Photosynthetic Performance and Urea Metabolism After Foliar Fertilization with Nickel and Urea in Cotton Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Growing Conditions
2.4. Foliar Fertilization with Urea and Nickel
2.5. Gas Exchange Measurement
2.6. Collection of Plant Material for Laboratory Analysis
2.7. Photosynthetic Pigments
2.8. Extraction and Measurement of Urease Enzyme Activity
2.9. Quantification of Urea Content
2.10. Extraction of Soluble Compounds
2.11. Quantification of Ammonia Content
2.12. Quantification of Ureide Content
2.13. Quantification of Amino Acid Content
2.14. Quantification of Protein Content
2.15. Quantification of Carbohydrate Content
2.16. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M.; Bhatt, R.; Fahad, S.; Hasanuzzaman, M. Agricultural Land Degradation: Processes and Problems Undermining Future Food Security. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Khan, I.A., Adnan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–61. [Google Scholar]
- Asghar, M.G.; Bashir, A. Protagonist of Mineral Nutrients in Drought Stress Tolerance of Field Crops. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Ferraz, C.A.M.; Fuzatto, M.G.; Gridi-Papp, I.L. Dados preliminares sôbre o emprêgo de adubos minerais nitrogenados em pulverização foliar no algodoeiro. Bragantia 1969, 28, 33–38. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Barretto, A.E.; Trivelin, P.C.O.; Victória, R.L. Absorção de uréia via foliar pelo algodoeiro em função do pH da solução. Pesqui. Agropecu. Bras. 1989, 25, 491–497. [Google Scholar]
- Carvalho, M.A.C.D.; Paulino, H.B.; Furlani-Júnior, E.; Buzetti, S.; Sá, M.E.D.; Athayde, M.L.F.D. Uso da adubação foliar nitrogenada e potássica no algodoeiro. Bragantia 2001, 60, 239–244. [Google Scholar] [CrossRef]
- Buriro, M.; Soomro, S.; Buriro, G.; Jogi, Q.; Muhaamad, N.; Kandhro Rais, N. Effect of foliar applied boron, zinc, and urea on growth and yield of cotton. Sci. Int. 2016, 28, 4113–4117. [Google Scholar]
- Ali, N. Review: Nitrogen utilization features in cotton crop. Am. J. Plant Sci. 2015, 6, 987–1002. [Google Scholar]
