Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Study Area
2.2. Genetic Materials of the Nopal Used in the Experiment
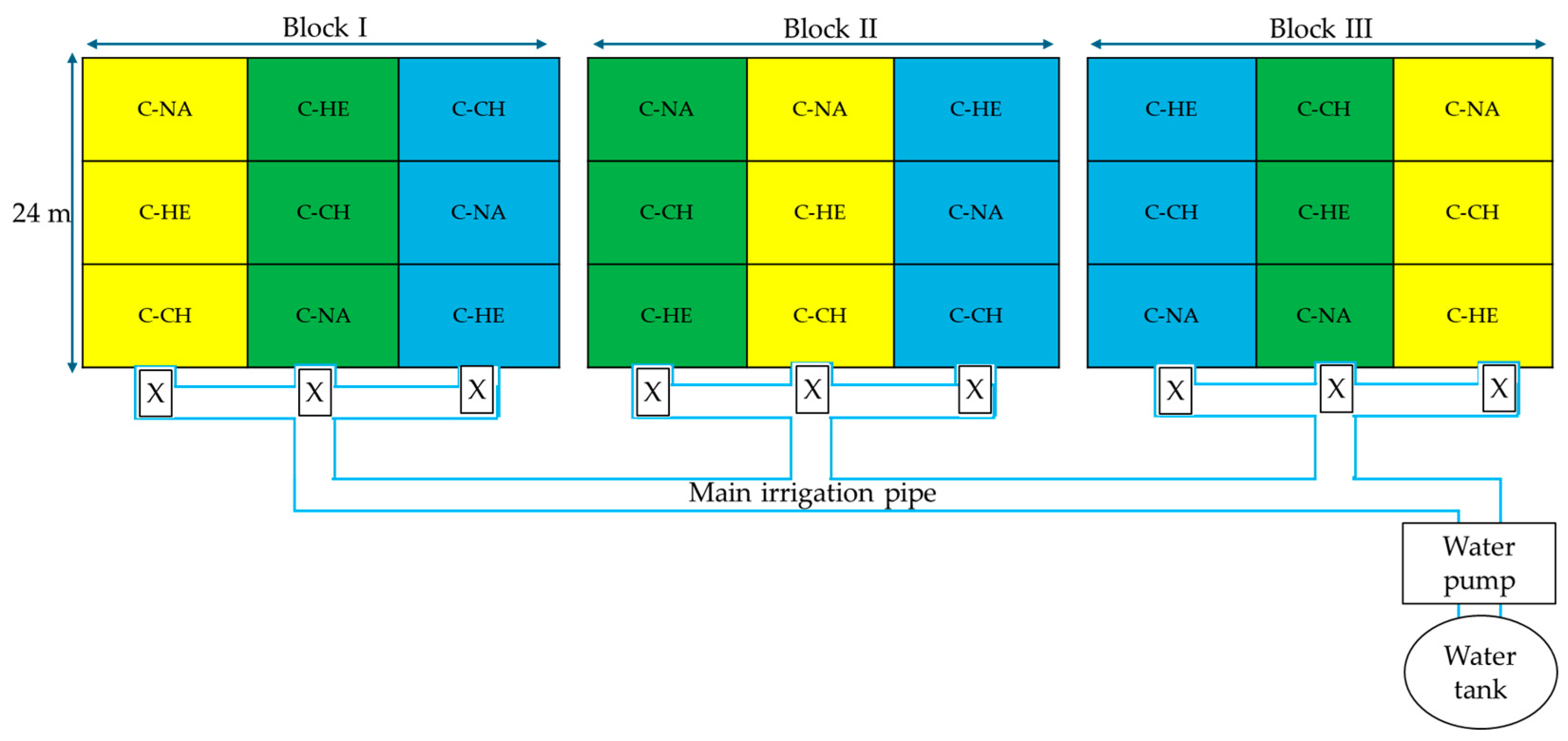
2.3. Experimental Design and Treatment Arrangements
2.4. Irrigation System Set Up
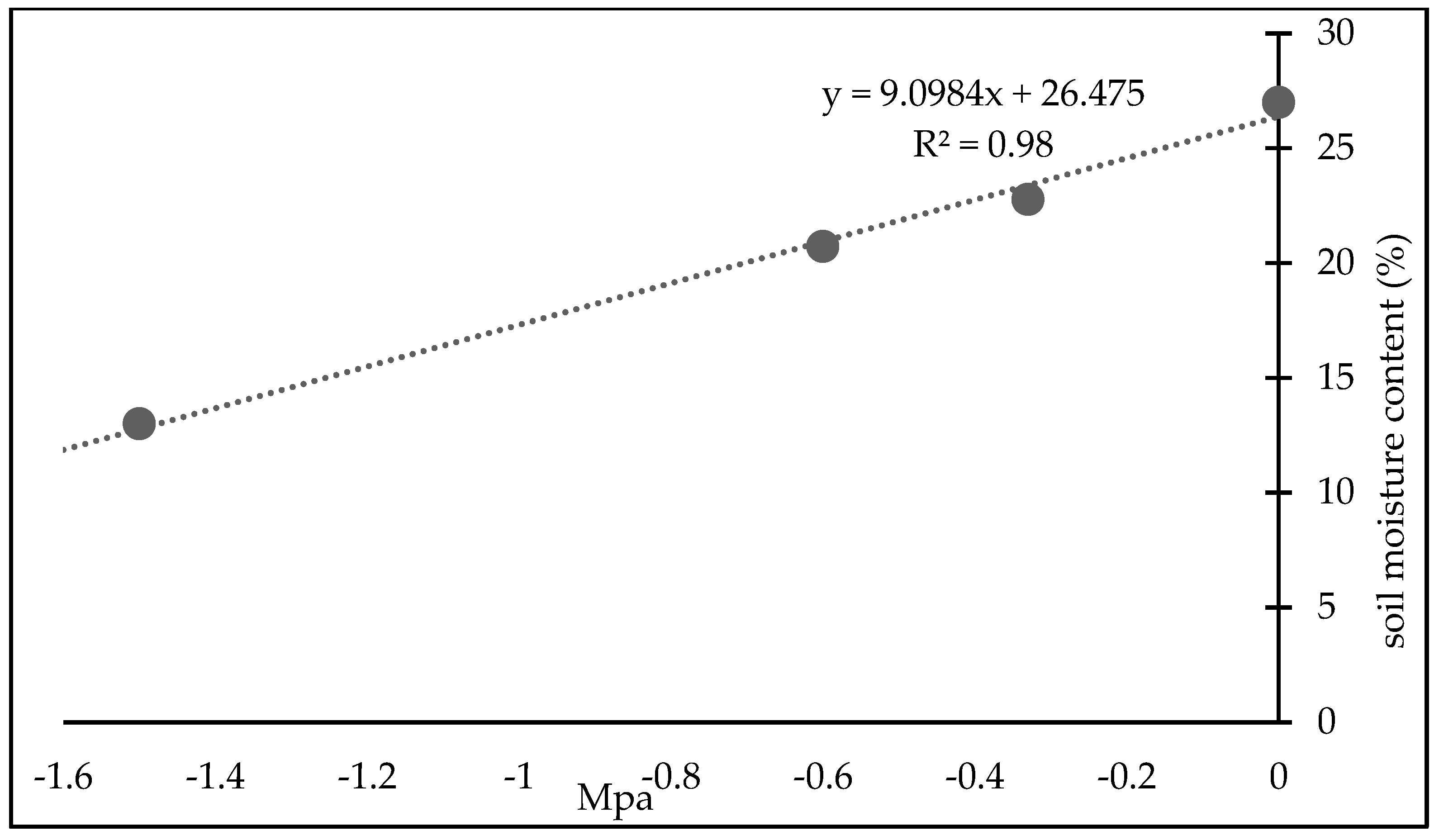
2.5. Variables
2.5.1. Physiological Variables Measured
2.5.2. Quality and Quantity of Mucilage Production
2.6. Data Analysis
3. Results
3.1. Description of Some Morphological Characteristics of the Nopal Morphotypes
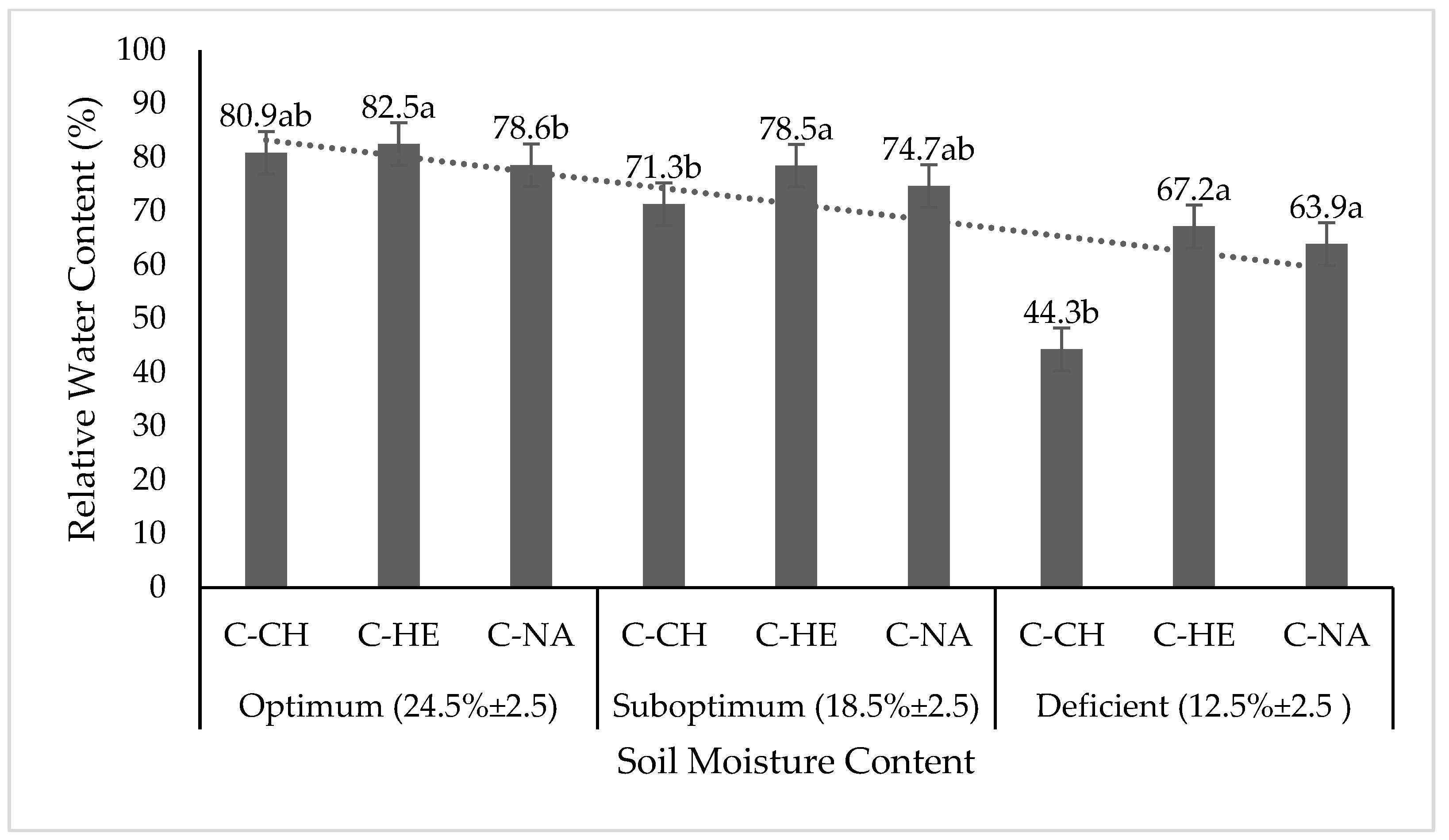
3.2. Measured, Chemical, and Physiological Variables
3.3. Quality and Mucilage Yield
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miralles, C.I.; Trasar-Cepeda, R.; Soria, R.; Ortega, M.; Lucas-Borja, E. Environmental and ecological