ε-Poly-l-lysine Suppressed Decay Development and Maintained Storage Quality in Guava Fruit by ROS Level Regulation and Antioxidant Ability Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Guava Fruit and Postharvest Treatment
2.2. Fruit Storability Assay
2.3. Determination of Appearance Quality
2.4. Measurement of Nutritional Quality Levels
2.5. Measurement of ROS and MDA Content
2.6. Antioxidant Enzyme Activity Assay
2.7. Statistical Analysis
3. Results
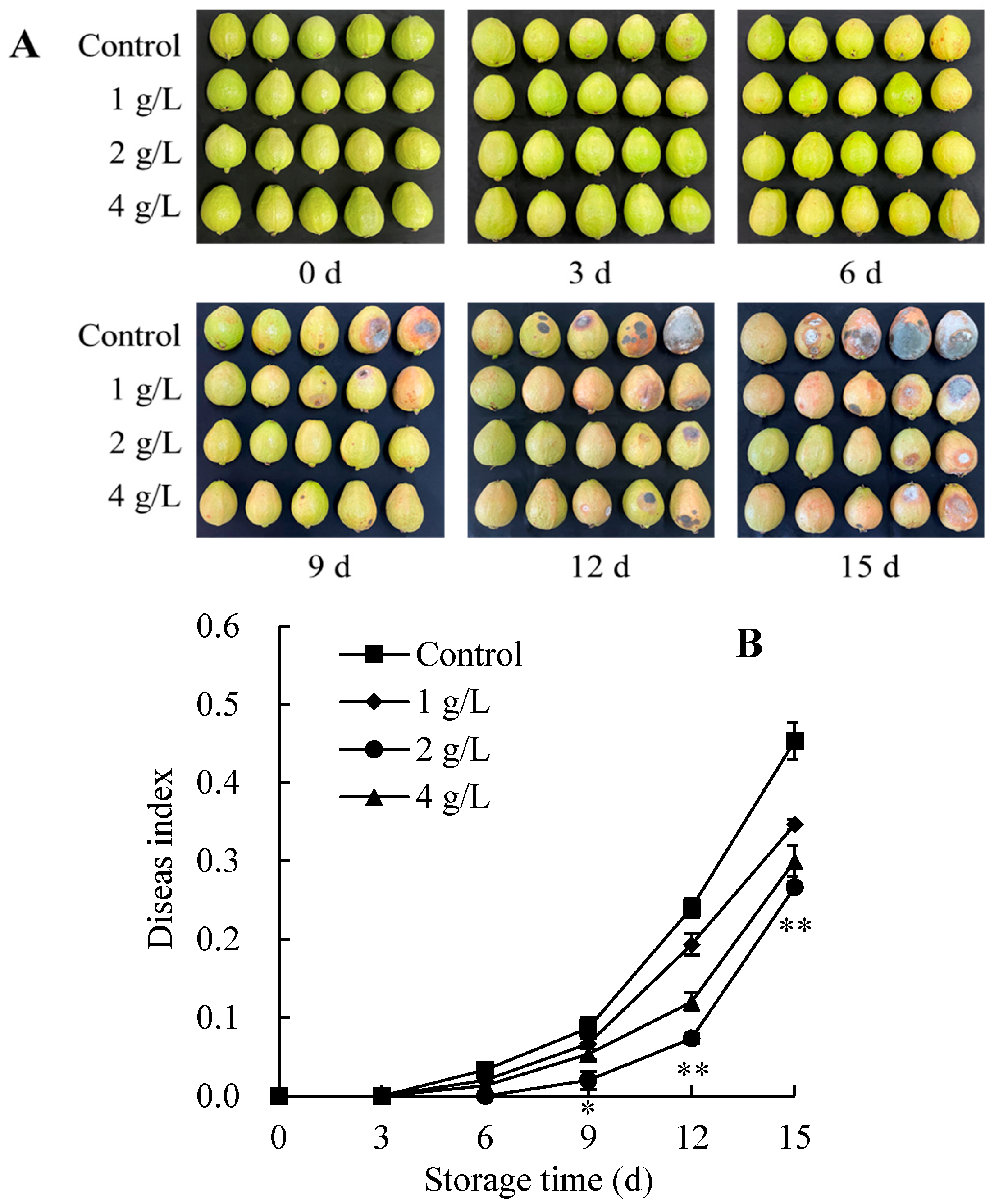
3.1. Changes in Decay Symptoms and Disease Index
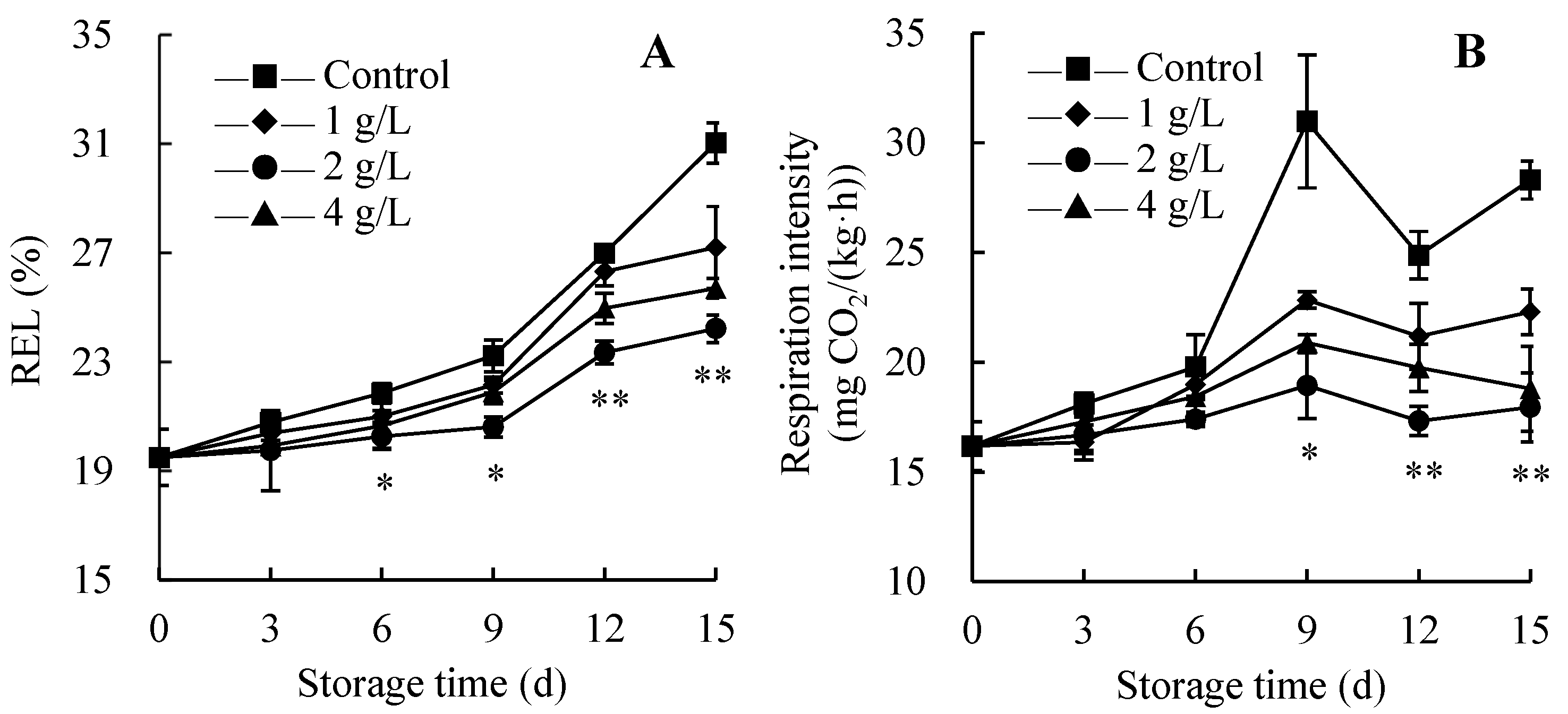
3.2. Changes in REL and Respiration Intensity
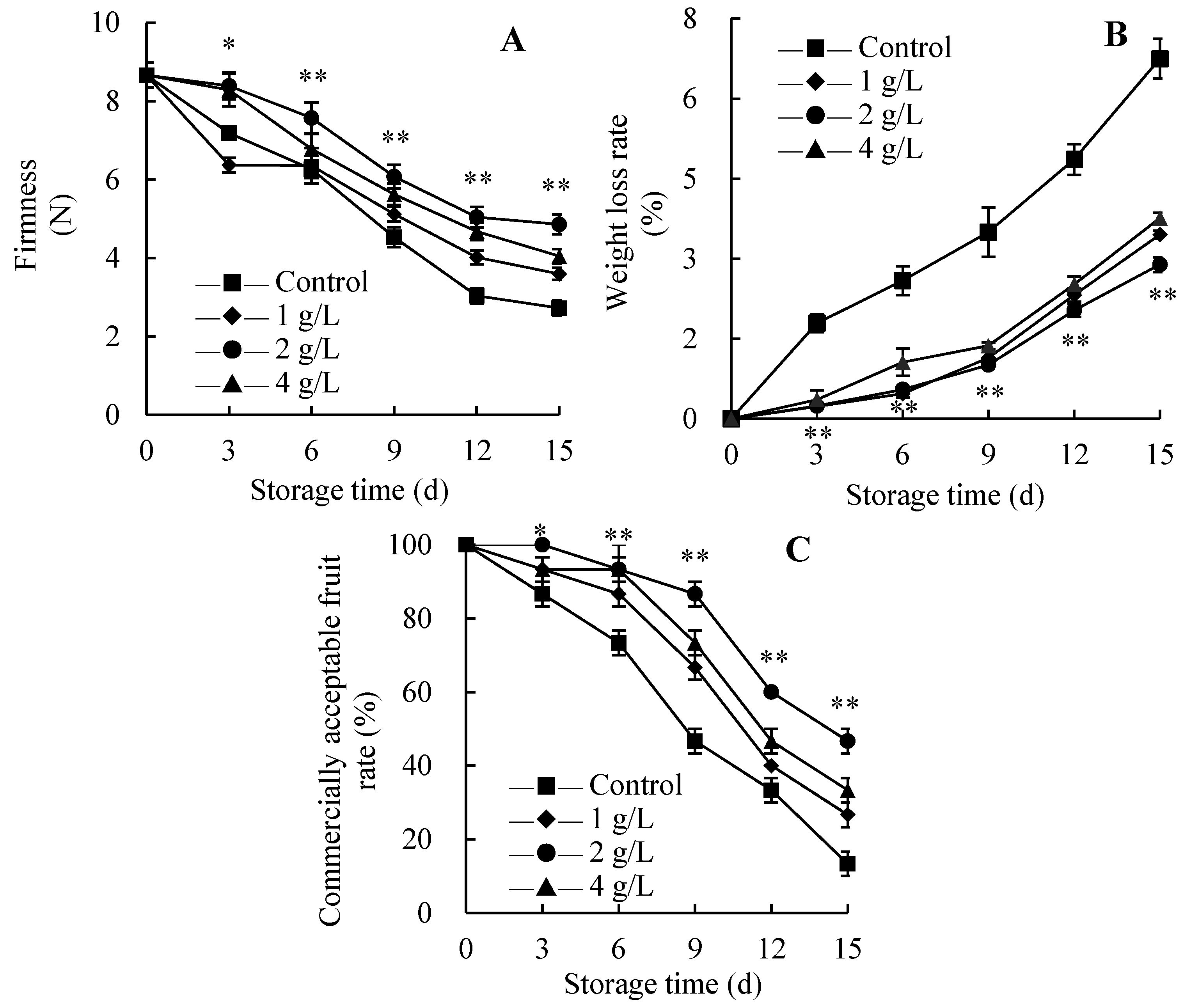
3.3. Changes in Firmness, Weight Loss Rate and Commercially Acceptable Fruit Rate
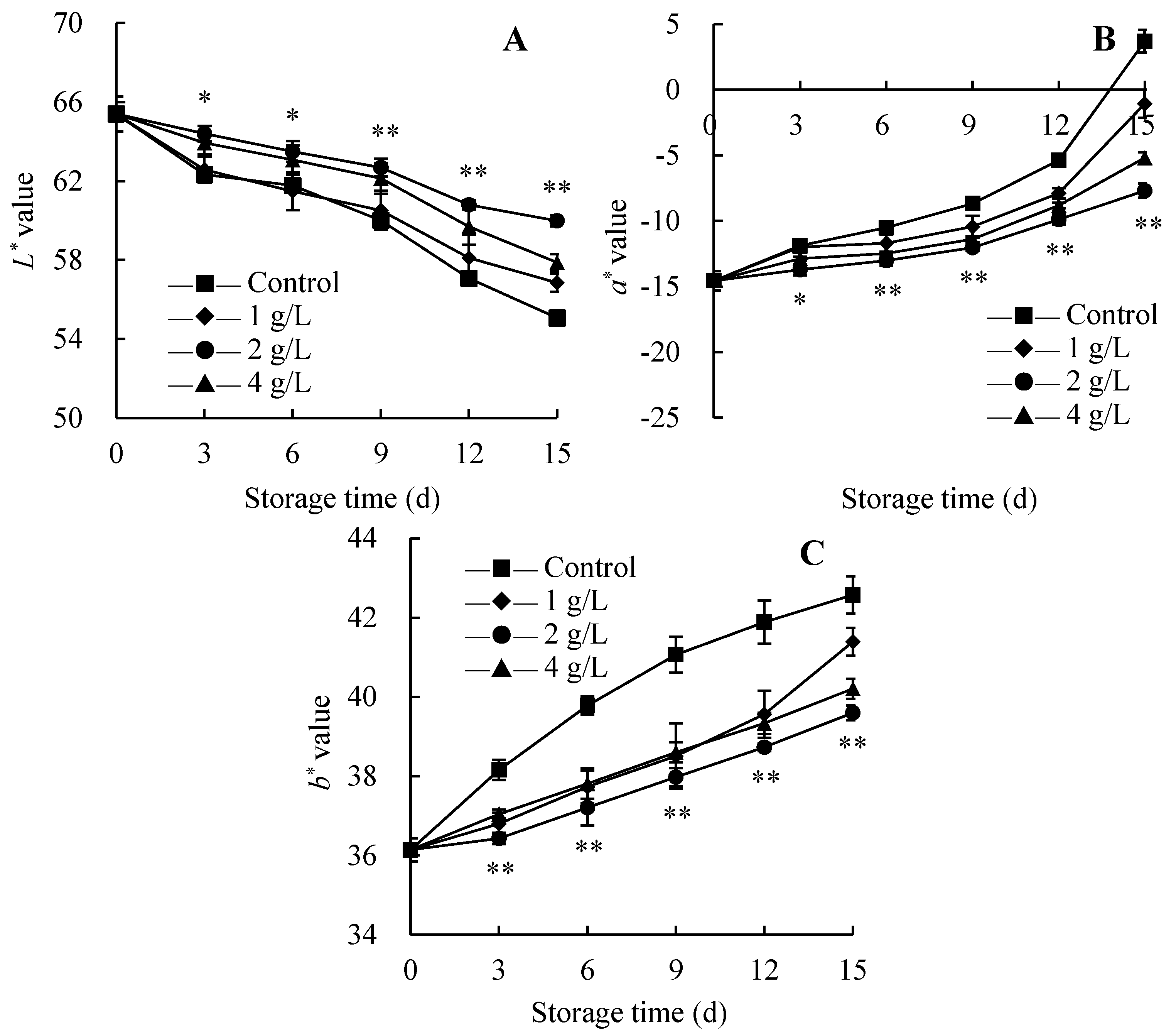
3.4. Changes in Appearance Quality
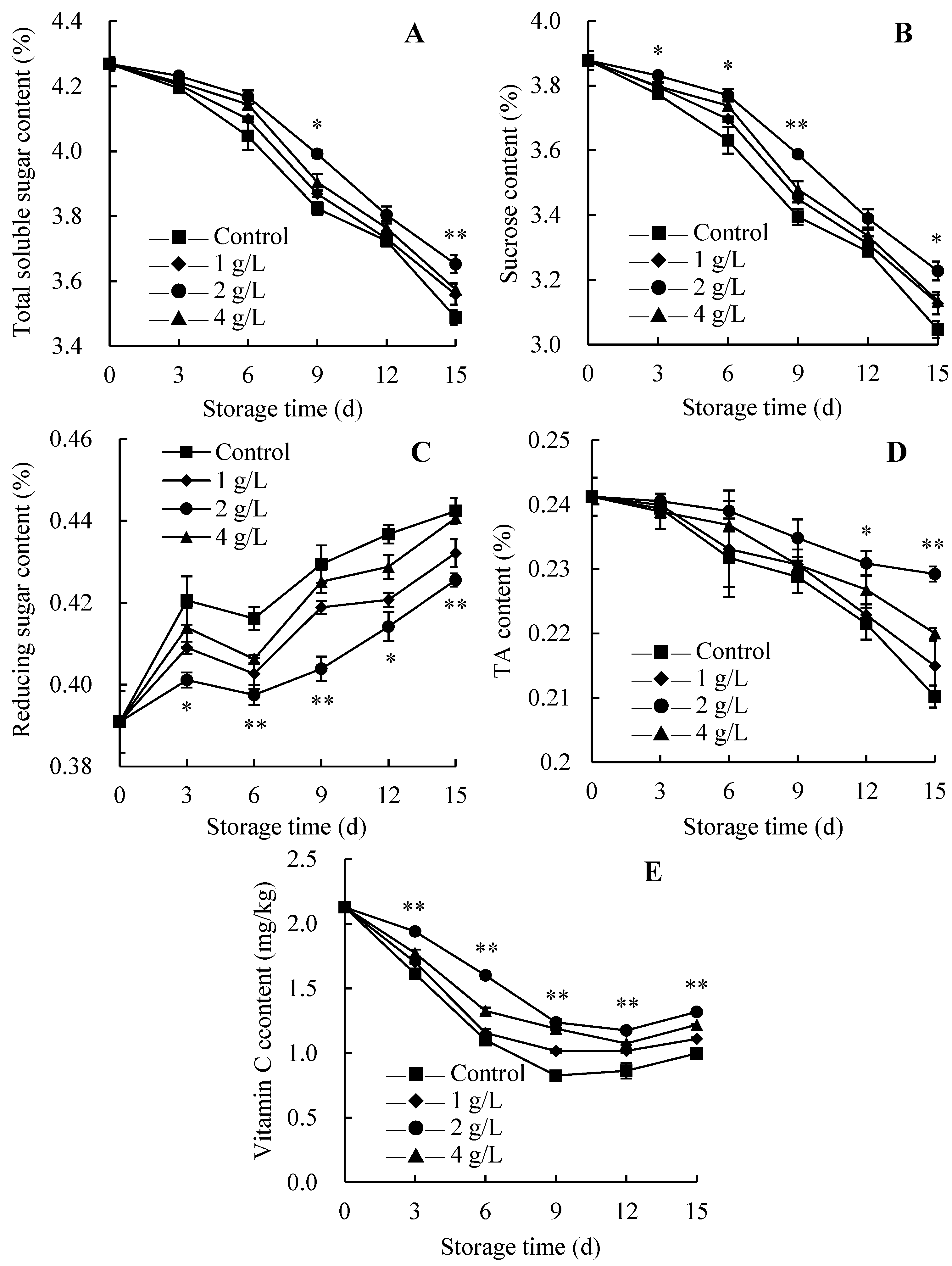
3.5. Changes in Nutritional Quality Levels
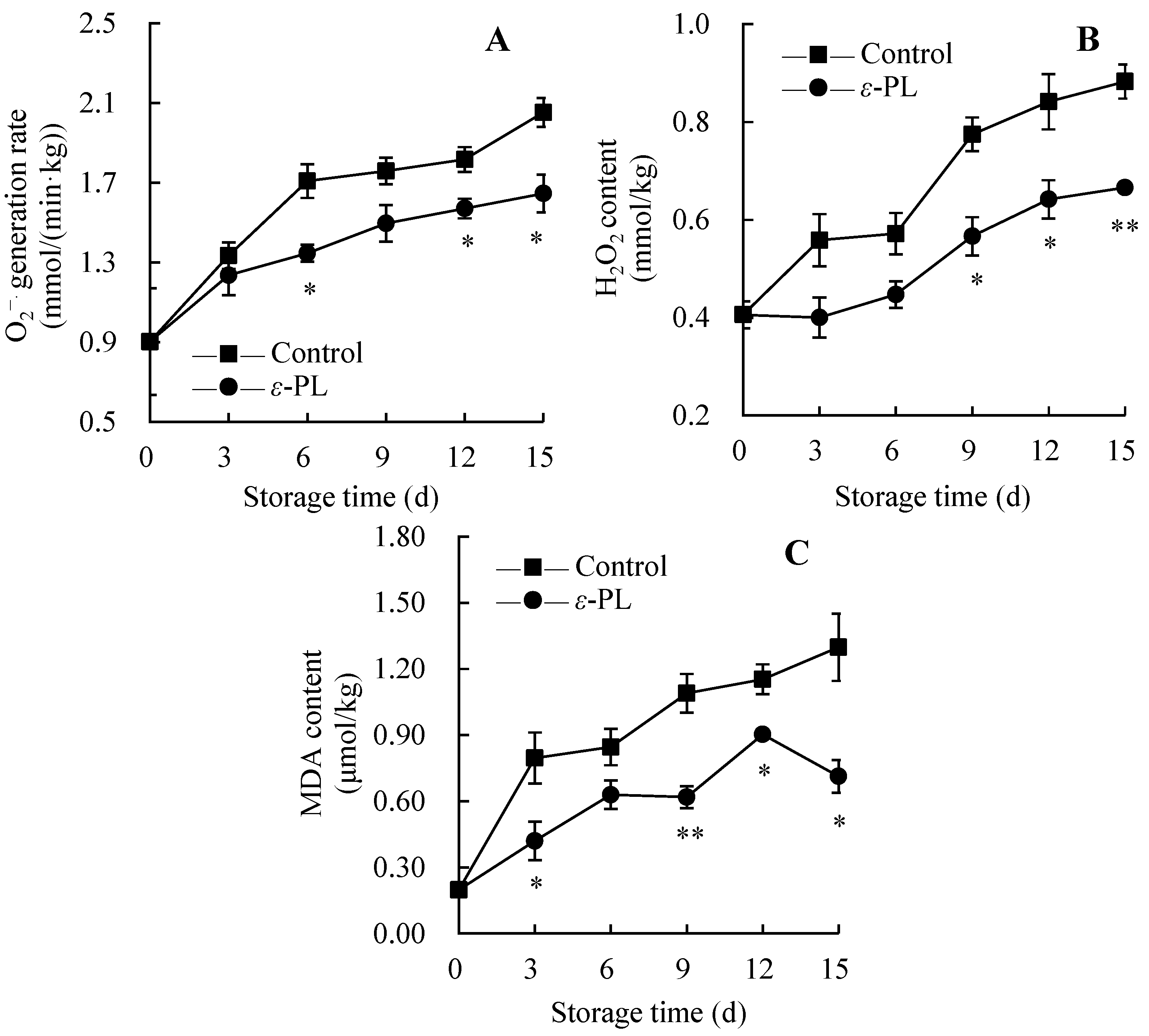
3.6. Changes in ROS and MDA Amounts
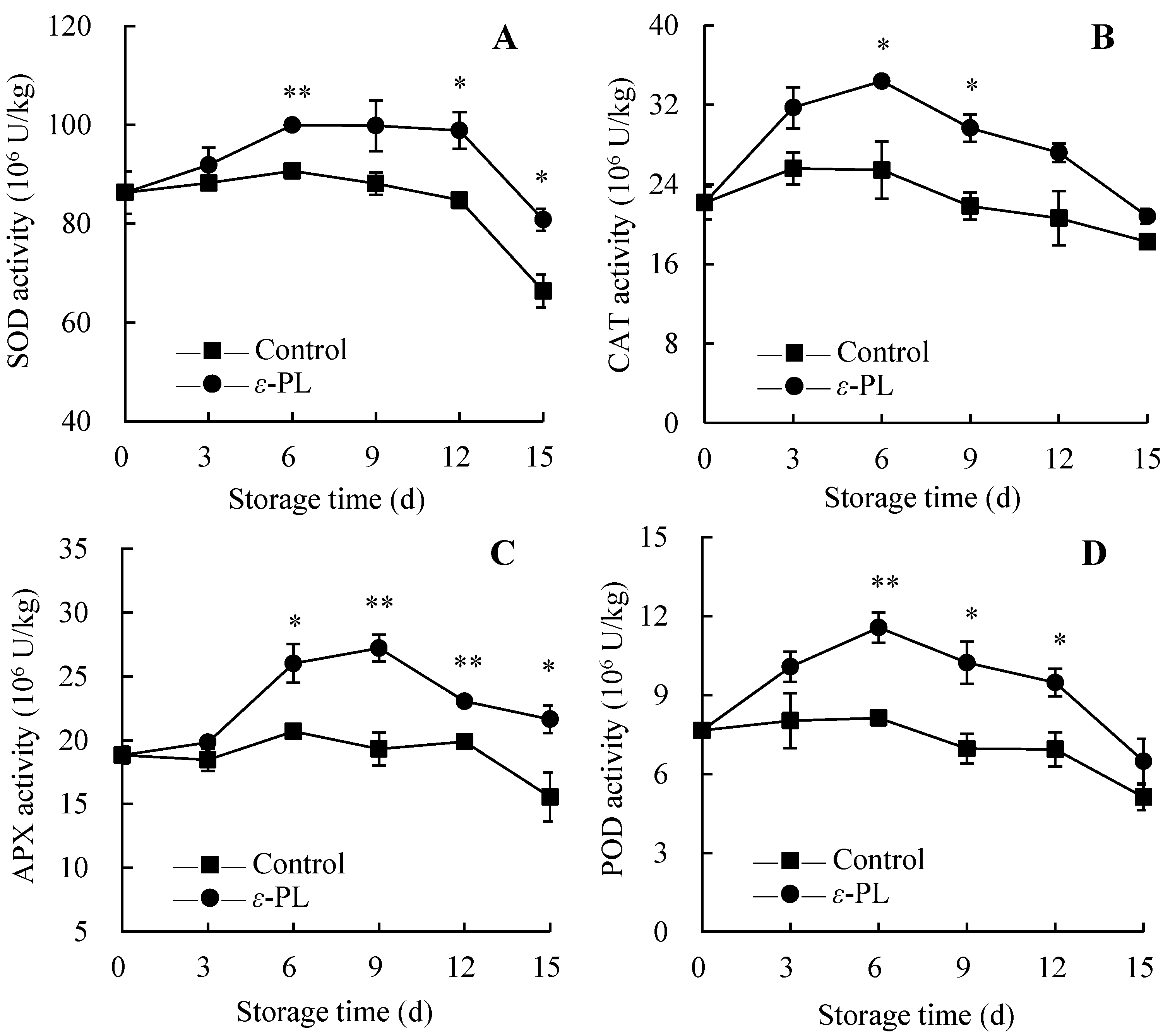
3.7. Changes in Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shu, C.; Kim-Lee, B.; Sun, X.X. Chitosan coating incorporated with carvacrol improves postharvest guava (Psidium guajava) quality. Horticulturae 2024, 10, 80. [Google Scholar] [CrossRef]
- Chen, H.B.; Feng, S.J.; Chen, Y.Z.; Jiang, X.J.; Lin, Y.Z.; Chen, Y.H. Postharvest immersion in slightly acidic electrolyzed water improves guava storability by regulating phenylpropane metabolism. Foods 2024, 13, 3850. [Google Scholar] [CrossRef]