- Dixon, N.E.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5.1.5). metalloenzyme simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar]
- Eskew, D.L.; Welch, R.M.; Cary, E.E. Nickel: An essential micronutrient for legumes and possibly all higher plants. Science 1983, 222, 621–623. [Google Scholar]
- Eskew, D.L.; Welch, R.M.; Norvell, W.A. Nickel in higher plants: Further evidence for an essential role. Plant Physiol. 1984, 76, 691–693. [Google Scholar]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A micronutrient essential for higher plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar]
- Fageria, N.K.; Baligar, V.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Witte, C.-P.; Tiller, S.A.; Taylor, M.A.; Davies, H.V. Leaf urea metabolism in potato urease activity profile and patterns of recovery and distribution of 15N after foliar urea application in wild-type and urease-antisense transgenics. Plant Physiol. 2002, 128, 1129. [Google Scholar] [PubMed]
- Walker, C.D.; Graham, R.D.; Madison, J.T.; Cary, E.E.; Welch, R.M. Effects of Ni deficiency on some nitrogen metabolites in cowpeas (Vigna unguiculata L. Walp). Plant Physiol. 1985, 79, 474–479. [Google Scholar]
- Bai, C.; Reilly, C.C.; Wood, B.W. Nickel deficiency disrupts metabolism of ureides, amino acids, and organic acids of young pecan foliage. Plant Physiol. 2006, 140, 433–443. [Google Scholar]
- Harasim, P.; Filipek, T. Nickel in the environment. J. Elem. 2015, 20, 525–534. [Google Scholar]
- Macedo, F.G.; Santos, E.F.; Lavres, J. Agricultural crop influences availability of nickel in the rhizosphere; a study on base cation saturations, Ni dosages and crop succession. Rhizosphere 2020, 13, 100182. [Google Scholar]
- Klucas, R.V.; Hanus, F.J.; Russell, S.A.; Evans, H.J. Nickel: A micronutrient element for hydrogen-dependent growth of rhizobium japonicum and for expression of urease activity in soybean leaves. Proc. Natl. Acad. Sci. USA 1983, 80, 2253–2257. [Google Scholar] [PubMed]
