Two-Sex Life Table Analysis of Frankliniella intonsa Reared on Nine Different Vegetable Crops in Guangxi, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.1.1. Insects
2.1.2. Crops
2.1.3. Test Set-Up
2.2. Method
2.3. Data Processing
3. Results
3.1. F. intonsa Growth and Development on Nine Crop Species
3.2. Adult Longevity and Fecundity of F. intonsa on Nine Plant Species
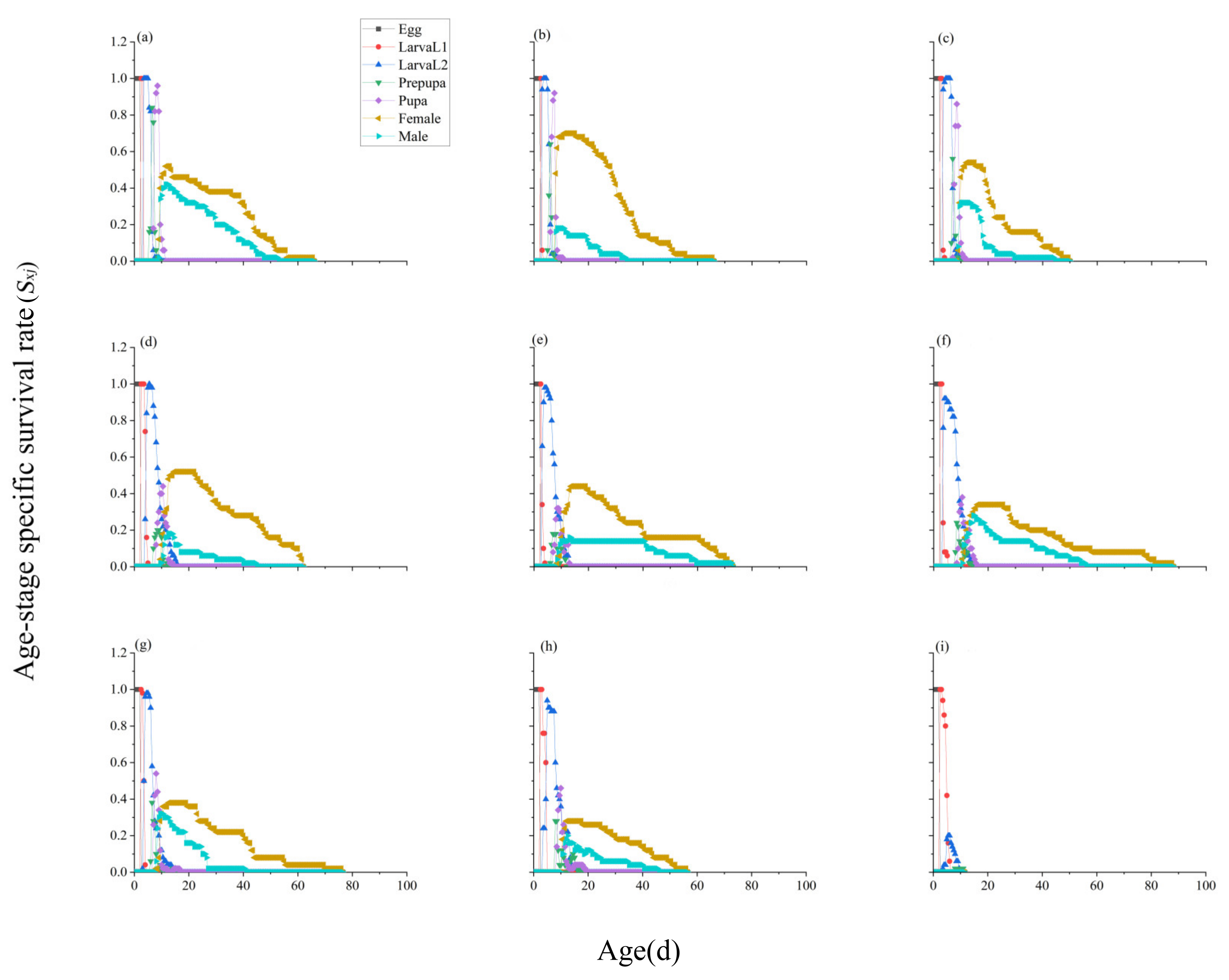
3.3. Age-Stage-Specific Survival Rate of F. intonsa
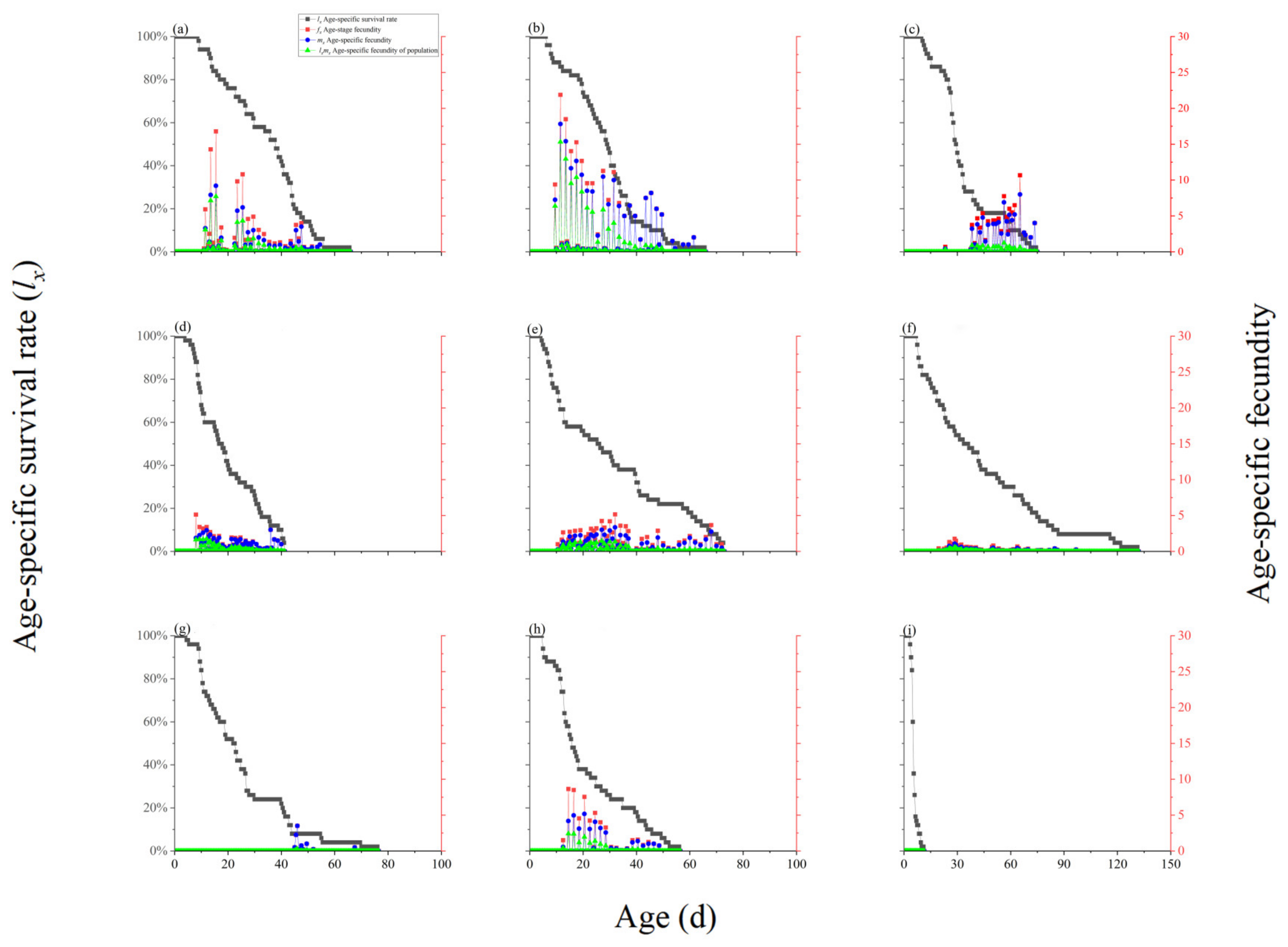
3.4. F. intonsa Age-Specific Survival and Fecundity
3.5. F. intonsa Population Life Table Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, H.; Chuai, Z.R.; Zhang, W.B. Research progress on the occurrence and control of Frankliniella intonsa. China Plant Prot. 2023, 43, 18–24+30. [Google Scholar] [CrossRef]
- Atakan, E.; Gençer, O. Influence of planting date on the relationship between populations of Frankliniella flower thrips and predatory bug Orius niger in cotton. J. Pest Sci. 2008, 81, 123–133. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, S.Y.; Seo, M.H.; Yoon, J.B. Cinnamyl alcohol: An attractant of the flower thrips Frankliniella intonsa. J. Asia-Pac. Entomol. 2022, 25, 101925. [Google Scholar] [CrossRef]
- Pobozniak, M. The occurrence of thrips (Thysanoptera) on food legumes (Fabaceae). J. Plant Dis. Protec. 2011, 118, 185–193. [Google Scholar] [CrossRef]
