyl C, in the FMT4 tea plantation compared to the others. Additionally, SOC mineralization was significantly higher in the MT150 tea plantation (15.23 g C kg−1 SOC) than in the MT3 (10.11 g C kg−1 SOC), MT30 (10.38 g C kg−1 SOC), and MT200 plantations (9.13 g C kg−1 SOC). Notably, although the MT200 tea plantation had a higher proportion of O-alkyl C (42.4%) than the MT3 and MT30 plantations (36.4%), and was similar to the MT150 plantation (43.1%), its SOC mineralization remained lower due to the higher clay content (278 g kg−1). Correlation analysis and random forest analysis further revealed that physical properties, particularly clay content, are the most significant factors regulating SOC mineralization, followed by the chemical structure, such as O-alkyl C and aromatic C, as well as other physicochemical properties like the carbon-to-nitrogen (C/N) ratio, and microbial properties like Gram-positive bacteria. In conclusion, our study highlights the complex interplay of soil physical properties and SOM chemical structure and microbial properties in regulating SOC mineralization, providing valuable insights for improving carbon management in tea plantations.
1. Introduction
Tea (Camellia sinensis L.) is a major cash crop, with over 70% of the global tea plantation area in China [1]. The stock of SOC in China’s tea plantations is approximately 124.58 Tg in the top soil layer [2], and their SOC density exceeds that of shrubland, grassland, and cropland [3,4]. Land-use change can induce differences in SOC mineralization due to changes in organic inputs [5,6] and soil chemical, physical, and microbial properties [7], which in turn, influence soil carbon cycling and functions [8]. Studies have indicated that land-use conversion to tea plantations has different effects on SOC content and SOC mineralization, which are influenced by the tea planting age [6,9,10]. For example, a five-year study found that SOC mineralization in the second year after conversion to tea plantations significantly decreased compared to the first year, and then exhibited increasing trends from the second to the fifth year [11]. Another study reported that SOC mineralization increased with tea planting age [12]. However, the regulatory mechanisms of SOC mineralization following the conversion of forests to tea plantations in China remain poorly understood, particularly regarding the contributions of chemical, physical, and microbial properties.
Chemical composition plays a crucial role in the mineralization of SOC [13]. When forests are converted into agricultural plantations, actions such as cutting and burning can occur, which may change microbial activity [14] and lead to the accumulation of relatively resistant black carbon, characterized by fused-ring aromatic C structures [15,16]. This can complicate SOC mineralization due to changes of its chemical composition [17]. Additionally, the age of tea plantations can influence soil microbial communities [18] and the chemical composition of SOC, thereby affecting its stability [19]. Several studies have indicated that SOC mineralization rates were negatively correlated with aromatic C content in soils [12,20]. However, it has also been shown that soils with stable C exhibited different mineralization rates [21,22]. These differences may be attributed to variations in soil microbial activity and physical properties [23,24]. Therefore, the impact of chemical composition on SOC mineralization in tea plantation ecosystems remains unclear.
The mineralization of SOC is influenced not only by chemical composition but also by physical protection and microbial activity [20,23]. Some studies have only examined the presence of aggregates that physically protect SOC in tea plantations [25,26], while additional studies have investigated the impact of chemical composition or microbial influences on SOC stability [10,12,19]. However, none of these studies have comprehensively assessed the relative contributions of these mechanisms. Therefore, to better understand the control mechanisms of SOC mineralization in tea plantations, an integrated assessment of the contributions from chemical, physical, and microbial factors is necessary.
In our study, soil samples were collected from the Mangjing tea plantation in Southwest Yunnan, which represented different methods of forest conversion to tea plantations and ages of tea plantings. The main objective was to comprehensively assess how the mineralization of SOC is affected by chemical, physical, and microbial factors in different tea plantations. We hypothesized the following: (1) tea plantations established through burning would exhibit lower SOC mineralization due to the accumulation of chemically recalcitrant aromatic carbon; (2) with the increase in tea plantation age, SOC mineralization gradually decreases, possibly due to the reduced content of easily decomposable organic carbon (such as O-alkyl C) in the soil; and (3) the relative contributions of SOC chemical composition, soil physical properties, and microbial communities to SOC mineralization would differ depending on the method of forest conversion and the age of the tea plantation.
2. Materials and Methods
2.1. Site Description and Soil Sampling
The study site is located in Mangjing Village, Huimin Township, Yunnan Province, China (22°09′ N, 100°03′ E), which is one of the core areas of the ancient tea plantations in Jingmai Mountain. The region has an average altitude of 1250–1595 m, an average annual temperature of 18–20 °C, and a mean annual rainfall of 1800 mm. The climate of the region is a subtropical mountain monsoon climate. The soil type is classified as Ultisol according to the USDA classification system [27]. The soil of the natural forest near the tea plantation was collected as the baseline soil, and its properties are as follows: pH of 5.02 ± 0.01, SOC of 45.52 ± 0.6 g kg−1, total nitrogen of 3.17 ± 0.1 g kg−1, C/N ratio of 13.43 ± 0.2, and soil particle size distribution (clay: 288.5 ± 2 g kg−1, silt: 272.6 ± 1 g kg−1, and sand: 439.0 ± 3 g kg−1). The mountains have been reclaimed by slash-and-burn for over a thousand years, resulting in coverage by old tea trees mixed with stands of broad-leaved subtropic trees. The native forest vegetation was initially removed through clearing and burning, followed by replanting the area with tea trees and arbor trees to establish a newly structured mixed woodland tea plantation. In other regions, selective thinning of the natural forest trees has been carried out to create a favorable environment for tea tree growth under the forest canopy, resulting in a typical tea plantation ecosystem. Soil samples were collected from areas where the forest has been mixed with tea trees for 200, 150, 30, and 3 years (referred to as MT200, MT150, MT30, and MT3, respectively) and from a post-fire mixed forest–tea plantation for 4 years (FMT4). No fertilizers are applied; the soil is ploughed and loosened once a year from November to March, and weeding is performed twice yearly, in August and December. The tea trees have not undergone high intensity pruning to maintain their natural growth state.
Each tea plantation was divided into three 20 m × 20 m quadrats, and soil samples from the 0–40 cm depth were collected within each quadrat using the five-point sampling method. Soil samples from each sampling point were combined to form a composite sample, ensuring the representativeness and homogeneity of the samples. The collected samples were divided into two portions: one was air-dried and passed through a 2 mm sieve for physicochemical analysis, and the other was stored at −20 °C for microbial analysis.