factors influencing soil functionality of biologically crusted soils by different lichen species in drylands. Sci. Total Environ. 2021, 794, 1484913. [Google Scholar] [CrossRef] [PubMed]
- Troyo, D.E.; Mercado, M.G.; Cruz, F.A.; Nieto, G.A.; Valdez, C.R.D.; García, H.J.L.; Murillo, A.B. Análisis de la sequía y desertificación mediante índices de aridez y estimación de la brecha hídrica en Baja California Sur, noroeste de México. Investig. Geográficas 2014, 85, 66–81. [Google Scholar] [CrossRef][Green Version]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Jorge, A.O.S.; Costa, A.S.G.; Oliveira, M.B.P.P. Adapting to Climate Change with Opuntia. Plants 2023, 12, 2907. [Google Scholar] [CrossRef]
- Bacarrillo-López, R.; Pedroza-Sandoval, A.; Inzunza-Ibarra, M.A.; Flores-Hernández, A.; Macías-Rodríguez, F.J. Productividad de forraje de variedades de nopal (Opuntia spp.) bajo diferentes regímenes de humedad del suelo. Ecosistemas Recur. Agropecu. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The Ecological Water-Use Strategies of Succulent Plants. Chapter 4. In Advances in Botanical Research; Jean-Claude Kader, J.-C., Delseny, M., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 55, pp. 179–225. [Google Scholar] [CrossRef]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, 890–896. [Google Scholar] [CrossRef] [PubMed]
- CNCUB (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad) Nopales. Diversidad Biológica 2021. Available online: http://www.biodiversidad.gob.mx/diversidad/alimentos/nopales (accessed on 12 October 2023).
