Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Records
2.2. Environment Variables
2.3. Model Construction
2.4. Model Evaluation and Habitat Suitability Classification
3. Results
3.1. Evaluation of Individual Models and Selection of the Ensemble Model
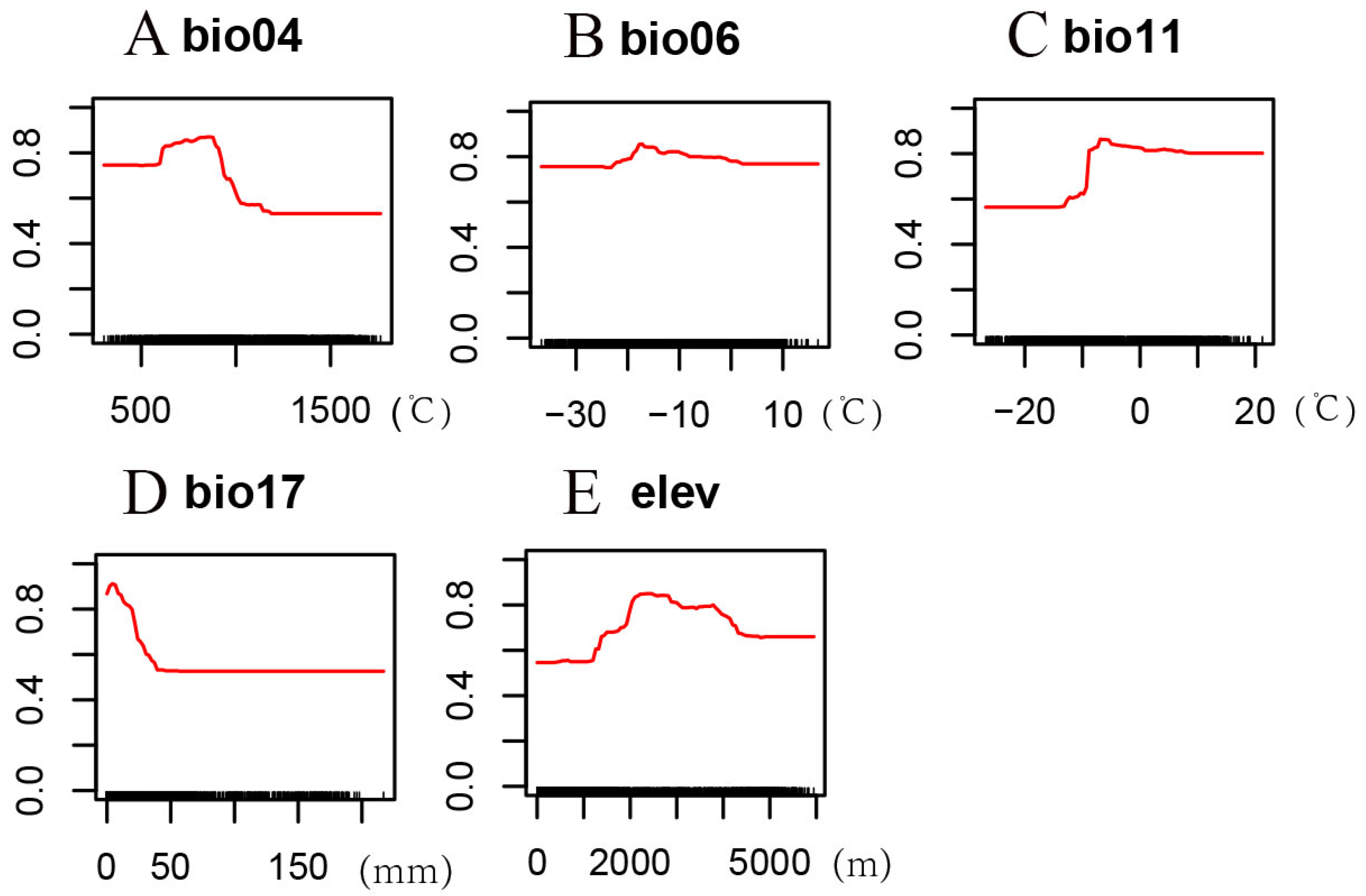
3.2. Environmental Factor Analysis
3.3. Current Climate Suitability Analysis
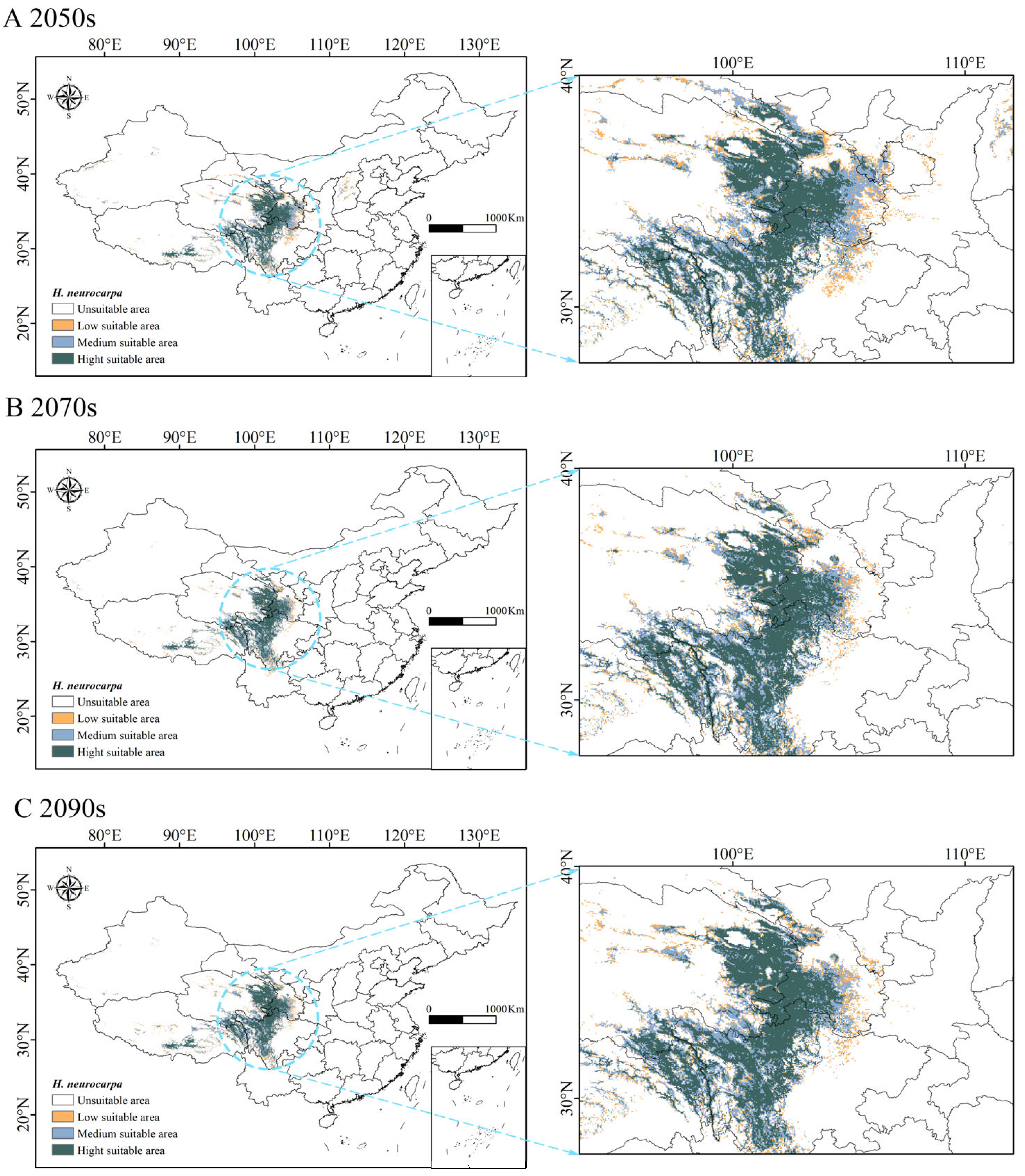
3.4. Prediction of Potential Suitable Habitat Under Future Climate Scenarios
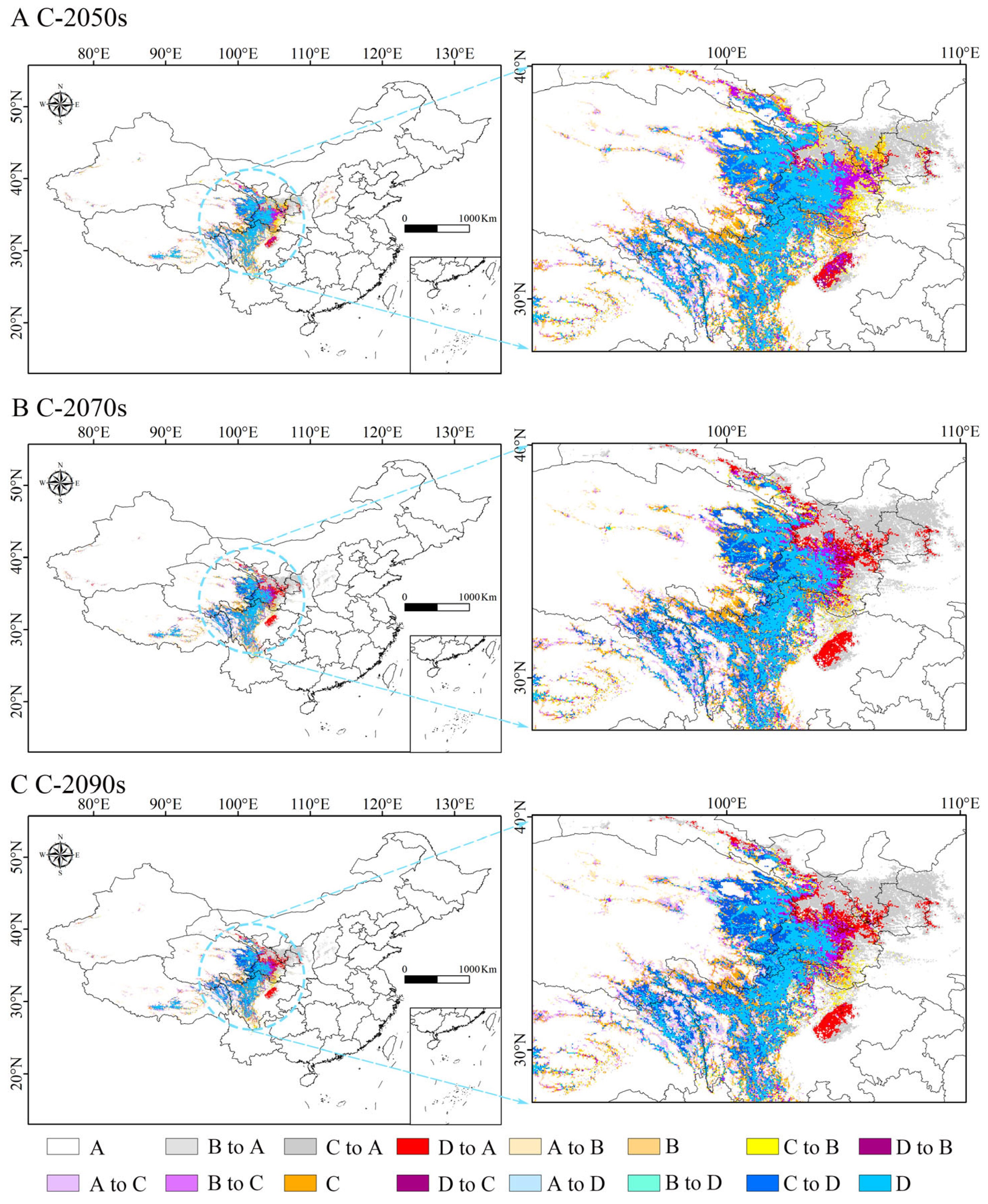
3.5. Shrinkage and Expansion of Potential Suitable Habitat for H. neurocarpa in the Future
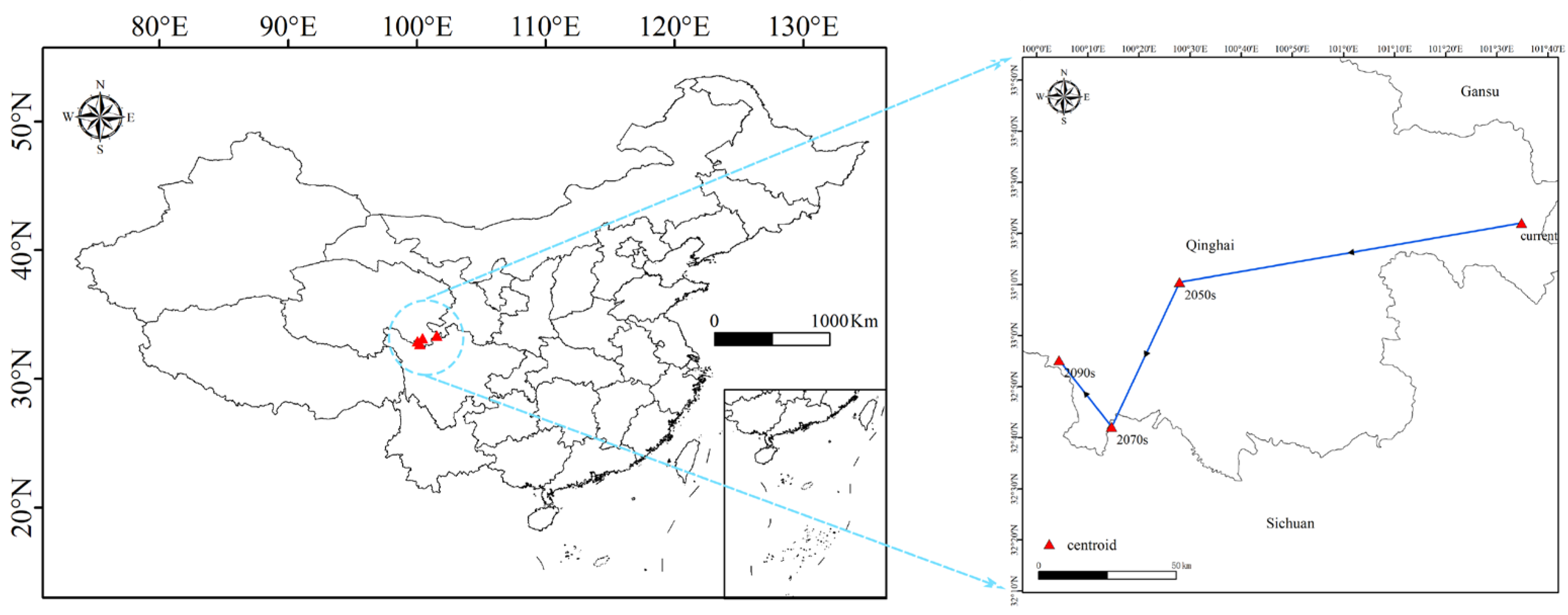
3.6. Centroid Shifts of H. neurocarpa Under Current and Future Scenarios
4. Discussion
4.1. Evaluation of the Integrated Model
4.2. Environmental Variables Affecting the Potential Distribution of H. neurocarpa
4.3. Distribution Changes of H. neurocarpa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, P.; Li, J.; Li, Y.; Xu, N.; Gao, Y.; Guo, L.; Huo, T.; Peng, C.; Meng, F. Habitat Protection and Planning for Three Ephedra Using the MaxEnt and Marxan Models. Ecol. Indic. 2021, 133, 108399. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Z.; Wang, Y.; Zhao, W. Spatial Distribution Characteristics of Suitable Planting Areas for Pyrus Species under Climate Change in China. Plants 2023, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Vanalli, C.; Casagrandi, R.; Gatto, M.; Bevacqua, D. Shifts in the Thermal Niche of Fruit Trees under Climate Change: The Case of Peach Cultivation in France. Agric. For. Meteorol. 2021, 300, 108327. [Google Scholar] [CrossRef]
- Sanczuk, P.; Verheyen, K.; Lenoir, J.; Zellweger, F.; Lembrechts, J.J.; Rodríguez-Sánchez, F.; Baeten, L.; Bernhardt-Römermann, M.; De Pauw, K.; Vangansbeke, P.; et al. Unexpected Westward Range Shifts in European Forest Plants Link to Nitrogen Deposition. Science 2024, 386, 193–198. [Google Scholar] [CrossRef]
- Mei, D.; Ma, X.; Fu, F.; Cao, F. Research Status and Development Prospects of Sea Buckthorn (Hippophae rhamnoides L.) Resources in China. Forests 2023, 14, 2461. [Google Scholar] [CrossRef]
- Ma, Q.-G.; He, N.-X.; Huang, H.-L.; Fu, X.-M.; Zhang, Z.-L.; Shu, J.-C.; Wang, Q.-Y.; Chen, J.; Wu, G.; Zhu, M.-N.; et al. Hippophae Rhamnoides L.: A Comprehensive Review on the Botany, Traditional Uses, Phytonutrients, Health Benefits, Quality Markers, and Applications. J. Agric. Food Chem. 2023, 71, 4769–4788. [Google Scholar] [CrossRef]
