Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat
Abstract
1. Introduction
2. Experimental Design and Methods
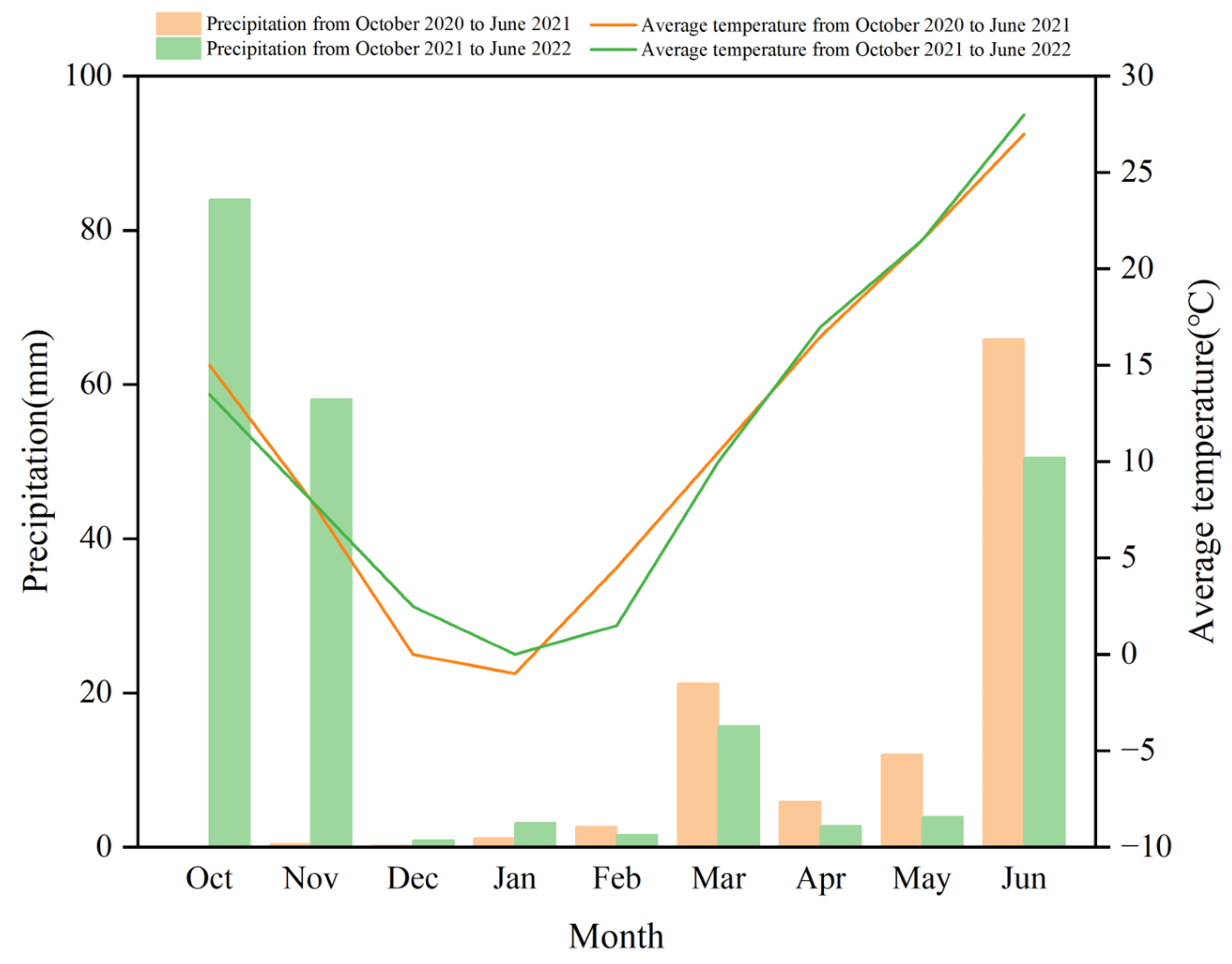
2.1. Site Description
2.2. Experimental Design
2.3. Sample Collection
2.4. Measurement Parameters and Methods
2.4.1. Rhizosphere Soil Physicochemical Properties
2.4.2. Rhizobacteria Community Profiling
2.5. Statistical Analysis
3. Results
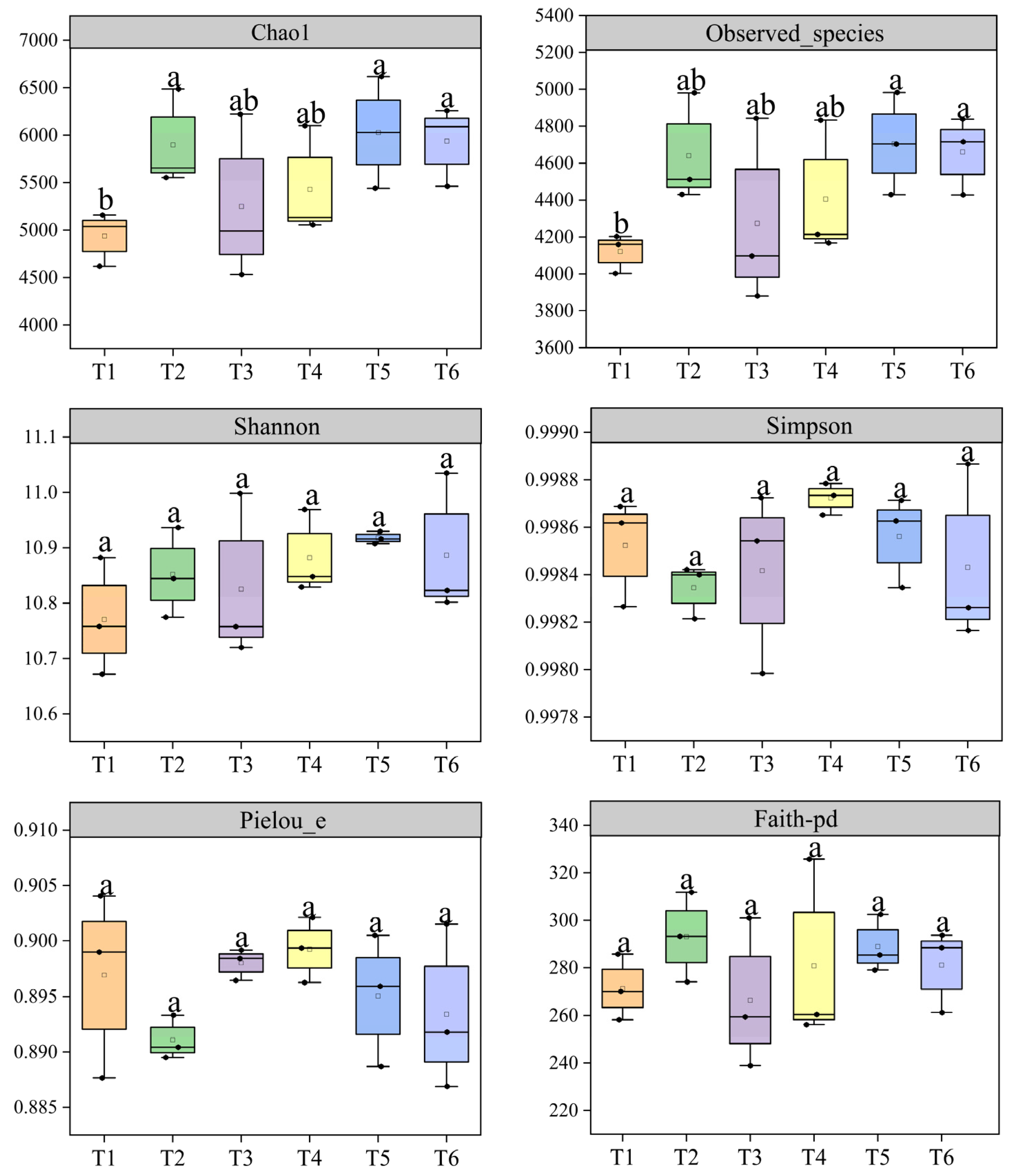
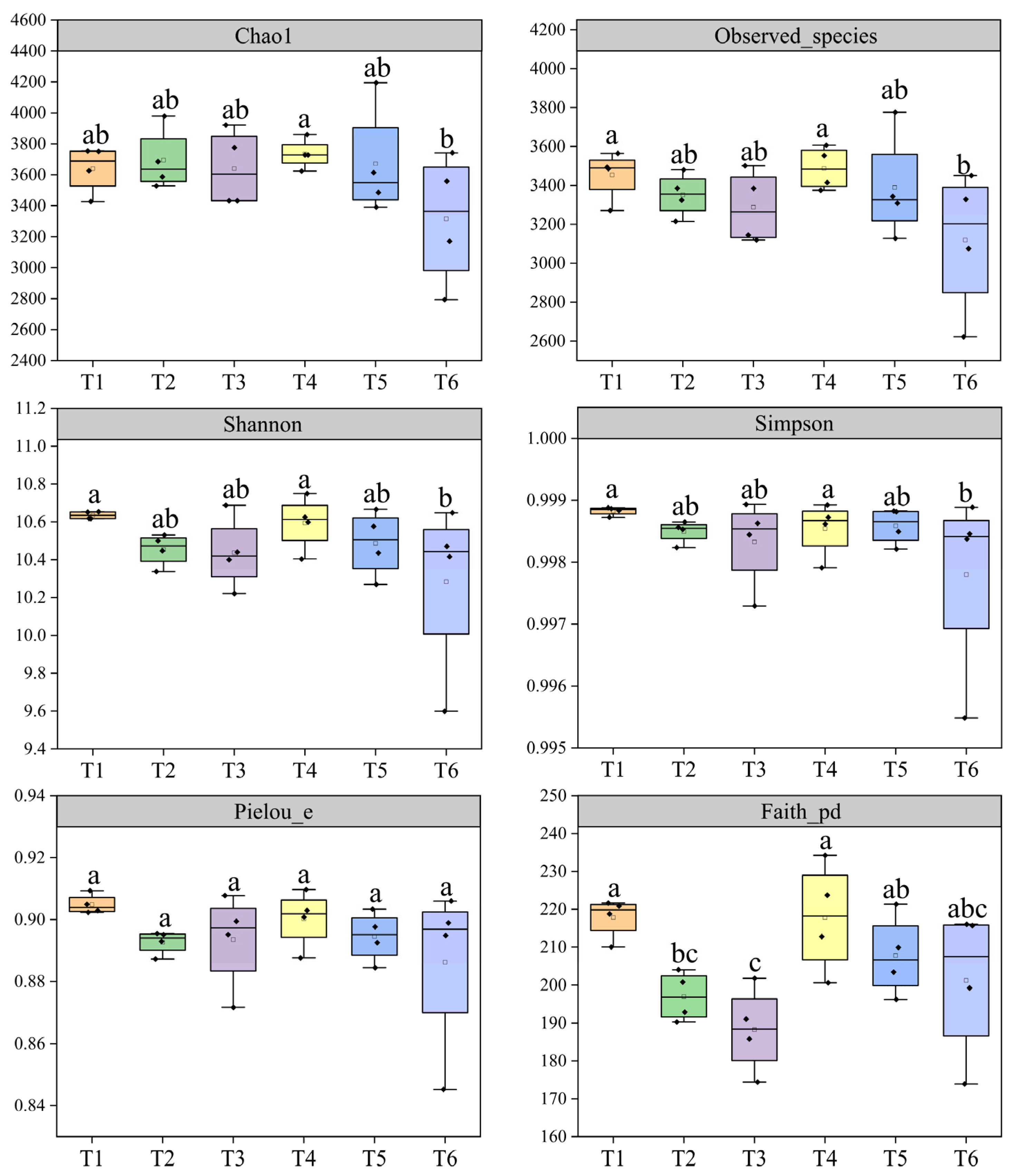
3.1. Rhizobacterial Diversity