- Dubeux, J.C.B.; Ferreira dos Santos, M.V.F.; da Cunha, M.V.; do Santos, D.C.; Souza, R.T.d.A.; de Mello, A.C.L.d.; de Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.B. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods 2023, 12, 2494. [Google Scholar] [CrossRef]
- Torres-Ponce, R.L.; Morales-Corral, D.; Ballinas-Casarrubias, M.d.L.; Nevárez-Morillon, G.V. El nopal: Planta del semidesierto con aplicaciones en farmacia, alimentos y nutrición animal. Rev. Mex. Cienc. Agrícolas 2015, 6, 1129–1142. [Google Scholar] [CrossRef]
- Majdoub, H.; Sadok, R.; Deratani, A. Polysaccharides from prickly pear peel and nopals of Opuntia ficus indica: Extraction, characterization, and polyelectrolyte behavior. Polym. Int. 2001, 50, 552–560. [Google Scholar] [CrossRef]
- Vargas-Rodríguez, L.; Arroyo, F.G.; Herrera MC, H.; Pérez, N.A.; García, V.M.I.; Rodríguez-Núñez, J.R. Propiedades físicas del mucílago de nopal. Acta Univ. 2016, 26, 8–11. [Google Scholar] [CrossRef]
- Inglese, P.; Mondragon, C.; Nefzaoui, A.; Saenz, C.; Taguchi, M.; Makkar, H.; Louhaichi, M. Ecología del cultivo, manejo y usos del nopal; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018. [Google Scholar]
- Luna-Zepién, E.A.; Zegbe, J.A.; Meza-Valézquez, J.A.; Minjares-Fuentes, R. Mucílago de nopal (Opuntia spp.) y su aplicación como aditivo alimentario: Una visión general. Rev. Fitotec. Mex. 2023, 46, 51–61. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández, M.P.; Guarda, A.; Galotto, M.J. Development of cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Rebah, F.B.; Siddeeg, S.M. Cactus an ecofriendly material for waste treatment. A review. J. Mater. Environ. Sci. 2017, 8, 1770–1782. [Google Scholar]
- Gheribi, R.; Khwaldia, K. Cactus mucilage for food packaging applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Sáenz, C.; Vásquez, M.; Trumper, S.; Fluxa, H.C. Extracción y Composición Química de Mucílago de Tuna (Opuntia ficus indica). II Congreso Internacional de la tuna y Cochinilla. Santiago, Chile. 1992, pp. 93–96. Available online: https://www.redalyc.org/pdf/416/41648312002.pdf (accessed on 14 February 2023).