- He, N.; Wang, Q.; Huang, H.; Chen, J.; Wu, G.; Zhu, M.; Shao, F.; Yan, Z.; Sang, Z.; Cao, L.; et al. A Comprehensive Review on Extraction, Structure, Detection, Bioactivity, and Metabolism of Flavonoids from Sea Buckthorn (Hippophae rhamnoides L.). J. Food Biochem. 2023, 2023, 4839124. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, Health Benefits, and Food Applications of Sea Buckthorn (Hippophae rhamnoides L.): A Comprehensive Review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Fang, L.; Li, J.; Han, S.; Li, Y.; Li, Y. Sex-Related Ecophysiological Responses of Hippophae rhamnoide Saplings to Simulate Sand Burial Treatment in Desertification Areas. Forests 2023, 14, 101. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Luo, D.; Quan, H.; Bianba, D.; Zhang, W. Comparative Drought Tolerance of Six Native Deciduous and Broad-Leaved Woody Plant Species Seedlings in the Qinghai–Tibet Plateau. Acta Physiol. Plant 2016, 38, 14. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, G.; Ma, Y. Development and Application of Microsatellite Markers in Hippophae rhamnoides Subsp. Sinensis Rousi (Hippophae rhamnoides L.) Based on Transcriptome Sequencing. Front. Genet. 2024, 15, 1373028. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Zhou, H.; Bai, Z.; Sun, K. The Study on Sea Buckthorn (Genus hippophae L.) Fruit Reveals Cell Division and Cell Expansion to Promote Morphogenesis. Plants 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, N.; Dong, Q.; Wang, H.; Wang, Y. Complete Chloroplast Genome Sequences of Hippophae Neurocarpa. Mitochondrial DNA Part B 2019, 4, 2048–2049. [Google Scholar] [CrossRef]
- Kou, Y.; Wu, Y.; Jia, D.; Li, Z.; Wang, Y. Range expansion, genetic differentiation, and phenotypic adaption of Hippophaë neurocarpa (Elaeagnaceae) on the Qinghai–Tibet Plateau. J. Syst. Evol. 2014, 52, 303–312. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A Review of Evidence about Use and Performance of Species Distribution Modelling Ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Franklin, J. Species Distribution Modelling Supports the Study of Past, Present and Future Biogeographies. J. Biogeogr. 2023, 50, 1533–1545. [Google Scholar] [CrossRef]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in Species Distribution Modelling in Context of Rare and Endemic Plants: A Systematic Review. Ecol. Process 2022, 11, 40. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is My Species Distribution Model Fit for Purpose? Matching Data and Models to Applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Thuiller, W. BIOMOD—Optimizing Predictions of Species Distributions and Projecting Potential Future Shifts under Global Change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Xian, X.; Zhu, J.; Chen, B.; Jia, T.; Wang, R.; Liu, W. Geographical Distribution Pattern and Ecological Niche of Solenopsis Invicta Buren in China under Climate Change. Diversity 2023, 15, 607. [Google Scholar] [CrossRef]
- Lantschner, M.V.; de la Vega, G.; Corley, J.C. Predicting the Distribution of Harmful Species and Their Natural Enemies in Agricultural, Livestock and Forestry Systems: An Overview. Int. J. Pest. Manag. 2019, 65, 190–206. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing Whether Ensemble Modelling Is Advantageous for Maximising Predictive Performance of Species Distribution Models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Liang, Y. Analysis of the Potential Distribution of Shoot Blight of Larch in China Based on the Optimized MaxEnt and Biomod2 Ensemble Models. Forests 2024, 15, 1313. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.; Yue, J.; Wang, Z.; Zou, H.; Ji, X.; Zhang, S.; Liu, Z. Prediction of Potential Suitable Areas and Priority Protection for Cupressus Gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef]
- Cong, Y.; Gu, Y.; Wang, W.J.; Wang, L.; Xue, Z.; Chen, Y.; Jin, Y.; Xu, J.; Li, M.-H.; He, H.S.; et al. The Interaction between Temperature and Precipitation on the Potential Distribution Range of Betula ermanii in the Alpine Treeline Ecotone on the Changbai Mountain. For. Ecosyst. 2024, 11, 100166. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Stöcklin, J.; Ranjitkar, S.; Sun, H.; Song, B. Global Warming Pushes the Distribution Range of the Two Alpine ‘Glasshouse’ Rheum Species North- and Upwards in the Eastern Himalayas and the Hengduan Mountains. Front. Plant Sci. 2022, 13, 925296. [Google Scholar] [CrossRef]
- Booth, T.H. Checking Bioclimatic Variables That Combine Temperature and Precipitation Data before Their Use in Species Distribution Models. Austral Ecol. 2022, 47, 1506–1514. [Google Scholar] [CrossRef]