- Mustafiz, A.; Ghosh, A.; Tripathi, A.K.; Kaur, C.; Ganguly, A.K.; Bhavesh, N.S.; Tripathi, J.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possess a single active site and functions in abiotic stress response. Plant J. 2014, 78, 951–963. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar]
- Hogan, M.E.; Swift, I.E.; Done, J. Urease assay and ammonia release from leaf tissues. Phytochemistry 1983, 22, 663–667. [Google Scholar] [CrossRef]
- Witte, C.-P.; Medina-Escobar, N. In-gel detection of urease with nitroblue tetrazolium and quantification of the enzyme from different crop plants using the indophenol reaction. Anal. Biochem. 2001, 290, 102–107. [Google Scholar]
- Khoshgoftarmanesh, A.H.; Hosseini, F.; Afyuni, M. Nickel supplementation effect on the growth, urease activity and urea and nitrate concentrations in lettuce supplied with different nitrogen sources. Sci. Hortic. 2011, 130, 381–385. [Google Scholar]
- Follmer, C. Insights into the role and structure of plant ureases. Phytochemistry 2008, 69, 18–28. [Google Scholar] [PubMed]
- Lopes, J.F.; Coelho, F.C.; Rabello, W.S.; Rangel, O.J.P.; Gravina, G.d.A.; Vieira, H.D. Produtividade e composição mineral do feijão em resposta às adubações com molibdênio e níquel. Rev. Ceres 2016, 63, 419–426. [Google Scholar]
- Macedo, L.O.; Favarin, J.L.; Tezotto, T.; Neto, A.P.; Andrade, S.A.L.; Mazzafera, P. Soil and foliar nickel application in coffee seedlings alters leaf nutrient balance. Agrochim. Int. J. Plant Chem. Soil Sci. Plant Nutr. Univ. Pisa 2020, 64, 167–180. [Google Scholar]
- Tsadilas, C.D.; Rinklebe, J.; Selim, H.M. Nickel in Soils and Plants; CRC Press: New York, NY, USA, 2019. [Google Scholar]
- Américo, G.H.P.; Américo-Pinheiro, J.H.P.; Furlani, E., Jr. Hormesis effect of dichlorophenoxy acetic acid sub-doses and mepiquat chloride on cotton plant. Planta Daninha 2017, 35, 1–9. [Google Scholar]
- Shareef, M.; Gui, D.; Zeng, F.; Waqas, M.; Ahmed, Z.; Zhang, B.; Iqbal, H.; Xue, J. Nitrogen leaching, recovery efficiency, and cotton productivity assessments on desert-sandy soil under various application methods. Agric. Water Manag. 2019, 223, 105716. [Google Scholar]
