Review on Mechanisms of Iron Accelerants and Their Effects on Anaerobic Digestion
Abstract
1. Introduction
2. Thermodynamic Barrier of Syntrophic Methanogenesis Involved in the Anaerobic Digestion
3. Pathways and Mechanisms of Iron Accelerants Affecting the Anaerobic Digestion
3.1. DIET of Syntrophic Methanogenic Microorganisms Mediated by Iron Accelerants
3.2. Strong Reducibility of Fe0 Promotes Anaerobic Methane Production
3.3. Nutritional Requirement of Iron Element of Anaerobic Microorganisms
4. Effects of Iron Accelerants on Anaerobic Digestion: Methane Production, Process Stability, and Microbial Community
4.1. Zero-Valent Iron (Fe0)
4.2. Fe3O4 and Magnetite
4.3. Fe2O3 and Hematite
4.4. Iron Salts and Other Iron Accelerants
5. Potential Engineering Application of Iron Accelerants in Anaerobic Digestion
6. Environmental Impact and Recovery of Iron Accelerants
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar]
- Adam, P.S.; Borrel, G.; Gribaldo, S. Evolutionary history of carbon monoxide dehydrogenase/acetyl-CoA synthase, one of the oldest enzymatic complexes. Proc. Natl. Acad. Sci. USA 2018, 115, E1166–E1173. [Google Scholar] [PubMed]
- Ajayi-Banji, A.A.; Rahman, S. Efficacy of magnetite (Fe3O4) nanoparticles for enhancing solid-state anaerobic co-digestion: Focus on reactor performance and retention time. Bioresour. Technol. 2021, 324, 124670. [Google Scholar]
- Akar, A.A.; Yousry, E.; Seif, R.; Allam, N.K. Optimizing biogas production with recycled iron nanoparticles: A sustainable approach to anaerobic digestion. Fuel 2025, 386, 134266. [Google Scholar]
- Ambuchi, J.J.; Zhang, Z.; Shan, L.; Liang, D.; Zhang, P.; Feng, Y. Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment. Water Res. 2017, 117, 87–94. [Google Scholar] [PubMed]
- Amen, T.W.M.; Eljamal, O.; Khalil, A.M.E.; Matsunaga, N. Evaluation of nano zero valent iron effects on fermentation of municipal anaerobic sludge and inducing biogas production. IOP Conf. Ser. Earth Environ. Sci. 2017, 67, 012004. [Google Scholar]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi)conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar]
- Bainotti, A.E.; Nishio, N. Growth kinetics of Acetobacterium sp. on methanol-formate in continuous culture. J. Appl. Microbiol. 2000, 88, 191–201. [Google Scholar]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar]
- Cai, C.; Leu, A.O.; Xie, G.-J.; Guo, J.; Feng, Y.; Zhao, J.-X.; Tyson, G.W.; Yuan, Z.; Hu, S. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. [Google Scholar]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Guivernau, M.; Burgos, L.; Riau, V.; Bonmatí, A. Nano zerovalent iron boosts methane content in biogas and reshapes microbial communities in long-term anaerobic digestion of pig slurry. Renew. Energy 2025, 239, 122133. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, C.; Zhu, J.; Jing, X.; Kong, F.; Zhang, C. Effects of waste rusted iron shavings on enhancing anaerobic digestion of food wastes and municipal sludge. J. Clean. Prod. 2020, 242, 118195. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for biogas production and H2S control from the anaerobic digestion of cattle manure: The influence of microscale waste iron powder and iron oxide nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, Z.; Hu, T.; He, S.; Liu, Y.; Jiang, J.; Zhao, Q.; Wei, L. A review of application of combined biochar and iron-based materials in anaerobic digestion for enhancing biogas productivity: Mechanisms, approaches and performance. Environ. Res. 2023, 234, 116589. [Google Scholar] [CrossRef]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of Magnetite Nanoparticles on Soybean Chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Greening, C.; Biswas, A.; Carere, C.R.; Jackson, C.J.; Taylor, M.C.; Stott, M.B.; Cook, G.M.; Morales, S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016, 10, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.F.; Lukoyanov, D.A.; Shaw, S.; Compton, P.; Tokmina-Lukaszewska, M.; Bothner, B.; Kelleher, N.; Dean, D.R.; Hoffman, B.M.; Seefeldt, L.C. Mechanism of N2 Reduction Catalyzed by Fe-Nitrogenase Involves Reductive Elimination of H2. Biochemistry 2018, 57, 701–710. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Hassanpourmoghadam, L.; Aminzadeh Goharrizi, B.; Torabian, A.; Bouteh, E.; Rittmann, B.E. Effect of Fe3O4 nanoparticles on anaerobic digestion of municipal wastewater sludge. Biomass Bioenergy 2023, 169, 106692. [Google Scholar] [CrossRef]
- Huang, W.; Yang, F.; Huang, W.; Wang, D.; Lei, Z.; Zhang, Z. Weak magnetic field significantly enhances methane production from a digester supplemented with zero valent iron. Bioresour. Technol. 2019, 282, 202–210. [Google Scholar]
- Hutchins, D.L.; Downey, J.P. Effective separation of magnetite nanoparticles within an industrial-scale pipeline reactor. Sep. Sci. Technol. 2020, 55, 2822–2829. [Google Scholar]
- Jadhav, P.; Khalid, Z.B.; Zularisam, A.W.; Krishnan, S.; Nasrullah, M. The role of iron-based nanoparticles (Fe-NPs) on methanogenesis in anaerobic digestion (AD) performance. Environ. Res. 2022, 204, 112043. [Google Scholar]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Lier, J.B.V. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2011, 14, 1646–1654. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, W. Enhanced anaerobic digestion using conductive materials through mediation of direct microbial interspecies electron transfer: A review. Fermentation 2023, 9, 884. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; He, X.; Liang, D.; Liu, X.; He, C.; Shen, D.; Jiao, Y. Investigation into the effects of different recycled magnetic additives on anaerobic co-digestion of sludge and straw. Fuel 2024, 358, 130245. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Pang, L.; Lu, Y.; Yang, P. Dosage effect of micron zero-valent iron during thermophilic anaerobic digestion of waste activated sludge: Performance and functional community. Environ. Res. 2023, 237, 116997. [Google Scholar]
- Liang, L.; Zhao, Z.; Zhou, H.; Zhang, Y. Insights into feasibility and microbial characterizations on simultaneous elimination of dissolved methane from anaerobic effluents and nitrate/nitrite reduction in a conventional anoxic reactor with magnetite. Water Res. 2024, 256, 121567. [Google Scholar] [PubMed]
- Liu, H.; Wen, J.; Liu, Q.; Li, R.; Lichtfouse, E.; Maurer, C.; Huang, J. Enhanced performances of anaerobic digestion processes treating organic wastes: Role of iron and carbon based nanomaterials. Surf. Interfaces 2023, 43, 103548. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Enhancing the performance and stability of the anaerobic digestion of sewage sludge by zero valent iron nanoparticles dosage. Bioresour. Technol. 2019, 275, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896–4906. [Google Scholar] [CrossRef]