- Alarcón-Aguilar, F.J. Hypoglycemic activity of two polysaccharides isolated from Opuntia ficus indica and Opuntia Streptacantha. Proc. West Pharmacol. Soc. 2003, 46, 139–142. [Google Scholar]
- Basurto, S.D.; Lorenzana Jiménez, M.; Magos, G.A. Utilidad del nopal para el control de la glucosa en la diabetes mellitus tipo 2. Rev. Fac. Med. 2006, 49, 157–162. [Google Scholar]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Galicia-Villanueva, S.; Escamilla-García, P.E.; Alvarado-Raya, H.; Aquino-González, L.V.; Serna-Álvarez, H.; Hernández-Cruz, L.M. Plantación experimental de nopal para evaluación de sistemas de fertilización y extracción de mucílago. Rev. Mex. Cienc. Agrícolas 2017, 8, 1087–1099. [Google Scholar]
- Naorem, A.; Patel, A.; Hassan, S.; Louhaichi, M.; Jayaraman, S. Global research landscape of cactus pear (Opuntia ficus-indica) in agricultural science. Front. Sustain. Food Syst. 2024, 8, 1354395. [Google Scholar] [CrossRef]
- Argentel, L.; González, L.M.; Ávila, C.; Aguilera, R. Comportamiento del contenido relativo de agua y la concentración de pigmentos fotosintéticos de variedades de trigo cultivadas en condiciones de salinidad. Cultiv. Trop. 2006, 27, 49–53. [Google Scholar]
- Medina, G.G.; Diaz, P.G.; López, H.J.; Ruíz, C.J.A.; Marín, S.M. Estadísticas climatológicas básicas del estado de Durango. (Período 1961–2003). Libro Técnico № 1. Campo Experimental Valle del Guadiana. CIRNOC-INIFAP. 2005. Available online: https://www.worldcat.org/title/estadisticas-climatologicas-basicas-del-estado-de-durango-periodo-1961-2003/oclc/651396737 (accessed on 25 July 2024).
- Arnoldo, F.H. El nopal (Opuntia spp.) en la región árida lagunera. Folleto de divulgación. Pronasol-Conacyt-Plan Nueva Laguna. Unidad Regional Universitaria de Zonas Áridas-Universidad Autónoma Chapingo. Bermejillo, Durango, México. Folleto Técnico, 1994, 13p. Available online: https://catalogo.bibliotecasguanajuato.gob.mx/cgi-bin/koha/opac-detail.pl?biblionumber=26600&shelfbrowse_itemnumber=70304# (accessed on 12 September 2023).
- Carranza-Sabás, J.A.; Peña-Valdivia, C.B.; Reyes-Agüero, J.A.; Luna-Cavazos, M. Flores-Hernández Morphological characterization in situ of Opuntia spp. cladode in Bermejillo, Durango. Rev. Chapingo Ser. Hortic. 2004, 10, 75–77. [Google Scholar] [CrossRef]
- Flores-Hernández, A.; Peña-Valdivia, C.B.; Hernández-Montiel, L.; Ramírez-Serrano, R.; Trejo-Calzada, R.; Meza-Herrera, C.A.; Preciado-Rangel, P.; Murillo-Amador, B. Isoenzyme characterization of nopal cultivars (Opuntia spp.). Ecosistemas Recur. Agropecu. 2016, 3, 75–89. [Google Scholar]
- Richards, L.A. Porous plate apparatus for measuring moisture retention and transmission by soil. Soil Sci. 1948, 66, 105–110. [Google Scholar] [CrossRef]
- Jiménez-Galindo, J.C.; Acosta-Gallegos, J.A. Rendimiento de frijol común (Phaseolus vulgaris L.) y tépari (Phaseolus acutifolius A. Gray) bajo el método riego-sequía en Chihuahua. Rev. Mex. Cienc. Agrícolas 2013, 4, 557–567. [Google Scholar]
- Kramer, P.J. Water Relation of Plants; Academic Press: New York, NY, USA, 1983; Available online: www.sciencedirect.com (accessed on 10 May 2023).
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolutions. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Wang, Y.T.; Strong, K.J. A two-year study monitoring several physical and chemical properties of field-grown Aloe barbadensis Miller leaves. Subtrop. Plant Sci. 1995, 47, 34–38. [Google Scholar]
- Pérez, N.A.; Charua, D.; Fernández, S. Extracción y purificación del mucílago y goma de nopal para su uso en conservación. Estud. Sobre Conserv. Restauración Museol. 2015, 2, 156–165. [Google Scholar]
- Fernández, V.; Bahamonde, H.A.; Peguero-Pina, E.; Gil-Pelegrin, D.; Sancho-Knapi, D.; Gil, L.; Goldbach, H.E.; Eicher, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef]
- Rusli, M.S.; Abidin ZH, Z.; Taha, R.M.; Arof, A.K. Spectroscopic studies of chlorophyll a from Opuntia ficus-indica. Mater. Res. Innov. 2009, 13, 266–268. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; 513p, Available online: https://books.google.com.mx/books/about/Physiological_Plant_Ecology.html?id=BgtzD4frr98C&redir_esc= (accessed on 12 October 2023).