- Rosolem, C.A.; van Mellis, V. Monitoring nitrogen nutrition in cotton. Rev. Bras. Ciência Solo 2010, 34, 1601–1607. [Google Scholar]
- Rosolem, C.A.; Echer, F.R.; Lisboa, I.P.; Barbosa, T.S. Acúmulo de nitrogênio, fósforo e potássio pelo algodoeiro sob irrigação cultivado em sistemas convencional e adensado. Rev. Bras. Ciência Solo 2012, 36, 457–466. [Google Scholar]
- Camargos, L.; Sodek, L. Nodule growth and nitrogen fixation of Calopogonium mucunoides L. show low sensitivity to nitrate. Symbiosis 2010, 51, 167–174. [Google Scholar]
- Pereira Bruno, I.; Moraes, M.F.; Damin, V.; Dourado-Neto, D.; Reichardt, K. Does nickel influence leaf nitrogen uptake in coffee seedlings? Braz. J. Agric. 2019, 94, 259–269. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; de Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd. ed; Potafos: Piracicaba, Brazil, 1997. [Google Scholar]
- Barcelos, J.; Furlani Junior, E.; Reis, H.; Putti, F.; Reis, A. Diagnóstico da exigência do algodoeiro em nitrogênio e níquel pela utilização do medidor portátil de clorofila [ Diagnosis of nitrogen and nickel requirements for cotton plants using a portable chlorophyll meter]. Rev. Bras. Eng. Biossistemas 2016, 10, 97–106. [Google Scholar]
- Rigon, J.P.G.; Neto, J.F.B.; Capuani, S.; Beltrão, N.E.M.; Silva, F.V.F. Utilização de nitrogênio e níquel durante o crescimento do Algodão. Enciclopedia Biosf. 2011, 7, 1019–1026. [Google Scholar]
- Kutman, B.Y.; Kutman, U.B.; Cakmak, I. Nickel-enriched seed and externally supplied nickel improve growth and alleviate foliar urea damage in soybean. Plant Soil 2013, 363, 61–75. [Google Scholar] [CrossRef]
- Einhardt, A.M.; Ferreira, S.; Oliveira, L.M.; Ribeiro, D.M.; Rodrigues, F.Á. Glyphosate and nickel differently affect photosynthesis and ethylene in glyphosate-resistant soybean plants infected by Phakopsora pachyrhizi. Physiol. Plant. 2020, 170, 592–606. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbrearas, J.F.; Coelho, M.R.; Almeida, J.A.; Filho, J.C.A.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Rio de Janeiro, Brazil, 2018; Volume 5. [Google Scholar]