- Lu, T.; Su, T.; Liang, X.; Wei, Y.; Zhang, J.; He, T. Dual character of methane production improvement and antibiotic resistance genes reduction by nano-Fe2O3 addition during anaerobic digestion of swine manure. J. Clean. Prod. 2022, 376, 134240. [Google Scholar] [CrossRef]
- Lytras, G.; Lytras, C.; Mathioudakis, D.; Papadopoulou, K.; Lyberatos, G. Food Waste Valorization Based on Anaerobic Digestion. Waste Biomass Valoriz. 2021, 12, 1677–1697. [Google Scholar] [CrossRef]
- Matsumura, H.; Faponle, A.S.; Hagedoorn, P.L.; Tosha, T.; de Visser, S.P.; Moënne-Loccoz, P. Mechanism of substrate inhibition in cytochrome-c dependent NO reductases from denitrifying bacteria (cNORs). J. Inorg. Biochem. 2022, 231, 111781. [Google Scholar] [CrossRef]
- Pang, L.; Chen, J.; Li, W.; Chatzisymeon, E.; Xu, K.; Yang, P. Particle size of zero-valent iron affects the risks from antibiotic resistance genes in waste activated sludge during anaerobic digestion. J. Hazard. Mater. 2025, 490, 137785. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-H.; Je Seong, H.; Sul, W.J.; Jin, K.-H.; Park, H.-D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef]
- Phelan, V.V.; Liu, W.T.; Pogliano, K.; Dorrestein, P.C. Microbial metabolic exchange--the chemotype-to-phenotype link. Nat. Chem. Biol. 2011, 8, 26. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Wang, T.; Ren, J.; Cao, Y.; Zhou, S. Impacts of ferric chloride, ferrous chloride and solid retention time on the methane-producing and physicochemical characterization in high-solids sludge anaerobic digestion. Renew. Energy 2019, 139, 1290–1298. [Google Scholar] [CrossRef]
- Qualhato, G.; Rocha, T.L.; de Oliveira Lima, E.C.; e Silva, D.M.; Cardoso, J.R.; Koppe Grisolia, C.; de Sabóia-Morais, S.M.T. Genotoxic and mutagenic assessment of iron oxide (maghemite-γ-Fe2O3) nanoparticle in the guppy Poecilia reticulata. Chemosphere 2017, 183, 305–314. [Google Scholar] [PubMed]
- Ragasri, S.; Vasa, T.N.; Sabumon, P.C. A mini review on effect of nano particles of Fe in the anaerobic digestion of waste activated sludge. Mater. Today Proc. 2021, 51, 1482–1488. [Google Scholar]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [PubMed]
- Ross, M.O.; Rosenzweig, A.C. A tale of two methane monooxygenases. JBIC J. Biol. Inorg. Chem. 2016, 22, 307–319. [Google Scholar]
- Scherer, P.; Lippert, H.; Wolff, G.J.B.T.E.R. Composition of the major elements and trace elements of 10 methanogenic bacteria determined by inductively coupled plasma emission spectrometry. Biol. Trace Elem. Res. 1983, 5, 149–163. [Google Scholar]
- Schmidt, J.E.; Ahring, B.K. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 1993, 59, 2546–2551. [Google Scholar]
- Schmidt, J.E.; Ahring, B.K. Interspecies Electron Transfer during Propionate and Butyrate Degradation in Mesophilic, Granular Sludge. Appl. Environ. Microbiol. 1995, 61, 2765. [Google Scholar]
- Shen, D.; Zhang, P.; Wu, S.-L.; Long, Y.; Wei, W.; Ni, B.-J. Enhanced biomethane production from waste activated sludge anaerobic digestion by ceramsite and amended Fe2O3 ceramsite. J. Environ. Manag. 2024, 351, 119973. [Google Scholar]
