The Effect of New Zeolite Composites from Fly Ashes Mixed with Leonardite and Lignite in Enhancing Soil Organic Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Soil Sampling Site
2.2. Materials Used in the Experiment
2.3. Description of the Pot Experiment Design
2.4. Description of the Experimental Area
2.5. Soil Sampling
2.6. Laboratory Analysis
2.6.1. Basal Soil Analysis
2.6.2. Carbon Fractions Determination
2.6.3. ATR-FTIR Spectroscopy Method
2.6.4. Water-Soluble Phenolic Compounds (WPC)
2.6.5. Statistical Analysis
3. Results and Discussion
3.1. The pH and Electrical Conductivity (EC) in Soil
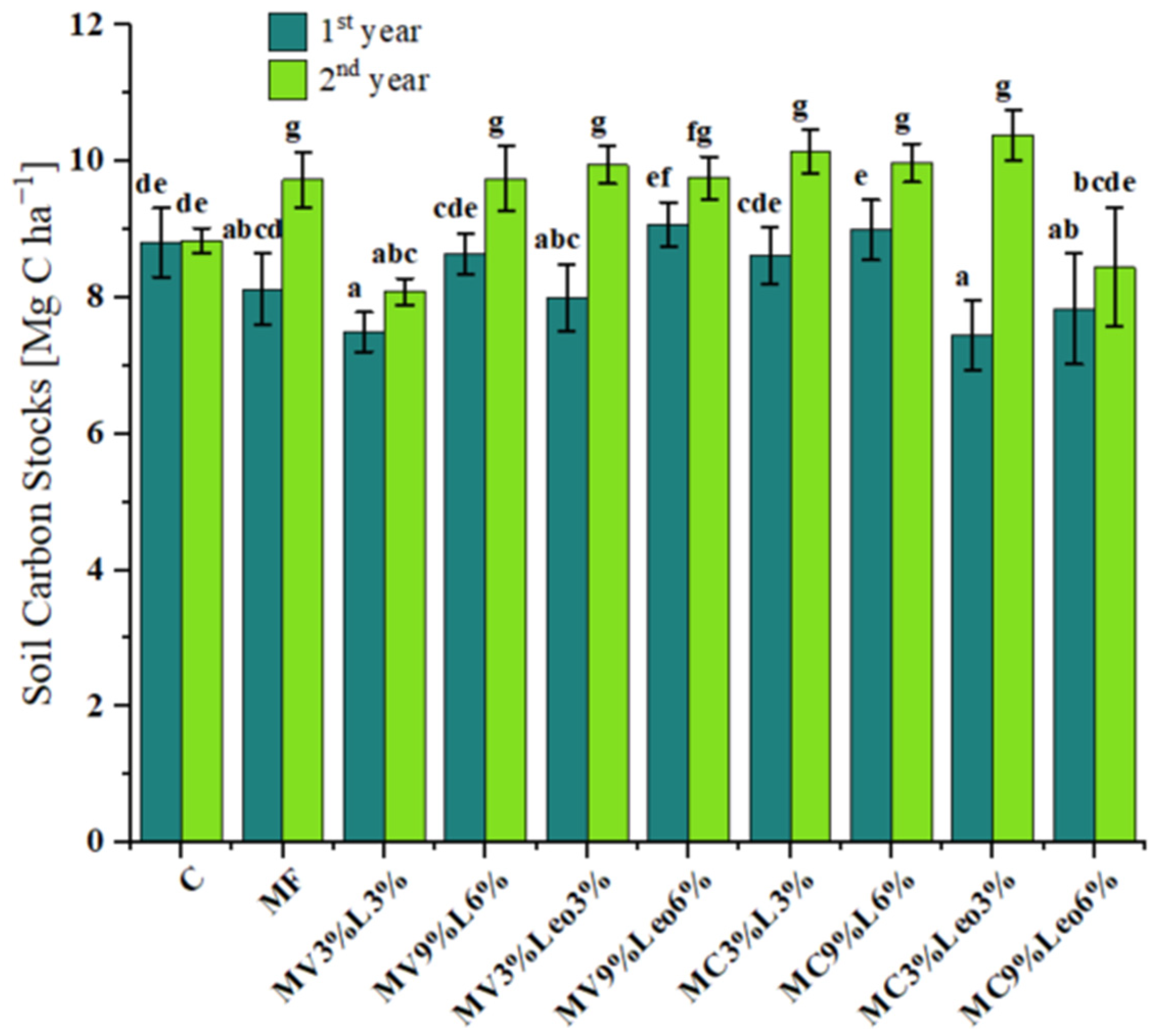
3.2. Total Carbon and Total Nitrogen Contents in Soil
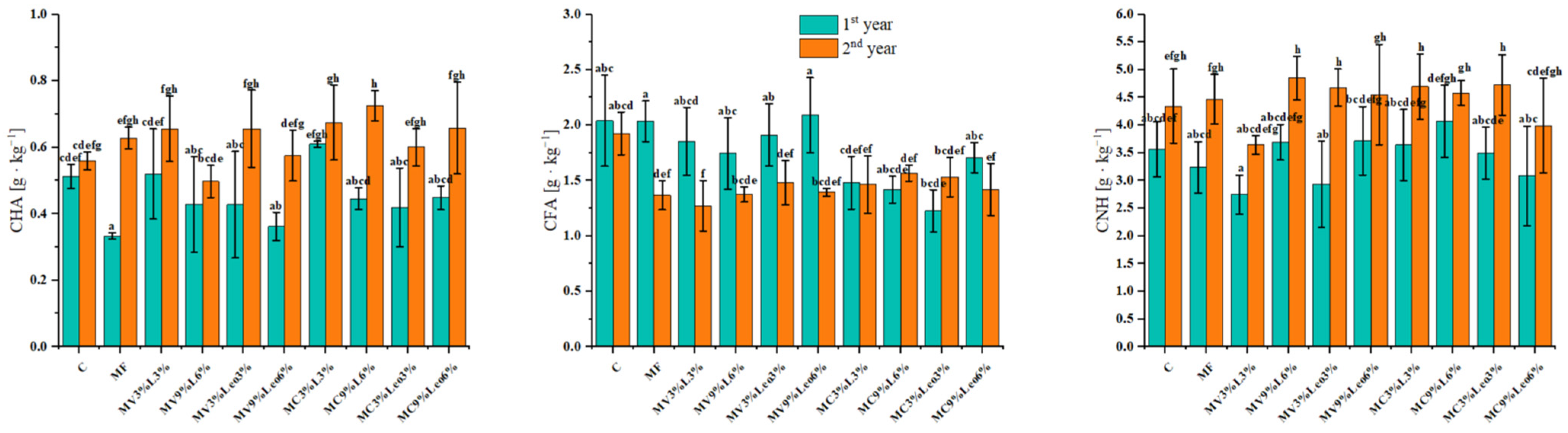
3.3. Content of Carbon Fractions in Soil
3.4. Humic Acid Carbon: Fulvic Acid Carbon Ratio
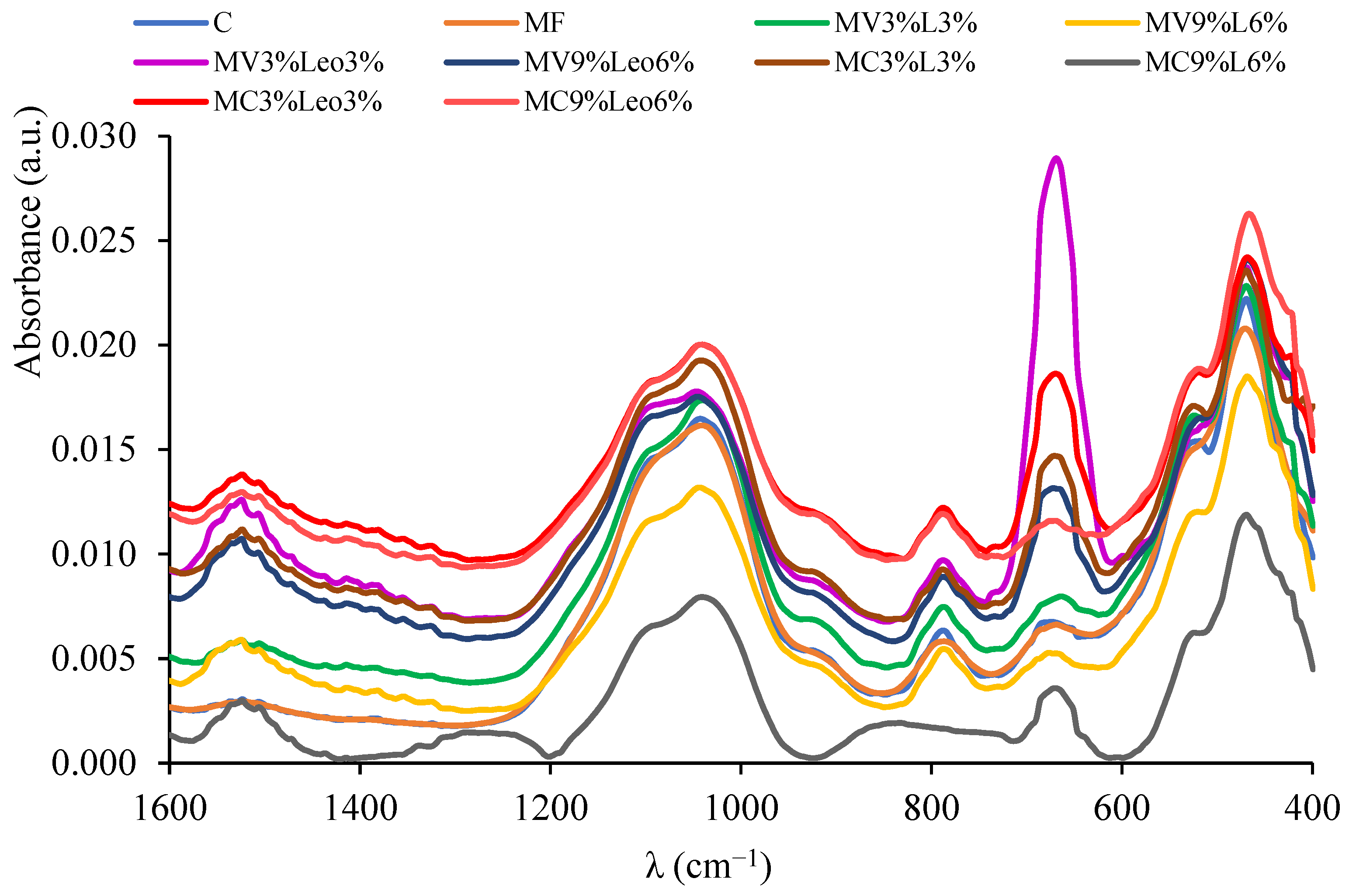
3.5. Analysis of ATR-FTIR Spectra of Soil Samples
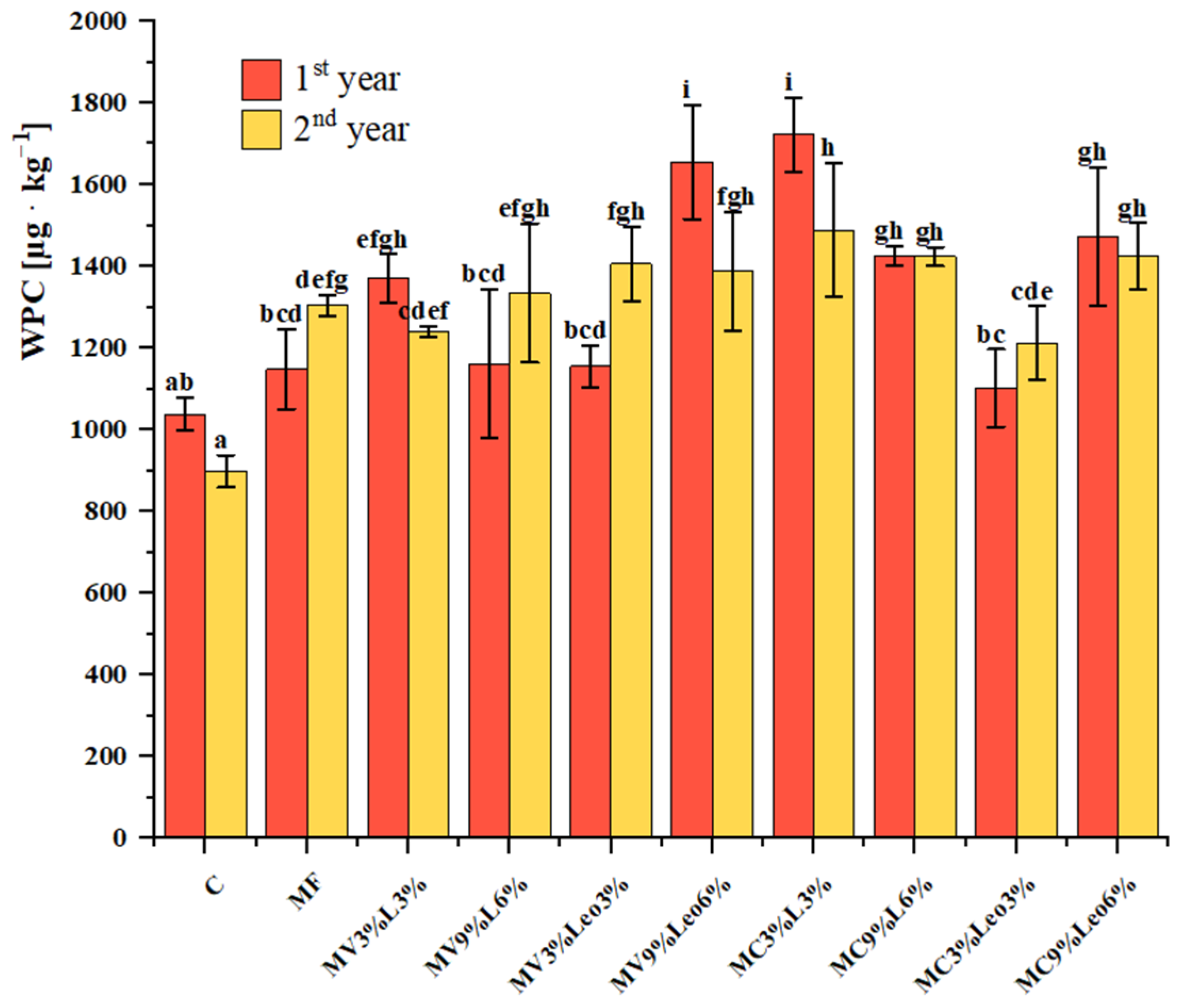
3.6. Water-Soluble Phenolic Compounds (WPC) in Soil
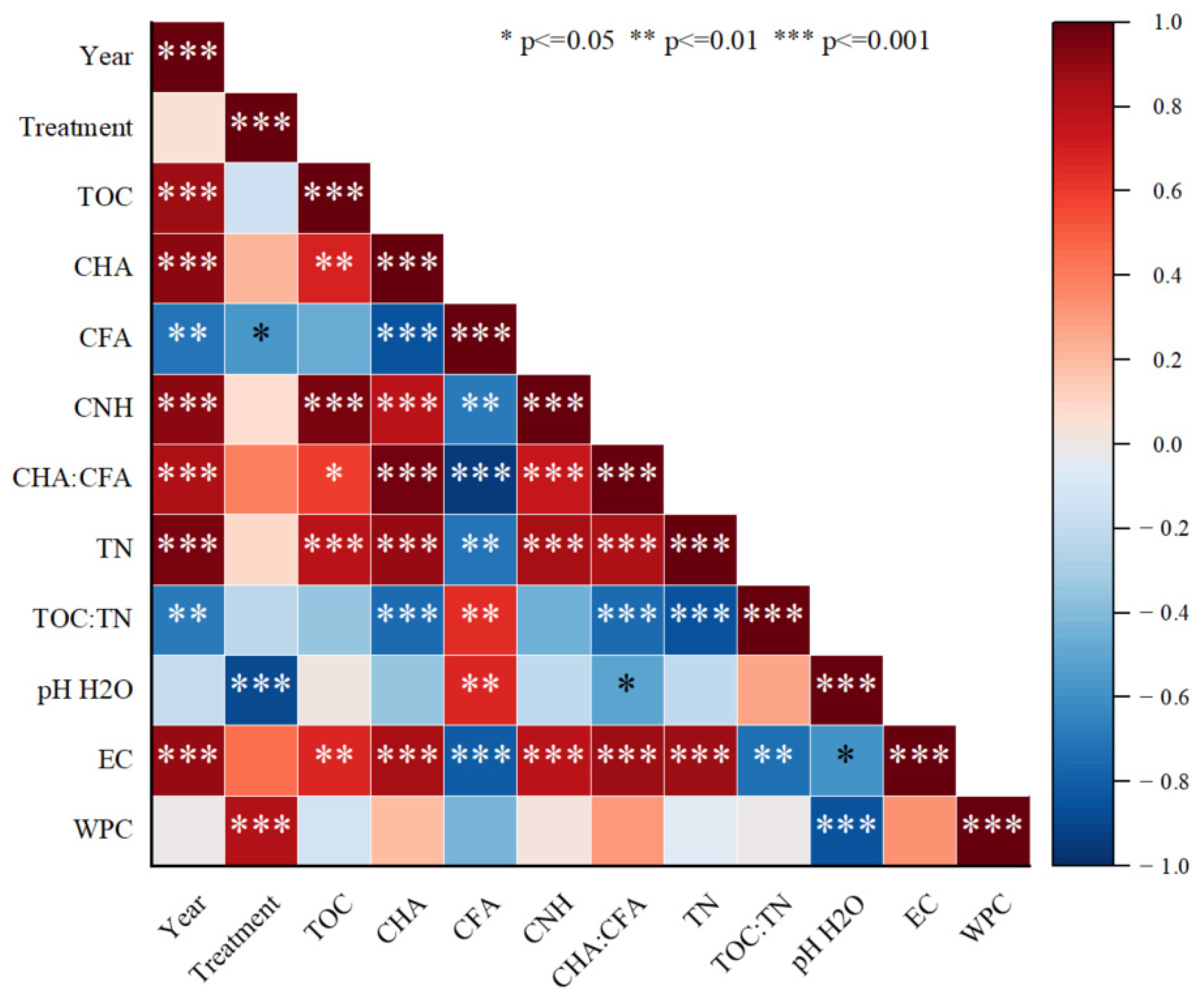
3.7. Pearson’s Correlation and Redundancy Analysis Among the Studied Parameters
4. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pandao, M.R.; Thakare, A.A.; Choudhari, R.J.; Navghare, N.R.; Sirsat, D.D.; Rathod, S.R. Soil Health and Nutrient Management. Int. J. Plant Soil Sci. 2024, 36, 873–883. [Google Scholar] [CrossRef]
- Bagnall, D.K.; Rieke, E.L.; Morgan, C.L.S.; Liptzin, D.L.; Cappellazzi, S.B.; Honeycutt, C.W. A Minimum Suite of Soil Health Indicators for North American Agriculture. Soil Secur. 2023, 10, 100084. [Google Scholar] [CrossRef]
- Maharjan, B.; Das, S.; Thapa, V.R.; Sharma Acharya, B. Soil Health Cycle. Agrosystems Geosci. Environ. 2024, 7, e20504. [Google Scholar] [CrossRef]
- Arriaga, F.J.; Guzman, J.; Lowery, B. Conventional Agricultural Production Systems and Soil Functions; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128054017. [Google Scholar]
- Wollenberg, E.; Vermeulen, S.J.; Girvetz, E.; Loboguerrero, A.M.; Ramirez-Villegas, J. Reducing Risks to Food Security from Climate Change. Glob. Food Secur. 2016, 11, 34–43. [Google Scholar] [CrossRef]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding Theworld. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, F.; Jia, X.; Liu, P.; Dong, S.; Zhang, J.; Zhao, B. The Combined Application of Organic and Inorganic Fertilizers Increases Soil Organic Matter and Improves Soil Microenvironment in Wheat-Maize Field. J. Soils Sediments 2020, 20, 2395–2404. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Jarosz, R.; Juda, M.; Mierzwa-Hersztek, M. Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil. Sustainability 2022, 14, 15004. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites Enhance Soil Health, Crop Productivity and Environmental Safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Zeolites and Their Potential Uses in Agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 113, pp. 219–241. ISBN 9780123864734. [Google Scholar]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The Use of Zeolites as an Addition to Fertilisers—A Review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, R.; Liu, Q.; Cao, Q.; Guo, R. Synthesis of Zeolite A from Fly Ash and Its Application in the Slow Release of Urea. Waste Manag. 2023, 158, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, Z.; Yu, L.; Wang, W. Fly Ash-Based Zeolites: From Waste to Value—A Comprehensive Overview of Synthesis, Properties, and Applications. Chem. Eng. Res. Des. 2024, 212, 240–260. [Google Scholar] [CrossRef]
- Kim Thi Tran, C.; Rose, M.T.; Cavagnaro, T.R.; Patti, A.F. Lignite Amendment Has Limited Impacts on Soil Microbial Communities and Mineral Nitrogen Availability. Appl. Soil Ecol. 2015, 95, 140–150. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Tastambek, K.T.; Sherelkhan, D.K.; Jussupova, D.B.; Altynbay, N.P. Low-Rank Coal as a Source of Humic Substances for Soil Amendment and Fertility Management. Agriculture 2021, 11, 1261. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Marcińska-Mazur, L.; Jarosz, R.; Mierzwa–Hersztek, M. Effect of Organic/Inorganic Composites as Soil Amendments on the Biomass Productivity and Root Architecture of Spring Wheat and Rapeseed. J. Environ. Manag. 2023, 344, 118628. [Google Scholar] [CrossRef]
- Głąb, T.; Jarosz, R.; Gondek, K.; Mierzwa-Hersztek, M. Maize Root Architecture and Biomass Productivity after Application of Organic and Inorganic Additives in Contaminated Soil. Ecol. Chem. Eng. 2024, 31, 75–87. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Zeolite Coated Urea Fertilizer Using Different Binders: Fabrication, Material Properties and Nitrogen Release Studies. Environ. Technol. Innov. 2019, 16, 100452. [Google Scholar] [CrossRef]