- Li, R.; Hu, X.; Li, Q.; Liu, L.; He, Y.; Chen, C. Gap Analysis of Firmiana danxiaensis, a Rare Tree Species Endemic to Southern China. Ecol. Indic. 2024, 158, 111606. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Shen, S.; Zhao, Y.; Hao, S.; Jiang, J.; Zhang, D. Analyzing the Distribution Patterns and Dynamic Niche of Magnolia grandiflora L. in the United States and China in Response to Climate Change. Front. Plant Sci. 2024, 15, 1440610. [Google Scholar] [CrossRef]
- Ullah, I.; Saleem, F.; Iyakaremye, V.; Yin, J.; Ma, X.; Syed, S.; Hina, S.; Asfaw, T.G.; Omer, A. Projected Changes in Socioeconomic Exposure to Heatwaves in South Asia Under Changing Climate. Earths Future 2022, 10, e2021EF002240. [Google Scholar] [CrossRef]
- Wang, B.; Jin, C.; Liu, J. Understanding Future Change of Global Monsoons Projected by CMIP6 Models. J. Clim. 2020, 33, 6471–6489. [Google Scholar] [CrossRef]
- Murali, G.; Iwamura, T.; Meiri, S.; Roll, U. Future Temperature Extremes Threaten Land Vertebrates. Nature 2023, 615, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jackson, D.A. Effects of Sample Size, Data Quality, and Species Response in Environmental Space on Modeling Species Distributions. Landsc. Ecol. 2023, 38, 4009–4031. [Google Scholar] [CrossRef]
- Gxasheka, M.; Gajana, C.S.; Dlamini, P. The Role of Topographic and Soil Factors on Woody Plant Encroachment in Mountainous Rangelands: A Mini Literature Review. Heliyon 2023, 9, e20615. [Google Scholar] [CrossRef]
- Zhang, H.; Zha, T.; Yu, Y.; Zhang, Z.; Zhang, X.; Zhang, H.; Ji, X. Functional Vegetation Community Responses to Soil and Topographic Factors in the Loess Plateau of China. Land. Degrad. Dev. 2023, 34, 5355–5372. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Zhang, Y.; Huang, J.; Li, G.; Du, S. The Influence of Climatic and Human-Induced Factors on the Spatial Distribution of Invasive Plant Species Richness across the Loess Plateau. Glob. Ecol. Conserv. 2024, 54, e03083. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Y.; He, Y. Molecular Epigenetic Mechanisms for the Memory of Temperature Stresses in Plants. J. Genet. Genom. 2022, 49, 991–1001. [Google Scholar] [CrossRef]
- Song, Z.; Song, X.; Pan, Y.; Dai, K.; Shou, J.; Chen, Q.; Huang, J.; Tang, X.; Huang, Z.; Du, Y. Effects of Winter Chilling and Photoperiod on Leaf-out and Flowering in a Subtropical Evergreen Broadleaved Forest in China. For. Ecol. Manag. 2020, 458, 117766. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Zhou, X.; Geng, X.; Guo, Y.; Zhang, Y. Progress in Plant Phenology Modeling under Global Climate Change. Sci. China Earth Sci. 2020, 63, 1237–1247. [Google Scholar] [CrossRef]
