Land Use Rather than Microplastic Type Determines the Diversity and Structure of Plastisphere Bacterial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Qualities of MPs
2.2. Experimental Design
2.3. Microplastic Collection and Treatment
2.4. Measurement of Soil Physicochemical Properties, DNA Extraction, and Amplicon Sequencing
2.5. Statistical Analysis
3. Results
3.1. Surface Morphology of Glass Ball and Microplastics
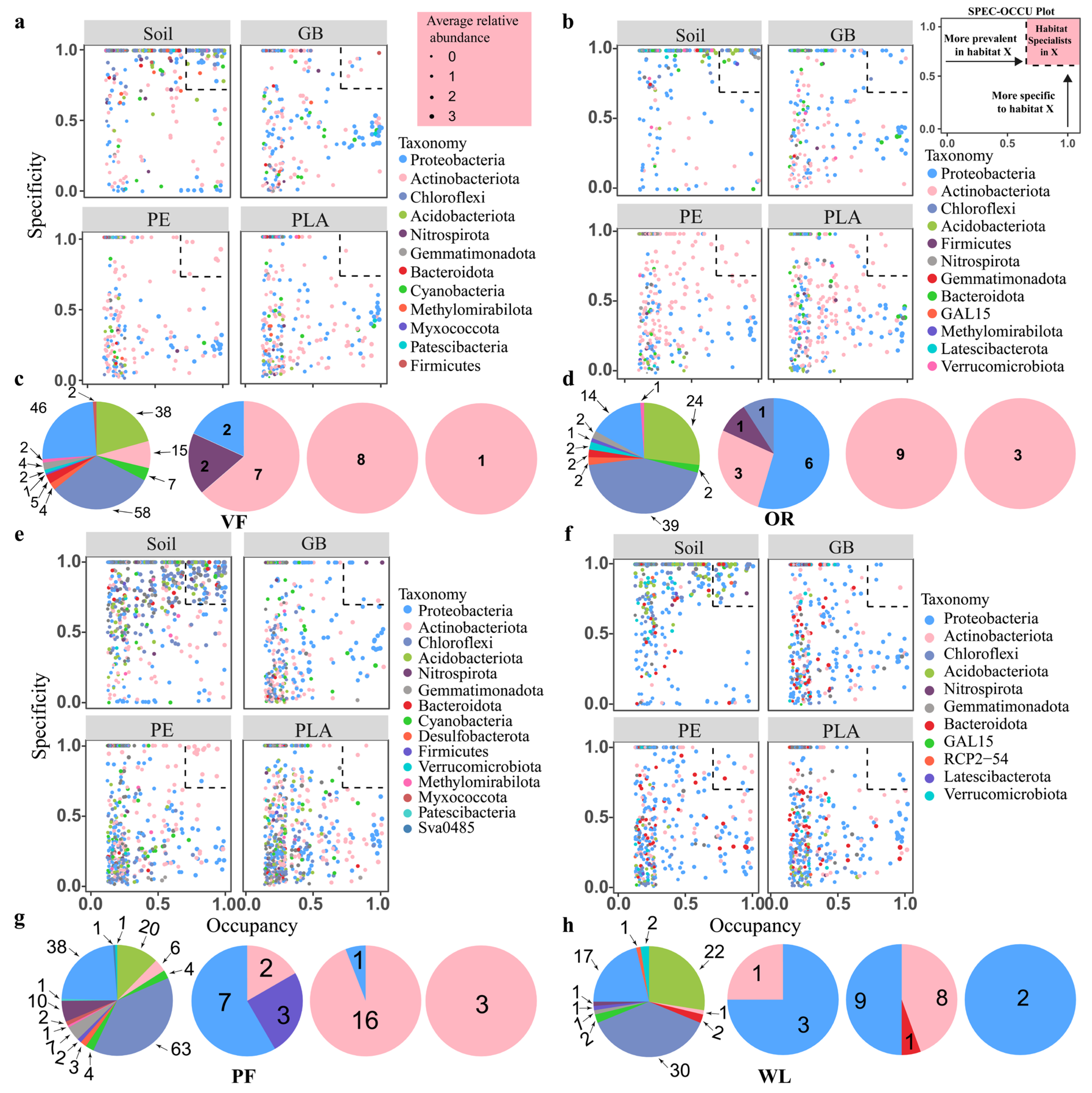
3.2. Bacterial Community Diversity and Composition
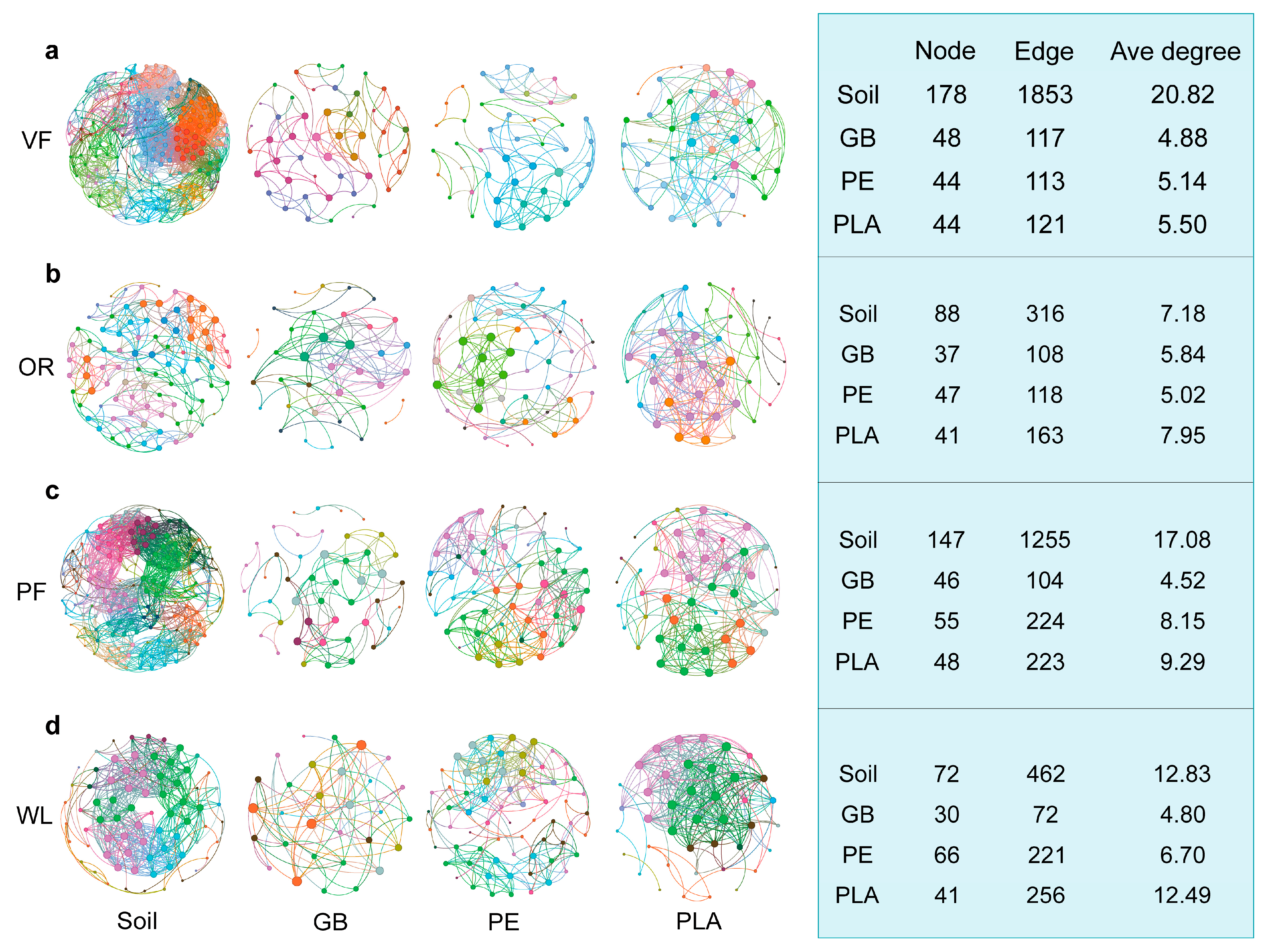
3.3. Co-Occurrence Network Characteristics of Bacterial Community
3.4. Microbial Community Assembly
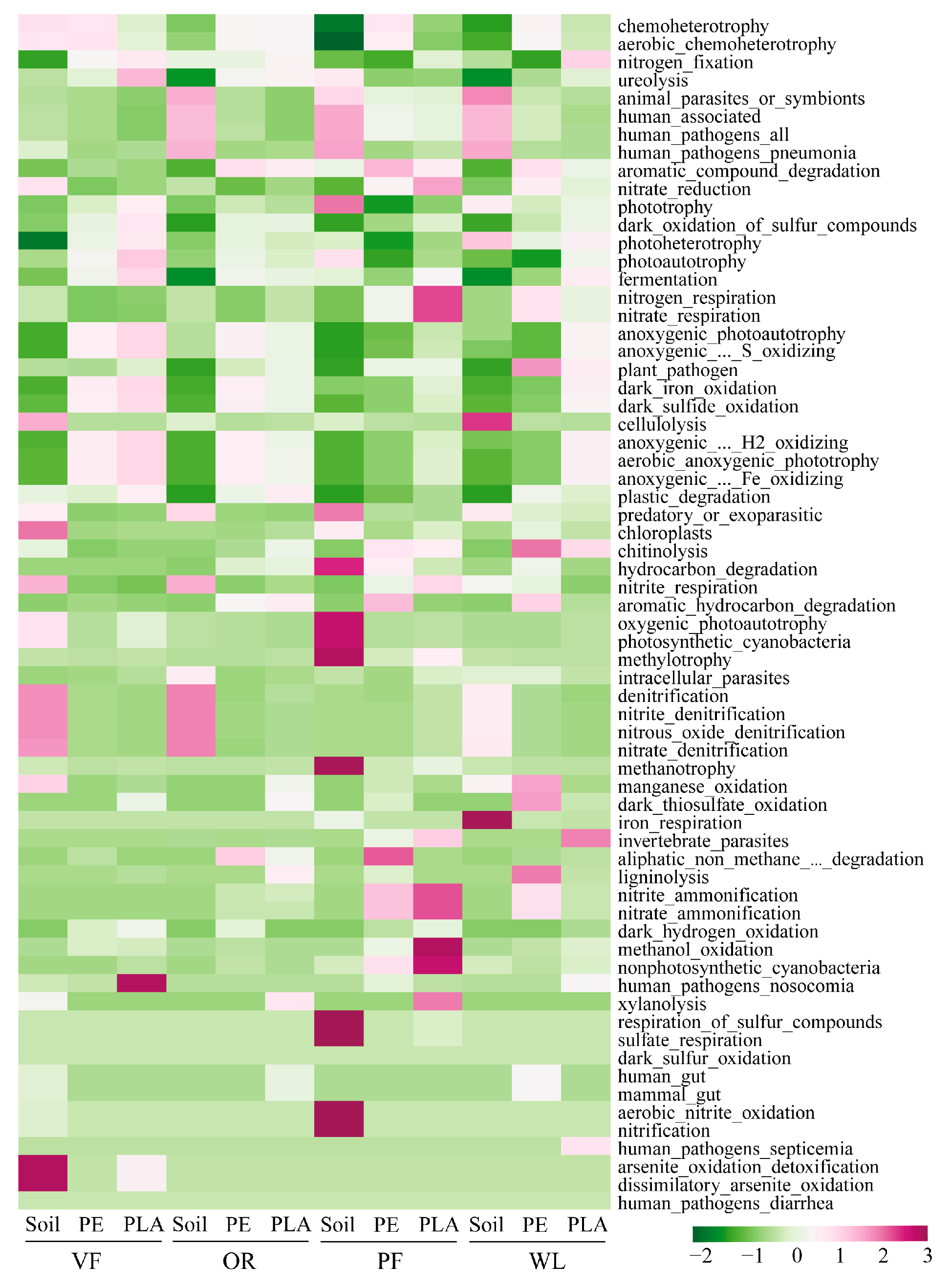
3.5. Functions of Bacterial Community in Plastisphere
4. Discussion
4.1. Changes in Bacterial Community in Plastisphere Across Land Use
4.2. Changes in Microbial Community Co-Occurrence Network and Assembly
4.3. Potential Functional Traits of Bacteria in Plastisphere
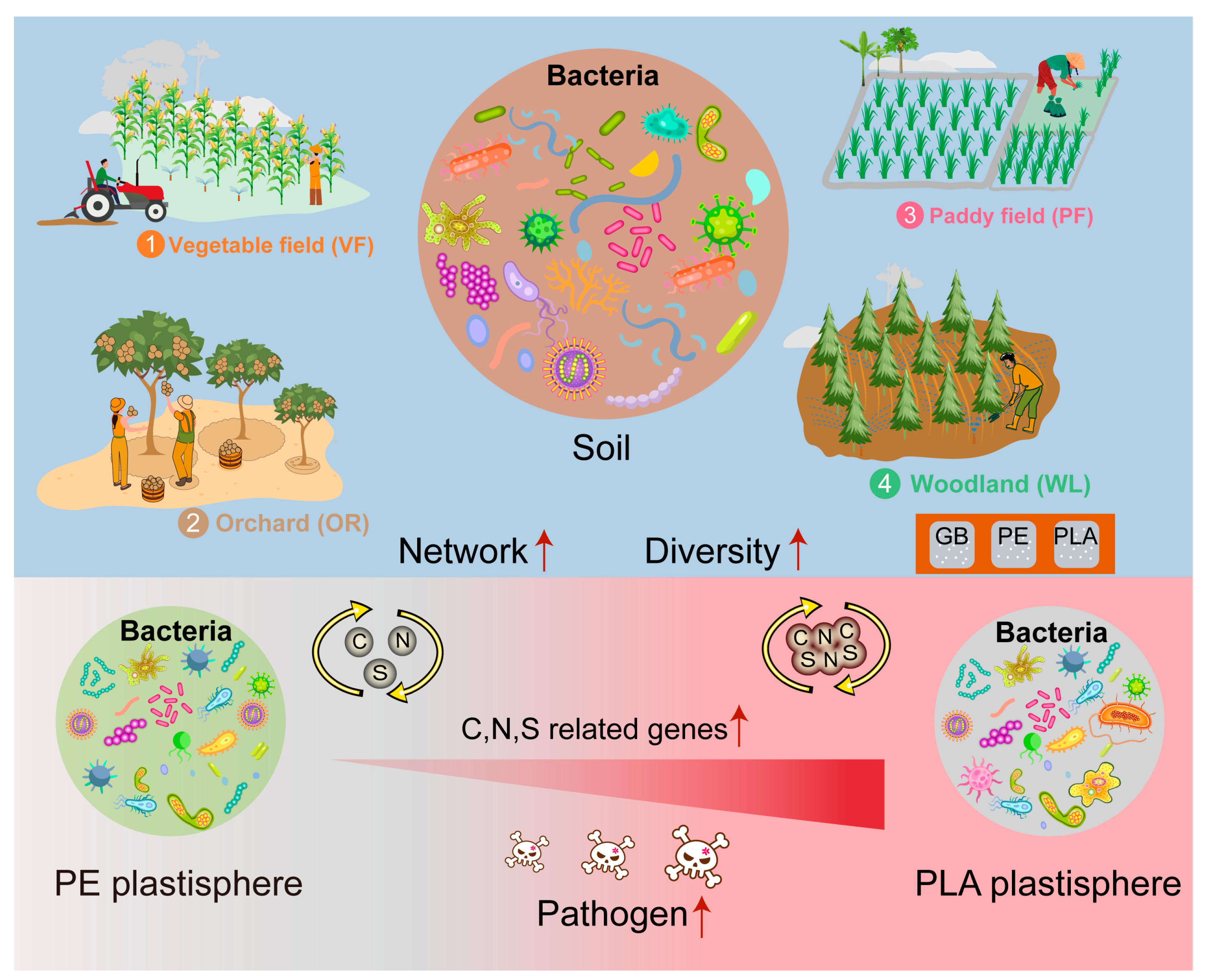
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in terrestrial ecosystem: Sources and migration in soil environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro-and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Sorasan, C.; Edo, C.; González-Pleiter, M.; Fernández-Piñas, F.; Leganés, F.; Rodríguez, A.; Rosal, R. Ageing and fragmentation of marine microplastics. Sci. Total Environ. 2022, 827, 154438. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-fragmentation of marine plastic waste and their environmental implications: A critical review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Y.; Wang, D.; Shan, Y.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; Ma, Y. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J. Hazard. Mater. 2022, 423, 127258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qi, X.; Yuan, Y.; Zhou, H.; Rong, X.; Dang, Z.; Yin, H. Deciphering the distinct successional patterns and potential roles of abundant and rare microbial taxa of urban riverine plastisphere. J. Hazard. Mater. 2023, 450, 131080. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef] [PubMed]