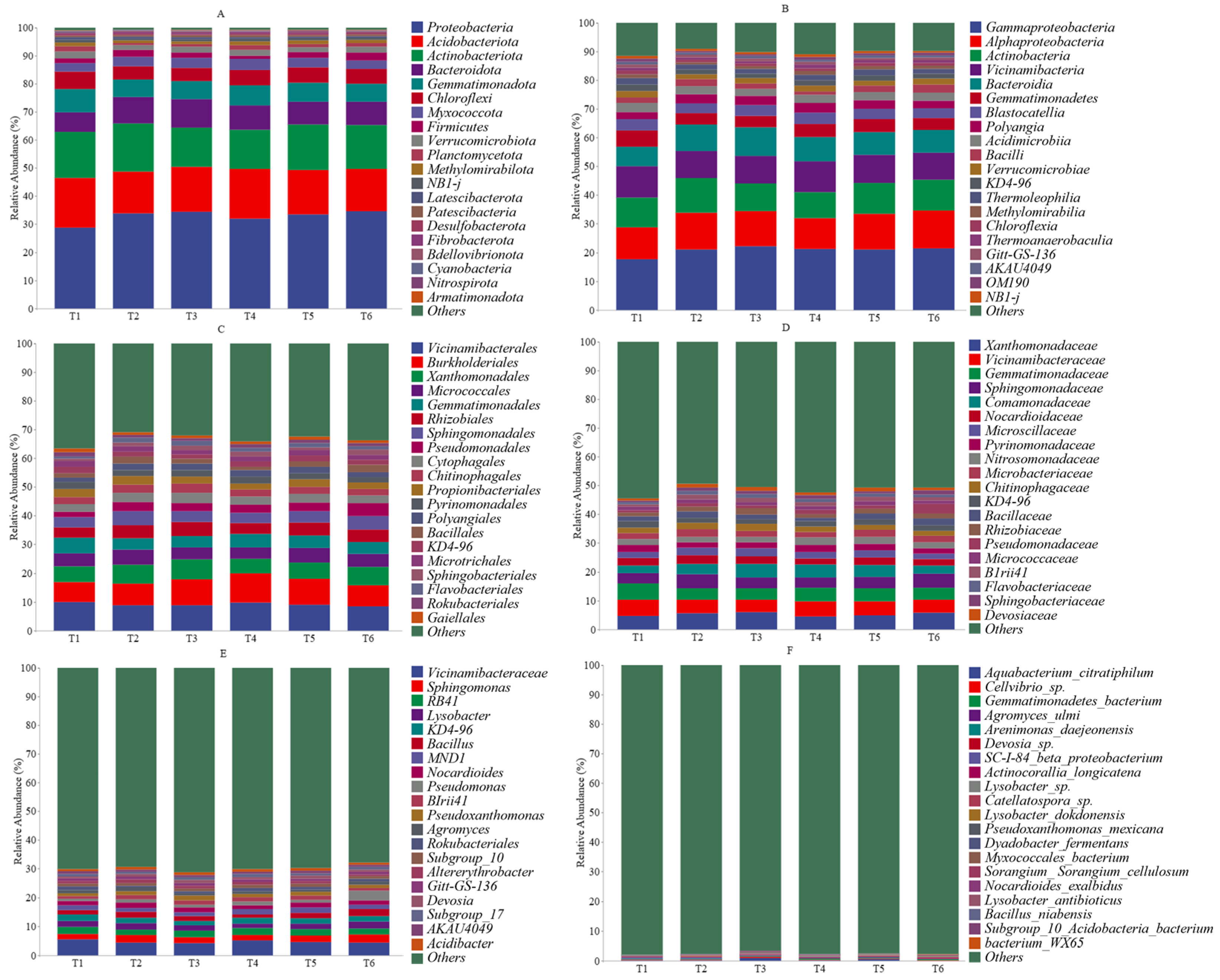
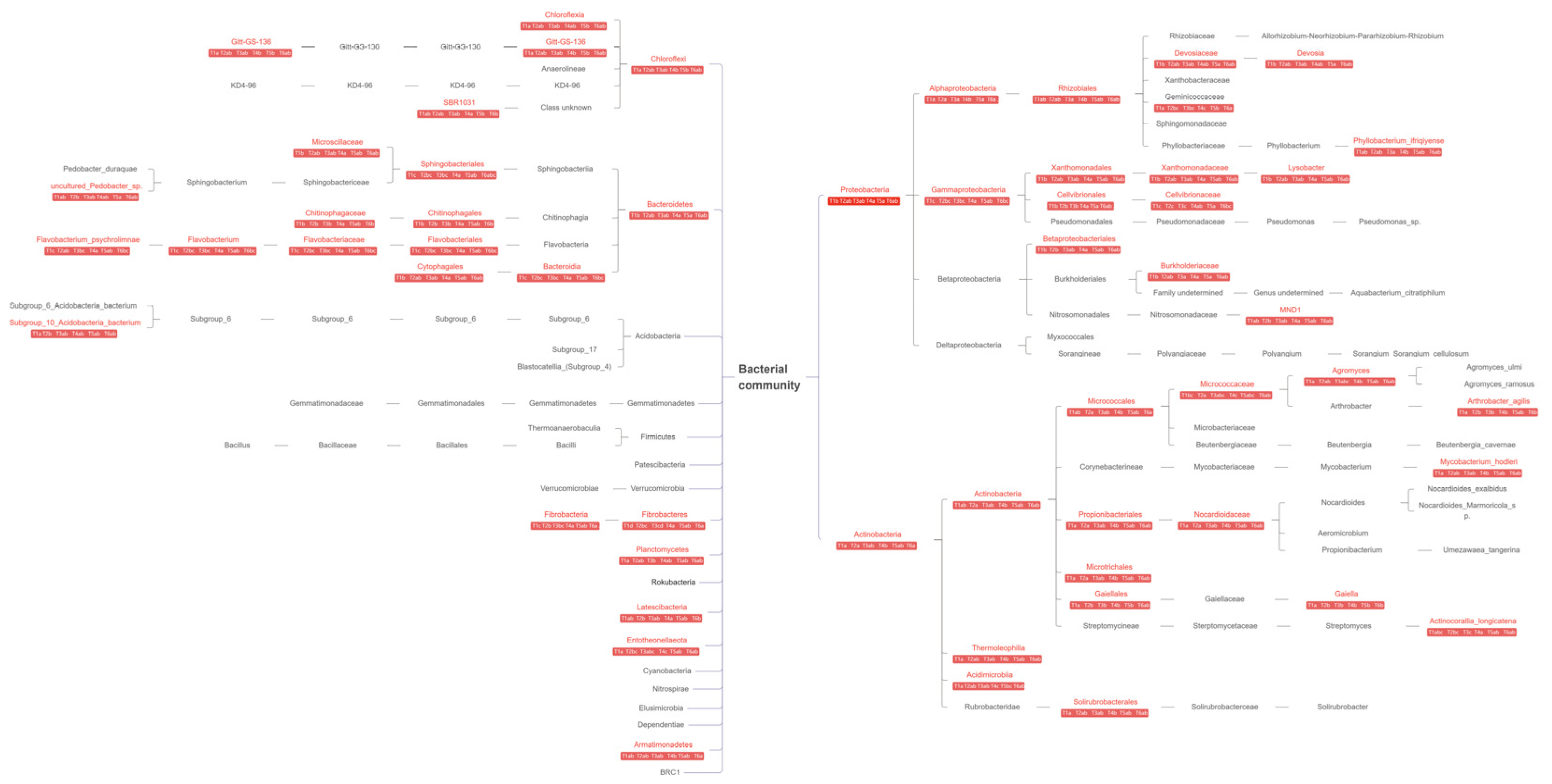
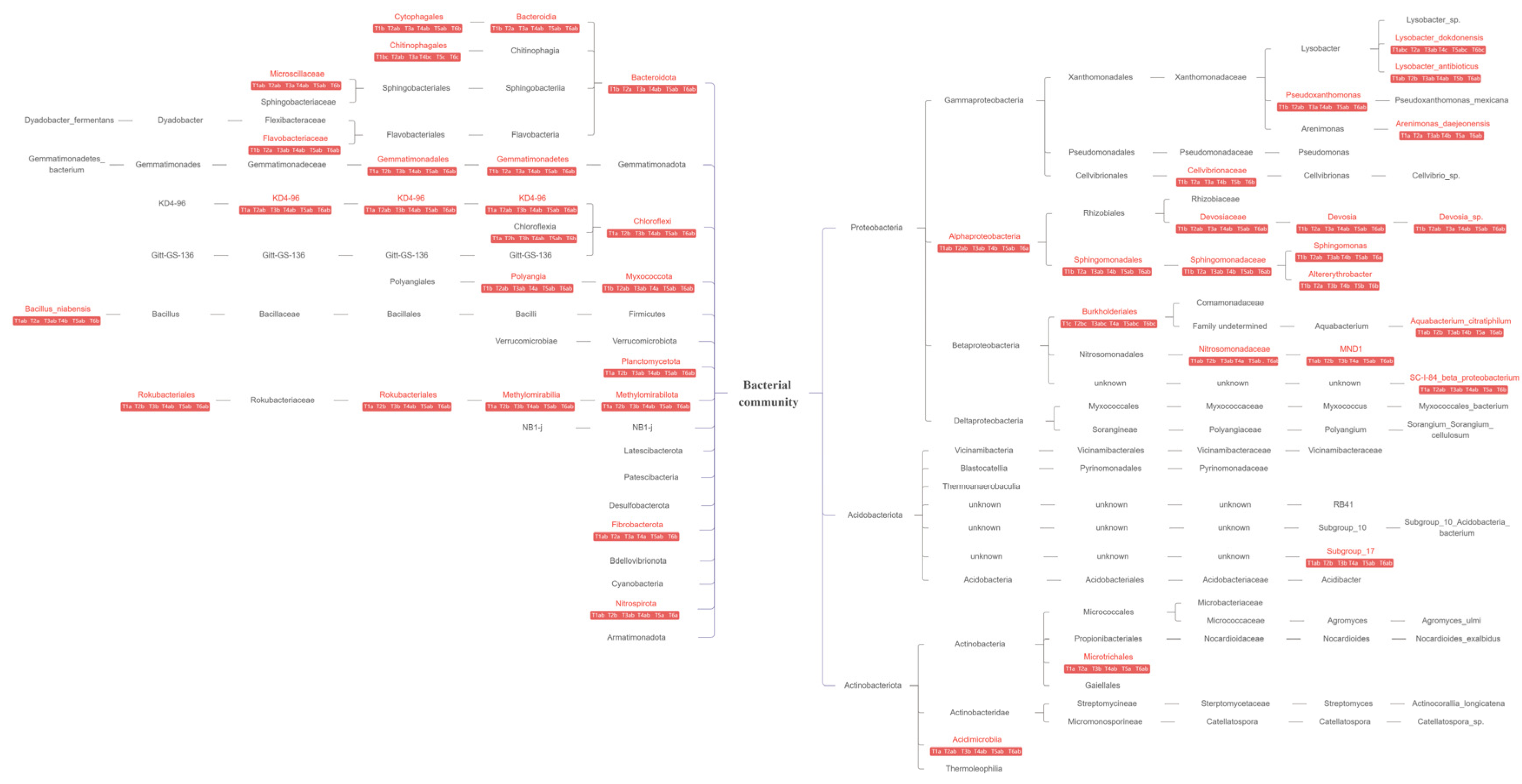
3.2. Dominant Rhizobacteria
3.3. Differences in Rhizobacterial Communities
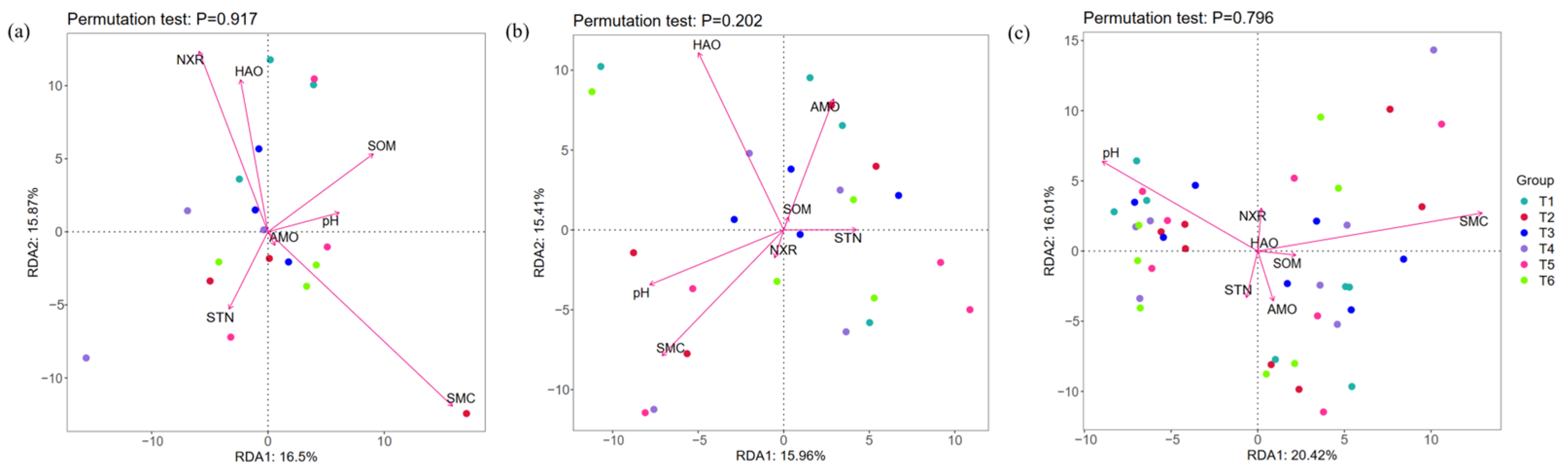
3.4. Relationship Between Rhizobacteria and Environment Factors
3.5. Yield Response
4. Discussion
4.1. Effect of Nitrogen Topdressing Regime on Rhizobacteria Diversity
4.2. Effect of Nitrogen Topdressing Regime on Rhizobacteria Community
