Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Trichoderma guizhouense NJAU4742
2.2. Random Mutation of Trichoderma guizhouense NJAU4742
2.3. Transcriptome Sequencing of the Wild Type and Mutant Strains Under Salt Stress
2.4. Construct Functional Fragments for Gene Knockout and Overexpression
2.5. Protoplast Transformation of Functional Fragments in Trichoderma guizhouense NJAU4742
2.6. Evaluate Intracellular Substrates Related to Salt Stress and Redox Status
2.7. Pot Experiments
2.8. Data Process and Statistical Analysis
3. Results
3.1. Screening of Irradiated Mutant Strains of Trichoderma guizhouense NJAU4742
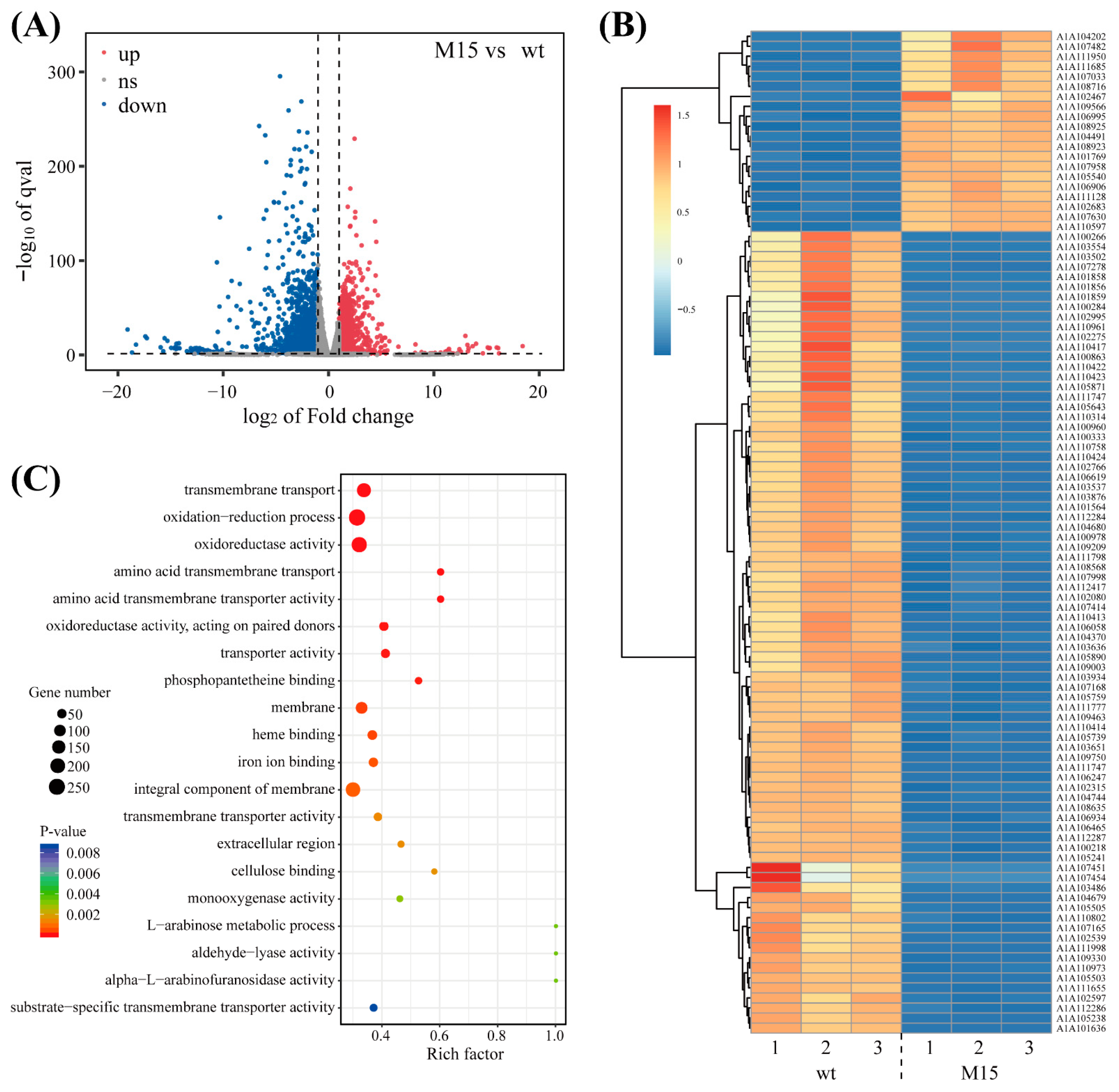
3.2. Transcriptome Sequencing Result of the Wild-Type and Mutant Strains Under Salt Stress
3.3. Na+ Efflux Pumps in NJAU4742 Alleviate Salt Stress
3.3.1. Na+/K+-ATPase Regulates the Intracellular Ion Balance of NJAU4742
3.3.2. The Variations of Intracellular Sodium Ions Within NJAU4742 Cells
3.4. Measurement of Intracellular Substances in Mutant Strains
3.4.1. Under Salt Stress, NJAU4742 Accumulates a Large Amount of Glycerol Intracellularly
3.4.2. Coenzyme I and SOD Relieve Oxidative Stress in NJAU4742 Cells
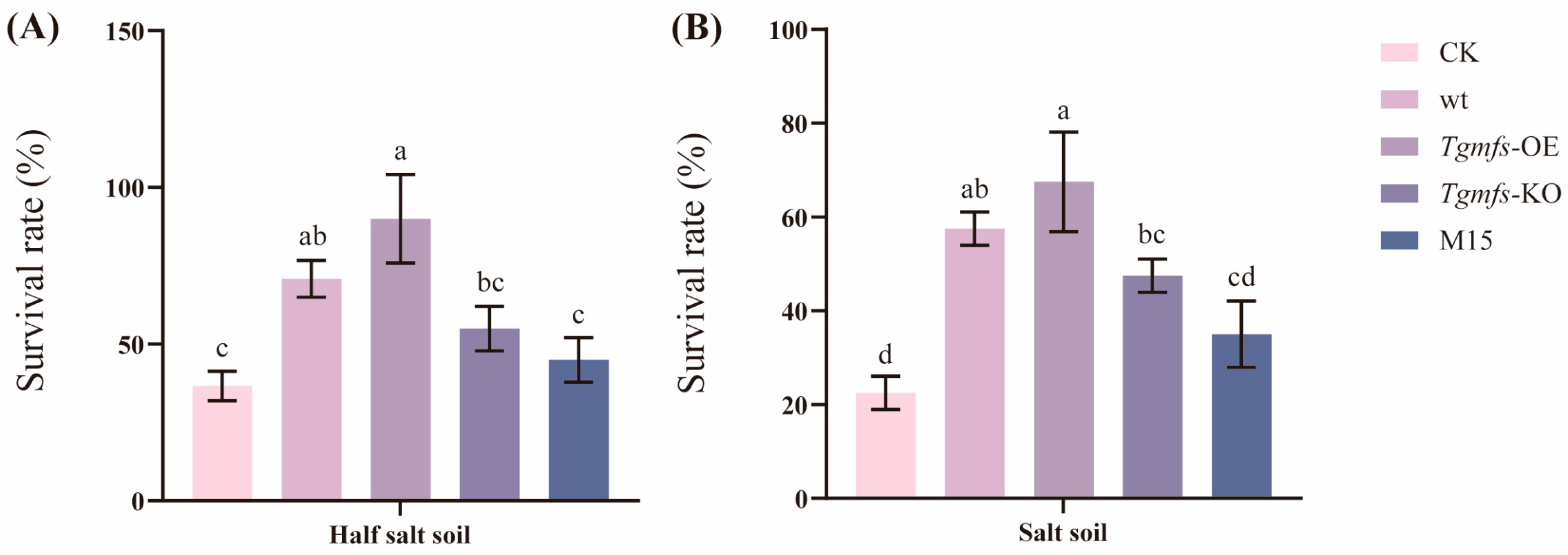
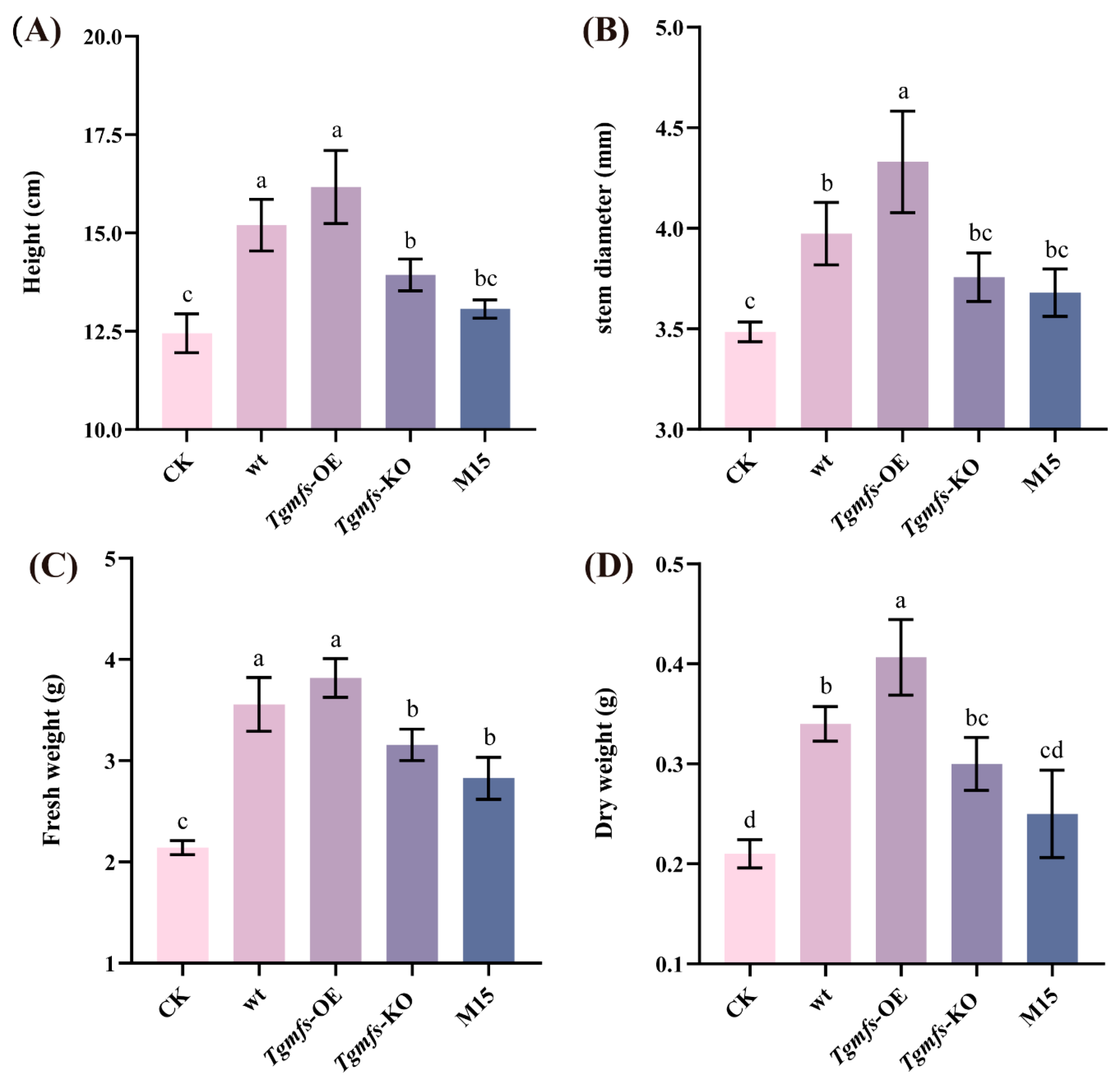
3.5. Effects of Salt-Tolerant Mutant Strain of Trichoderma on Tomato Biomass and Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021, 130, 913–925. [Google Scholar] [CrossRef]
- Gonzalez, J.A.B.; Carrillo-Gonzalez, R.; Gonzalez-Chavez, M.d.C.A.; Sanchez, E.C.; Maruri, D.T. Selection of Salinity-Adapted Endorhizal Fungal Consortia from Two Inoculum Sources and Six Halophyte Plants. J. Fungi 2023, 9, 893. [Google Scholar] [CrossRef]
- Perez-Llano, Y.; Caridad Rodriguez-Pupo, E.; Druzhinina, I.S.; Chenthamara, K.; Cai, F.; Gunde-Cimerman, N.; Zalar, P.; Gostinčar, C.; Kostanjšek, R.; Folch-Mallol, J.L.; et al. Stress Reshapes the Physiological Response of Halophile Fungi to Salinity. Cells 2020, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Schillaci, M.; Balestrini, R. Mitigating the impact of soil salinity: Recent developments and future strategies. Ital. J. Agron. 2023, 18, 2173. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitas, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef]