- Syranidou, E.; Kalogerakis, N. Interactions of microplastics, antibiotics and antibiotic resistant genes within WWTPs. Sci. Total Environ. 2022, 804, 150141. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, J.; Li, G.; Rillig, M.C.; Zhu, Y. Soil plastispheres as hotspots of antibiotic resistance genes and potential pathogens. ISME J. 2022, 16, 521–532. [Google Scholar] [CrossRef]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2021, 757, 143875. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro) plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef]
- Agarwal, S. Biodegradable polymers: Present opportunities and challenges in providing a microplastic-free environment. Macromol. Chem. Phys. 2020, 221, 2000017. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological degradation of plastics and microplastics: A recent perspective on associated mechanisms and influencing factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Ya, H.; Xing, Y.; Zhang, T.; Lv, M.; Jiang, B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Appl. Soil Ecol. 2022, 180, 104623. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Ok, Y.S.; Tsang, D.C.; Hou, D. Soil plastisphere: Exploration methods, influencing factors, and ecological insights. J. Hazard. Mater. 2022, 430, 128503. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, J.; Wang, X.; Ding, C.; Wang, J. Deciphering the mechanisms shaping the plastisphere microbiota in soil. Msystems 2022, 7, e322–e352. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, X.; Yu, M. Microbial colonization and degradation of marine microplastics in the plastisphere: A review. Front. Microbiol. 2023, 14, 1127308. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Kim, J.; Yu, Y.; Choi, J. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Tan, W. The “neighbor avoidance effect” of microplastics on bacterial and fungal diversity and communities in different soil horizons. Environ. Sci. Ecotechnol. 2021, 8, 100121. [Google Scholar] [CrossRef]