2.2. Soil Properties Analysis
2.2.1. Basic Physical and Chemical Properties
Soil pH was measured using a pH meter with a soil to water ratio of 1:2.5. The soil organic carbon (SOC), total nitrogen content (TN), δ13C, and δ15N were determined using an elemental analyzer and isotope ratio mass spectrometry (Isoprime Elementar, Frankfurt, Germany). The soil mineral specific surface area (SSA) was measured using the N2 Brunauer–Emmett–Teller (BET) method [28]. Particle size distribution was determined using the pipette method based on Stokes’ law [29]. Free oxides (Fed and Ald) were extracted by the citrate–dithiocarbonate (CDB) method. Amorphous iron and aluminum (Feo and Alo) were obtained by the ammonium oxalate method. Iron and aluminum associated with organic matter (Fep and Alp) were extracted using pyrophosphate [30]. The extraction process was repeated three times and the concentrations of iron and aluminum in the supernatant were measured by ICP-OES (Agilent, Palo Alto, CA, USA).
2.2.2. Chemical Structure of SOC
The chemical structure of the SOC samples was determined by 13C solid-state nuclear magnetic resonance (NMR) spectroscopy [31]. Before NMR measurements, the samples were treated with 2% hydrofluoric acid (HF) to remove paramagnetic compounds and concentrate organic carbon. In particular, 50 mL of 2% HF were added to 5 g of soil and shaken at 120–150 rpm. The treatment was performed in a series of steps: 1 h for 5 times, 16 h for 3 times, and 64 h for 1 time. After each treatment, the supernatant was removed by centrifugation, and the residue was retained for further analysis [32]. We used 13C cross-polarization/total sideband suppression (CP/TOSS) and CP/TOSS with dipolar dephasing techniques to qualitatively generate whole spectra and sub-spectra that selected the signals of non-protonated carbons and mobile groups. The experiments were conducted using a Bruker AVANCE 400 spectrometer (Bruker Biospin, Rheinstetten, Germany) at 100 MHz for 13C with a 4 mm double-resonance MAS probe head, at a spin rate of 5 kHz and a CP time of 1 ms, with a 1H 90° pulse length of 4 us and a recycle delay of 0.8 s. The different C functional groups are based on the previous literature [33,34].
Two indices for assessing SOC stability were calculated: (i) the alkyl C to O-alkyl C ratio, which serves as a sensitive indicator of the extent of SOC decomposition [35], and (ii) the hydrophobic C to hydrophilic C ratio, which evaluates the degree of SOC hydrophobicity [36]. The hydrophobic C to hydrophilic C ratio is calculated by summing the alkyl C and aromatic C and dividing it by the sum of the O-alkyl C and carbonyl C. Lower ratios of alkyl C to O-alkyl C and hydrophobic C to hydrophilic C are often indicators of decreased SOC stability [35,37].
2.2.3. Soil Microbial Analysis
Soil microbial phospholipid fatty acids (PLFAs) were measured following the protocol described in reference [38]. Briefly, aliquots of 3 g freeze-dried soil samples were treated twice with a chloroform–methanol–citrate buffer (1:2:0.8) to extract lipids. Phospholipids were separated from neutral lipids and glycolipids using solid-phase extraction columns (SPE-Si, Supelco Inc., Bellefonte, PA, USA). Phospholipids were then converted into fatty acid methyl esters (FAMEs) through mild methanolysis in a 1 M KOH methanol solution. The extracted FAMEs were analyzed using the MIDI Sherlock Microbial Identification System on a gas chromatograph (Agilent 6890N, Agilent Technologies Inc., Santa Clara, CA, USA) with an FID detector and an Agilent 19091B-102 column. Peaks were identified using the Sherlock Microbial Identification System Version 6.2 (MIDI, Inc., Newark, DE, USA), with non-adecanoic acid (19:0) used as an internal standard and an average recovery rate of the internal standard of 81.64% [39].
Based on microbial fatty acid biomarkers from the existing literature, the fatty acids in the samples were assigned to different microbial groups. The PLFAs were categorized into different microbial groups including Gram-positive bacteria (G+), Gram-negative bacteria (G–), general bacteria (G.B.), fungi, and actinomycetes [40]. G+ encompassed PLFAs such as i14:0, a15:0, i15:0, i16:0, a17:0, i17:0, i18:0, and i19:0 [39,40]; G– included 14:1 ω5c, 15:1 ω5c, 16:1 ω7c, 16:1 ω9c, 17:1 ω8c, 18:1 ω5c, 18:1 ω7c, 17:0 cy ω7c, 19:0 cy ω7c, 20:1 ω9c, 21:1 ω4c, and 20:1 ω8c [41]; G.B., 16:0, 17:0, 18:0, and 20:0 [42]; fungi, 16:1 ω5c and 18:2 ω6c [43]; and actinomycetes, 10Me16:0, 10Me17:0, and 10Me18:0 [44]. The prefixes “a”, “i”, and “Me” denote anteiso, iso, and mid-chain methyl branching, respectively, while the prefix “cy” indicates a cyclopropyl ring.
2.3. SOC Mineralization
Five grams of air-dried and sieved soil subsamples (<2 mm) were pre-incubated in 20 mL glass vials at 25 °C with 60% water holding capacity for one week to stimulate microbial activity. Three replicates were set up for each tea garden soil sample. After this initial period, the samples were incubated for another 15 days in an artificial climate chamber (MGC-350HP, Shanghai, China) at a constant temperature of 25 °C. The glass vials were sufficient for three replicates during each gas sampling. Additionally, three blank glass vials without soil were included to determine the background concentration of CO2.
Gas sampling occurred at 1, 2, 3, 5, 8, and 15 days after the beginning of incubation. Gas samples were collected from the headspace of each glass vial using a syringe to measure the concentration of CO2 with a gas chromatograph (Agilent 7890A, Agilent Technologies Inc., Santa Clara, CA, USA). Before gas sampling, the incubated glass vials were opened to facilitate the exchange of headspace air and then sealed with airtight lids for 12 h. After each sampling, the glass vials were opened and flushed with air for 30 min. Cumulative SOC mineralization was normalized by the initial SOC content for each tea plantation [45].