- Araújo, M.B.; Ferri-Yáñez, F.; Bozinovic, F.; Marquet, P.A.; Valladares, F.; Chown, S.L. Heat Freezes Niche Evolution. Ecol. Lett. 2013, 16, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xue, D.; Zhang, X.; Fu, Y. Estimation of Daily Mean Land Surface Temperature over the Qinghai–Tibet Plateau Based on an RTM-DTC Model. Atmosphere 2023, 14, 1559. [Google Scholar] [CrossRef]
- Hou, D.; Li, N.; Qu, X.; Dong, S.; Guo, K.; Liu, C. Divergent Responses of Nutrient Biogeographical Patterns in Different Shrub Types across the Arid Region of Qinghai-Tibet Plateau. BMC Plant Biol. 2024, 24, 1150. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, P.C. DeepSAGE Based Differential Gene Expression Analysis under Cold and Freeze Stress in Seabuckthorn (Hippophae rhamnoides L.). PLoS ONE 2015, 10, e0121982. [Google Scholar] [CrossRef]
- Kashyap, P.; Deswal, R. A Novel Class I Chitinase from Hippophae rhamnoides: Indications for Participating in ICE-CBF Cold Stress Signaling Pathway. Plant Science 2017, 259, 62–70. [Google Scholar] [CrossRef]
- Yao, S.; Akram, M.A.; Hu, W.; Sun, Y.; Sun, Y.; Deng, Y.; Ran, J.; Deng, J. Effects of Water and Energy on Plant Diversity along the Aridity Gradient across Dryland in China. Plants 2021, 10, 636. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Liu, J.; Yang, G.; Lv, Z.; Zhang, J.; He, C. Transcripts and ABA-Dependent Signaling in Response to Drought Stress in Hippophae rhamnoides L. Trees 2020, 34, 1033–1045. [Google Scholar] [CrossRef]
- Sun, Z.; Han, J. Effect of Soft Rock Amendment on Soil Hydraulic Parameters and Crop Performance in Mu Us Sandy Land, China. Field Crops Res. 2018, 222, 85–93. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Shi, F.; Chai, L.; Zhao, S.; Ding, M.; Liao, Q. Nonlinear Effects of Thermokarst Lakes on Peripheral Vegetation Greenness across the Qinghai-Tibet Plateau Using Stable Isotopes and Satellite Detection. Remote Sens. Environ. 2022, 280, 113215. [Google Scholar] [CrossRef]
- Shang, P.; Shen, B.; Zeng, B.; Bi, L.; Qu, M.; Zheng, Y.; Ye, Y.; Li, W.; Zhou, X.; Yang, X.; et al. Integrated Transcriptomic and Metabolomics Analysis of the Root Responses of Orchardgrass to Submergence Stress. Int. J. Mol. Sci. 2023, 24, 2089. [Google Scholar] [CrossRef]