- Su, K.; Li, L.; Wang, Q.; Cao, R. A Review on the Interspecies Electron Transfer of Methane Production in Anaerobic Digestion System. Fermentation 2023, 9, 467. [Google Scholar] [CrossRef]
- Su, L.; Shi, X.; Guo, G.; Zhao, A.; Zhao, Y. Stabilization of sewage sludge in the presence of nanoscale zero-valent iron (nZVI): Abatement of odor and improvement of biogas production. J. Mater. Cycles Waste Manag. 2013, 15, 461–468. [Google Scholar]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Zhang, M.; Xiong, P.; Liu, L.; Bao, Y.; Zhao, Z. Link between characteristics of Fe(III) oxides and critical role in enhancing anaerobic methanogenic degradation of complex organic compounds. Environ. Res. 2021, 194, 110498. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Jin, S.; Chen, H.; Xu, X.; Wang, Z.; Xing, B.; Zhu, L. Responsiveness extracellular electron transfer (EET) enhancement of anaerobic digestion system during start-up and starvation recovery stages via magnetite addition. Bioresour. Technol. 2019, 272, 162–170. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.; Liang, X.; Cheng, M.; Ren, L. Effects of nanoscale zero-valent iron on the performance and the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2019, 293, 122092. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Li, Y.; Su, Y.; Wu, D.; Xie, B. Enhanced anaerobic digestion performance of food waste by zero-valent iron and iron oxides nanoparticles: Comparative analyses of microbial community and metabolism. Bioresour. Technol. 2023, 371, 128633. [Google Scholar] [CrossRef]
- Wang, P.; Wu, D.; Su, Y.; Xie, B. Mitigated dissemination of antibiotic resistance genes by nanoscale zero-valent iron and iron oxides during anaerobic digestion: Roles of microbial succession and regulation. J. Hazard. Mater. 2024, 473, 134636. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Lv, N.; Pan, X.; Cai, G.; Ning, J.; Zhu, G. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion. Bioresour. Technol. 2021, 324, 124671. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Liu, M.; Gu, L.; Xu, L.; Li, J.; Ao, L. Enhancement of anaerobic digestion of high salinity food waste by magnetite and potassium ions: Digestor performance, microbial and metabolomic analyses. Bioresour. Technol. 2023, 388, 129769. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.M.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Wu, L.; Shen, Z.; Zhou, Y.; Zuo, J. Stimulating anaerobic digestion to degrade recalcitrant organic pollutants: Potential role of conductive materials-led direct interspecies electron transfer. J. Environ. Manag. 2023, 344, 118337. [Google Scholar]
- Wu, Z.; Ji, S.; Li, Y.-Y.; Liu, J. A review of iron use and recycling in municipal wastewater treatment plants and a novel applicable integrated process. Bioresour. Technol. 2023, 379, 129037. [Google Scholar]
- Xu, X.-J.; Yan, J.; Yuan, Q.-K.; Wang, X.-T.; Yuan, Y.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Enhanced methane production in anaerobic digestion: A critical review on regulation based on electron transfer. Bioresour. Technol. 2022, 364, 128003. [Google Scholar]
- Xu, Y.; Wang, M.; Yu, Q.; Zhang, Y. Enhancing methanogenesis from anaerobic digestion of propionate with addition of Fe oxides supported on conductive carbon cloth. Bioresour. Technol. 2020, 302, 122796. [Google Scholar]