- Menaka, M.; Asrey, R.; Singh, D.; Patel, V.B.; Meena, N.K.; Vinod, B.R.; Ahamad, S. Preserving functional properties and inhibiting postharvest peel browning in guava during cold storage via 24-epibrassinolide application. Postharvest Biol. Technol. 2024, 216, 113033. [Google Scholar] [CrossRef]
- Gull, S.; Ejaz, S.; Ali, S.; Ali, M.M.; Hussain, S.; Sardar, H.; Azam, M.; Nawaz, A.; Naz, S.; Maqbool, M. A novel edible coating based on Albizia [Albizia lebbeck (L.) Benth.] gum delays softening and maintains quality of harvested guava fruits. Int. J. Biol. Macromol. 2024, 277, 134096. [Google Scholar] [CrossRef]
- Zhang, Y. Post-harvest cold shock treatment enhanced antioxidant capacity to reduce chilling injury and improves the shelf life of guava (Psidium guajava L.). Front. Sustain. Food Syst. 2024, 8, 1297056. [Google Scholar] [CrossRef]
- Chen, H.B.; Lin, H.T.; Jiang, X.J.; Lin, M.S.; Fan, Z.Q. Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment. Food Chem. X 2022, 14, 100297. [Google Scholar] [CrossRef]
- Chen, N.H.; Wei, W.; Yang, Y.Y.; Chen, L.; Shan, W.; Chen, J.Y.; Lu, W.J.; Kuang, J.F.; Wu, C.J. Postharvest physiology and handling of guava fruit. Foods 2024, 13, 805. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Hussain, B.; Ejaz, S.; Sardar, H. Carboxymethyl cellulose coating maintains quality of harvested aonla fruit by regulating oxidative stress and ascorbate-glutathione cycle. Postharvest Biol. Technol. 2024, 207, 112621. [Google Scholar] [CrossRef]