- Saldana, C.; Villava, C.; Ramirez-Villarreal, J.; Morales-Tlalpan, V.; Campos-Guillen, J.; Chavez-Servin, J.; García-Gasca, T. Rapid and reversible cell volume changes in response to osmotic stress in yeast. Braz. J. Microbiol. 2021, 52, 895–903. [Google Scholar] [CrossRef]
- Proft, M.; Struhl, K. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell 2004, 118, 351–361. [Google Scholar] [CrossRef]
- Shirvanyan, A.; Mirzoyan, S.; Trchounian, K. Relationship between proton/potassium fluxes and central carbon catabolic pathways in different Saccharomyces cerevisiae strains under osmotic stress conditions. Process Biochem. 2023, 133, 309–318. [Google Scholar] [CrossRef]
- Wang, C.; Xiang, Y.; Qian, D. Current progress in plant V-ATPase: From biochemical properties to physiological functions. J. Plant Physiol. 2021, 266, 153525. [Google Scholar] [CrossRef]
- Anschutz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, C.; Wang, S.-M. Coordination of AtHKT1;1 and AtSOS1 facilitates Na+ and K+ homeostasis in Arabidopsis thaliana under salt stress. J. Plant Biol. 2014, 57, 282–290. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Wang, Q.; Liu, Y.; Li, T.; Liu, D.; Shen, Q. Tr-milRNA1 Contributes to Lignocellulase Secretion under Heat Stress by Regulating the Lectin-Type Cargo Receptor Gene Trvip36 in Trichoderma guizhouence NJAU 4742. J. Fungi 2021, 7, 997. [Google Scholar] [CrossRef]
- Li, Y.; Shao, J.; Fu, Y.; Chen, Y.; Wang, H.; Xu, Z.; Feng, H.; Xun, W.; Liu, Y.; Zhang, N.; et al. The volatile cedrene from Trichoderma guizhouense modulates Arabidopsis root development through auxin transport and signalling. Plant Cell Environ. 2022, 45, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.; Yao, Y.; Zhu, F.; Wang, W.; Chang, X. The influence of different concentrations of bio-organic fertilizer on cucumber Fusarium wilt and soil microflora alterations. PLoS ONE 2017, 12, e0171490. [Google Scholar] [CrossRef]
- Cai, F.; Chen, W.; Wei, Z.; Pang, G.; Li, R.; Ran, W.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Pang, G.; Cai, F.; Li, R.; Zhao, Z.; Li, R.; Gu, X.; Shen, Q.; Chen, W. Trichoderma-enriched organic fertilizer can mitigate microbiome degeneration of monocropped soil to maintain better plant growth. Plant Soil 2017, 416, 181–192. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H., Jr. The major facilitator superfamily (MFS) revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Hinchliffe, P.; Greene, N.P.; Paterson, N.G.; Crow, A.; Hughes, C.; Koronakis, V. Structure of the periplasmic adaptor protein from a major facilitator superfamily (MFS) multidrug efflux pump. FEBS Lett. 2014, 588, 3147–3153. [Google Scholar] [CrossRef]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and General Transport Mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar] [CrossRef]
- Li, P.; Gu, Y.; Li, J.; Xie, L.; Li, X.; Xie, J. Mycobacterium tuberculosis Major Facilitator Superfamily Transporters. J. Membr. Biol. 2017, 250, 573–585. [Google Scholar] [CrossRef]
- Nino-Gonzalez, M.; Novo-Uzal, E.; Richardson, D.N.; Barros, P.M.; Duque, P. More Transporters, More Substrates: The Arabidopsis Major Facilitator Superfamily Revisited. Mol. Plant 2019, 12, 1182–1202. [Google Scholar] [CrossRef]
- Sand, M.; de Berardinis, V.; Mingote, A.; Santos, H.; Goettig, S.; Mueller, V.; Averhoff, B. Salt adaptation in Acinetobacter baylyi: Identification and characterization of a secondary glycine betaine transporter. Arch. Microbiol. 2011, 193, 723–730. [Google Scholar] [CrossRef]
- Frawley, E.R.; Crouch, M.-L.V.; Bingham-Ramos, L.K.; Robbins, H.F.; Wang, W.; Wright, G.D.; Fang, F.C. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2013, 110, 12054–12059. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.; Cabedo, M.; Rull, B.; Yenush, L.; Serrano, R.; Mulet, J.M. Role of the yeast multidrug transporter Qdr2 in cation homeostasis and the oxidative stress response. FEMS Yeast Res. 2013, 13, 97–106. [Google Scholar] [CrossRef]
- Paul, S.; Alegre, K.O.; Holdsworth, S.R.; Rice, M.; Brown, J.A.; McVeigh, P.; Kelly, S.M.; Law, C.J. A single-component multidrug transporter of the major facilitator superfamily is part of a network that protects Escherichia coli from bile salt stress. Mol. Microbiol. 2014, 92, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Li, X.; Guo, L.; Li, C.; Lai, J.; Han, Y.; Ye, W.; Miao, Y.; Deng, M.; et al. A gene cluster for polyamine transport and modification improves salt tolerance in tomato. Plant J. 2024, 120, 1706–1723. [Google Scholar] [CrossRef]