- Nan, J.; Chen, X.; Chen, C.; Lashari, M.S.; Deng, J.; Du, Z. Impact of Flue Gas Desulfurization Gypsum and Lignite Humic Acid Application on Soil Organic Matter and Physical Properties of a Saline-Sodic Farmland Soil in Eastern China. J. Soils Sediments 2016, 16, 2175–2185. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Marcińska-Mazur, L.; Jarosz, R.; Juda, M.; Lošák, T.; Mierzwa-Hersztek, M. Effect of Soil Application of Zeolite-Carbon Composite, Leonardite and Lignite on the Microorganisms. Ecol. Chem. Eng. 2022, 29, 553–563. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Jarosz, R.; Marcińska-Mazur, L.; Gondek, K.; Lahori, A.H.; Szara-Bąk, M.; Lošák, T.; Szerement, J.; Mokrzycki, J.; Karcz, R.; et al. The Impact of Mineral and Organic Supplements on the Abundance of Selected Groups of Culturable Microorganisms in Soil Contaminated with Heavy Metals. J. Elem. 2023, 28, 595–617. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S. Effects of the Lignite Bioorganic Fertilizer on Greenhouse Gas Emissions and Pathways of Nitrogen and Carbon Cycling in Saline-Sodic Farmlands at Northwest China. J. Clean. Prod. 2022, 334, 130080. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The Effect of Leonardite-Derived Amendments on Soil Microbiome Structure and Potato Yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Ratanaprommanee, C.; Chinachanta, K.; Chaiwan, F.; Shutsrirung, A. Chemical Characterization of Leonardite and Its Potential Use as Soil Conditioner and Plant Growth Enhancement. Asia-Pac. J. Sci. Technol. 2017, 22, 1–10. [Google Scholar]
- IUSS Working Group WRB IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022; Volume 13, ISBN 9798986245119. [Google Scholar]
- Mokrzycki, J.; Franus, W.; Panek, R.; Sobczyk, M.; Rusiniak, P.; Szerement, J.; Jarosz, R.; Marcińska-Mazur, L.; Bajda, T.; Mierzwa-Hersztek, M. Zeolite Composite Materials from Fly Ash: An Assessment of Physicochemical and Adsorption Properties. Materials 2023, 16, 2142. [Google Scholar] [CrossRef]
- Szerement, J.; Jurek, K.; Mokrzycki, J.; Jarosz, R.; Oleszczuk, P.; Mierzwa-Hersztek, M. Zeolite Composites from Fly Ashes Mixed with Leonardite as a Useful Addition to Fertilizer for Accelerating the PAHs Degradation in Soil. Soil Tillage Res. 2023, 230, 105701. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, Z.; Zheng, L.C.; Yi, X.Y. Remediation of Soil Co-Contaminated with Pyrene and Cadmium by Growing Maize (Zea Mays L.). Int. J. Environ. Sci. Technol. 2009, 6, 249–258. [Google Scholar] [CrossRef]