- Du, M.R.; Lian, L.D.; Zhang, Y.C.; Lin, H.; Wang, J. Roles of ROS metabolism and phenylpropanoid pathway in quality maintenance of postharvest Pleurotus eryngii under hyperoxia stress. Postharvest Biol. Technol. 2024, 207, 112617. [Google Scholar] [CrossRef]
- Jiang, K.N.; Zhang, X.H.; Li, T.Y.; Liu, J.Q.; Liu, M.J.; Han, S.K. Gibberellin and shikimic acid enhance ascorbic acid accumulation and ROS scavenging ability to delay the senescence of postharvest jujube fruit. Postharvest Biol. Technol. 2025, 222, 113340. [Google Scholar] [CrossRef]
- Cai, S.Y.; Zhang, Z.Q.; Wang, J.L.; Fu, Y.; Zhang, Z.K.; Khan, M.R.; Cong, X.L. Effect of exogenous melatonin on postharvest storage quality of passion fruit through antioxidant metabolism. LWT-Food Sci. Technol. 2024, 194, 115835. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Chen, G.; Lin, H.T.; Lin, M.S.; Wang, H.; Lin, Y.F. Chitosan postharvest treatment suppresses the pulp breakdown development of longan fruit through regulating ROS metabolism. Int. J. Biol. Macromol. 2020, 165, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.Z.; Liu, Q.Q.; Li, M.L.; Feng, S.J.; Lin, M.Y.; Chen, Y.H.; Lin, H.T. Slightly acidic electrolyzed water treatment enhances the quality attributes and the storability of postharvest litchis through regulating the metabolism of reactive oxygen species. Food Chem. X 2024, 23, 101644. [Google Scholar] [CrossRef]