4.3. Rhizobacteria Responses to Rhizosphere Soil Environment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| T1 | No nitrogen application |
| T2 | Single topdressing applications of 120 kg ha−1 at the jointing stage |
| T3 | Single topdressing applications of 80 kg ha−1 at the jointing stage |
| T4 | Split topdressing applications combining 80 kg ha−1 at jointing with 40 kg ha−1 at the booting stage |
| T5 | Split topdressing applications combining 80 kg ha−1 at jointing with 40 kg ha−1 at the flowering stage |
| T6 | Split topdressing applications combining 80 kg ha−1 at jointing with 40 kg ha−1 at 10th day post-anthesis |
| SMC | Soil moisture content |
| STN | Soil total nitrogen |
| SOM | Soil organic matter |
| AMO | Ammonia monooxygenase |
| HAO | Hydroxylamine oxidoreductase |
| NXR | Nitrite oxidoreductase |
References
- Lv, T.; Peng, S.; Liu, B.; Liu, Y.; Ding, Y. Planting suitability of China’s main grain crops under future climate change. Field Crops Res. 2023, 302, 109112. [Google Scholar] [CrossRef]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, S.; Xin, S.; Tong, B.; Wang, S.; Ma, W.; Wei, J. Effects of nitrogen application rate on yield, quality and soil nitrogen balance of winter wheat. J. Hebei Agric. Univ. 2019, 42, 9–15. [Google Scholar] [CrossRef]
- Wang, L.; Wu, W.; Li, R.; Hu, J.; Yan, S.; Shao, Q.; Xu, F.; Zhang, C.; Zhou, Y.; Li, W. Effects of nitrogen rate on grain quality and nitrogen utilization of weak gluten wheat. Acta Agric. Zhejiang 2021, 33, 777–784. Available online: http://www.zjnyxb.cn/CN/Y2021/V33/I5/777 (accessed on 20 December 2024).