- Troshchynska, Y.; Bleha, R.; Synytsya, A.; Štětina, J. Chemical Composition and Rheological Properties of Seed Mucilages of Various Yellow-and Brown-Seeded Flax (Linum usitatissimum L.) Cultivars. Polymers 2022, 14, 2040. [Google Scholar] [CrossRef]
- Time, A.; Garrido, M.; Acevedo, E. Water relations and growth response to drought stress of Prosopis tamarugo Phil. A review. J. Soil Sci. Plant Nutr. 2018, 18, 329–343. [Google Scholar] [CrossRef]
- Feng, W.; Lindner, H.; Robbins, N.E., 2nd; Dinneny, J.R. Growing Out of Stress: The Role of Cell- and Organ-Scale Growth Control in Plant Water-Stress Responses. Plant Cell 2016, 28, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Mota-Ituarte, M.; Pedroza-Sandoval, A.; Minjares-Fuentes, R.; Trejo-Calzada, R.; Zegbe Jorge, A.; Quezada-Rivera, J.J. Water deficit and salinity modify some morphometric, physiological, and productive attributes of Aloe vera (L.). Bot. Sci. 2023, 101, 463–475. [Google Scholar] [CrossRef]
- Aguilar-Beccerril, G.; Peña-Valdivia, C.B. Alteraciones fisiológicas provocadas por sequía en nopal (Opuntia ficus-indica). Rev. Fitotec. Mex. 2006, 29, 231–237. [Google Scholar]
- Moradi, P. Key plant products and common mechanisms utilized by plants in water deficit stress responses. Bot. Sci. 2016, 94, 657–671. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E.; Zañudo-Hernández, J.; Muñoz-Urias, A.; Robles-Murguía, C. Ecofisiología de tallos jóvenes (cladodios) de Opuntia ficus-indica en condiciones húmeda y seca. Gayana. Botánica 2012, 69, 232–239. [Google Scholar]
- Lüttge, U. One Morphotype, Three Physiotypes: Sympatric Species of Clusia with Obligate C3 Photosynthesis, Obligate CAM and C3-CAM Intermediate Behavior. Plant Biol. 1999, 1, 138–148. [Google Scholar] [CrossRef]
- Betancourt-Domínguez, M.A.; Hernández-Pérez, T.; García-Saucedo, P.; Cruz-Hernández, A.; Paredes-López, O. Physic-chemical changes in cladodes (nopalitos) from cultivated and wild cacti (Opuntia spp.). Plant Foods Hum. Nutr. 2006, 61, 115–119. [Google Scholar] [CrossRef]
- Ievinsh, G. Water Content of Plant Tissues: So Simple That Almost Forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef]
- González-Espíndola, L.Á.; Pedroza-Sandoval, A.; Trejo-CaAlllzada, R.; Jacobo-Salcedo, M.d.R.; García de los Santos, G.; Quezada-Rivera, J.J. Relative Water Content, Chlorophyll Index, and Photosynthetic Pigments on Lotus corniculatus L. in Response to Water Deficit. Plants 2024, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, I.; Aguilar, I.; Pérez de Vida, F.; Molina, F.; Gutiérrez, L.; Rosas, J.E. Genotype by environment interaction characterization and its modeling with random regression to climatic variables in two rice breeding populations. Crop Sci. 2023, 63, 2020–2040. [Google Scholar] [CrossRef]
- Calatayud, A.; Barreno, E. Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments, and lipid peroxidation. Plant Physiol. Biochem. 2004, 42, 549–555. [Google Scholar] [CrossRef]