- Zhao, H.D.; Liu, B.D.; Zhang, W.L.; Cao, J.K.; Jiang, W.B. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Xie, P.D.; Yang, Y.Y.; Li, Y.; Wang, T.; Bai, B.T.; Prusky, D.; Li, Y.C.; Bi, Y. Preharvest phenylalanine spraying alleviates chilling injury in harvested muskmelons by maintaining reactive oxygen species homeostasis. Food Chem. 2025, 466, 142198. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.Y.; Sun, T.; An, J.; Ding, Y.; Zhang, W.W.; Liu, F.J.; Wang, C. Sodium nitroprusside (SNP) treatment increases the postharvest resistance of apple fruit to Alternaria alternata by enhancing antioxidant enzyme activity. Physiol. Mol. Plant. P 2024, 129, 102199. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Q.; Gao, X.Q.; Wang, W.J.; Zeng, K.F. Fatty acid metabolism and C9 aldehyde biosynthesis are involved in ε-poly-l-lysine-induced citrus fruit resistance to Penicillium digitatum. Pestic. Biochem. Phys. 2023, 196, 105614. [Google Scholar] [CrossRef]
- Song, R.; Wang, X.H.; Jiao, L.; Jiang, H.Y.; Yuan, S.; Zhang, L.; Shi, Z.X.; Fan, Z.C.; Meng, D.M. Epsilon-poly-l-lysine alleviates brown blotch disease of postharvest Agaricus bisporus mushrooms by directly inhibiting Pseudomonas tolaasii and inducing mushroom disease resistance. Pestic. Biochem. Phys. 2024, 199, 105759. [Google Scholar] [CrossRef]