- Hurej, M.; Kucharczyk, H.; Twardowski, J.P.; Kozak, M. Thrips (Thysanoptera) associated with two morphological forms of andean lupin (Lupinus angustifolius). Biologia 2015, 70, 935–942. [Google Scholar] [CrossRef]
- Li, M.; Peng, Z.; Guo, C. Age-Stage, Two-Sex Life Tables of Megalurothrips usitatus (Bagnall) and Frankliniella intonsa (Trybom) on different bean pods under laboratory conditions: Implications for their competitive interactions. Insects 2024, 15, 1003. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, Y.; Wang, J.P. Study on the following effects of natural enemies on Frankliniella intonsa and Brevipalpus obovatus in tea plantations. Plant Prot. 2018, 44, 99–106. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, J.; Chen, Q.; He, J.; Li, X.; Zhang, J.; Liu, Z.; Wu, Y.; Li, X.; Wang, Y. Chromosome-level genome assembly of the flower thrips Frankliniella intonsa. Sci. Data 2023, 10, 844. [Google Scholar] [CrossRef]
- Tian, H.J.; Yu, Y.; Chen, Y.X. Evaluation of the lethal effects of three insecticides on eggs and larvae of Frankliniella intonsa. Fujian Agric. Sci. Technol. 2024, 55, 48–54. [Google Scholar] [CrossRef]
- Announcement No. 654 of the Ministry of agriculture and rural affairs of the people’s republic of China. In Gazette of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2023; pp. 103–104.