- Maki-Díaz, G.; Peña-Valdivia, C.B.; García-Nava, R.; Arévalo-Galarza, M.L.; Calderón-Zavala, G.; Anaya-Rosales, S. Características físicas y químicas de nopal verdura (Opuntia ficus-indica) para exportación y consumo nacional. Agrociencia 2015, 49, 31–51. [Google Scholar]
- Razzak, S.; Aouji, M.; Zirari, M.; Benchehida, H.; Taibi, M.; Bengueddour, R.; Wondmie, G.F.; Ibenmoussa, S.; Bin Jardan, Y.A.; Taboz, Y. Nutritional composition, functional and chemical characterization of Moroccan Opuntia ficus-indica cladode powder. Int. J. Food Prop. 2024, 27, 1167–1179. [Google Scholar] [CrossRef]
- Xolocotzi-Acoltzi, S.; Pedroza-Sandoval, A.; García-De los Santos, G.; Álvarez-Vázquez, P.; Gramillo-Ávila, I. Growth, Productivity, Yield Components and Seasonality of Different Genotypes of Forage Clover Lotus corniculatus L. under Varied Soil Moisture Contents. Plants 2024, 13, 1407. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, L.; Martínez-Damián, M.T.; Barrientos-Priego, A.F.; Aguilar-Gallegos, N.; Gallegos-Vásquez, C. Potencial de oscurecimiento enzimático de variedades de nopalitos. J. Prof. Assoc. Cactus Dev. 2007, 9, 165–184. [Google Scholar]
- Rodríguez-Félix, A.; Cantwell, M. Developmental changes in composition and quality of prickly pear cactus cladodes (nopalitos). Plant Food Hum. Nutr. 1988, 38, 83–93. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14 (Suppl. S1), S165–S183. [Google Scholar] [CrossRef]
- Tomkowiak, A.; Jamruszka, T.; Bocianowski, J.; Sobiech, A.; Jarzyniak, K.; Lenort, M.; Mikołajczyk, S.; Żurek, M. Transcriptomic Characterization of Genes Harboring Markers Linked to Maize Yield. Genes 2024, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Rodríguez, N.S.; Pedroza-Sandoval, A.; Zegbe, J.A.; Trejo-Calzada, R. Indicadores de productividad y calidad de gel de sábila en condiciones de estrés salino. Rev. Fitotec. Mex. 2020, 43, 181–187. [Google Scholar] [CrossRef]
- González-Altozano, P.; Castel, J.L. Effects of regulated deficit irrigation in ‘Clementina de Nules’ citrus trees growth, yield and fruit quality. Acta Hortic. 2000, 537, 749–758. [Google Scholar] [CrossRef]
- Verreynne, J.S.; Rabe, E.; Theron, K.I. The effect of combined deficit irrigation and summer trunk girdling on the internal fruit quality of ‘Marisol’ Clementines. Sci. Hortic. 2001, 91, 25–37. [Google Scholar] [CrossRef]
- Hernandez, G.O.; Briones, V.O. Crassulacean acid metabolism photosynthesis incolumnar cactus seedlings during ontogeny: The effectof light on nocturnal acidity accumulation andchlorophyll fluorescence. Am. J. Bot. 2007, 94, 1344–1351. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Jardim, A.M.D.R.F.; Santos, H.R.B.; Alves, H.K.M.N.; Ferreira-Silva, S.L.; de Souza, L.S.B.; Júnior, G.D.N.A.; de Sá Souza, M.; de Araújo, G.G.L.; de Souza, C.A.A.; da Silva, T.G.F. Genotypic differences relative photochemical activity, inorganic and organic solutes and yield performance in clones of the forage cactus under semi-arid environment. Plant Physiol. Biochem. 2021, 162, 421–430. [Google Scholar] [CrossRef]
- Parent, B.; Hache, Z.C.; Redondo, E.; Simonneau, T.; Chaumont, F.; Tardieu, F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef]