- Yang, H.; Liu, L.; Shu, Z.; Zhang, W.; Huang, C.; Zhu, Y.; Li, S.; Wang, W.; Li, G.; Zhang, Q.; et al. Magnetic iron oxide nanoparticles: An emerging threat for the environment and human health. J. Environ. Sci. 2025, 152, 188–202. [Google Scholar]
- Yang, Y.; Cheng, X.; Rene, E.R.; Qiu, B.; Hu, Q. Effect of iron sources on methane production and phosphorous transformation in an anaerobic digestion system of waste activated sludge. Bioresour. Technol. 2024, 395, 130315. [Google Scholar]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar]
- Yao, B.; Liu, M.; Tang, T.; Hu, X.; Yang, C.; Chen, Y. Enhancement of anaerobic digestion of ciprofloxacin wastewater by nano zero-valent iron immobilized onto biochar. Bioresour. Technol. 2023, 385, 129462. [Google Scholar]
- Ye, J.; Hu, A.; Ren, G.; Chen, M.; Tang, J.; Zhang, P.; Zhou, S.; He, Z. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud. Water Res. 2018, 134, 54–62. [Google Scholar]
- You, G.; Wang, C.; Wang, P.; Chen, J.; Gao, Y.; Li, Y.; Xu, Y. Long-term transformation of nanoscale zero-valent iron explains its biological effects in anaerobic digestion: From ferroptosis-like death to magnetite-enhanced direct electron transfer networks. Water Res. 2023, 241, 120115. [Google Scholar]
- Yu, B.; Lou, Z.; Zhang, D.; Shan, A.; Yuan, H.; Zhu, N.; Zhang, K. Variations of organic matters and microbial community in thermophilic anaerobic digestion of waste activated sludge with the addition of ferric salts. Bioresour. Technol. 2015, 179, 291–298. [Google Scholar]
- Yu, M.; Shao, H.; Wang, P.; Ren, L. Metagenomic analysis reveals the mechanisms of biochar supported nano zero-valent iron in two-phase anaerobic digestion of food waste: Microbial community, CAZmey, functional genes and antibiotic resistance genes. J. Environ. Manag. 2024, 366, 121763. [Google Scholar]
- Yuan, T.; Ko, J.H.; Zhou, L.; Gao, X.; Xu, Q. Iron oxide alleviates acids stress by facilitating syntrophic metabolism between Syntrophomonas and methanogens. Chemosphere 2020, 247, 125866. [Google Scholar]
- Yusuf, H.H.; Xiaofang, P.; Ye, Z.-L.; Abdelwahab, T.A.M.; Fodah, A.E.M. A novel strategy for enhancing high solid anaerobic digestion of fecal slag and food waste using percolate recirculation and dosage of nano zero-valent iron. Water Res. 2024, 267, 122477. [Google Scholar] [PubMed]
- Zhang, B.; Zhao, Z.; Ma, R.; Chen, N.; Kong, Z.; Lei, Z.; Zhang, Z. Unveiling the mechanisms of Fe(III)-loaded chitosan composite (CTS-Fe) in enhancing anaerobic digestion of waste activated sludge. J. Environ. Sci. 2024, 138, 200–211. [Google Scholar]
- Zhang, H.; Fu, Z.; Guan, D.; Zhao, J.; Wang, Y.; Zhang, Q.; Xie, J.; Sun, Y.; Guo, L.; Wang, D. A comprehensive review on food waste anaerobic co-digestion: Current situation and research prospect. Process Saf. Environ. Prot. 2023, 179, 546–558. [Google Scholar]
- Zhang, J.; Zhang, Y.; Quan, X.; Liu, Y.; An, X.; Chen, S.; Zhao, H. Bioaugmentation and functional partitioning in a zero valent iron-anaerobic reactor for sulfate-containing wastewater treatment. Chem. Eng. J. 2011, 174, 159–165. [Google Scholar]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Bioresour. Technol. 2019, 275, 172–182. [Google Scholar]