- Salam, A.K.; Rizki, D.O.; Santa, I.T.D.; Supriatin, S.; Septiana, L.M.; Sarno, S.; Niswati, A. The Biochar-Improved Growth-Characteristics of Corn ( Zea Mays L.) in a 22-Years Old Heavy-Metal Contaminated Tropical Soil. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1034. [Google Scholar] [CrossRef]
- Kalembasa, S. Quick Method of Determination of Organic Carbon in Soil. Pol. J. Soil Sci. 1991, 24, 17–22. [Google Scholar]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Garcia-Pausas, J.; Rabissi, A.; Rovira, P.; Romanyà, J. Organic Fertilisation Increases C and N Stocks and Reduces Soil Organic Matter Stability in Mediterranean Vegetable Gardens. Land Degrad. Dev. 2017, 28, 691–698. [Google Scholar] [CrossRef]
- Jarosz, R.; Mierzwa-Hersztek, M.; Gondek, K.; Kopeć, M.; Lošák, T.; Marcińska-Mazur, L. Changes in Quantity and Quality of Organic Matter in Soil after Application of Poultry Litter and Poultry Litter Biochar—5-Year Field Experiment. Biomass Convers. Biorefinery 2022, 12, 2925–2934. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Conte, P.; Haberhauer, G.; Gerzabek, M.H. Increased Soil Organic Carbon Sequestration through Hydrophobic Protection by Humic Substances. Soil Biol. Biochem. 2002, 34, 1839–1851. [Google Scholar] [CrossRef]
- Aziz, Y.; Shah, G.A.; Rashid, M.I. ZnO Nanoparticles and Zeolite Influence Soil Nutrient Availability but Do Not Affect Herbage Nitrogen Uptake from Biogas Slurry. Chemosphere 2019, 216, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Akhtar, K.; Khan, A.; Nizamani, G.S. Agricultural Practices Can Reduce Soil Greenhouse Gas Emissions: Challenges and Future Perspectives. In Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 263–274. ISBN 9781000462135. [Google Scholar]
- Brust, G.E. Management Strategies for Organic Vegetable Fertility; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128120606. [Google Scholar]
- Paul, E.A. The Nature and Dynamics of Soil Organic Matter: Plant Inputs, Microbial Transformations, and Organic Matter Stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A.; Clark, S.B. Humic and Fulvic Acids and Organic Colloidal Materials in the Environment. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 651. [Google Scholar] [CrossRef]
- Truc, M.T.; Yoshida, M. Effect of Zeolite on the Decomposition Resistance of of Organic Matter in Tropical Soils under Global Warming. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 2011, 5, 664–668. [Google Scholar]