2.4. Statistical Analysis
Analyses of variance were performed using Duncan’s test in SPSS (version 26, SPSS Inc., Chicago, IL, USA) to identify significant differences in soil physicochemical properties and microbial community composition among tea plantations. Pearson correlation analyses were conducted in Origin (version 2020, OriginLab Corp., Northampton, MA, USA) to explore the relationships between other physicochemical properties, physical properties, microbial properties, chemical structures, and the cumulative SOC mineralization. Principal component analysis (PCA) was also conducted in Origin 2020 to identify variations in SOC mineralization based on forest conversion to tea plantation methods and tea planting ages. Additionally, a random forest model was implemented using the A3, rfPermute, and randomForest packages in R (version 4.1.2, R Core Team, 2022) to identify the major factors influencing SOC mineralization. Figures were created using Origin 2020 software.
3. Results
3.1. Soil Physicochemical and Microbial Properties
The soil properties were significantly affected by the methods of forest conversion to tea plantations. Soils in these tea plantations were acidic, with pH values from 4.40 ± 0.03 to 5.73 ± 0.01. Notably, the FMT4 tea plantation had a significantly higher SOC content (27.1 ± 0.0 g kg−1), C/N ratio (12.1 ± 0.1 g kg−1), δ13C (−20.6 ± 0.0), and SSA (28.3 ± 0.1 m2 g−1) (p < 0.05), as well as higher clay (308 ± 7 g kg−1) and silt (287 ± 4 g kg−1) contents, compared to other tea plantations (Table 1). Additionally, the FMT4 tea plantation (Figure 1) exhibited significantly higher levels of Fed, Ald, and Alo compared to the MT3, MT30, and MT200 tea plantations (p < 0.05).

Table 1.
Soil physicochemical properties of different tea plantations.
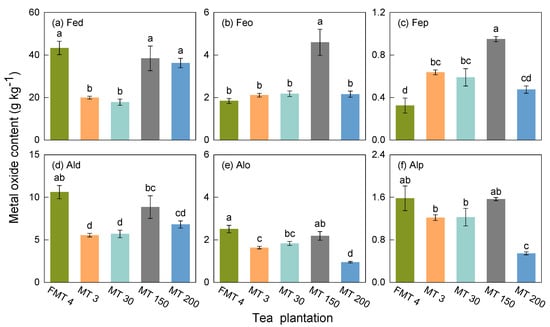
Figure 1.
The content of the different iron and aluminum oxides extracted using dithionite citrate bicarbonate (Fed and Ald), acid ammonium oxalate (Feo and Alo), and sodium pyrophosphate (Fep and Alp), in different tea plantations. Bars represent the standard error of the means (n = 3). Lowercase letters over the columns indicate significant differences at the p < 0.05 level between tea plantations.
Soil properties were also influenced by the age of tea planting. The SOC, TN, and SSA contents increased from the MT3 to the MT150 tea plantations but decreased in the MT200 tea plantation (Table 1). The clay content in the MT200 tea plantation (278 ± 3 g kg−1) was 13.1% to 33.3% higher than in the MT3, MT30, and MT150 (185–241 g kg−1) tea plantations. Furthermore, the content of iron (Fed, Feo, and Fep) and aluminum (Ald and Alo) oxides in the MT150 tea plantations was higher than in the MT3 and MT30 tea plantations (Figure 1). There were no differences in the metal oxide content between the MT3 and MT30 tea plantations (p > 0.05).
Microbial biomass and community composition did not vary significantly among these tea plantations, except for the MT200 tea plantation, which had a lower bacterial to fungal ratio than the other tea plantations (Figure 2). The relative abundance of G+ was higher in the MT150 tea plantation compared to the MT3 and MT200 tea plantations.
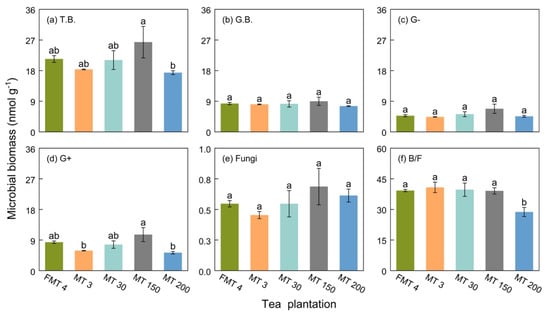
Figure 2.
The PLFA contents in the soil of different tea plantations. Lowercase letters above the bars indicate significance at p < 0.05 between tea plantations. T.B., total biomass of the PLFAs, G.B., general bacteria, G–, Gram-negative bacteria, G+, Gram-positive bacteria, B/F, the ratio of bacteria to fungi.
3.2. Chemical Composition of SOC Identified by 13C NMR
The highest peaks in the spectra were observed for O–alkyl C and alkyl C, followed by aromatics, NCH/OCH3, and COO/N–C=O, except for the FMT4 tea plantation, which had the highest aromatics peak (Figure 3).
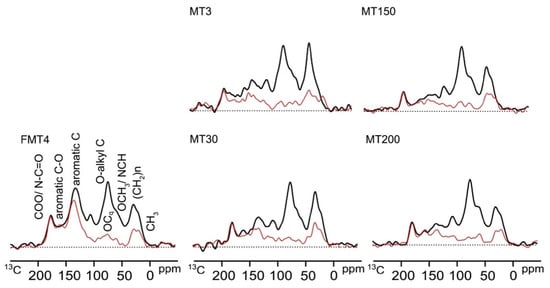
Figure 3.
Spectra of SOC derived by 13C CP/TOSS NMR (black lines) and dipolar-dephased techniques (red lines) in different tea plantations.
The SOC in the FMT4 tea plantation contained more aromatic C (33.4% vs. 14.0–21.3%) and COO/N-C=O (7.8% vs. 4.7–6.0%), and less alkyl C (17.0% vs. 20.8–27.0%) and O-alkyl C (32.8% vs. 36.4–43.1%) than the other tea plantations (Table 2). The SOC in the MT150 tea plantation had relatively fewer alkyl C and aromatic C, and more O-alkyl C than those in the MT3 and MT30 tea plantations. There were no differences in the chemical composition of SOC between the MT3 and MT30 tea plantations. The SOC in the MT150 tea plantation contained more alkyl C (25.1% vs. 20.8%) and less aromatic C (14.1% vs. 18.3%) than that in the MT200 tea plantation. Additionally, the alkyl C/O-alkyl C ratio followed the order MT3 > MT30 > MT150 > FMT4 > MT200, while the hydrophobic C/hydrophilic C ratio was FMT4 > MT3 > MT30 > MT150 = MT200.