- Jian, S.; Wu, Z.; Hu, C.; Zhang, X. Sap Flow in Response to Rainfall Pulses for Two Shrub Species in the Semiarid Chinese Loess Plateau. J. Hydrol. Hydromech. 2016, 64, 121–132. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Z.; Liu, S.; Centritto, M.; Cao, X.; Zhang, M.; Zhao, G. Photosynthetic Capacity of Male and Female Hippophae Rhamnoides Plants along an Elevation Gradient in Eastern Qinghai-Tibetan Plateau, China. Tree Physiol. 2021, 41, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhou, L.; Qi, S.; Jin, M.; Hu, J.; Lu, J. Effect of Habitat Factors on the Understory Plant Diversity of Platycladus Orientalis Plantations in Beijing Mountainous Areas Based on MaxEnt Model. Ecol. Indic. 2021, 129, 107917. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Insect Temperature–Body Size Trends Common to Laboratory, Latitudinal and Seasonal Gradients Are Not Found across Altitudes. Funct. Ecol. 2018, 32, 948–957. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Duan, B.; Korpelainen, H.; Li, C. Adaptability to Elevated Temperature and Nitrogen Addition Is Greater in a High-Elevation Population than in a Low-Elevation Population of Hippophae rhamnoides. Trees 2011, 25, 1073–1082. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Sushko, S.; Selezneva, A.; Ananyeva, N.; Zhuravleva, A.; Kudeyarov, V.; Makarov, M.; Blagodatsky, S. Soil Microbial Activity along an Altitudinal Gradient: Vegetation as a Main Driver beyond Topographic and Edaphic Factors. Appl. Soil Ecol. 2021, 168, 104197. [Google Scholar] [CrossRef]
- Ma, Q.; Li, X.; Wu, S.; Zeng, F. Potential Geographical Distribution of Stipa Purpurea across the Tibetan Plateau in China under Climate Change in the 21st Century. Glob. Ecol. Conserv. 2022, 35, e02064. [Google Scholar] [CrossRef]
- Chen, K.; Wang, B.; Chen, C.; Zhou, G. MaxEnt Modeling to Predict the Current and Future Distribution of Pomatosace filicula under Climate Change Scenarios on the Qinghai–Tibet Plateau. Plants 2022, 11, 670. [Google Scholar] [CrossRef]
- Huang, R.; Du, H.; Wen, Y.; Zhang, C.; Zhang, M.; Lu, H.; Wu, C.; Zhao, B. Predicting the Distribution of Suitable Habitat of the Poisonous Weed Astragalus Variabilis in China under Current and Future Climate Conditions. Front. Plant Sci. 2022, 13, 921310. [Google Scholar] [CrossRef]