- Alimi, O.S.; Claveau-Mallet, D.; Kurusu, R.S.; Lapointe, M.; Bayen, S.; Tufenkji, N. Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: What are we missing? J. Hazard. Mater. 2022, 423, 126955. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Gao, F.; Li, J.; Zheng, L.; Sun, C.; He, C.; Wang, Z.; Qu, L. Marine microplastic-associated bacterial community succession in response to geography, exposure time, and plastic type in China’s coastal seawaters. Mar. Pollut. Bull. 2019, 145, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, J.; Yang, F.; Lei, Y.; Zhang, Q.; Cheng, X. Alterations in soil microbial community composition and biomass following agricultural land use change. Sci. Rep. 2016, 6, 36587. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Marshall, C.W.; Cheng, M.; Xu, H.; Li, H.; Yang, X.; Zheng, T. Changes in land use driven by urbanization impact nitrogen cycling and the microbial community composition in soils. Sci. Rep. 2017, 7, 44049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Wu, N.; Li, W.; Song, X.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on plastic debris: The localization of immigrant bacterial communities. Water Res. 2021, 193, 116883. [Google Scholar] [CrossRef]
- Forero-López, A.D.; Brugnoni, L.I.; Abasto, B.; Rimondino, G.N.; Lassalle, V.L.; Ardusso, M.G.; Nazzarro, M.S.; Martinez, A.M.; Spetter, C.V.; Biancalana, F. Plastisphere on microplastics: In situ assays in an estuarine environment. J. Hazard. Mater. 2022, 440, 129737. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Roig, B.; Gonzalez, C.; Thomas, O. Simple UV/UV-visible method for nitrogen and phosphorus measurement in wastewater. Talanta 1999, 50, 751–758. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on water quality detection by uv-vis spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 2006, 1695. [Google Scholar]
- Newman, M.E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Li, N.; Qu, J.; Yang, J. Microplastics distribution and microbial community characteristics of farmland soil under different mulch methods. J. Hazard. Mater. 2023, 445, 130408. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef]