Table 2.
Relative proportions of functional groups of SOC determined by 13C CP/TOSS and dipolar-dephased techniques in different tea plantations.
3.3. SOC Mineralization Under Different Tea Plantations
The mineralization rate of SOC in these tea plantations decreased over the incubation period and then reached a stable state on the eighth day (Figure 4a). During the first three days of incubation, the SOC mineralization rate in the FMT4 tea plantation was lower than in other plantations, except for the MT200 tea plantation. The SOC mineralization rate in the MT150 tea plantation remained the highest among these tea plantations throughout the incubation period.
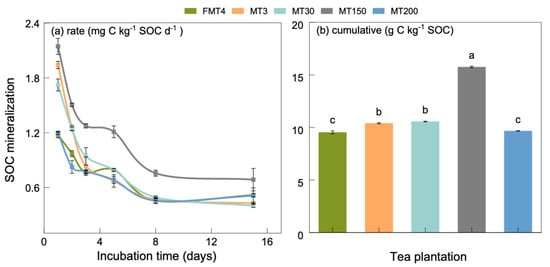
Figure 4.
The SOC mineralization from different tea plantations during 15-day incubation. Bars indicate the standard error of the means (n = 3). Lowercase letters in (b) indicate significant differences at the p < 0.05 level between plantations.
Significant differences (p < 0.05) were observed in the accumulation of SOC mineralization in these tea plantations after a 15-day incubation period (Figure 4b). The cumulative SOC mineralization in the FMT4 tea plantation was 9.5 g C kg−1 SOC, which was 5.9% to 37.5% lower than those of the MT3, MT30, and MT150 tea plantations, respectively, and was similar to that of the MT200 tea plantation. There was no significant difference in SOC mineralization accumulation between the MT3 and MT30 plantations (p > 0.05).
3.4. Key Factors Controlling SOC Mineralization
Principal component analysis (PCA) showed that the soil properties of tea plantations were differentiated by the methods of forest conversion to tea plantations and the ages of tea plantations (Figure 5). The first two principal components (PC1 and PC2) explained 65.7% of the total variance. The PC scores of tea plantations with different forest conversion to tea plantation methods were differentiated on PC1 (Figure 5a). The FMT4 tea plantation was characterized by clay and silt content, aromatic C, and COO/N-C=O, whereas the other tea plantations were characterized by sand content and alkyl C, N-alkyl/methoxyl C, and O-alkyl C (Figure 5b). Figure 5a shows that the tea plantations with different ages were differentiated on PC2. The MT150 tea plantation was characterized by SOC, TN, Fe–Al oxides, and microbial properties such as G+ and the B/F ratio, whereas the other tea plantations were characterized by pH and A-C/O-C. Significant negative correlations between PC1 and cumulative mineralization, and positive correlations between PC2 and cumulative mineralization, were found (Figure 5c,d).
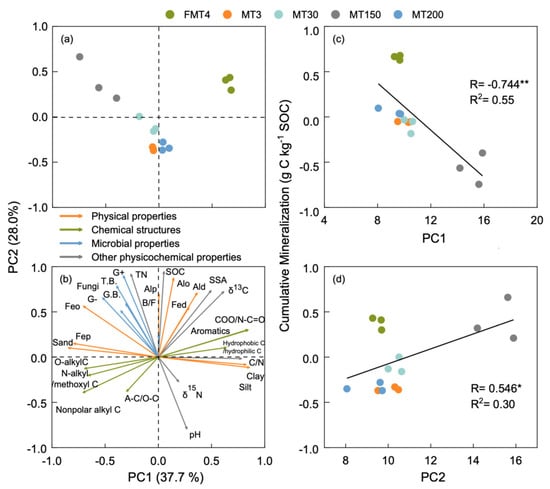
Figure 5.
Principal component analysis scores (a) and loadings (b) of various physicochemical and microbiological characteristics in different tea plantations, and linear regressions of PC1 (c) and PC2 (d) in relation to the cumulative mineralization of SOC. PC1 and PC2 are the first two components of the PCA based on 29 soil properties. * and ** indicate significant differences at p < 0.05 and 0.01. T.B., total biomass of the PLFAs, G.B., general bacteria, G–, Gram-negative bacteria, G+, Gram-positive bacteria, B/F, the ratio of bacteria to fungi, SSA, specific surface area, TN, total nitrogen, A-C/O-C, the ratio of alkyl C to O-alkyl C.
The cumulative SOC mineralization was negatively correlated with the soil C/N ratio, pH, clay and silt content, and the relative abundance of aromatic C and COO/N-C=O (Figure 6). However, SOC mineralization was positively correlated with TN, Feo, Fep, sand, O-alkyl C, T.B., G+ and G−.
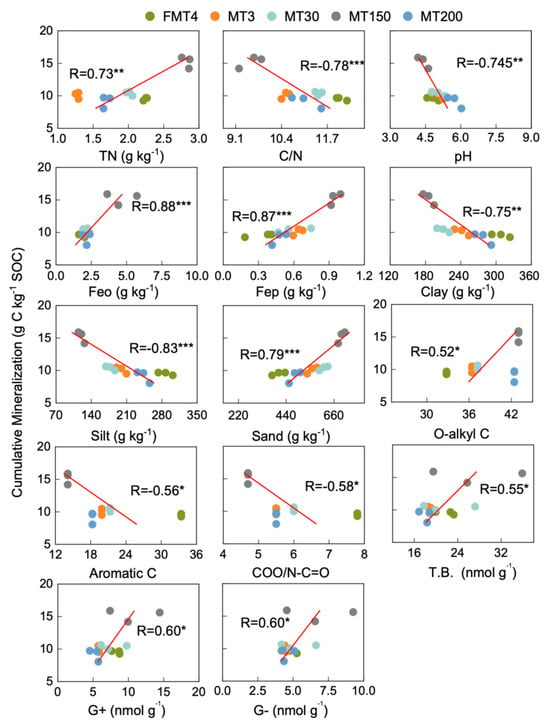
Figure 6.
Pearson correlations between basic soil properties, physical properties, microbial properties, chemical structures, and the cumulative mineralization of SOC. TN, total nitrogen, T.B., total biomass of the PLFAs, G+, Gram-positive bacteria, G–, Gram-negative bacteria. *, **, and *** indicate significant differences at p < 0.05, 0.01, and 0.001.