- Huang, H.W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Hong, P.; Dash, C.K.; Ghafar, M.A.; Haq, I.U.; Lu, L.; Zhou, C.; Wu, Q.; Wang, L. Demography and population projection of Tetranychus urticae (Tetranychidae) on Phaseolus vulgaris (Fabaceae) colonized by entomopathogenic fungal endophytes. Insects 2024, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Chi Hsin, C.H.; Fu, J.W.; You, M.S. Age-stage, two-sex life table and its application in population ecology and integrated pest management. Acta Entomol. Sin. 2019, 62, 55–262. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhang, Y.L.; Quandahor, P. Oviposition preference and age-stage, two-sex life table analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different maize varieties. Insects 2023, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. Lethal and Sublethal effects of Cyromazine on the biology of Musca domestica based on the age–Stage, two-Sex life table theory. Toxics 2023, 12, 2. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Xie, W.; Wu, Q.; Xu, B.; Wang, S.; Zhu, X.; Wang, S.; Zhang, Y. Effects of temperature on the age-stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2015, 108, 126–134. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Zheng, X.; Gong, R.; Cao, X.; Yang, L. An age-stage, two-sex life table for Megalurothrips usitatus feeding on eight different crop plants. Agronomy 2024, 14, 2283. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; Fujian Academy of Agricultural Sciences: Fuzhou, China, 2025; Available online: https://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 12 January 2025).