- Liu, G.; Bai, Z.; Cui, G.; He, W.; Kong, Z.; Ji, G.; Gong, H.; Li, D. Effects of land use on the soil microbial community in the Songnen Grassland of Northeast China. Front. Microbiol. 2022, 13, 865184. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, C.; Jiang, L.; Song, M.; Zhang, D.; Li, J.; Li, Y.; Ostle, N.J.; Zhang, G. Land-use changes alter soil bacterial composition and diversity in tropical forest soil in China. Sci. Total Environ. 2020, 712, 136526. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Ji, S.; Chang, M.; Wang, L.; Gan, Y.; Liu, J. The ecology of the plastisphere: Microbial composition, function, assembly, and network in the freshwater and seawater ecosystems. Water Res. 2021, 202, 117428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, Y.; Zhu, J.; Cai, Z.; Zhou, J. The structure and assembly mechanisms of plastisphere microbial community in natural marine environment. J. Hazard. Mater. 2022, 421, 126780. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jia, W.; Xu, L.; Zhang, M.; Huang, Y. The plastisphere of biodegradable and conventional microplastics from residues exhibit distinct microbial structure, network and function in plastic-mulching farmland. J. Hazard. Mater. 2023, 442, 130011. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Wang, Y.; Cheng, Q. A review of the occurrence and degradation of biodegradable microplastics in soil environments. Sci. Total Environ. 2023, 904, 166855. [Google Scholar] [CrossRef]
- Bao, R.; Fu, D.; Fan, Z.; Peng, X.; Peng, L. Aging of microplastics and their role as vector for copper in aqueous solution. Gondwana Res. 2022, 108, 81–90. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an emerging environmental pollutant in agricultural soils: Effects on ecosystems and human health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do poly (lactic acid) microplastics instigate a threat? A perception for their dynamic towards environmental pollution and toxicity. Sci. Total Environ. 2022, 832, 155014. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.X.; Yang, J. How to build a microplastics-free environment: Strategies for microplastics degradation and plastics recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef]