- Jia, K.; Liu, J.L.; Shen, B. Yield effect change of fertilizers in the past 14 years and optimized fertilization of winter wheat in north of China. Plant Nutr. Fertil. Sci. 2020, 26, 2032–2042. Available online: https://www.plantnutrifert.org/article/doi/10.11674/zwyf.20381 (accessed on 20 December 2024).
- Wang, T.; Cheng, H. Research progress of nitrogen fertilizer application and its environmental effects in agricultural production. J. Heilongjiang Environ. 2020, 33, 58–59. [Google Scholar]
- Yang, M.; Wu, W.; Zhang, J.; Fu, B.; Peng, Z.; Xue, C. Effects of nitrogen topdressing timing on grain yield, quality and nitrogen utilization of strong gluten wheat. J. Hebei Agric. Univ. 2020, 43, 11–18. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Z.; Sheng, K.; Li, X.; Zhao, Z. Effect of nitrogen dressing application in different period on the yield and grain quality of strong gluten wheat. Chin. Agric. Sci. Bull. 2015, 31, 26–30. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, D.; Chang, H.; Wang, D.; Wang, Y.; Yang, Y.; Liu, X.; Wang, Y.; Shi, S.; Zhao, G. Response of nitrogen accumulation and translocation after anthesis in strong gluten wheat to nitrogen topdressing period and proportion. Crops 2023, 6, 114–120. [Google Scholar] [CrossRef]
- Pan, Y. Effects of Elevated Atmospheric CO2 Concentration and Split N application on Yield and Quality of Wheat. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2023. [Google Scholar]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecoll Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Chen, J.; Sharifi, R.; Khan, M.S.S.; Islam, F.; Bhat, J.A.; Kui, L.; Majeed, A. Wheat microbiome: Structure, dynamics, and role in improvingperformance under stress environments. Front. Microbiol. 2022, 12, 821546. [Google Scholar]
- Samain, E.; Van Tuinen, D.; Jeandet, P.; Aussenac, T.; Selim, S. Biological control of septoria leaf blotch and growth promotion in wheat by Paenibacillus sp. strain B2 and Curtobacterium plantarum strain EDS. Biol. Control 2017, 114, 87–96. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Jin, L.; Sun, L.; Wang, Q.; Zhang, L.; Chen, Y. Arbuscular mycorrhizal fungi in the rhizosphere soil of poisonous plants depressed the growth of pasture grasses in the Tibetan Plateau Alpine meadow. Ecosyst. Health Sustain. 2019, 5, 226–236. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, N.; Zhang, Z.; Zhang, Q.; Yang, Y.; Yu, Z.; Sun, L.; Lu, T.; Qian, H. Shaping effects of rice, wheat, maize, and soybean seedlings on their rhizosphere microbial community. Environ. Sci. Pollut. Res. 2023, 30, 35972–35984. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Jan, S.; Bhat, M.A.; Kamal, M.A.; Rahman, S.; Shah, A.A.; Jan, A.T. Plant growth promoting rhizobacteria in plant health: A perspective study of the underground interaction. Plants 2023, 12, 629. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.N.; Liu, B.B.; Dai, W.C.; Cai, H.M.; Zheng, B.Q.; Li, J.C. Effects of late spring coldness during the anther differentiation period on bacterial community structure in wheat rhizosphere. Chin. J. Agrometeorol. 2024, 45, 756. Available online: https://zgnyqx.ieda.org.cn/CN/Y2024/V45/I7/756 (accessed on 22 December 2024).
- Li, J.; Huang, S.; Wei, C.; Nong, Y.; Chen, Y.; Liao, C.; Qin, X. Effects of different nitrogen levels on tea rhizosphere microbial community and its role in adjusting soil nutrients. Acta Agric. Boreali-Sin. 2019, 34, 281–288. Available online: http://www.hbnxb.net/CN/Y2019/V34/IS1/281 (accessed on 22 December 2024).
- Qu, Q.; Zhang, Z.; Peijnenburg, W.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food. Chem. 2020, 68, 5024–5038. Available online: https://pubmed.ncbi.nlm.nih.gov/32255613/ (accessed on 22 December 2024).
- Xu, R.Y.; Zuo, M.X.; Yuan, Y.L.; Sun, J.; Gu, W.J.; Lu, Y.S.; Xie, K.Z.; Xu, P.Z. Effects of nitrogen fertilizer dosage optimization on nitrogen uptake content and utilization efficiency and microbial function genes of nitrogen cycle in sweet corn. J. Southern Agric. 2020, 51, 2919–2926. [Google Scholar] [CrossRef]
- Cui, P.; Wu, A.; Wang, J.; Dong, E.; Nan, J.; Bai, W.; Jiao, X. Effect of different fertilization treatments on soil microbial function diversity in rhizosphere of sorghum. Acta Agric. Boreali-Sin. 2018, 33, 195–202. Available online: http://www.hbnxb.net/CN/Y2018/V33/I5/195 (accessed on 22 December 2024).