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zheng, Y.Q.; Chen, B. Effects of different hosts on the development and fecundity of Phthorimaea operculella based on age-stage, two-sex life table. J. Plant Prot. 2020, 47, 488–496. [Google Scholar] [CrossRef]
- Tok, B.; Kaydan, M.B.; Mustu, M.; Ulusoy, M.R. Development and life table parameters of Phenacoccus madeirensis Green (Hemiptera: Pseudococcidae) on four ornamental plants. Neotrop. Entomol. 2016, 45, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, Y.M.; Zang, L.S. Adaptability of Trialeurodes vaporariorum to three important host plants based on the age-stage two-sex life table. J. Econ. Entomol. 2023, 45, 442–450. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Agosta, S.J. On ecological fitting, plant–insect associations, herbivore host shifts, and host plant selection. Oikos 2006, 114, 556–565. [Google Scholar] [CrossRef]
- Srinivasan, R. Introduction: Host plant choice and feeding ecology of insects. Entomol. Exp. Appl. 2019, 167, 288–291. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Espelie, K.E. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 1995, 40, 171–194. [Google Scholar] [CrossRef]
- Formisano, L.; Miras-Moreno, B.; Ciriello, M.; El-Nakhel, C.; Corrado, G.; Lucini, L.; Colla, G.; Rouphael, Y. Trichoderma and phosphite elicited distinctive secondary metabolite signatures in zucchini squash plants. Agronomy 2021, 11, 1205. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zhang, A.; Tan, M.; Yan, S.; Jiang, D. Transfer of heavy metals along the food chain: A review on the pest control performance of insect natural enemies under heavy metal stress. J. Hazard. Mater. 2024, 478, 135587. [Google Scholar] [CrossRef]
| Hostplant | Developmental Duration/Days | |||||
|---|---|---|---|---|---|---|
| Egg | 1st Instar | 2nd Instar | Prepupa | Pupa | Egg-Adult | |
| Cowpea | 2.5 ± 0.00 a (50) | 1.00 ± 0.00 d (50) | 2.92 ± 0.06 e (49) | 1.01 ± 0.03 a (48) | 2.15 ± 0.08 a (47) | 9.65 ± 0.08 d (47) |
| Green bean | 2.5 ± 0.00 a (50) | 0.53 ± 0.01 f (50) | 2.85 ± 0.07 e (47) | 0.73 ± 0.04 bc (47) | 1.60 ± 0.03 cd (44) | 8.22 ± 0.07 e (44) |
| Soybean | 2.5 ± 0.00 a (50) | 1.04 ± 0.02 d (50) | 3.67 ± 0.08 d (46) | 0.67 ± 0.03 c (45) | 1.75 ± 0.04 bc (43) | 9.65 ± 0.08 d (43) |
| Catjang cowpea | 2.5 ± 0.00 a (50) | 1.96 ± 0.04 b (50) | 4.26 ± 0.17 c (39) | 0.80 ± 0.03 bc (39) | 1.74 ± 0.05 bc (35) | 11.28 ± 0.19 b (35) |
| Courgette | 2.5 ± 0.00 a (50) | 0.73 ± 0.05 e (50) | 4.83 ± 0.23 b (33) | 0.84 ± 0.05 b (32) | 1.73 ± 0.07 bc (30) | 10.65 ± 0.28 c (30) |
| Wax gourd | 2.5 ± 0.00 a (50) | 1.23 ± 0.07 c (50) | 6.08 ± 0.23 a (37) | 0.83 ± 0.04 b (34) | 1.77 ± 0.05 bc (31) | 12.32 ± 0.26 a (31) |
| Bitter gourd | 2.5 ± 0.00 a (50) | 1.24 ± 0.04 c (49) | 3.43 ± 0.21 de (39) | 0.76 ± 0.04 bc (38) | 1.50 ± 0.04 d (35) | 9.15 ± 0.15 d (35) |
| Cucumber | 2.5 ± 0.00 a (50) | 2.05 ± 0.09 b (49) | 5.11 ± 0.39 b (39) | 0.77 ± 0.06 bc (33) | 1.85 ± 0.07 b (28) | 11.76 ± 0.40 b (28) |
| Chieh-qua | 2.5 ± 0.00 a (50) | 2.37 ± 0.16 a (12) | - | - | - | - |
| F | - | 104.646 | 32.609 | 9.871 | 13.249 | 5.508 |