- Zhang, W.; Li, L.; Wang, X.; Xing, W.; Li, R.; Yang, T.; Lv, D. Role of trace elements in anaerobic digestion of food waste: Process stability, recovery from volatile fatty acid inhibition and microbial community dynamics. Bioresour. Technol. 2020, 315, 123796. [Google Scholar]
- Zhang, W.; Xing, W.; Li, R. Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Bioresour. Technol. 2018, 265, 82–92. [Google Scholar]
- Zhang, W.; Zhang, L.; Li, A. Anaerobic co-digestion of food waste with MSW incineration plant fresh leachate: Process performance and synergistic effects. Chem. Eng. J. 2015, 259, 795–805. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renew. Energy 2020, 146, 2724–2735. [Google Scholar]
- Zhao, Z.; Li, Y.; Zhang, Y. Engineering enhanced anaerobic digestion: Benefits of ethanol fermentation pretreatment for boosting direct interspecies electron transfer. Energy 2021, 228, 120643. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; An, Y.; Huang, L.; Zhang, G. Enhancing anaerobic digestion of waste activated sludge with iron modified tea-based biochar via improving electron transfer and metabolic activity. Renew. Energy 2025, 242, 122458. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, F.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure. Bioresour. Technol. 2022, 346, 126438. [Google Scholar]
- Zhu, R.; Chen, Y.; Zhao, T.; Jiang, Q.; Wang, H.; Zheng, L.; Shi, D.; Zhai, J.; He, Q.; Gu, L. Enhanced mesophilic anaerobic co-digestion of waste sludge and food waste by using hematite (α-Fe2O3) supported bentonite as additive. Bioresour. Technol. 2020, 313, 123603. [Google Scholar] [CrossRef]



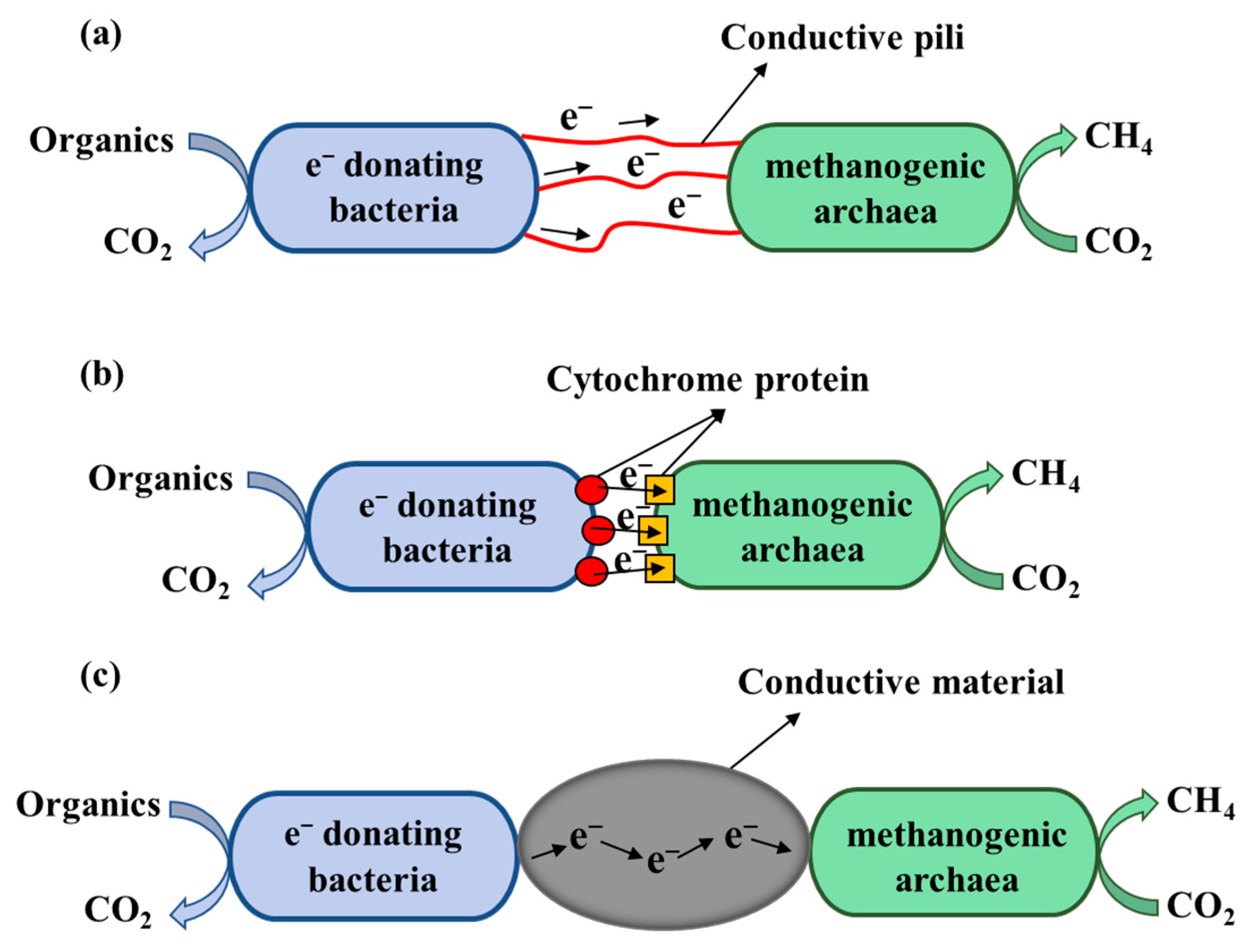
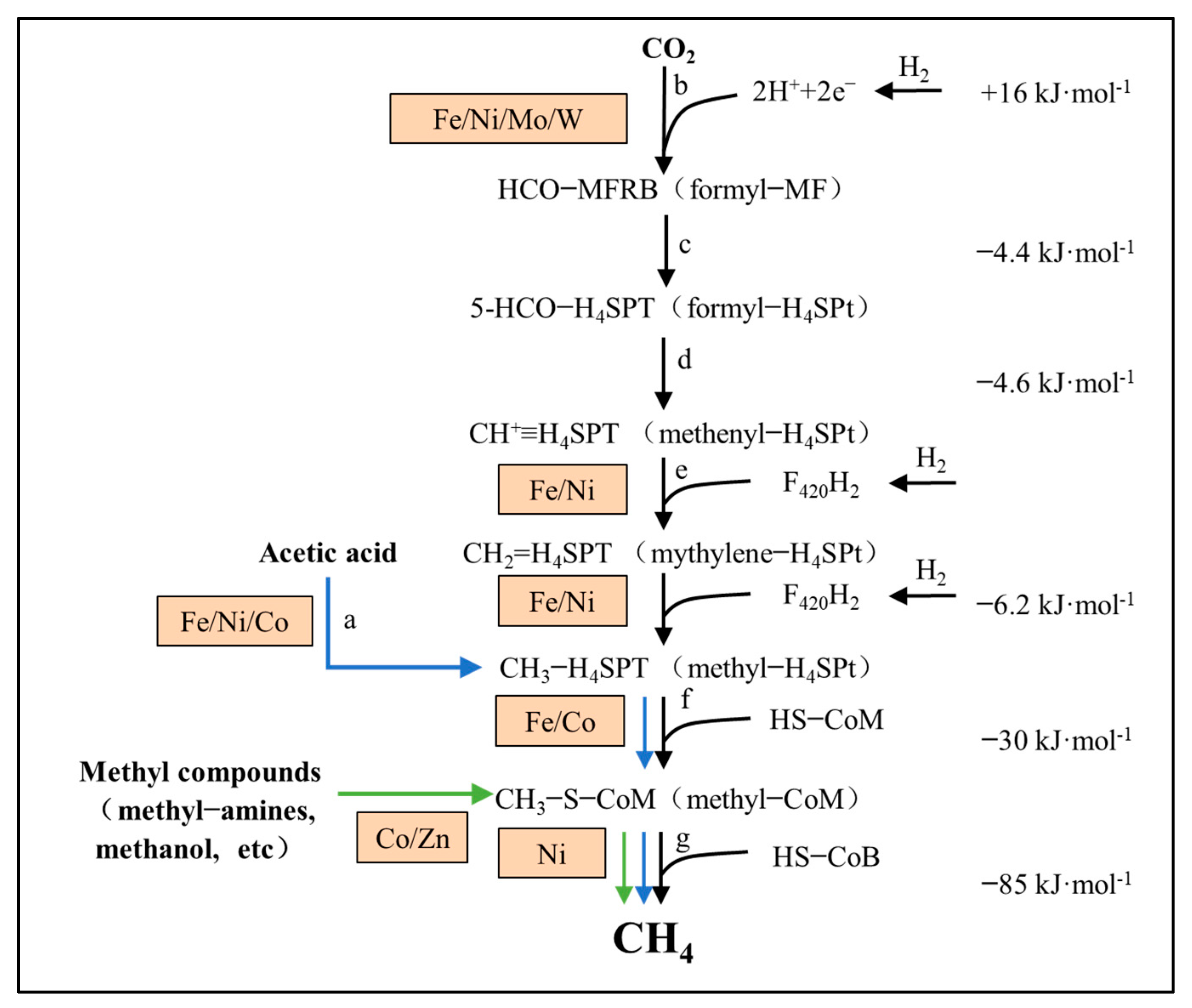
| Substrate | Acetogenesis Reaction | Standard Gibbs Free Energy ΔG0′ (kJ/mol) |
|---|---|---|
| Propionate | CH3CH2COO− + 3H2O→CH3COO− + HCO3− + 3H2 + H+ | +76.1 |
| Butyrate | CH3CH2CH2COO− + 2H2O→2CH3COO− + 2H2 + H+ | +48.1 |
| Ethanol | CH3CH2OH + H2O→CH3COO− + 2H2 + H+ | +9.6 |
| Element | Enzyme | Microorganism | Reference |
|---|---|---|---|
| Fe | Carbon monoxide dehydrogenase | Methanogen/Acetogen | [37] |
| Superoxide Dismutase | Methanogen | [38] | |
| Hydrogenase | Desulfovibrio/Esherichia coli | [39] | |
| Acetyl coenzyme A synthetase | Moorella thermoacetica | [40] | |
| NO reductase | Pseudomonospore denitrifying bacteria | [41] | |
| Nitrite reductase | Pseudomonas stutzeri | [42] | |
| Nitrate reductase | Paracoccus denitrificans | [42] | |
| Nitrogenase | [43] | ||
| Methane monooxygenase | [44] |
| Substrate | Operating Mode | Particle Size of Fe0 | Dosage | Inoculum Ratio (Substrate: Inoculum, on vs. Basis) | Reaction Temperature | Reaction Time | Effects on Anaerobic Digestion | Reference |
|---|---|---|---|---|---|---|---|---|
| Swine manure | Batch | 500 nm | 5 g/L | 1:1 | 35 °C | 30 days | Methane yield increased by 17.16%; relative abundance of bacteria Bacteroidia and Gammaproteobacteria increased by 19.4% and 10.3%, respectively; relative abundance of archaea Methanothrix and Methanolinea increased by 19.8% and 16.2%, respectively. | [47] |
| Fresh manure | Batch | 9 nm | 5–20 mg/L | - | 37 °C | 50 days | Biogas yield was 1.44–1.45 folds of that in the control trial; methane production was 1.38–1.59 folds of that in the control trial. The growth rate of gas production was proportional to the dosage of Fe0. | [49] |