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Boil. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Yang, Q. Effects of Nitrogen Fertilizer Application on Root Exudatesand Microbial Diversity of Tomato. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2022. [Google Scholar]
- Li, M.S. Changes of Microbial Population Structure in Corn Rhizosphere Underdifferent Nitrogen Application Levels. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2018. [Google Scholar]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O. Perspectives for sustainable agriculture from the microbiome in plant rhizosphere. Plant Biotechnol. Rep. 2021, 15, 259–278. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soils, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Liu, S.S. The Plastic Responses of Winter Wheat Rhizosphere Microbial Community to Nitrogen Fertilizer Management and Their Subsequent Effects on Yield and Quality. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2023. [Google Scholar]
- Wang, D.; Ren, H. Microbial community in buckwheat rhizosphere with different nitrogen application rates. PeerJ 2023, 11, e15514. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, Y.; Guan, C.; Guan, M. Effects of nitrogen application rate on rhizosphere microbial diversity in oilseed Rape (Brassica napus L.). Agronomy 2021, 11, 1539. [Google Scholar] [CrossRef]
- Liang, M.; Meng, W.; Chen, Z.; Shen, Y.; Liu, Y.; Shen, Y.; Liu, Z.; Nan, Z.; Xu, J.; Zhang, Z. Effects of nitrogen application levels on microbial community structure and diversity in peanut rhizosphere soil. Shandong Agric. Sci. 2023, 55, 78–83. [Google Scholar] [CrossRef]
- Wang, S.Z. The Research on the Effects of Different Nitrogen Fertilizer Amount and Model on Soil Microorganism in Winter Wheat Season. Master’s Thesis, Henan University, Zhengzhou, China, 2019. [Google Scholar]
- Constancias, F.; Prévost-Bouré, N.C.; Terrat, S.; Aussems, S.; Nowak, V.; Guillemin, J.P.; Bonnotte, A.; Biju-Duval, L.; Navel, A.; Martins, J.M.; et al. Microscale evidence for a high decrease of soil bacterial density and diversity by cropping. Agron. Sustain. Dev. 2014, 34, 831–840. [Google Scholar] [CrossRef]
- Li, X.; Jiao, Y.; Dai, G.; Yang, M.; Wen, H. Soil bacterial community diversity under different degrees of saline-alkaline in the Hetao Area of Inner Mongolia. China Environ. Sci. 2016, 36, 249–260. [Google Scholar] [CrossRef]
- Liu, W.; Ling, N.; Guo, J.; Ruan, Y.; Zhu, C.; Shen, Q.; Guo, S. Legacy effects of 8-year nitrogen inputs on bacterial assemblage in wheat rhizosphere. Biol. Fert. Soil. 2020, 56, 583–596. [Google Scholar] [CrossRef]
- Bapiri, A.; Bååth, E.; Rousk, J. Drying–rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb. Ecol 2010, 60, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, G. Effects of irrigation and nitrogen treatments on nitrogen utilization and soil microbial community in winter wheat. Ph.D. Thesis, Henan Agricultural University, Zhengzhou, China, 2019. [Google Scholar]
- Fu, B.y.; Zhang, J.h.; Yang, M.x.; Xu, H.s.; Peng, Z.p.; Xue, C. Effects of nitrogen topdressing timing on microbial community sreucture in strong gluten wheat rhizosphere soil. J. Hebei Agric. Univ. 2022, 45, 1–8. [Google Scholar] [CrossRef]
- Li, X.M.; Long, J.J.; Zhou, Y.; Wang, Y.; Wang, H.Q. Application and research progress offoliarfertilizer. J. Anhui Agric. Sci. 2017, 45, 127–130. [Google Scholar] [CrossRef]
- Xian, W.; Zhang, X.; Li, W. Research status and prospect on bacterial phylum Chloroflexi. Acta Microbiol. Sin. 2020, 60, 1801–1820. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Cai, W.; Li, G.; Mei, Y.; Yang, S. Different amounts of nitrogen fertilizer applications alter the bacterial diversity and community structure in the rhizosphere soil of sugarcane. Front. Microbiol. 2021, 12, 721441. [Google Scholar] [CrossRef]
- Jiang, W.; Xue, G.; Bai, H.; Du, J.; Zhu, C.; Li, J.; Song, Q.; Ji, S.; Wang, L. Effects of different treatments on the bacterial community composition and diversity in cucumber continuous cropping soil. J. North. Agric. 2022, 50, 28–37. [Google Scholar]
- Yu, M.; Su, W.Q.; Huang, L.; Parikh, S.J.; Tang, C.; Dahlgren, R.A.; Xu, J. Bacterial community structure and putative nitrogen-cycling functional traits along a charosphere gradient under waterlogged conditions. Soil Boil. Biochem. 2021, 162, 108420. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Tan, W.; Di, H.; Xu, J.; Li, Y. High manure load reduces bacterial diversity and network complexity in a paddy soil under crop rotations. Soil Ecol. Lett. 2020, 2, 104–119. [Google Scholar] [CrossRef]
- Zhang, J.H. Effects of Late Foliar N Application on Yield, Quality and N Uptake and Utilization of Wheat. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2022. [Google Scholar]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome. 2019, 7, 136. [Google Scholar] [CrossRef]