- An, Q.; Zheng, N.; Pan, J.; Ji, Y.; Wang, S.; Li, X.; Chen, C.; Peng, L.; Wang, B. Association between plant microbiota and cadmium uptake under the influence of microplastics with different particle sizes. Environ. Int. 2024, 190, 108938. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Dey, S.; Rout, A.K.; Behera, B.K.; Ghosh, K. Plastisphere community assemblage of aquatic environment: Plastic-microbe interaction, role in degradation and characterization technologies. Environ. Microbiome 2022, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Liu, Y.; Li, L.; Li, Y.; Vogts, A.; Luo, Y.; Waniek, J.J. Structural and functional characteristics of microplastic associated biofilms in response to temporal dynamics and polymer types. Bull. Environ. Contam. Toxicol. 2021, 107, 633–639. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Han, S.; Wang, Y.; Duan, Y.; Liu, T.; Hou, L.; Zhang, Z.; Li, L.; Lin, Y. Polyethylene mulching film degrading bacteria within the plastisphere: Co-culture of plastic degrading strains screened by bacterial community succession. J. Hazard. Mater. 2023, 442, 130045. [Google Scholar] [CrossRef]
- Cui, B.; Fu, S.; Hao, X.; Zhou, D. Synergistic effects of simultaneous coupling ozonation and biodegradation for coking wastewater treatment: Advances in COD removal, toxic elimination, and microbial regulation. Chemosphere 2023, 318, 137956. [Google Scholar] [CrossRef]
- Luo, J.; Banerjee, S.; Ma, Q.; Liao, G.; Hu, B.; Zhao, H.; Li, T. Organic fertilization drives shifts in microbiome complexity and keystone taxa increase the resistance of microbial mediated functions to biodiversity loss. Biol. Fertil. Soils 2023, 59, 441–458. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. Cu (II) adsorption on poly (lactic acid) microplastics: Significance of microbial colonization and degradation. Chem. Eng. J. 2022, 429, 132306. [Google Scholar] [CrossRef]