- Ibañez, T.B.; Santos, L.F.M.; Lapaz, A.M.; Ribeiro, I.V.; Ribeiro, F.V.; Reis, A.R.; Moreira, A.; Heinrichs, R. Sulfur modulates yield and storage proteins in soybean grains. Sci. Agric. 2021, 78, e20190020. [Google Scholar] [CrossRef]
- Quaggio, J.A.; van Raij, B.; Malavolta, E. Alternative use of the SMP-buffer solution to determine lime requirement of soils. Commun. Soil Sci. Plant Anal. 1985, 16, 245–260. [Google Scholar] [CrossRef]
- Munger, P.; Bleiholder, H.; Hack, H.; Hess, M.; Stauß, R.; van den Boom, T.; Weber, E. Phenological Growth Stages of the Cotton Plant (Gossypium hirsutum L.): Codification and Description according to the BBCH Scale1. J. Agron. Crop Sci. 1998, 180, 143–149. [Google Scholar] [CrossRef]
- Hiscox, J.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- McCullough, H. The determination of ammonia in whole blood by a direct colorimetric method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef]
- Kyllingsbæk, A. Extraction and colorimetric determination of urea in plants. Acta Agric. Scand. 1975, 25, 109–112. [Google Scholar] [CrossRef]
- Kojima, S.; Bohner, A.; Gassert, B.; Yuan, L.; Wirén, N. AtDUR3 represents the major transporter for high-affinity urea transport across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 2007, 52, 30–40. [Google Scholar] [PubMed]
- Bieleski, R.L.; Turner, N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Lapaz, A.M.; Camargos, L.S.; Yoshida, C.H.P.; Firmino, A.C.; de Figueiredo, P.A.M.; Aguilar, J.V.; Nicolai, A.B.; Silva de Paiva, W.D.; Cruz, V.H.; Tomaz, R.S. Response of soybean to soil waterlogging associated with iron excess in the reproductive stage. Physiol. Mol. Biol. Plants 2020, 26, 1635–1648. [Google Scholar] [PubMed]
- Van Der Drift, C.; De Windt, F.E.; Vogels, G.D. Allantoate hydrolysis by allantoate amidohydrolase. Arch. Biochem. Biophys. 1970, 136, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar]