- Schlemper, T.R.; Leite, M.F.A.; Lucheta, A.R.; Shimels, M.; Bouwmeester, H.J.; van Veen, J.A.; Kuramae, E.E. Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol. Ecol. 2017, 93, 1–11. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, A.; Waheed, A.; Hakeem, K.R.; Mohamed, H.I.; Basit, A.; Toor, M.D.; Liu, Y.H.; Li, L.; Li, W.J. Navigating climate change: Exploring the dynamics between plant–soil microbiomes and their impact on plant growth and productivity. Glob. Change Biol. 2025, 31, e70057. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Yuan, Y.; Zuo, J.; Sun, L.; Tao, J. Microbiota associated with the rhizosphere of Paeonia lactiflora Pall.(ornamental cultivar). Appl. Soil Ecol. 2022, 169, 104214. [Google Scholar] [CrossRef]
- Bogino, P.; Abod, A.; Nievas, F.; Giordano, W. Water-limiting conditions alter the structure and biofilm-forming ability of bacterial multispecies communities in the alfalfa rhizosphere. PLoS ONE 2013, 8, e79614. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Xu, C.S.; He, H.H.; Zeng, Q.; Chen, N.; Li, X.L.; Ren, T.-B.; Ji, X.M.; Liu, G.S. Effect of biochar on soil enzyme activity & the bacterial community and its mechanism. Environ. Sci. 2021, 42, 422–432. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Wang, S.; Yu, L.; Armanbek, G.; Yu, J.; Jiang, L.; Yuan, D.; Guo, Z.; Zhang, H.; et al. Towards a more labor-saving way in microbial ammonium oxidation: A review on complete ammonia oxidization (comammox). Sci. Total Environ. 2022, 829, 154590. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; van Kessel, M.A.; Lücker, S. Complete nitrification: Insights into the ecophysiology of comammox Nitrospira. Appl. Microbiol. Biotechnol. 2019, 103, 177–189. [Google Scholar] [CrossRef]
- Xu, J.Y.; Mao, Y.P. From canonical nitrite oxidizing bacteria to complete ammonia oxidizer: Discovery and advances. Microbiol. China 2019, 46, 879–890. [Google Scholar] [CrossRef]
- Guo, N.; Li, R.; Bai, W.; Ma, J.; Li, A.; Qiao, H.; Liu, D.; Guo, Y.; Li, F. Responses of flax growth and rhizosphere bacterial communities to different dosage of organic fertilizer. Acta Agric. Boreali-Sin. 2025, 40, 146–156. [Google Scholar] [CrossRef]
- Ren, H.F.; Wang, L.; Hao, X.Y.; Zhang, D.S.; Zong, Z.Y.; Li, P. Response of soil bacteria and fungi communities in wheat fields to climate change under slow release fertilizer treatment. Soils Fertil. Sci. China 2022, 10, 50–63. [Google Scholar] [CrossRef]
- Li, R.; Gao, Y.; Chen, Q.; Li, Z.; Gao, F.; Meng, Q.; Li, T.; Liu, A.; Wang, Q.; Wu, L.; et al. Blended controlled-release nitrogen fertilizer with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat. Soil Till. Res. 2021, 212, 105045. [Google Scholar] [CrossRef]
- He, J.X. Community Structure of Ammonia-Oxidizing Bacteria During Long-Term Fertilizer Application in the Loess Plateau. Master’s Thesis, Lanzhou University, Lanzhou, China, 2011. [Google Scholar]
- Han, S.; Zeng, L.; Luo, X.; Xiong, X.; Wen, S.; Wang, B.; Chen, W.; Huang, Q. Shifts in Nitrobacter-and Nitrospira-like nitrite-oxidizing bacterial communities under long-term fertilization practices. Soil Boil. Biochem. 2018, 124, 118–125. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Caruso, T. Soil microbial community responses to climate extremes: Resistance, resilience and transitions to alternative states. Philos. Trans. R. Soc. B 2020, 375, 20190112. [Google Scholar] [CrossRef]
- Ku, Y.S.; Liao, Y.J.; Chiou, S.P.; Lam, H.M.; Chan, C. From trade-off to synergy: Microbial insights into enhancing plant growth and immunity. Plant Biotechnol. J. 2024, 22, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.; Martiny, A.C.; Brodie, E.; Chase, A.B.; Rodríguez-Verdugo, A.; Treseder, K.K.; Allison, S.D. Investigating the eco-evolutionary response of microbiomes to environmental change. Ecol. Lett. 2023, 26, S81–S90. [Google Scholar] [CrossRef]


| Treatment | Basal | Jointing Stage | Booting Stage | Flowering Stage | 10th Day Post-Anthesis |
|---|---|---|---|---|---|
| T1 | 0 | 0 | |||
| T2 | 90 | 120 | |||
| T3 | 90 | 80 | |||
| T4 | 90 | 80 | 40 | ||
| T5 | 90 | 80 | 40 | ||
| T6 | 90 | 80 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Wang, Y.; Liu, S.; Wu, W.; Wang, W.; Liu, M.; Xiao, H.; Dong, J.; Ren, H.; Xu, H.; et al. Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat. Agriculture 2025, 15, 794. https://doi.org/10.3390/agriculture15070794
An Y, Wang Y, Liu S, Wu W, Wang W, Liu M, Xiao H, Dong J, Ren H, Xu H, et al. Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat. Agriculture. 2025; 15(7):794. https://doi.org/10.3390/agriculture15070794
Chicago/Turabian StyleAn, Yu, Yang Wang, Shuangshuang Liu, Wei Wu, Weiming Wang, Mengmeng Liu, Hui Xiao, Jing Dong, Hongjie Ren, Huasen Xu, and et al. 2025. "Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat" Agriculture 15, no. 7: 794. https://doi.org/10.3390/agriculture15070794
APA StyleAn, Y., Wang, Y., Liu, S., Wu, W., Wang, W., Liu, M., Xiao, H., Dong, J., Ren, H., Xu, H., & Xue, C. (2025). Impact of Split Nitrogen Topdressing on Rhizobacteria Community of Winter Wheat. Agriculture, 15(7), 794. https://doi.org/10.3390/agriculture15070794