- Mishra, S.; Swain, S.; Sahoo, M.; Mishra, S.; Das, A.P. Microbial colonization and degradation of microplastics in aquatic ecosystem: A review. Geomicrobiol. J. 2022, 39, 259–269. [Google Scholar] [CrossRef]
- Sun, X.; Xiang, H.; Xiong, H.; Fang, Y.; Wang, Y. Bioremediation of microplastics in freshwater environments: A systematic review of biofilm culture, degradation mechanisms, and analytical methods. Sci. Total Environ. 2023, 863, 160953. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Gao, Q.; Zhao, J.; Feng, J.; van Nostrand, J.D.; Yang, Y.; Zhou, J. Biogeographic patterns of microbial association networks in paddy soil within Eastern China. Soil Biol. Biochem. 2020, 142, 107696. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Cao, N.; Duan, C.; Ding, C.; Huang, Y.; Wang, J. Biodegradable microplastics enhance soil microbial network complexity and ecological stochasticity. J. Hazard. Mater. 2022, 439, 129610. [Google Scholar] [CrossRef]
- Fan, P.; Yu, H.; Xi, B.; Tan, W. A review on the occurrence and influence of biodegradable microplastics in soil ecosystems: Are biodegradable plastics substitute or threat? Environ. Int. 2022, 163, 107244. [Google Scholar] [CrossRef]
- Liu, N.; Hu, H.; Ma, W.; Deng, Y.; Wang, Q.; Luo, A.; Meng, J.; Feng, X.; Wang, Z. Relative importance of deterministic and stochastic processes on soil microbial community assembly in temperate grasslands. Microorganisms 2021, 9, 1929. [Google Scholar] [CrossRef]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial community responses to anthropogenically induced environmental change: Towards a systems approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef]
- Graham, E.B.; Stegen, J.C. Dispersal-based microbial community assembly decreases biogeochemical function. Processes 2017, 5, 65. [Google Scholar] [CrossRef]
- Caruso, T.; Chan, Y.; Lacap, D.C.; Lau, M.C.; McKay, C.P.; Pointing, S.B. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J. 2011, 5, 1406–1413. [Google Scholar] [CrossRef]