- Dou, Y.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.Y.; Zhang, X.Y.; Zhao, L.N.; Wang, K.L.; Zhang, H.Y. Transcriptome analysis provides insights into potential mechanisms of epsilon-poly-L-lysine inhibiting Penicillium expansum invading apples. Postharvest Biol. Technol. 2024, 207, 112622. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.Y.; Zhang, X.Y.; Zhang, H.Y. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol. Technol. 2021, 171, 111346. [Google Scholar] [CrossRef]
- Lia, S.F.; Zhang, L.H.; Liu, M.P.; Wang, X.Y.; Zhao, G.Y.; Zong, W. Effect of poly-ε-lysine incorporated into alginate-based edible coatings on microbial and physicochemical properties of fresh-cut kiwifruit. Postharvest Biol. Technol. 2017, 134, 114–121. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Chen, L.L.; Chen, J.Y.; Jiang, X.J.; Zheng, J.S.; Chen, H.B. Effect of ε-poly-L-lysine on postharvest diseases and disease-resistant substance metabolism in passion fruits. Food Sci. 2024, 45, 142–149. [Google Scholar]
- Huang, T.; Liu, G.S.; Zhu, L.S.; Liu, J.L.; Xiang, Y.; Xu, X.B.; Zhang, Z.K. Mitigation of chilling injury in mango fruit by methyl jasmonate is associated with regulation of antioxidant capacity and energy homeostasis. Postharvest Biol. Technol. 2024, 211, 112801. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Li, N.; Lin, H.T.; Lin, M.S.; Chen, Y.H.; Wang, H.; Ritenour, M.A.; Lin, Y.F. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef]