Soil properties that significantly correlated with SOC mineralization were further profiled with random forest modeling (Figure 7). Random forest analysis showed that fourteen factors considered in this study significantly affected (p < 0.05) SOC mineralization. Among them, the physical properties such as clay, sand, Fep, Feo, and silt were more important in SOC mineralization than the chemical structures such as O-alkyl C, aromatic C, and COO/N-C=O, and the other physicochemical properties such as C/N.
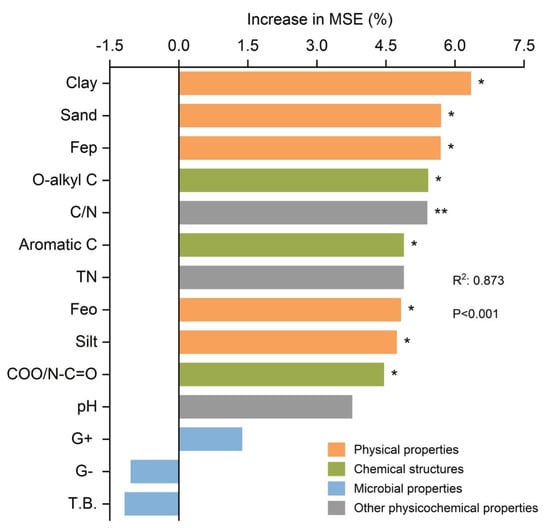
Figure 7.
Relative effects of random forest model variables on the cumulative mineralization of SOC in tea plantations. The random forest mean predictor importance is represented as the percentage increase in the mean variance error (MSE) of the clay, sand, Fep, O-alkyl C, C/N, aromatic C, TN, Feo, silt, COO/N=O, pH, G+, G-, and T.B content variables on SOC mineralization. The color differences in the bars indicate variations in soil properties. * and ** indicate significant differences at p < 0.05 and 0.01. TN, total nitrogen, T.B., total biomass of the PLFAs, G+, Gram-positive bacteria, G–, Gram-negative bacteria.
4. Discussion
4.1. Effects of Forest Conversion to Tea Plantation Methods on SOC Mineralization
Cumulative SOC mineralization was significantly lower in the FMT4 tea plantation compared to the other tea plantations, such as MT3, MT30, and MT150 (Figure 4). This difference was affected by variations in the chemical structure of SOC, which exhibited higher proportions of aromatic C and COO/N-C=O, and lower proportions of O-alkyl C in the FMT4 tea plantation (Table 2, Figure 6). The FMT4 tea plantation was established after being reclaimed by clear-cutting and burning, whereas the other plantations were reclaimed through partial cutting. The process of fire-induced burning promotes the accumulation of aromatic C in the soil, which is more resistant to decomposition by microorganisms, thereby decreasing SOC mineralization rates. Several studies have found that following fire events, the content of aromatic C significantly increases [16,46,47]. Additionally, fire can destroy some organic matter in the soil, reducing the fraction of readily degradable O-alkyl C and subsequently affecting SOC decomposition [48]. The increased aromaticity and hydrophobicity of SOC in the FMT4 tea plantation can further explain its lower mineralization rates compared to the other tea plantations, with the exception of the MT 200 plantation. Therefore, different methods of forest reclamation can affect SOC mineralization.
4.2. Effect of Planting Time on SOC Mineralization
Planting time primarily influences SOC mineralization by increasing the proportion of O-alkyl C in soil. A higher proportion of O-alkyl C, mainly associated with the carbon signal of carbohydrate compounds such as glucose, cellulose, and hemicellulose, may result from long-term inputs of plant litter or root exudates [49,50]. Glucose, cellulose, and hemicellulose are generally considered labile carbon compounds [49]; therefore, soils rich in O-alkyl C typically show greater susceptibility to microbial degradation [31], leading to higher SOC mineralization [12]. However, the interaction between soil texture (clay content) and nutrient factors (N content and C/N ratio) disrupts the relationship between the chemical structure of SOC and its mineralization and stabilization, leading to different impacts on SOC mineralization in the MT150 and MT200 tea plantations. Because the MT150 plantation exhibited the greatest nitrogen availability (C/N = 9.6), the abundance of Gram-positive bacteria (G+, 10.59 ± 2.06 %) was significantly higher than in MT3 and MT200 (p < 0.05). Previous studies show that G+ taxa specialize in degrading aromatic C and other recalcitrant compounds [51], whereas the relative abundance of Gram-negative bacteria (G−) was consistently lower across plantations. Under acidic conditions (pH 4.4–5.7), the thicker peptidoglycan cell walls of G+ confer greater acid tolerance, allowing them to out-compete G− [52].
The MT150 tea plantation, alongside higher O-alkyl C, also had higher total nitrogen levels and a lower C/N ratio than the other tea plantations (Table 1). The higher nitrogen availability likely supported the growth of microorganisms, particularly Gram-positive bacteria (Figure 2d). In addition, the MT150 tea plantation had a lower clay and silt content. Lower clay and silt content may reduce SOC protection by decreasing physical encapsulation and adsorption. These factors led to the MT150 plantation exhibiting the highest cumulative SOC mineralization among all the tea plantations (Figure 4).
Although the MT200 tea plantation has a higher O-alkyl C proportion than the MT3 and MT30 plantations, it exhibited higher clay and silt content and lower total N levels. The higher clay and silt content, along with lower N levels, disrupt the relationship between the chemical structure of SOC and its mineralization. Clay particles physically encapsulate easily degradable O-alkyl C, thereby limiting microbial decomposition and utilization [53]. The lower nitrogen content weakens the microbial community, further inhibiting SOC decomposition. As a result, the MT200 tea plantation exhibits relatively lower SOC mineralization despite its higher O-alkyl C proportion. This evidence highlights the importance of clay and silt in physically protecting SOC, as well as the limiting role of N in organic carbon mineralization.