- Doni, S.; Gispert, M.; Peruzzi, E.; Macci, C.; Mattii, G.B.; Manzi, D.; Masini, C.M.; Grazia, M. Impact of Natural Zeolite on Chemical and Biochemical Properties of Vineyard Soils. Soil Use Manag. 2021, 37, 832–842. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.; Zhang, Z.; Hu, C.; He, Y.; Huang, K.; Pang, Q.; Hu, G. Response of Soil Organic Carbon and Its Fractions to Natural Vegetation Restoration in a Tropical Karst Area, Southwest China. Front. For. Glob. Change 2023, 6, 1172062. [Google Scholar] [CrossRef]
- Murindangabo, Y.T.; Kopecký, M.; Konvalina, P.; Ghorbani, M.; Perná, K.; Nguyen, T.G.; Bernas, J.; Baloch, S.B.; Hoang, T.N.; Eze, F.O.; et al. Quantitative Approaches in Assessing Soil Organic Matter Dynamics for Sustainable Management. Agronomy 2023, 13, 1776. [Google Scholar] [CrossRef]
- The World Bank Enhancing Carbon Stocks and Reducing CO2 Emissions in Agriculture and Natural Resource Management Projects; World Bank: Washington, DC, USA, 2012.
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar]
- Zhang, W.; Xu, M.; Wang, X.; Huang, Q.; Nie, J.; Li, Z.; Li, S.; Hwang, S.W.; Lee, K.B. Effects of Organic Amendments on Soil Carbon Sequestration in Paddy Fields of Subtropical China. J. Soils Sediments 2012, 12, 457–470. [Google Scholar] [CrossRef]
- Singh, B.P.; Setia, R.; Wiesmeier, M.; Kunhikrishnan, A. Agricultural Management Practices and Soil Organic Carbon Storage; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128127667. [Google Scholar]
- Rutkowska, A.; Piku, D. Effect of Crop Rotation and Nitrogen Fertilization on the Quality and Quantity of Soil Organic Matter. Soil Process. Curr. Trends Qual. Assess. 2013, 249, 267. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Rosenqvist, J.; Jonsson, C.M.; Wold, S.; Blomberg, E.; Wallinder, I.O. Influence of Humic Acid and Dihydroxy Benzoic Acid on the Agglomeration, Adsorption, Sedimentation and Dissolution of Copper, Manganese, Aluminum and Silica Nanoparticles-A Tentative Exposure Scenario. PLoS ONE 2018, 13, e0192553. [Google Scholar] [CrossRef] [PubMed]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Organic Matter and Mineral Composition of Silicate Soils: FTIR Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection Modalities. Agronomy 2021, 11, 1879. [Google Scholar] [CrossRef]
- Volkov, D.S.; Krivoshein, P.K.; Proskurnin, M.A. Detonation Nanodiamonds: A Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection Ftir Spectroscopies. Nanomaterials 2020, 10, 2501. [Google Scholar] [CrossRef]