- Li, L.; Zhang, R.; Jia, L. Climatic Characteristics of Tibetan Plateau Vortex Precipitation Based on Observations. Int. J. Climatol. 2022, 42, 9237–9252. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Luo, W.; Duan, T.; Chen, Q. Characteristics of Hourly Extreme Precipitation over the Eastern Extension of the Tibetan Plateau. Atmosphere 2024, 15, 170. [Google Scholar] [CrossRef]
- Nie, Y.; Sun, J. Increase in Summer Precipitation over the Sichuan Basin in Recent Decades and Possible Causes. Int. J. Climatol. 2023, 43, 4269–4285. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, D.; Tian, L.; Zhang, H. Aeolian Activities and Protective Effects of Artificial Plants in Re-Vegetated Sandy Land of Qinghai Lake, China. Chin. Geogr. Sci. 2020, 30, 1129–1142. [Google Scholar] [CrossRef]
- Meinshausen, M.; Lewis, J.; McGlade, C.; Gütschow, J.; Nicholls, Z.; Burdon, R.; Cozzi, L.; Hackmann, B. Realization of Paris Agreement Pledges May Limit Warming Just below 2 °C. Nature 2022, 604, 304–309. [Google Scholar] [CrossRef]
- Soley-Guardia, M.; Alvarado-Serrano, D.F.; Anderson, R.P. Top Ten Hazards to Avoid When Modeling Species Distributions: A Didactic Guide of Assumptions, Problems, and Recommendations. Ecography 2024, 2024, e06852. [Google Scholar] [CrossRef]


| Code | Variable | Unit |
|---|---|---|
| bio01 | Annual Mean Temperature | °C |
| bio03 | Isothermality | % |
| bio04 | Temperature Seasonality | °C |
| bio05 | Max Temperature of Warmest Month | °C |
| bio06 | Min Temperature of Coldest Month | °C |
| bio09 | Mean Temperature of Driest Quarter | °C |
| bio11 | Mean Temperature of Coldest Quarter | °C |
| bio17 | Precipitation of Driest Quarter | mm |
| hf | Human Footprint Index | / |
| elev | Elevation | m |
| aspect | Aspect | ° |
| slope | Slope | ° |
| usda | USDA Soil Texture Classification | / |
| gm_lc | Land Cover Type | / |
| gm_ve | Vegetation Cover Percentage | % |
| ph_water | Potential of Water | / |
| d1_swr | Annual Average Soil Moisture Status Category | / |
| annual_mean_uv-b | Annual Average UV Radiation | kJ/m2 |
| Code | Contribution Value | Contribution Rate (%) |
|---|---|---|
| bio04 | 0.56 | 19.34% |
| bio17 | 0.34 | 11.65% |
| bio11 | 0.33 | 11.59% |
| bio06 | 0.32 | 11.20% |
| elev | 0.31 | 10.61% |
| bio01 | 0.28 | 9.61% |
| annual_mean_uv_b | 0.19 | 6.64% |
| bio05 | 0.15 | 5.09% |
| bio03 | 0.10 | 3.61% |
| hf | 0.09 | 3.05% |
| bio09 | 0.08 | 2.62% |
| d1_usda | 0.03 | 1.15% |
| d1_ph_water | 0.03 | 0.97% |
| aspect | 0.03 | 0.91% |
| gm_lc | 0.02 | 0.77% |