4.3. Control Mechanisms of SOC Mineralization
The cumulative mineralization of SOC is negatively correlated with C/N, pH, clay, silt, aromatic C, and COO/N-C=O, while it shows a positive correlation with TN, Feo, Fep, sand, O-alkyl C, T.B., G+, and G− (Figure 6). Among these factors, the most important factors regulating SOC mineralization are soil physical properties, such as clay, sand, Fep, Feo, and silt content. This is followed by chemical structure factors, like O-alkyl C, aromatic C, and COO/N-C=O, as well as other physicochemical properties (C/N and TN) and microbial properties (Figure 7). Our findings indicate a positive impact of iron oxides on SOC mineralization, which contradicts results from other studies suggesting that iron oxide binds to SOC to form stable complexes, thereby reducing SOC mineralization [54,55]. At the MT150 tea plantation, the levels of SOC mineralization, Feo, and Fep content were the highest among all the tea plantations studied. Notably, the pH in this tea plantation was lower compared to the others. As the soil acidifies, the valence state of iron changes, which may result in the release of organic carbon, previously sequestered by iron oxides, into the soil solution as soluble carbon [56]. This might explain why we observed a positive relationship between iron oxides and SOC mineralization in this study.
Previous studies in tea plantations have reported the effects of nitrogen on SOC mineralization [57,58], as well as the influence of chemical composition [12] and the regulation of clay on SOC mineralization [53,59]. However, most of these studies have tended to analyze individual factors in isolation, lacking a systematic and integrative comparison. Furthermore, research on the regulatory roles of chemical composition and mineral factors in SOC mineralization remains relatively limited in tea plantation soils, whereas more extensive investigations have been conducted in other soil types, such as forest and grassland soils [20,60]. Our research provides valuable evidence that highlights the importance of these factors in tea plantations. A comparative analysis of these regulatory factors will not only help clarify their individual and interactive roles but also contribute to a more comprehensive understanding of SOC turnover processes in tea plantation soils.
5. Conclusions
The physical properties of soil, particularly clay content, are the most critical factors regulating SOC mineralization. These are followed by the chemical structure (such as O-alkyl C), physicochemical properties (e.g., C/N ratio), and microbial properties (e.g., G+). The forest conversion to tea plantation method and age significantly influenced the chemical composition of SOC, which in turn affects SOC mineralization. Compared with the partial cutting method (MT3, MT30, and MT150), tea plantations converted by clear-cutting and burning (FMT4) decreased the SOC mineralization and improved the SOC stability through higher proportions of aromatic C and carbonyl/carboxyl C, and lower proportions of O-alkyl C compounds. Additionally, with an increase of tea plantation age, SOC stability decreased due to the higher proportion of O-alkyl C and nitrogen content, as well as the growth of G+. Notably, although the MT200 plantation had a higher proportion of O-alkyl C than the MT3 and MT30 plantations, and similar to the MT150 plantation, its SOC mineralization remained lower due to its higher clay content. Long-term cultivation of a tea plantation (soil age >30 years) significantly increased the content of labile organic matter, soil nitrogen concentration, and the biomass of Gram-positive bacteria (excluding the MT200 strain), which accelerated soil organic carbon (SOC) mineralization. However, this organic carbon destabilization can be mitigated by enhancing clay content and increasing the proportion of recalcitrant organic matter. These findings demonstrate that SOC stability in long-term tea plantation soils can be improved through clay supplementation (e.g., soil amendments) and application of recalcitrant organic materials (e.g., well-decomposed compost). To further elucidate the implications of SOC stability on tea plantation productivity, future research should investigate the relationship between SOC stability and the productivity of tea plantations, while also evaluating the potential effects of SOC stability on tea plant growth, yield, and soil fertility.
Author Contributions
D.X.: writing—review and editing, writing—original draft, visualization, validation, software, methodology, investigation, formal analysis, data curation. B.S.N.: formal analysis, data curation. X.C.: writing—review and editing, validation. S.Y.: supervision, project administration. Y.Z.: writing—review and editing, validation, supervision, project administration, investigation, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [No. 41930761 to BZ, No. 42177284 to YLZ and No. 42107330 to CX].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank Qinghua Wang for sampling Ultisol from Yunnan, and Bin Zhang for suggestions for the earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SOC | Soil organic carbon |
| C/N | Carbon-to-nitrogen |
| TN | Total nitrogen content |
| SSA | Specific surface area |
| T.B. | Total biomass of the PLFAs |
| G.B. | General bacteria |
| G– | Gram-negative bacteria |
| G+ | Gram-positive bacteria |
| B/F | The ratio of bacteria to fungi |
| A-C/O-C | The ratio of alkyl C to O-alkyl C |
References
- FAO; CAAS. Carbon Neutral Tea Production in China—Three Pilot Case Studies; FAO: Rome, Italy, 2021; ISBN 978-92-5-134373-9. [Google Scholar]
- National Bureau of Statistics. China Statistical Yearbook 2024; China Statistics Press: Beijing, China, 2024; ISBN 978-7-5056-3527-2. [Google Scholar]
- Tang, X.; Zhao, X.; Bai, Y.; Tang, Z.; Wang, W.; Zhao, Y.; Wan, H.; Xie, Z.; Shi, X.; Wu, B.; et al. Carbon Pools in China’s Terrestrial Ecosystems: New Estimates Based on an Intensive Field Survey. Proc. Natl. Acad. Sci. USA 2018, 115, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, B.S.; Li, F.; Li, X.; Wang, Z.; Hou, J.; Cao, R.; Yang, W. Soil Organic Carbon Stock in China’s Tea Plantations and Their Great Potential of Carbon Sequestration. J. Clean. Prod. 2023, 421, 138485. [Google Scholar] [CrossRef]