- Dudek, M.; Kabała, C.; Łabaz, B.; Mituła, P.; Bednik, M.; Medyńska-Juraszek, A. Mid-Infrared Spectroscopy Supports Identification of the Origin of Organic Matter in Soils. Land 2021, 10, 215. [Google Scholar] [CrossRef]
- Mylotte, R.; Verheyen, V.; Reynolds, A.; Dalton, C.; Patti, A.F.; Chang, R.R.; Burdon, J.; Hayes, M.H.B. Isolation and Characterisation of Recalcitrant Organic Components from an Estuarine Sediment Core. J. Soils Sediments 2015, 15, 211–224. [Google Scholar] [CrossRef]
- Müller, C.M.; Pejcic, B.; Esteban, L.; Piane, C.D.; Raven, M.; Mizaikoff, B. Infrared Attenuated Total Reflectance Spectroscopy: An Innovative Strategy for Analyzing Mineral Components in Energy Relevant Systems. Sci. Rep. 2014, 4, 6764. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Nawrocka, A.; Pińkowska, H.; Bajda, T.; Stanek-Tarkowska, J.; Szostek, M. FT-IR Analysis and the Content of Phenolic Compounds in Exogenous Organic Matter Produced from Plant Biomass. J. Elem. 2019, 24, 879–896. [Google Scholar] [CrossRef]
- Ziolkowska, A.; Debska, B.; Banach-Szott, M. Transformations of Phenolic Compounds in Meadow Soils. Sci. Rep. 2020, 10, 19330. [Google Scholar] [CrossRef]
- Min, K.; Freeman, C.; Kang, H.; Choi, S.U. The Regulation by Phenolic Compounds of Soil Organic Matter Dynamics under a Changing Environment. BioMed Res. Int. 2015, 2015, 825098. [Google Scholar] [CrossRef]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Liptzin, D.; Norris, C.E.; Cappellazzi, S.B.; Mac Bean, G.; Cope, M.; Greub, K.L.H.; Rieke, E.L.; Tracy, P.W.; Aberle, E.; Ashworth, A.; et al. An Evaluation of Carbon Indicators of Soil Health in Long-Term Agricultural Experiments. Soil Biol. Biochem. 2022, 172, 108708. [Google Scholar] [CrossRef]
- Rubin, R.; Oldfield, E.; Lavallee, J.; Griffin, T.; Mayers, B.; Sanderman, J. Climate Mitigation through Soil Amendments: Quantification, Evidence, and Uncertainty. Carbon Manag. 2023, 14, 2217785. [Google Scholar] [CrossRef]
| Symbol | Treatment Description |
|---|---|
| C | Control soil without any additivities |
| MF | Soil with addition of only mineral (NPK) fertilization |
| MV3%L3% | Soil with 94% NPK, 3% zeolite–vermiculite composite and 3% lignite |
| MV9%L6% | Soil with 85% NPK, 9% zeolite–vermiculite composite and 6% lignite |
| MV3%Leo3% | Soil with 94% NPK, 3% zeolite–vermiculite composite and 3% leonardite |
| MV9%Leo6% | Soil with 85% NPK, 9% zeolite–vermiculite composite and 6% leonardite |