| df (n1,n2) | - | 8400 | 7321 | 7308 | 7285 | 7285 |
| p | - | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Hostplant | Longevity/Days | Sex Ratio (F/M) | Fecundity | |
|---|---|---|---|---|
| Female Adult | Male Adult | |||
| Cowpea | 29.03 ± 2.70 ab (26) | 21.95 ± 2.58 b (21) | 26:21 | 87.50 ± 6.84 b (26) |
| Green bean | 25.74 ± 1.87 bc (35) | 14.00 ± 2.54 bcd (9) | 35:9 | 148.68 ± 14.88 a (35) |
| Soybean | 18.07 ± 2.06 c (27) | 11.12 ± 1.86 cd (16) | 27:16 | 23.22 ± 6.89 de (27) |
| Catjang cowpea | 30.65 ± 2.71 ab (26) | 13.22 ± 4.06 bcd (9) | 26:9 | 48.46 ± 5.45 cd (26) |
| Courgette | 32.90 ± 4.13 ab (22) | 37.81 ± 5.98 a (8) | 22:8 | 73.86 ± 10.44 bc (22) |
| Wax gourd | 35.94 ± 5.09 a (17) | 20.53 ± 3.89 bc (14) | 17:14 | 21.06 ± 3.01 de (17) |
| Bitter gourd | 29.84 ± 3.75 ab (19) | 13.03 ± 1.92 bcd (16) | 19:16 | 1.89 ± 1.02 e (19) |
| Cucumber | 27.78 ± 3.11 ab (14) | 9.67 ± 2.65 d (14) | 14:14 | 49.50 ± 9.46 cd (14) |
| Chieh-qua | - | - | - | - |
| F | 3.128 | 6.838 | - | 26.049 |
| df (n1,n2) | 7178 | 7.99 | - | 7178 |
| p | 0.004 | 0.000 | - | 0.000 |
| Hostplant | Net Reproductive Rate | Intrinsic Rate of Increase/d−1 | Finite Rate of Increase/d−1 | Mean Generation Time/d |
|---|---|---|---|---|
| Cowpea | 45.5100 ± 0.0224 b | 0.2228 ± 0.0000 b | 1.2496 ± 0.0000 b | 17.0896 ± 0.0012 g |
| Green bean | 104.0416 ± 0.0444 a | 0.3112 ± 0.0000 a | 1.3652 ± 0.0000 a | 14.8967 ± 0.0007 h |
| Soybean | 12.5365 ± 0.0127 f | 0.0756 ± 0.0000 g | 1.0786 ± 0.0000 g | 32.7307 ± 0.0050 b |
| Catjang cowpea | 25.2152 ± 0.0140 d | 0.1585 ± 0.0000 d | 1.1718 ± 0.0000 d | 20.2962 ± 0.0022 e |
| Courgette | 32.6065 ± 0.0216 c | 0.1650 ± 0.0000 c | 1.1796 ± 0.0001 c | 21.0293 ± 0.0033 d |
| Wax gourd | 7.1653 ± 0.0055 g | 0.0871 ± 0.0000 f | 1.0911 ± 0.0000 f | 22.2977 ± 0.0018 c |
| Bitter gourd | 0.7300 ± 0.0012 h | −0.0101 ± 0.0000 h | 0.9900 ± 0.0000 h | 49.4894 ± 0.0398 a |
| Cucumber | 13.7354 ± 0.0126 e | 0.1320 ± 0.0001 e | 1.1413 ± 0.0001 e | 19.5130 ± 0.0020 f |
| Chieh-qua | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, R.; Huang, L.; Wang, H.; Cao, X.; Liu, H.; Yang, L. Two-Sex Life Table Analysis of Frankliniella intonsa Reared on Nine Different Vegetable Crops in Guangxi, China. Agriculture 2025, 15, 862. https://doi.org/10.3390/agriculture15080862
Gong R, Huang L, Wang H, Cao X, Liu H, Yang L. Two-Sex Life Table Analysis of Frankliniella intonsa Reared on Nine Different Vegetable Crops in Guangxi, China. Agriculture. 2025; 15(8):862. https://doi.org/10.3390/agriculture15080862
Chicago/Turabian StyleGong, Rui, Lifei Huang, Huanting Wang, Xuemei Cao, Hongquan Liu, and Lang Yang. 2025. "Two-Sex Life Table Analysis of Frankliniella intonsa Reared on Nine Different Vegetable Crops in Guangxi, China" Agriculture 15, no. 8: 862. https://doi.org/10.3390/agriculture15080862
APA StyleGong, R., Huang, L., Wang, H., Cao, X., Liu, H., & Yang, L. (2025). Two-Sex Life Table Analysis of Frankliniella intonsa Reared on Nine Different Vegetable Crops in Guangxi, China. Agriculture, 15(8), 862. https://doi.org/10.3390/agriculture15080862