- Jia, J.; Yu, D.; Zhou, W.; Zhou, L.; Bao, Y.; Meng, Y.; Dai, L. Variations of Soil Aggregates and Soil Organic Carbon Mineralization across Forest Types on the Northern Slope of Changbai Mountain. Acta Ecol. Sin. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, Z.; Li, T.; Zhang, X.; He, S.; Wang, Y.; Liu, T.; Li, W. Dynamics of Soil Organic Carbon Mineralization in Tea Plantations Converted from Farmland at Western Sichuan, China. PLoS ONE 2017, 12, e0185271. [Google Scholar] [CrossRef]
- Li, W. Study on the Organic Carbon Dynamics and Its Mineralization Characteristics with Tea Plantations Soils. Master’s Thesis, Sichuan Agricultural University, Yaan, China, 2016. [Google Scholar]
- Tchienkoua, M.; Zech, W. Organic Carbon and Plant Nutrient Dynamics under Three Land Uses in the Highlands of West Cameroon. Agric. Ecosyst. Environ. 2004, 104, 673–679. [Google Scholar] [CrossRef]
- Zou, S.; Huang, C.; Feng, T.; Chen, Y.; Bai, X.; Li, W.; He, B. Effects of Woodland Conversion to Tea Plantations and Tea Planting Age on Soil Organic Carbon Accrual in Subtropical China. Forests 2024, 15, 1862. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Yu, P.; Fu, W.; Morrison, L.; Lin, H.; Meng, M.; Zhou, X.; Lv, Y.; Wu, J. Converting Evergreen Broad-Leaved Forests into Tea and Moso Bamboo Plantations Affects Labile Carbon Pools and the Chemical Composition of Soil Organic Carbon. Sci. Total Environ. 2020, 711, 135225. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Li, Y.; Liu, X.; Wang, Y.; Qin, J.; Wu, J. Effects of Land-Use Conversion from Masson Pine Forests to Tea Plantations on Net Ecosystem Carbon and Greenhouse Gas Budgets. Agric. Ecosyst. Environ. 2021, 320, 107578. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J.; Li, Y.; Zhang, L.; Hu, Q.; Li, Z.; Yang, X.; Li, J.; Wu, T.; Mao, Y.; et al. Changes in Soil Organic Carbon Stocks and Mineralization Following the Replacement of Secondary Evergreen Broadleaf Forests with Tea (Camellia Sinensis L.) Plantations. Soil Use Manag. 2024, 40, e13125. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. Analytical Approaches for Characterizing Soil Organic Matter. Org. Geochem. 2000, 31, 609–625. [Google Scholar] [CrossRef]
- Köster, K.; Aaltonen, H.; Berninger, F.; Heinonsalo, J.; Köster, E.; Ribeiro-Kumara, C.; Sun, H.; Tedersoo, L.; Zhou, X.; Pumpanen, J. Impacts of Wildfire on Soil Microbiome in Boreal Environments. Curr. Opin. Environ. Sci. Health 2021, 22, 100258. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, L.-J.; Yao, S.-H.; Mao, J.-D.; Schmidt-Rohr, K.; Olk, D.C.; Cao, X.-Y.; Cui, J.-F.; Zhang, B. Distinct Changes in Composition of Soil Organic Matter with Length of Cropping Time in Subsoils of a Phaeozem and Chernozem. Eur. J. Soil Sci. 2018, 69, 868–878. [Google Scholar] [CrossRef]
- Neff, J.C.; Harden, J.W.; Gleixner, G. Fire Effects on Soil Organic Matter Content, Composition, and Nutrients in Boreal Interior Alaska. Can. J. For. Res. 2005, 35, 2178–2187. [Google Scholar] [CrossRef]
- Certini, G.; Nocentini, C.; Knicker, H.; Arfaioli, P.; Rumpel, C. Wildfire Effects on Soil Organic Matter Quantity and Quality in Two Fire-Prone Mediterranean Pine Forests. Geoderma 2011, 167–168, 148–155. [Google Scholar] [CrossRef]
- Han, W.; Kemmitt, S.J.; Brookes, P.C. Soil Microbial Biomass and Activity in Chinese Tea Gardens of Varying Stand Age and Productivity. Soil Biol. Biochem. 2007, 39, 1468–1478. [Google Scholar] [CrossRef]
- He, S.; Zheng, Z.; Zhu, R. Long-Term Tea Plantation Effects on Composition and Stabilization of Soil Organic Matter in Southwest China. CATENA 2021, 199, 105132. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions—A Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Guenet, B.; Juarez, S.; Bardoux, G.; Abbadie, L.; Chenu, C. Evidence That Stable C Is as Vulnerable to Priming Effect as Is More Labile C in Soil. Soil Biol. Biochem. 2012, 52, 43–48. [Google Scholar] [CrossRef]
- Yin, X.; Yu, X.; Qin, L.; Jiang, M.; Lu, X.; Zou, Y. Reclamation Leads to Loss of Soil Organic Carbon and Molecular Complexity: Evidence from Natural to Reclaimed Wetlands. Soil Tillage Res. 2025, 248, 106436. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, J.; Jia, W.; Wang, S.; Liu, H.; Liu, W.; Zhang, Q.; Yang, G.; Han, X.; Ren, G. Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China. Plants 2024, 13, 2455. [Google Scholar] [CrossRef]
- Díaz, E.; Jiménez, J.I.; Nogales, J. Aerobic Degradation of Aromatic Compounds. Curr. Opin. Biotechnol. 2013, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, T.; Zheng, Z. Tea Plantation Age Effects on Soil Aggregate-Associated Carbon and Nitrogen in the Hilly Region of Western Sichuan, China. Soil Tillage Res. 2018, 180, 91–98. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Z.; Li, T.; Zhang, X.; Wang, Y.; Yu, H.; He, S.; Liu, T. Effect of Tea Plantation Age on the Distribution of Soil Organic Carbon Fractions within Water-Stable Aggregates in the Hilly Region of Western Sichuan, China. CATENA 2015, 133, 198–205. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022; ISBN 978-1998295005. [Google Scholar]
- Feng, W.; Plante, A.F.; Aufdenkampe, A.K.; Six, J. Soil Organic Matter Stability in Organo-Mineral Complexes as a Function of Increasing C Loading. Soil Biol. Biochem. 2014, 69, 398–405. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods, 9th ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1986; ISBN 978-0-89118-864-3. [Google Scholar]
- Parfitt, R.L.; Childs, C.W. Estimation of Forms of Fe and Al—A Review, and Analysis of Contrasting Soils by Dissolution and Mossbauer Methods. Soil Res. 1988, 26, 121–144. [Google Scholar] [CrossRef]