| MC3%L3% | Soil with 94% NPK, 3% zeolite–carbon composite and 3% lignite |
| MC9%L6% | Soil with 85% NPK, 9% zeolite–carbon composite and 6% lignite |
| MC3%Leo3% | Soil with 94% NPK, 3% zeolite–carbon composite and 3% leonardite |
| MC9%Leo6% | Soil with 85% NPK, 9% zeolite–carbon composite and 6% leonardite |
| Treatment | pH 1st Year | pH 2nd Year | EC 1st Year µS · cm−1 | EC 2nd Year µS · cm−1 | T 1st Year mmol(+) · kg−1 | T 2nd Year mmol(+) · kg−1 |
|---|---|---|---|---|---|---|
| C | 5.91 d ± 0.10 | 5.97 d ± 0.12 | 365.8 a ± 58.6 | 341.3 a ± 9.2 | 106.5 c ± 1.8 | 129.8 d ± 5.1 |
| MF | 5.28 abc ± 0.06 | 5.34 c ± 0.16 | 426.7 b ± 46.9 | 762.7 j ± 20.8 | 93.7 a ± 3.6 | 137.6 ef ± 3.2 |
| MV3%L3% | 5.22 abc ± 0.12 | 5.17 abc ± 0.13 | 564.7 g ± 53.3 | 709.5 i ± 15.1 | 94.6 a ± 1.3 | 133.3 def ± 1.8 |
| MV9%L6% | 5.30 abc ± 0.07 | 5.25 abc ± 0.18 | 330.3 a ± 14.6 | 640.7 h ± 49.1 | 100.7 abc ± 3.8 | 131.1 de ± 0.8 |
| MV3%Leo3% | 5.31 bc ± 0.16 | 5.24 abc ± 0.13 | 525.5 efg ± 28.2 | 699.3 i ± 52.9 | 99.6 abc ± 2.9 | 131.9 def ± 1.8 |
| MV9%Leo6% | 5.23 abc ± 0.05 | 5.09 a ± 0.14 | 499.5 def ± 55.1 | 1111.7 l ± 33.0 | 101.9 bc ± 3.9 | 132.0 def ± 1.0 |
| MC3%L3% | 5.12 ab ± 0.12 | 5.15 abc ± 0.13 | 544.3 fg ± 11.8 | 708.3 ij ± 23.8 | 94.4 a ± 3.7 | 131.7 def ± 5.2 |
| MC9%L6% | 5.24 abc ± 0.06 | 5.27 abc ± 0.18 | 448.7 bcd ± 4.2 | 698.0 i ± 43.6 | 99.0 ab ± 4.0 | 138.5 f ± 5.6 |
| MC3%Leo3% | 5.25 abc ± 0.16 | 5.10 a ± 0.05 | 441.0 bcd ± 9.1 | 1263.3 m ± 62.7 | 104.8 bc ± 3.4 | 138.4 f ± 4.0 |
| MC9%Leo6% | 5.27 abc ± 0.05 | 5.15 abc ± 0.13 | 484.0 cde ± 18.0 | 824.5 k ± 19.6 | 100.4 abc ± 9.1 | 126.6 d ± 9.0 |
| Treatment | TOC 1st Year | TOC 2nd Year | TN 1st Year | TN 2nd Year | TOC:TN 1st Year | TOC:TN 2nd Year |
|---|---|---|---|---|---|---|
| g · kg−1 DM | g · kg−1 DM | g · kg−1 DM | g · kg−1 DM | |||
| C | 6.12 abcdefgh ± 0.44 | 6.23 gh ± 0.81 | 0.477 bc ± 0.050 | 0.512 cd ± 0.041 | 12.9 abcde ± 1.5 | 13.4 abcde ± 1.5 |
| MF | 5.61 abcd ± 0.36 | 6.42 defgh ± 0.41 | 0.435 abc ± 0.082 | 0.449 abc ± 0.093 | 14.3 bcde ± 2.3 | 15.0 cde ± 4.6 |
| MV3%L3% | 5.13 a ± 0.28 | 5.57 abcd ± 0.13 | 0.402 ab ± 0.062 | 0.585 de ± 0.048 | 12.8 abcd ± 0.7 | 9.6 a ± 1.0 |
| MV9%L6% | 5.87 abcdef ± 0.29 | 6.72 efg ± 0.33 | 0.401 ab ± 0.082 | 0.524 cde ± 0.055 | 15.0 cde ± 2.8 | 13.0 abcde ± 2.1 |
| MV3%Leo3% | 5.27 abc ± 0.73 | 6.82 gh ± 0.24 | 0.376 a ± 0.051 | 0.509 cd ± 0.063 | 14.2 bcde ± 2.7 | 13.5 bcde ± 1.7 |