| Food waste | Batch | 40 nm | 2 g/L | 2:1 | 35 °C | 15 days | Biogas yield increased by 62.58%; methane yield increased by 35.47%. | [46] |
| Food waste | Batch | 300–600 nm | 2–10 g/L | 1:1 | 37 °C | 65 days | Methane yield increased by 2.7–8.5%. | [54] |
| Food waste | Batch | 110 nm | 20–60 mg/L | 1.5:1 | 35 °C | 23 days | Methane production did not show significant change. | [50] |
| Artificial wastewater | Batch | 100 nm | 5 g/L | - | 37 °C | 54 h | Methane yield increased by 23.9%. | [45] |
| Activated sludge | Batch | 150 μm | 25–250 mg/g TS | - | 55 °C | 32 days | Methane yield reached 0.8–12 folds of that in the control trial. | [8] |
| Activated sludge | Batch | < 100 nm | 250 mg/L | - | 37 °C | 14 days | Biogas yield increased by 25.23%. | [51] |
| Activated sludge | Batch | 40–60 nm | 5–9 mg/g VS | 0.5:1 | 36 °C | 40 days | Biogas yield rose with the increasing concentration of Fe0; the greatest increment of biogas yield reached 135%. | [52] |
| Activated sludge | Batch | 20 nm | 0.1% | - | 37 °C | 17 days | The concentration of H2S decreased by 98.0%; biogas yield increased by 30.4%; methane yield increased by 40.4%. | [7] |
| Activated sludge | Batch | 50 nm | 1% | - | 37 °C | 12 days | Methane yield decreased by 29.7%. | [53] |
| Iron Accelerant | Substrate | Operating Mode | Particle Size of Fe3O4 and Magnetite | Dosage | Inoculum Ratio (Substrate: Inoculum, on vs. Basis) | Reaction Temperature | Reaction Time | Effects on Anaerobic Digestion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | Poultry litter | Batch | - | 15 mg/L | 1:1 | 35 °C | 79 days | Methane yield increased by 27.5%. | [58] |
| Nano Fe3O4 | Dairy manure (55.7%) and corn stover (7.2%) | Batch | 15–20 nm | 20 mg/L | 2.5:1 | 35 °C | 80 days | Reaction time reduced by 27 days; methane yield increased. | [56] |
| Nano Fe3O4 | Fresh manure | Batch | 7 nm | 5–20 mg/L | - | 37 °C | 50 days | Biogas yield rose to 1.63–1.66 folds of that in the control trial; methane yield reached 1.82–1.96 folds of that in the control trial. | [49] |
| Nano Fe3O4 | Food waste | Batch | 29.5 nm | 25–80 mg/L | 1.5:1 | 35 °C | 28 days | Methane yield increased by 7–50.8%. | [50] |
| Nano Fe3O4 | Activated sludge | Batch | 20–30 nm | 20–200 mg/L | - | 36 °C | 12 days | Methane yield increased by 1.1–1.6 folds compared to the control. | [55] |
| Nano Fe3O4 | Activated sludge | Batch | 12–18 nm and 50–100 nm | 40–250 mg/L | 1.5:1 | 37 °C | 25 days | 120 mg/L of Fe3O4 (12–18 nm) enhanced methane yield by 1.7 folds compared to the control; 250 mg/L of Fe3O4 (50–100 nm) increased methane yield by 1.4 folds compared to the control. | [61] |
| Waste iron powder (85% Fe3O4) | Dairy manure | Batch | <20 μm | 1000 mg/L | 1:3 | 38 °C | 30 days | H2S concentration decreased by 77.24%; methane yield increased by 56.89%. | [59] |
| Magnetite | Food waste | Batch | 300–600 nm | 2–10 g/L | 1:1 | 37 °C | 65 days | Methane yield enhanced by 3.2–6%. | [54] |
| Magnetite | High salinity food waste | Semi-continuous | - | 2 g/L | - | 35 °C | 108 days | Methane yield decreased by 18.9%. | [62] |
| Magnetite | Activated sludge | Batch | 20 nm | 1% | - | 37 °C | 12 days | Methane yield decreased by 11.5%. | [53] |