- Giri, M.; Dhonde, M.B.; Tumbare, A.D. Effect of split and foliar application of nitrogen on leaf nitrogen concentration, SPAD index and photosynthesis in Bt. Cotton (Gossypium hirsutum L.). SAARC J. Agric. 2017, 14, 1–11. [Google Scholar]
- Pettigrew, W.T.; McCarty, J.C.; Vaughn, K.C. Leaf senescence-like characteristics contribute to cotton’s premature photosynthetic decline. Photosynth. Res. 2000, 65, 187–195. [Google Scholar]
- Wientjes, E.; Philippi, J.; Borst, J.W.; van Amerongen, H. Imaging the photosystem i/photosystem ii chlorophyll ratio inside the leaf. Biochim. Biophys. Acta. 2017, 1858, 259–265. [Google Scholar]
- Gheibi, M.N.; Malakouti, M.J.; Kholdebarin, B.; Ghanati, F.; Teimouri, S.; Sayadi, R. Significance of nickel supply for growth and chlorophyll content of wheat supplied with urea or ammonium nitrate. J. Plant Nutr. 2009, 32, 1440–1450. [Google Scholar] [CrossRef]
- Gerendas, J.; Zhu, Z.; Sattelmacher, B. Influence of N and Ni supply on nitrogen metabolism and urease activity in rice (Oryza sativa L.). J. Exp. Bot. 1998, 83, 65–71. [Google Scholar] [CrossRef]
- Polacco, J.C.; Mazzafera, P.; Tezotto, T. Opinion: Nickel and urease in plants: Still many knowledge gaps. Plant Sci. 2013, 199–200, 79–90. [Google Scholar] [CrossRef]
- Polacco, J.C.; Holland, M.A. Roles of Urease in Plant Cells. In International Review of Cytology; Jeon, K.W., Jarvik, J., Eds.; Academic Press: New York, NY, USA, 1993; pp. 65–103. [Google Scholar]
- Winkler, R.G.; Polacco, J.C.; Eskew, D.L.; Welch, R.M. Nickel is not required for apourease synthesis in soybean seeds. Plant Physiol. 1983, 72, 262–263. [Google Scholar] [PubMed]
- Garnica, M.; Houdusse, F.; Zamarreño, A.M.; Garcia-Mina, J.M. Nitrate modifies the assimilation pattern of ammonium and urea in wheat seedlings. J. Sci. Food Agric. 2010, 90, 357–369. [Google Scholar]
- Matiz, A.; Mioto, P.T.; Aidar, M.P.M.; Mercier, H. Utilization of urea by leaves of bromeliad Vriesea gigantea under water deficit: Much more than a nitrogen source. Biol. Plant. 2017, 61, 751–762. [Google Scholar]
- Thomas, R.; Schrader, L. Ureide metabolism higher plants. Phytochemistry 1981, 20, 361–371. [Google Scholar]