- Ming, X.; Xiang, L. (Eds.) Genetic Analysis of Several Traits Related to Salt Tolerance in Tomato. In Proceedings of the 3rd Conference on Key Technology of Horticulture, Shenyang, China, 17–18 December 2011. [Google Scholar]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Wu, F.; Chen, Z.; Xu, X.; Xue, X.; Zhang, Y.; Sui, N. Halotolerant Bacillus sp. strain RA coordinates myo-inositol metabolism to confer salt tolerance to tomato. J. Integr. Plant Biol. 2024, 66, 1871–1885. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tan, M.-Z.; Wang, R.-X.; Ye, F.-T.; Chen, Y.-P.; Luo, X.-M.; Feng, J.-X. Combination of genetic engineering and random mutagenesis for improving production of raw-starch-degrading enzymes in Penicillium oxalicum. Microb. Cell Factories 2022, 21, 272. [Google Scholar] [CrossRef]
- Miao, Y.; Xia, Y.; Kong, Y.; Zhu, H.; Mei, H.; Li, P.; Feng, H.; Xun, W.; Xu, Z.; Zhang, N.; et al. Overcoming diverse homologous recombinations and single chimeric guide RNA competitive inhibition enhances Cas9-based cyclical multiple genes coediting in filamentous fungi. Environ. Microbiol. 2021, 23, 2937–2954. [Google Scholar] [CrossRef]
- Choudhary, K.; Shih, N.P.; Deng, F.; Ledda, M.; Li, B.; Aviran, S. Metrics for rapid quality control in RNA structure probing experiments. Bioinformatics 2016, 32, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, L.; Wang, X.; Wang, Z.; Li, P.; Dai, J.; Zhang, H.; Xie, Y. Nitratireductor luteus sp. nov. isolated from saline-alkali land. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2023, 116, 221–229. [Google Scholar] [CrossRef]
- Oren, A. Life in Hypersaline Environments. In Their World: A Diversity of Microbial Environments; Hurst, C.J., Ed.; Advances in Environtal Microbiology; Springer: Cham, Switzerland, 2016; pp. 301–339. [Google Scholar]
- Garg, N.; Manchanda, G. Role of Arbuscular Mycorrhizae in the Alleviation of Ionic, Osmotic and Oxidative Stresses Induced by Salinity in Cajanus cajan (L.) Millsp (pigeonpea). J. Agron. Crop Sci. 2009, 195, 110–123. [Google Scholar] [CrossRef]
- Hagemann, M. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol. Rev. 2011, 35, 87–123. [Google Scholar] [CrossRef]
- Vindelov, J.; Arneborg, N. Saccharomyces cerevisiae and Zygosaccharomyces mellis exhibit different hyperosmotic shock responses. Yeast 2002, 19, 429–439. [Google Scholar] [CrossRef]