| MV9%Leo6% | 6.17 cdefgh ± 0.34 | 6.52 efgh ± 0.89 | 0.377 a ± 0.050 | 0.609 e ± 0.062 | 16.6 e ± 2.7 | 10.7 ab ± 0.5 |
| MC3%L3% | 5.73 abcde ± 0.56 | 6.84 gh ± 0.40 | 0.377 a ± 0.096 | 0.525 cde ± 0.050 | 15.7 de ± 2.8 | 13.1 abcde ± 1.2 |
| MC9%L6% | 5.94 abcdefg ± 0.64 | 6.87 h ± 0.19 | 0.452 abc ± 0.058 | 0.595 de ± 0.032 | 13.2 abcde ± 0.5 | 11.6 abc ± 0.8 |
| MC3%Leo3% | 5.14 a ± 0.35 | 6.86 h ± 0.66 | 0.401 ab ± 0.004 | 0.595 de ± 0.069 | 12.8 abcd ± 0.9 | 11.7 abc ± 2.4 |
| MC9%Leo6% | 5.24 ab ± 0.81 | 6.06 bcdefgh ± 0.94 | 0.402 ab ± 0.050 | 0.515 cd ± 0.029 | 14.3 bcde ± 4.3 | 11.8 abc ± 1.6 |
| CHA:CFA | ||
|---|---|---|
| Treatment | 1st year | 2nd year |
| C | 0.260 abc ± 0.060 | 0.293 abcd ± 0.039 |
| MF | 0.165 a ± 0.018 | 0.463 def ± 0.055 |
| MV3%L3% | 0.294 abcd ± 0.126 | 0.536 f ± 0.163 |
| MV9%L6% | 0.264 abc ± 0.147 | 0.362 bcde ± 0.033 |
| MV3%Leo3% | 0.239 ab ± 0.136 | 0.458 def ± 0.152 |
| MV9%Leo6% | 0.176 a ± 0.030 | 0.413 bcdef ± 0.060 |
| MC3%L3% | 0.420 cdef ± 0.069 | 0.475 ef ± 0.132 |
| MC9%L6% | 0.316 abcde ± 0.038 | 0.464 def ± 0.048 |
| MC3%Leo3% | 0.355 bcde ± 0.145 | 0.399 bcdef ± 0.081 |
| MC9%Leo6% | 0.263 abc ± 0.021 | 0.489 ef ± 0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, R.; Kowalska, J.B.; Gondek, K.; Bejger, R.; Mielnik, L.; Lahori, A.H.; Mierzwa-Hersztek, M. The Effect of New Zeolite Composites from Fly Ashes Mixed with Leonardite and Lignite in Enhancing Soil Organic Matter. Agriculture 2025, 15, 786. https://doi.org/10.3390/agriculture15070786
Jarosz R, Kowalska JB, Gondek K, Bejger R, Mielnik L, Lahori AH, Mierzwa-Hersztek M. The Effect of New Zeolite Composites from Fly Ashes Mixed with Leonardite and Lignite in Enhancing Soil Organic Matter. Agriculture. 2025; 15(7):786. https://doi.org/10.3390/agriculture15070786
Chicago/Turabian StyleJarosz, Renata, Joanna Beata Kowalska, Krzysztof Gondek, Romualda Bejger, Lilla Mielnik, Altaf Hussain Lahori, and Monika Mierzwa-Hersztek. 2025. "The Effect of New Zeolite Composites from Fly Ashes Mixed with Leonardite and Lignite in Enhancing Soil Organic Matter" Agriculture 15, no. 7: 786. https://doi.org/10.3390/agriculture15070786
APA StyleJarosz, R., Kowalska, J. B., Gondek, K., Bejger, R., Mielnik, L., Lahori, A. H., & Mierzwa-Hersztek, M. (2025). The Effect of New Zeolite Composites from Fly Ashes Mixed with Leonardite and Lignite in Enhancing Soil Organic Matter. Agriculture, 15(7), 786. https://doi.org/10.3390/agriculture15070786