- Ge, Y.H.; Chen, Y.R.; Li, C.Y.; Wei, M.L.; Li, X.H.; Li, S.; Lu, S.L.; Li, J.R. Effect of trisodium phosphate dipping treatment on the quality and energy metabolism of apples. Food Chem. 2019, 274, 324–329. [Google Scholar] [CrossRef]
- Li, B.B.; Zang, Y.S.; Xun, J.P.; Wang, X.F.; Lu, H.D.; Qi, J.L.; Wang, X.; Xi, Z.M. 24-Epibrassinolide improves quality and resistance against Botrytis cinerea of harvest table grapes through modulating reactive oxygen species homeostasis. Postharvest Biol. Technol. 2024, 215, 113016. [Google Scholar] [CrossRef]
- Shen, X.Y.; Liu, Y.; Zeng, Y.; Zhao, Y.Q.; Bao, Y.Q.; Shao, X.F.; Wu, Z.G.; Zheng, Y.H.; Jin, P. Hydrogen sulfide attenuates chilling injury in loquat fruit by alleviating oxidative stress and maintaining cell membrane integrity. Food Chem. 2025, 463, 141094. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, Y.; Li, J.; Gu, X.Y.; Zhao, L.N.; Li, B.; Wang, K.L.; Yang, Q.Y.; Zhang, H.Y. Pichia caribbica improves disease resistance of cherry tomatoes by regulating ROS metabolism. Biol. Control 2022, 169, 104870. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhao, L.N.; Chen, Y.Q.; Dhanasekaran, S.; Chen, X.F.; Zhang, X.Y.; Yang, X.Z.; Wu, M.Y.; Song, Y.D.; Zhang, H.Y. Study on the control effect and physiological mechanism of Wickerhamomyces anomalus on primary postharvest diseases of peach fruit. Int. J. Food Microbiol. 2024, 413, 110575. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, F.; Yang, Q.; Tang, J.R.; Chen, L.; Shi, Z.J.; He, X.H.; Deng, J. Carboxymethyl chitosan different durations induces disease resistance of grapefruit by modulating ascorbate-glutathione cycle and cell wall metabolism. Postharvest Biol. Technol. 2024, 211, 112845. [Google Scholar] [CrossRef]
- Liu, Z.C.; Zhang, X.Y.; Zhu, S.H.; Huang, D.D. Strigolactone preserves fresh-cut apple quality during shelf life. Agriculture 2024, 14, 1588. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Ma, H.J.; Ren, X.Y.; Shi, H.M.; Liu, Z.X.; Liang, J.R. Exogenous glucose reduces the incidence of black rot disease in apricot (Prunus armeniaca L.) by regulating energy metabolism and ROS. Sci. Hortic. 2023, 313, 111903. [Google Scholar] [CrossRef]
- Liu, Z.G.; Reymick, O.O.; Feng, Z.; Duan, B.; Tao, N.G. Phenylalanine enhances the efficiency of sodium dehydroacetate to control citrus fruit decay by stimulating reactive oxygen metabolism and phenylpropanoid pathway. Postharvest Biol. Technol. 2025, 222, 113392. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wu, Y.J.; Zhang, X.; Zhu, X.; Hou, Y.Y.; Chen, J.Y.; Cui, K.B.; Li, X.W.; Wu, W.X. Methyl jasmonate attenuates chilling injury of prune fruit by maintaining ROS homeostasis and regulating GABA metabolism and energy status. Postharvest Biol. Technol. 2025, 220, 113303. [Google Scholar] [CrossRef]