- Purahong, W.; Wahdan, S.F.M.; Heinz, D.; Jariyavidyanont, K.; Sungkapreecha, C.; Tanunchai, B.; Sansupa, C.; Sadubsarn, D.; Alaneed, R.; Heintz-Buschart, A. Back to the future: Decomposability of a biobased and biodegradable plastic in field soil environments and its microbiome under ambient and future climates. Environ. Sci. Technol. 2021, 55, 12337–12351. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, Y. Microplastics increase soil microbial network complexity and trigger diversity-driven community assembly. Environ. Pollut. 2023, 333, 122095. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Q.; Chen, M.; Li, H.; Liao, H.; Pu, Q.; Zhu, Y.; Cui, L. Temporal dynamics of antibiotic resistome in the plastisphere during microbial colonization. Environ. Sci. Technol. 2020, 54, 11322–11332. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, L.; Bai, X.; Zhang, G.; Zhang, M.; Huang, Y. Potential environmental risks of field bio/non-degradable microplastic from mulching residues in farmland: Evidence from metagenomic analysis of plastisphere. J. Hazard. Mater. 2024, 465, 133428. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Pan, S.; Li, G.; Liu, H.; Xiu, W.; Gong, L.; Zhao, J.; Zhang, G.; Yang, D. Can microplastics mediate soil properties, plant growth and carbon/nitrogen turnover in the terrestrial ecosystem? Ecosyst. Health Sustain. 2022, 8, 2133638. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Gupta, R.; Tiwari, A. Communities of microbial enzymes associated with biodegradation of plastics. J. Polym. Environ. 2013, 21, 575–579. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Shen, J.; Xu, F.; Hou, H.; Xie, C.; Wang, B.; An, S. Mechanism of polyethylene and biodegradable microplastic aging effects on soil organic carbon fractions in different land-use types. Sci. Total Environ. 2024, 912, 168961. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Peng, Y.; Zhang, Z.; Fan, Z.; Wang, J.; Wang, X. Microbes drive metabolism, community diversity, and interactions in response to microplastic-induced nutrient imbalance. Sci. Total Environ. 2023, 877, 162885. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Hirano, S.S.; Upper, C.D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—A pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 2000, 64, 624–653. [Google Scholar] [CrossRef] [PubMed]