- Mathers, N.J.; Xu, Z. Solid-State 13C NMR Spectroscopy: Characterization of Soil Organic Matter under Two Contrasting Residue Management Regimes in a 2-Year-Old Pine Plantation of Subtropical Australia. Geoderma 2003, 114, 19–31. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Clarke, P.; Taylor, J.A.; Oades, J.M.; Newman, R.H. The Removal of Magnetic Materials from Surface Soils—A Solid State 13C CP/MAS NMR Study. Aust. J. Soil Res. 1994, 32, 1215–1229. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Yao, S.-H.; Cao, X.-Y.; Schmidt-Rohr, K.; Olk, D.C.; Mao, J.-D.; Zhang, B. Structural Evidence for Soil Organic Matter Turnover Following Glucose Addition and Microbial Controls over Soil Carbon Change at Different Horizons of a Mollisol. Soil Biol. Biochem. 2018, 119, 63–73. [Google Scholar] [CrossRef]
- Mao, J.; Cao, X.; Chen, N. Characterization of Biochars Using Advanced Solid-State 13C Nuclear Magnetic Resonance Spectroscopy. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 47–55. ISBN 978-1-4614-3348-4. [Google Scholar]
- Baldock, J.; Oades, J.; Nelson, P.; Skene, T.; Golchin, A.; Clarke, P. Assessing the Extent of Decomposition of Natural Organic Materials Using Solid-State 13C NMR Spectroscopy. Aust. J. Soil Res. 1997, 35, 1061–1084. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, C.; Zhang, K.; Shi, S.; Guo, J.; Gao, F.; Liu, J.; Wang, J.; Liu, Y. The Influence of Tree Species on Soil Organic Carbon Stability under Three Temperate Forests in the Baihua Mountain Reserve, China. Global Ecol. Conserv. 2021, 26, e01454. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, S.; Zhu, P.; Gao, H.; Song, X.; Wang, L. Effect of Long-Term Application of Organic Fertilizer on Structural Characteristics of Humin in BlackSoil—A Solid-State 13C NMR Study. Sci. Agric. Sin. 2009, 42, 2223–2228. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of Soil Microbial Communities: Effects of Agricultural Management, Season, and Soil Type on Phospholipid Fatty Acid Profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, N.; Wang, J.; Yao, H.; Qiu, Q.; Chapman, S.J. High Turnover Rate of Free Phospholipids in Soil Confirms the Classic Hypothesis of PLFA Methodology. Soil Biol. Biochem. 2019, 135, 323–330. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Liao, H.; Zheng, C.; Li, J.; Liu, L.; Zhang, M. Dynamics of Soil Microbial Communities Following Vegetation Succession in a Karst Mountain Ecosystem, Southwest China. Sci Rep. 2019, 9, 2160. [Google Scholar] [CrossRef]
- Veum, K.S.; Lorenz, T.; Kremer, R.J. Phospholipid Fatty Acid Profiles of Soils under Variable Handling and Storage Conditions. Agron. J. 2019, 111, 1090–1096. [Google Scholar] [CrossRef]
- Ma, Q.; Kuzyakov, Y.; Pan, W.; Tang, S.; Chadwick, D.R.; Wen, Y.; Hill, P.W.; Macdonald, A.; Ge, T.; Si, L.; et al. Substrate Control of Sulphur Utilisation and Microbial Stoichiometry in Soil: Results of 13C, 15N, 14C, and 35S Quad Labelling. ISME J. 2021, 15, 3148–3158. [Google Scholar] [CrossRef]
- Joergensen, R. Phospholipid Fatty Acids in Soil—Drawbacks and Future Prospects. Biol. Fertil. Soils 2021, 58, 1–6. [Google Scholar] [CrossRef]
- Zelles, L. Fatty Acid Patterns of Phospholipids and Lipopolysaccharides in the Characterisation of Microbial Communities in Soil: A Review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Weiglein, T.L.; Strahm, B.D.; Bowman, M.M.; Gallo, A.C.; Hatten, J.A.; Heckman, K.A.; Matosziuk, L.M.; Nave, L.E.; Possinger, A.R.; SanClements, M.D.; et al. Key Predictors of Soil Organic Matter Vulnerability to Mineralization Differ with Depth at a Continental Scale. Biogeochemistry 2022, 157, 87–107. [Google Scholar] [CrossRef]
- Bird, M.I.; Wynn, J.G.; Saiz, G.; Wurster, C.M.; McBeath, A. The Pyrogenic Carbon Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 273–298. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Harden, J.; Georgiou, K.; Hemes, K.S.; Malhotra, A.; Nolan, C.J.; Jackson, R.B. Fire Effects on the Persistence of Soil Organic Matter and Long-Term Carbon Storage. Nat. Geosci. 2022, 15, 5–13. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The Effect of Fire on Soil Organic Matter—A Review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate Organic Matter as a Functional Soil Component for Persistent Soil Organic Carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The Ratio of Gram-Positive to Gram-Negative Bacterial PLFA Markers as an Indicator of Carbon Availability in Organic Soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the Acid Test: Responses of Gram-Positive Bacteria to Low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Wang, S.; Yao, H.; Huang, X.; Yang, Y.; Zheng, B.; Lin, S.; Wang, W. Research Progress on Soil Organic Carbon Pools Components, Influencing Factors and Stability of Tea Plantation. Res. Environ. Sci. 2024, 37, 1104–1115. [Google Scholar] [CrossRef]
- Han, Z.; Wu, X.; Liang, A.; Li, S.; Gao, H.; Song, X.; Liu, X.; Jia, A.; Degré, A. Conservation Tillage Enhances the Sequestration and Iron-Mediated Stabilization of Aggregate-Associated Organic Carbon in Mollisols. CATENA 2024, 243, 108197. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, S. Amorphous Iron Oxides Protect Aggregate-Associated Organic Carbon from Microbial Utilization and Decomposition Evidenced from the Natural Abundance of 13C. Soil Tillage Res. 2023, 227, 105623. [Google Scholar] [CrossRef]
- Hall, S.J.; Silver, W.L. Iron Oxidation Stimulates Organic Matter Decomposition in Humid Tropical Forest Soils. Glob. Change Biol. 2013, 19, 2804–2813. [Google Scholar] [CrossRef]
- Juang, K.-W.; Chen, C.-P. Changes in Soil Organic Carbon and Nitrogen Stocks in Organic Farming Practice and Abandoned Tea Plantation. Bot. Stud. 2023, 64, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Wang, F.; You, Z.M.; Wu, Z.D.; Jiang, F.Y.; Zhang, W.J. Effect of Nitrogen Fertilization on Organic Carbon Mineralization in Soils at Tea Plantations. Fujian J. Agric. Sci. 2014, 29, 1092–1097. [Google Scholar]
- Kong, Z.; Zhang, L. Soil Carbon Sequestration Capacity and Accumulation Characteristics of Different Organic Carbon Fractions in Tea Gardens in Northwest Zhejiang. J. Zhejiang Univ. (Agric. Life Sci. Ed.) 2016, 42, 209–219. [Google Scholar]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).