| Iron Accelerant | Substrate | Operating Mode | Particle Size of Fe2O3 and Hematite | Dosage | Inoculum Ratio (Substrate: Inoculum, on vs. Basis) | Reaction Temperature | Reaction Time | Effects on Anaerobic Digestion | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | Beet sugar industrial wastewater | Batch | 20 nm | 750 mg/L | - | 36 °C | 74 days | Methane yield increased by 28.9%; chemical oxygen demand concentration decreased by 21.8%. | [65] |
| Red mud (45.46% of hematite) | Activated sludge | Batch | 50–300 µm | 20 g/L | - | 35 °C | 32 days | Methane yield enhanced by 35.52%. | [67] |
| Fe2O3 | Food waste | Batch | 300–600 nm | 2–10 g/L | 1:1 | 37 °C | 65 days | Methane yield increased by 4.4–6.7%. | [54] |
| Fe2O3 | Dairy manure | Batch | 20–40 nm | 1000 mg/L | - | 38 °C | 30 days | Methane yield rose by 21.11%; H2S concentration significantly decreased. | [59] |
| Fe2O3 | Swine manure | Batch | 30 nm | 75–350 mmol/L | 3:1 | 37 °C | 30 days | Anaerobic digestion reaction was enhanced by Fe2O3 in the first twelve days but then methane production was inhibited by Fe2O3; the inhibition level was proportional to the dosage of Fe2O3; methane yield decreased by 7.8%. | [66] |
| Iron Accelerant | Substrate | Operating Mode | Dosage | Inoculum Ratio (Substrate: Inoculum, on vs. Basis) | Reaction Temperature | Reaction Time | Effects on Anaerobic Digestion | Reference |
|---|---|---|---|---|---|---|---|---|
| FeCl3 | Activated sludge | Batch | 200 mg/L | - | 55 °C | 48 days | Biogas yield increased by 79.6%. | [68] |
| FeCl2 | Dewatered sludge | Batch | 100–1000 mg/L | 2:1 | 35 °C | 27 days | Methane yield enhanced by 6.4%. | [69] |
| FeCl3 | Dewatered sludge | Batch | 100–800 mg/L | 2:1 | 35 °C | 27 days | Methane increased by 28.9%. | [69] |
| Fe2O3-carbon cloth | Sodium propionate (30 mM) | Batch | - | - | 37 °C | 24 days | Methane yield rose by 15.4%; propionate degradation rate increased by 19.67%. | [70] |
| Fe2O3-ceramsite | Activated sludge | Batch | 10 g/L | 2:1 | 35 °C | 55 days | Methane yield increased to 1.4 folds of that in the control trial. | [71] |
| Rusted iron (FeOOH-Fe2O3) | Food waste and municipal sludge | Batch | - | - | 35 °C | 36 days | Methane yield increased by 64.4%; the peak value of methane production rate enhanced by 12.2%. | [73] |
| α-Fe2O3-bentonite | Food waste | Batch | 0.5–3.75 g/g VS | 2:1 | 37 °C | 45 days | Biogas yield increased by 60.6–232.1%; methane yield rose to 3.3–12.3 folds of that in the control trial. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, W.; Xing, W.; Li, R. Review on Mechanisms of Iron Accelerants and Their Effects on Anaerobic Digestion. Agriculture 2025, 15, 728. https://doi.org/10.3390/agriculture15070728
Wang H, Zhang W, Xing W, Li R. Review on Mechanisms of Iron Accelerants and Their Effects on Anaerobic Digestion. Agriculture. 2025; 15(7):728. https://doi.org/10.3390/agriculture15070728
Chicago/Turabian StyleWang, Han, Wanli Zhang, Wanli Xing, and Rundong Li. 2025. "Review on Mechanisms of Iron Accelerants and Their Effects on Anaerobic Digestion" Agriculture 15, no. 7: 728. https://doi.org/10.3390/agriculture15070728
APA StyleWang, H., Zhang, W., Xing, W., & Li, R. (2025). Review on Mechanisms of Iron Accelerants and Their Effects on Anaerobic Digestion. Agriculture, 15(7), 728. https://doi.org/10.3390/agriculture15070728