- Werner, A.K.; Witte, C.-P. The biochemistry of nitrogen mobilization: Purine ring catabolism. Trends Plant Sci. 2011, 16, 381–387. [Google Scholar]
- Mothes, K. The metabolism of urea and ureides. Can. J. Bot. 2011, 39, 1785–1807. [Google Scholar]
- Raso, M.J.; Muñoz, A.; Pineda, M.; Piedras, P. Biochemical characterization of an allantoate-degrading enzyme from French bean (Phaseolus vulgaris): The requirement of phenylhydrazine. Planta 2007, 226, 1333–1342. [Google Scholar]
- Delfim, J.; Dameto, L.S.; Moraes, L.A.C.; Moreira, A. Nitrogen and Nickel Foliar Application on Grain yield, Yield Components, and Quality of Soybean. Commun. Soil Sci. Plant Anal. 2022, 53, 1226–1234. [Google Scholar] [CrossRef]
- Zhran, M.; Moursy, A.; Lynn, T.M.; Fahmy, A. Effect of urea fertilization on growth of broad bean (Vicia faba L.) under various nickel (Ni) levels with or without acetic acid addition, using 15N-labeled fertilizer. Environ. Geochem. Health 2021, 43, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Kochenborger, A.C.; Orioli Júnior, V.; Silva, G.A.; Sargentim, M.M.; Torres, J.L.R. Aplicação foliar de ureia, níquel e sacarose em estádio reprodutivo da soja. Nativa 2024, 11, 82–89. [Google Scholar] [CrossRef]
- Mendes, N.A.C.; Cunha, M.L.O.; Bosse, M.A.; Silva, V.M.; Moro, A.L.; Agathokleous, E.; Vicente, E.F.; Reis, A.R.D. Physiological and biochemical role of nickel in nodulation and biological nitrogen fixation in Vigna unguiculata L. Walp. Plant Physiol. Biochem. 2023, 201, 107869. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.V.; Lapaz, A.M.; Sanches, C.V.; Yoshida, C.H.P.; Camargos, L.S.; Furlani-Júnior, E. Application of 2,4-D hormetic dose associated with the supply of nitrogen and nickel on cotton plants. J. Environ. Sci. Health Part B 2021, 56, 852–859. [Google Scholar] [CrossRef]
- Aguilar, J.V.; Ferreira, T.C.; Bomfim, N.C.P.; Mendes, T.F.S.; Lapaz, A.M.; Brambilla, M.R.; Coscione, A.R.; Souza, L.A.; Furlani-Júnior, E.; Camargos, L.S. Different responses to phenological stages: A role for nickel in growth and physiology of herbaceous cotton. Plant Growth Regul. 2023, 101, 663–678. [Google Scholar] [CrossRef]