| Nopal Morphotypes | Soil Moisture Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OSMC (22–27) | SSMC (16–21) | DSMC (10–15) | |||||||
| Chl a | Chl b | TChl | Chl a | Chl b | TChl | Chl a | Chl b | TChl | |
| C-CH | 9.44 a ±0.08 | 5.3 a ±0.56 | 14.74 a ±0.47 | 10.2 ab ±0.07 | 5.87 a ±0.02 | 16.11 a ±0.10 | 5.31 a ±0.29 | 4.80 a ±0.65 | 10.11 a ±0.94 |
| C-HE | 9.34 a ±0.05 | 4.7 a ±0.0 | 14.03 a ±0.05 | 10.1 b ±0.05 | 4.72 b ±0.00 | 14.86 b ±0.05 | 5.20 a ±0.18 | 4.44 a ±0.07 | 9.64 a ±0.24 |
| C-NA | 9.52 a ±0.09 | 5.0 a ±0.57 | 14.55 a ±0.53 | 10.3 a ±0.13 | 4.74 b ±0.01 | 15.10 ab ±0.14 | 5.27 a ±0.04 | 4.40 a ±0.07 | 9.67 a ±0.06 |
| Nopal Morphotypes | Soil Moisture Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OSMC (22–27) | SSMC (16–21) | DSMC (10–15) | |||||||
| pH | MMC (%) | MY (mL kg−1 FW) | pH | MMC (%) | MY (mL kg−1 FW) | pH | MMC (%) | MY (mL kg−1 FW) | |
| C-CH | 4.76 b ±0.03 | 98.2 a ±0.18 | 800.0 a ±0.0 | 4.55 a ±0.03 | 97.6 a ±0.03 | 712.6 a ±2.5 | 4.45 a ±0.03 | 97.1 a ±0.07 | 552.3 b ±7.5 |
| C-HE | 4.81 ab ±0.08 | 98.2 a ±0.01 | 789.3 c ±1.1 | 4.57 a ±0.05 | 97.5 a ±0.02 | 701.3 b ±4.1 | 4.49 a ±0.07 | 96.9 b ±0.11 | 571.0 a ±7.9 |
| C-NA | 5.02 a ±0.08 | 98.2 a ±0.06 | 795.6 b ±1.1 | 4.57 a ±0.03 | 97.5 a ±0.03 | 706.0 b ±5.2 | 4.48 a ±0.02 | 97.0 ab ±0.02 | 573.3 a ±5.7 |
| Nopal Morphotypes | Soil Moisture Content (%) | |||||
|---|---|---|---|---|---|---|
| OSMC (22–27) | SSMC (16–21) | DSMC (10–15) | ||||
| Ash Content (%) | Total Solids (%) | Ash Content (%) | Total Solids (%) | Ash Content (%) | Total Solids (%) | |
| C-CH | 2.44 a ±0.00 | 6.0 a ±0.00 | 2.56 a ±0.02 | 6.0 a ±0.00 | 4.14 a ±0.05 | 6.0 a ±0.00 |
| C-HE | 2.42 a ±0.07 | 6.3 a ±0.57 | 2.52 a ±0.02 | 6.3 a ±0.57 | 4.11 a ±0.02 | 6.0 a ±0.00 |
| C-NA | 2.36 a ±0.07 | 5.6 a ±0.57 | 2.51 a ±0.02 | 6.0 a ±0.00 | 4.08 a ±0.00 | 6.0 a ±0.00 |
| MMC | AC | MY | TS | Chl a | Chl b | TChl | |
|---|---|---|---|---|---|---|---|
| MY | 0.95721 <0.0001 | −0.94340 ** <0.0001 | 1.00000 | 0.00227 0.9910 | 0.85278 ** <0.0001 | 0.42214 * 0.0283 | 0.84003 ** <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroza-Sandoval, A.; González-Espíndola, L.Á.; Gramillo-Ávila, I.; Miranda-Rojas, J.A. Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions. Agriculture 2025, 15, 593. https://doi.org/10.3390/agriculture15060593
Pedroza-Sandoval A, González-Espíndola LÁ, Gramillo-Ávila I, Miranda-Rojas JA. Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions. Agriculture. 2025; 15(6):593. https://doi.org/10.3390/agriculture15060593
Chicago/Turabian StylePedroza-Sandoval, Aurelio, Luis Ángel González-Espíndola, Isaac Gramillo-Ávila, and José Antonio Miranda-Rojas. 2025. "Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions" Agriculture 15, no. 6: 593. https://doi.org/10.3390/agriculture15060593
APA StylePedroza-Sandoval, A., González-Espíndola, L. Á., Gramillo-Ávila, I., & Miranda-Rojas, J. A. (2025). Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions. Agriculture, 15(6), 593. https://doi.org/10.3390/agriculture15060593