- Hong, M.; Zhou, L.; Zhang, H.; Huang, L.H.; He, M.Y. Melatonin treatment delays postharvest senescence of ‘Dayagan’ hybrid citrus fruit by enhancing reactive oxygen species-scavenging capacity and preserving higher unsaturated fatty acid content. Postharvest Biol. Technol. 2025, 222, 113400. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Li, L.; Wen, M.; Luo, F.; Tan, M.; Lin, Y.; Chen, H. ε-Poly-l-lysine Suppressed Decay Development and Maintained Storage Quality in Guava Fruit by ROS Level Regulation and Antioxidant Ability Enhancement. Agriculture 2025, 15, 654. https://doi.org/10.3390/agriculture15060654
An Y, Li L, Wen M, Luo F, Tan M, Lin Y, Chen H. ε-Poly-l-lysine Suppressed Decay Development and Maintained Storage Quality in Guava Fruit by ROS Level Regulation and Antioxidant Ability Enhancement. Agriculture. 2025; 15(6):654. https://doi.org/10.3390/agriculture15060654
Chicago/Turabian StyleAn, Yingying, Li Li, Mingming Wen, Feng Luo, Mei Tan, Yuzhao Lin, and Hongbin Chen. 2025. "ε-Poly-l-lysine Suppressed Decay Development and Maintained Storage Quality in Guava Fruit by ROS Level Regulation and Antioxidant Ability Enhancement" Agriculture 15, no. 6: 654. https://doi.org/10.3390/agriculture15060654
APA StyleAn, Y., Li, L., Wen, M., Luo, F., Tan, M., Lin, Y., & Chen, H. (2025). ε-Poly-l-lysine Suppressed Decay Development and Maintained Storage Quality in Guava Fruit by ROS Level Regulation and Antioxidant Ability Enhancement. Agriculture, 15(6), 654. https://doi.org/10.3390/agriculture15060654