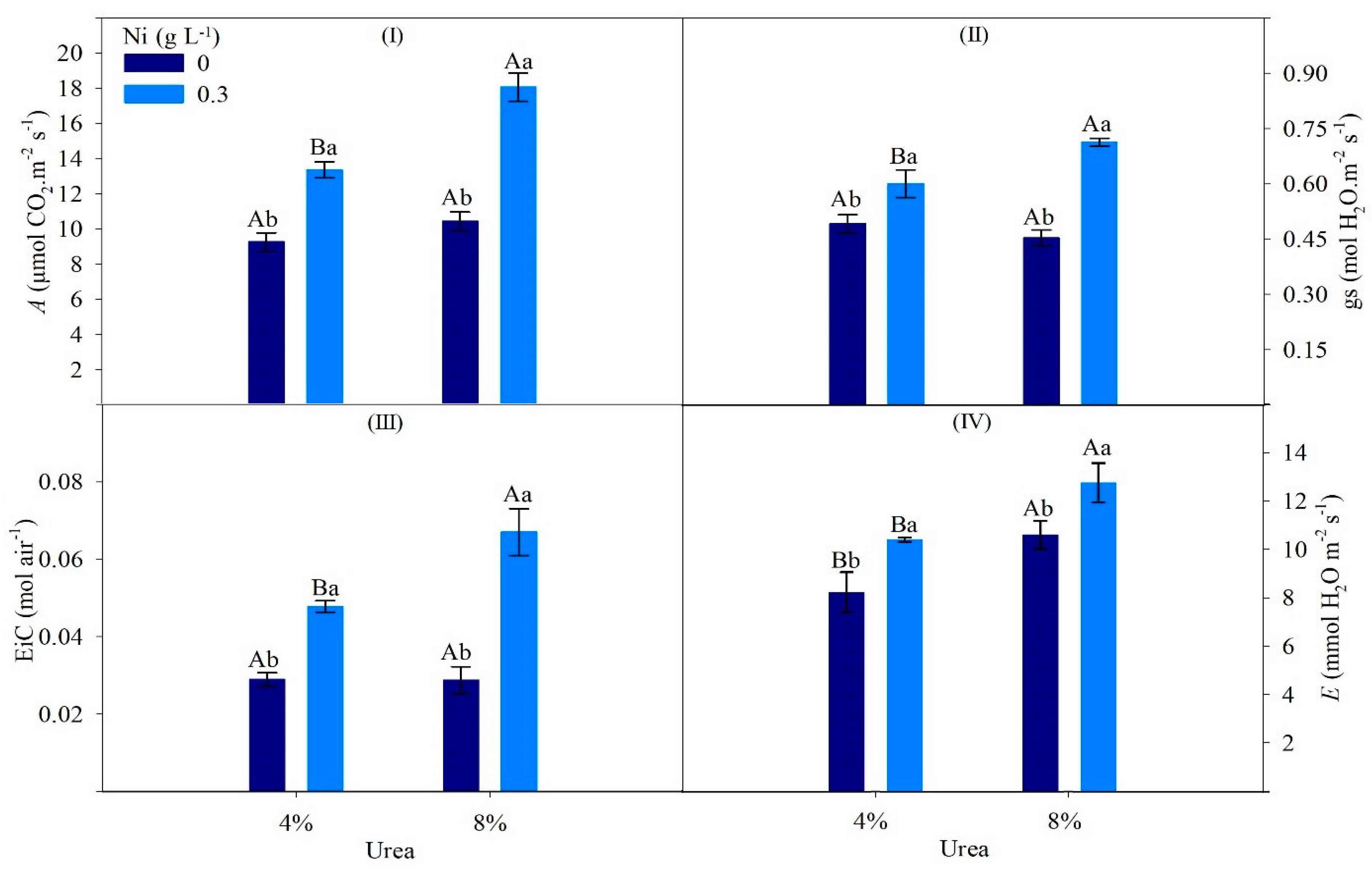
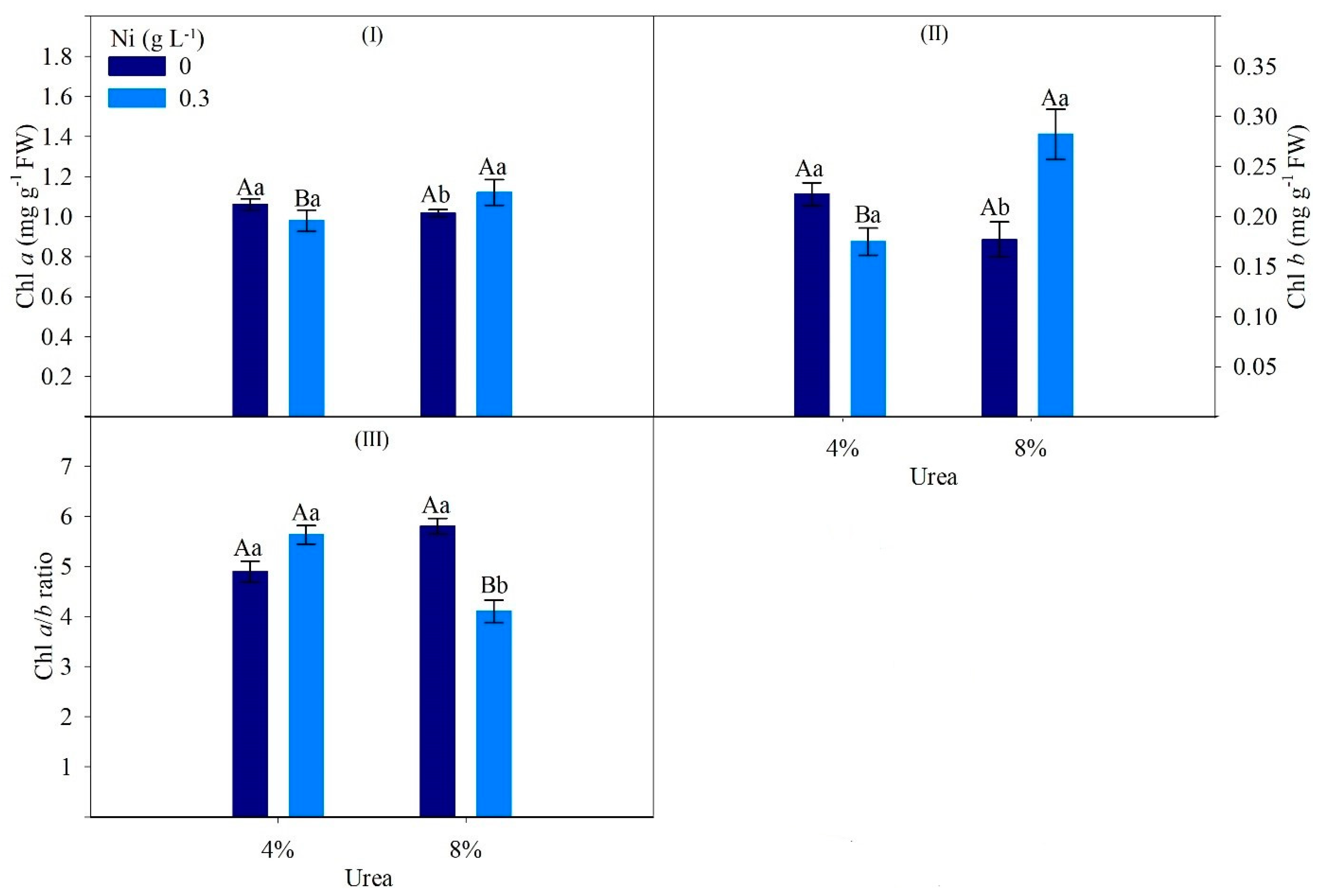
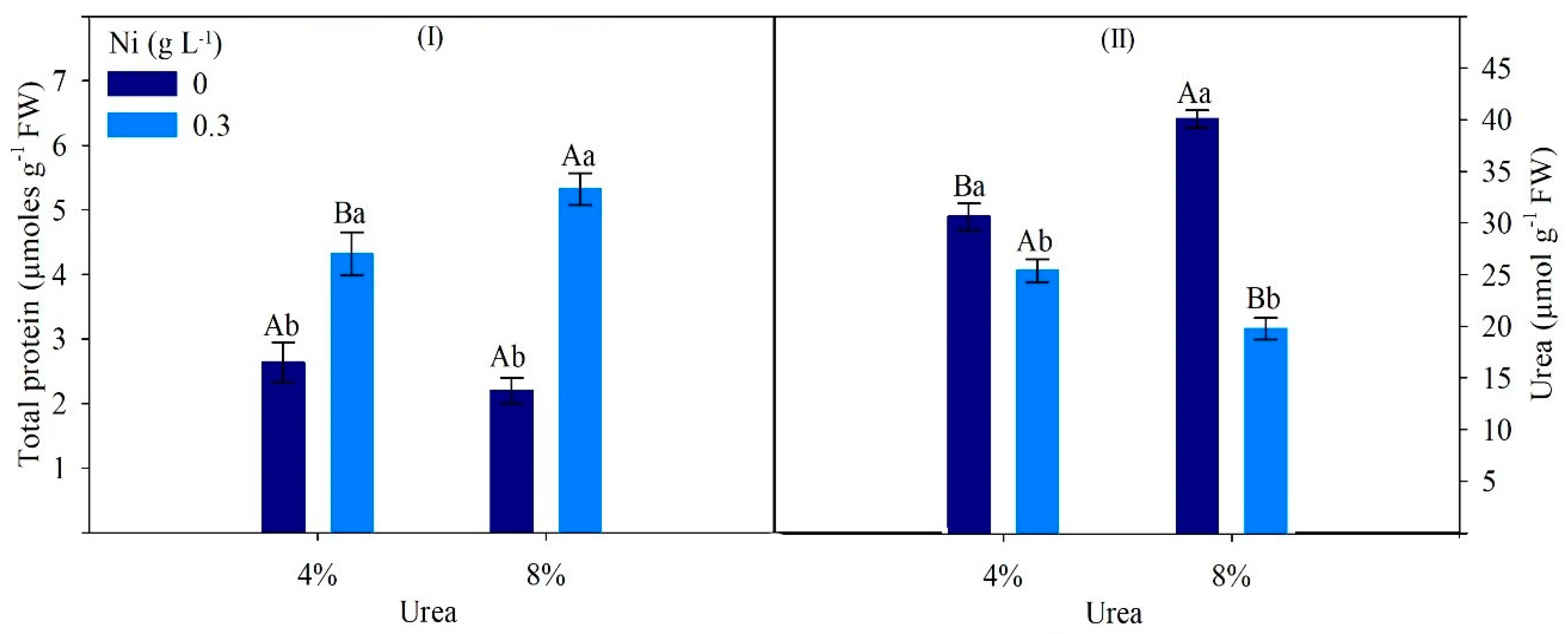

| Factor | Ci | TSC | CAR | Urease | Ammonia | Ureides | Amino Acids |
|---|---|---|---|---|---|---|---|
| Urea | ns | ** | ns | ns | ** | * | *** |
| Ni | ** | ns | ns | *** | ns | *** | *** |
| Urea × Ni | ns | ns | ns | ns | ns | ns | ns |
| Urea (%) | |||||||
| 4 | 300.5 | 1.95 b | 0.75 | 5.65 | 0.51 b | 13.17 b | 11.70 b |
| 8 | 322.0 | 2.38 a | 0.72 | 6.20 | 0.79 a | 13.95 a | 23.15 a |
| Ni (g L−1) | |||||||
| 0 | 346,0 a | 2.15 | 0.77 | 2.74 b | 0.57 | 11.81 b | 15.38 b |
| 0.3 | 276.5 b | 2.18 | 0.71 | 9.10 a | 0.73 | 15.31 a | 19.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, J.V.; Lapaz, A.d.M.; Bomfim, N.C.P.; Mendes, T.F.S.; Souza, L.A.; Furlani Júnior, E.; Camargos, L.S. Photosynthetic Performance and Urea Metabolism After Foliar Fertilization with Nickel and Urea in Cotton Plants. Agriculture 2025, 15, 699. https://doi.org/10.3390/agriculture15070699
Aguilar JV, Lapaz AdM, Bomfim NCP, Mendes TFS, Souza LA, Furlani Júnior E, Camargos LS. Photosynthetic Performance and Urea Metabolism After Foliar Fertilization with Nickel and Urea in Cotton Plants. Agriculture. 2025; 15(7):699. https://doi.org/10.3390/agriculture15070699
Chicago/Turabian StyleAguilar, Jailson Vieira, Allan de Marcos Lapaz, Nayane Cristina Pires Bomfim, Thalita Fischer Santini Mendes, Lucas Anjos Souza, Enes Furlani Júnior, and Liliane Santos Camargos. 2025. "Photosynthetic Performance and Urea Metabolism After Foliar Fertilization with Nickel and Urea in Cotton Plants" Agriculture 15, no. 7: 699. https://doi.org/10.3390/agriculture15070699
APA StyleAguilar, J. V., Lapaz, A. d. M., Bomfim, N. C. P., Mendes, T. F. S., Souza, L. A., Furlani Júnior, E., & Camargos, L. S. (2025). Photosynthetic Performance and Urea Metabolism After Foliar Fertilization with Nickel and Urea in Cotton Plants. Agriculture, 15(7), 699. https://doi.org/10.3390/agriculture15070699