- Tanigawa, M.; Kihara, A.; Terashima, M.; Takahara, T.; Maeda, T. Sphingolipids Regulate the Yeast High-Osmolarity Glycerol Response Pathway. Mol. Cell. Biol. 2012, 32, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Kumari, J.; Rathore, M.S. Na+/K+-ATPase a Primary Membrane Transporter: An Overview and Recent Advances with Special Reference to Algae. J. Membr. Biol. 2020, 253, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Dwivedi, M. Overexpression, Isolation, Purification, and Crystallization of NhaA. In Methods Enzymology; Shukla, A.K., Ed.; Membrane Proteins—Engineering, Purification and Crystallization; Elsevier: Amsterdam, The Netherlands, 2015; Volume 557, pp. 135–148. [Google Scholar]
- Holtmann, G.; Bakker, E.P.; Uozumi, N.; Bremer, E. KtrAB and KtrCD: Two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 2003, 185, 1289–1298. [Google Scholar] [CrossRef]
- Rashid, A.N.; Janda, M.; Mahmud, M.Z.; Valentova, O.; Lenka, B.; Alekber, Q.A. Influence of Salt Stress on the FLG22-Induced ROS Production in Arabidopsis thaliana Leaves. Pak. J. Bot. 2021, 53, 1605–1610. [Google Scholar] [CrossRef]
- Swapnil, P.; Yadav, A.K.; Srivastav, S.; Sharma, N.K.; Srikrishna, S.; Rai, A.K. Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Res.-Biomass Biofuels Bioprod. 2017, 23, 88–95. [Google Scholar]
- Wei, M.; Zhuang, Y.; Li, H.; Li, P.; Huo, H.; Shu, D.; Huang, W.; Wang, S. The cloning and characterization of hypersensitive to salt stress mutant, affected in quinolinate synthase, highlights the involvement of NAD in stress-induced accumulation of ABA and proline. Plant J. 2020, 102, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Srivastava, S.; Lokhande, V.H.; D’Souza, S.F.; Suprasanna, P. Salt stress reveals differential antioxidant and energetics responses in glycophyte (Brassica juncea L.) and halophyte (Sesuvium portulacastrum L.). Front. Environ. Sci. 2015, 3, 19. [Google Scholar] [CrossRef]
- Zhu, H.; Li, T.; Li, C.; Liu, Y.; Miao, Y.; Liu, D.; Shen, Q. Intracellular kynurenine promotes acetaldehyde accumulation, further inducing the apoptosis in soil beneficial fungi Trichoderma guizhouense NJAU4742 under acid stress. Environ. Microbiol. 2023, 25, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kato, T.; Watakabe, S.; Song, W.; Aikawa, S.; Furukawa, K. The respiratory chain provides salt stress tolerance by maintaining a low NADH/NAD+ ratio in Zymomonas mobilis. Microbiology 2015, 161, 2384–2394. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, D.; Wang, Z.; Guo, H.; Wang, Y.; Wang, X.; Dong, X. Oxidative stress response in atrazine-degrading bacteria exposed to atrazine. J. Hazard. Mater. 2012, 229, 434–438. [Google Scholar] [CrossRef]
- Gandhi, A.; Reichelt, M.; Goyal, D.; Vadassery, J.; Oelmueller, R. Trichoderma harzianum Protects the Arabidopsis Salt Overly Sensitive 1 Mutant Against Salt Stress. J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Yu, Y.-N.; Gao, R.-W.; Wang, H.; Zhang, J.; Li, R.; Long, X.-H.; Shen, Q.-R.; Chen, W.; Cai, F. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, X.; Zhou, Y.; Ma, S.; Wang, Y.; Li, Z.; Zhao, D.; Yang, Y.; Zhang, H.; Meng, C.; et al. Purines enrich root-associated Pseudomonas and improve wild soybean growth under salt stress. Nat. Commun. 2024, 15, 3520. [Google Scholar] [CrossRef]
- Margarita, S.; Sviatoslav, M.; Yelena, O.; Makhpal, Y. Can salt-adapted microorganisms alleviate salt stress in plants and enhance their non-specific resilience? Front. Agron. 2023, 5, 1287108. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Z.; Zhou, L.; Xu, H.; Liu, C.; Yan, X. Advances in Identifying the Mechanisms by Which Microorganisms Improve Barley Salt Tolerance. Life 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, H.; Li, T.; Wu, H.; Xia, Y.; Huang, Q.; Liu, D.; Shen, Q. Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions. Agriculture 2025, 15, 610. https://doi.org/10.3390/agriculture15060610
Mei H, Li T, Wu H, Xia Y, Huang Q, Liu D, Shen Q. Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions. Agriculture. 2025; 15(6):610. https://doi.org/10.3390/agriculture15060610
Chicago/Turabian StyleMei, Huiling, Tuo Li, Haiyan Wu, Yanwei Xia, Qiwei Huang, Dongyang Liu, and Qirong Shen. 2025. "Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions" Agriculture 15, no. 6: 610. https://doi.org/10.3390/agriculture15060610
APA StyleMei, H., Li, T., Wu, H., Xia, Y., Huang, Q., Liu, D., & Shen, Q. (2025). Transcriptomic Profiling Reveals Key Gene in Trichoderma guizhouense NJAU4742 Enhancing Tomato Tolerance Under Saline Conditions. Agriculture, 15(6), 610. https://doi.org/10.3390/agriculture15060610