| slope | 0.02 | 0.75% |
| gm_ve | 0.01 | 0.44% |
| d1_swr | 0 | 0 |
| Environmental Variables | Suitable Range | Optimum Value |
|---|---|---|
| bio04 | 618.25–915.56 °C | 821.18 °C |
| bio06 | −19.07–−7.86 °C | −17.15 °C |
| bio11 | −8.70–8.62 °C | −6.21 °C |
| bio17 | 0.55–19.64 mm | 5.62 mm |
| elev | 2045.63–3033.28 m | 2381.00 m |
| Decade | Predicted Area (×103 km2) | Comparison with Current Distribution (%) | ||||
|---|---|---|---|---|---|---|
| Poorly Suitable Area | Moderately Suitable Area | Highly Suitable Area | Poorly Suitable Area | Moderately Suitable Area | Highly Suitable Area | |
| Current | 115.09 | 363.72 | 238.94 | |||
| 2050s | 127.33 | 248.8 | 290.12 | 10.64% | −31.59% | 21.42% |
| 2070s | 91.91 | 205.16 | 277.45 | −20.14% | −43.59% | 16.12% |
| 2090s | 100.97 | 210.35 | 292.2 | −12.26% | −42.17% | 22.29% |
| Decade | Predicted Area (×103 km2) | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B to A | C to A | D to A | A to B | B | C to B | D to B | |
| 2050s | 8488.19 | 52.29 | 95.99 | 10.02 | 59.06 | 19.77 | 37.92 | 10.57 |
| 2070s | 8491.86 | 62.67 | 146.30 | 37.40 | 48.72 | 12.17 | 19.53 | 11.49 |
| 2090s | 8464.74 | 63.09 | 144.44 | 36.94 | 57.99 | 10.36 | 20.26 | 12.36 |
| Decade | Predicted Area (×103 km2) | |||||||
| A to C | B to C | C | D to C | A to D | B to D | C to D | D | |
| 2050s | 45.56 | 36.32 | 127.76 | 39.17 | 2.19 | 6.70 | 102.05 | 179.18 |
| 2070s | 50.69 | 32.15 | 92.48 | 29.83 | 3.73 | 8.09 | 105.40 | 160.23 |
| 2090s | 65.45 | 30.80 | 83.04 | 31.06 | 6.82 | 10.83 | 115.97 | 158.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, T.; Liu, Q.; Xu, D.; He, Z.; Zhuo, Z. Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling. Agriculture 2025, 15, 722. https://doi.org/10.3390/agriculture15070722
Gan T, Liu Q, Xu D, He Z, Zhuo Z. Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling. Agriculture. 2025; 15(7):722. https://doi.org/10.3390/agriculture15070722
Chicago/Turabian StyleGan, Tingjiang, Quanwei Liu, Danping Xu, Zhipeng He, and Zhihang Zhuo. 2025. "Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling" Agriculture 15, no. 7: 722. https://doi.org/10.3390/agriculture15070722
APA StyleGan, T., Liu, Q., Xu, D., He, Z., & Zhuo, Z. (2025). Assessing the Impact of Climate Change on Hippophae neurocarpa in China Using Biomod2 Modeling. Agriculture, 15(7), 722. https://doi.org/10.3390/agriculture15070722