- Lipps, S.M.; Samac, D.A. Pseudomonas viridiflava: An internal outsider of the Pseudomonas syringae species complex. Mol. Plant Pathol. 2022, 23, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Córdova, P.; Rivera-González, J.P.; Rojas-Martínez, V.; Fiore, N.; Bastías, R.; Zamorano, A.; Vera, F.; Barrueto, J.; Díaz, B.; Ilabaca-Díaz, C. Phytopathogenic Pseudomonas syringae as a threat to agriculture: Perspectives of a promising biological control using bacteriophages and microorganisms. Horticulturae 2023, 9, 712. [Google Scholar] [CrossRef]
- Liu, R.; Liang, J.; Yang, Y.; Jiang, H.; Tian, X. Effect of polylactic acid microplastics on soil properties, soil microbials and plant growth. Chemosphere 2023, 329, 138504. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Z.; Zhang, S.; Zhuang, W.; Shi, Z.; Liu, Z.; Wei, H.; Zhang, J. Land Use Rather than Microplastic Type Determines the Diversity and Structure of Plastisphere Bacterial Communities. Agriculture 2025, 15, 778. https://doi.org/10.3390/agriculture15070778
Wang Y, Zhang Z, Zhang S, Zhuang W, Shi Z, Liu Z, Wei H, Zhang J. Land Use Rather than Microplastic Type Determines the Diversity and Structure of Plastisphere Bacterial Communities. Agriculture. 2025; 15(7):778. https://doi.org/10.3390/agriculture15070778
Chicago/Turabian StyleWang, Yangyang, Zixuan Zhang, Shuang Zhang, Wanlin Zhuang, Zhaoji Shi, Ziqiang Liu, Hui Wei, and Jiaen Zhang. 2025. "Land Use Rather than Microplastic Type Determines the Diversity and Structure of Plastisphere Bacterial Communities" Agriculture 15, no. 7: 778. https://doi.org/10.3390/agriculture15070778
APA StyleWang, Y., Zhang, Z., Zhang, S., Zhuang, W., Shi, Z., Liu, Z., Wei, H., & Zhang, J. (2025). Land Use Rather than Microplastic Type Determines the Diversity and Structure of Plastisphere Bacterial Communities. Agriculture, 15(7), 778. https://doi.org/10.3390/agriculture15070778







