Abstract
The adoption of integrated production systems may be an alternative for improving soil health and increasing production. The aim of this study was to evaluate changes in soil fertility and microbial metabolism, as well as the impact on soybean productivity, in different conservation systems in contrast to the conventional system, after four years of adopting integrated systems. The experimental design used was a randomized block design with seven treatments and three replications. The treatments included different species of forage grasses, the no-tillage soybean–maize system in succession, and conventional planting. It was found that after four years of using integrated systems, the changes in soil health were small, indicating that these effects are seen over the long term. Soil chemistry showed that the use of forage grasses is essential for improving fertility, with a focus on phosphorus, potassium, magnesium, sulfur, base sum, and cation exchange capacity, which is reflected in the high soybean productivity in treatments with forage grasses, especially the use of Paiaguás and Piatã grasses. Even with slow changes in soil health, adopting integrated systems is an important practice for tropical sandy soils, as visible improvements in fertility were observed, which are reflected in productivity gains.
1. Introduction
Global population growth has increased the demand for food, challenging agriculture to achieve high productivity without compromising the environment. In this context, ensuring food security requires strategies to optimize crop quality and yield [1].
In tropical climate countries, a significant portion of agriculture occurs on highly weathered, acidic, and low-fertility soils, such as sandy soils. These soils have significant limitations, including low cation exchange capacity (CEC) and reduced water retention. However, these constraints can be mitigated by increasing soil organic matter (OM) content, which provides numerous benefits, including improvements in the soil’s physical, chemical, and biological properties. OM plays a central role in soil fertility, structure, and microbial activity [2,3].
In this regard, to increase soil organic matter content, tropical grasses stand out among the various cover crop species evaluated for sustainable systems [4]. These plants exhibit high biomass production, competitive ability against native vegetation, drought resistance, and adaptation to different soil types, including sandy soils [5]. Furthermore, they tolerate soil compaction and have a slower residue decomposition rate compared to legumes due to their higher C/N ratio. They are also notable for their significant root production and distribution within the phytomass [6,7].
The residue from these grasses acts as a barrier against high temperatures while promoting soil moisture retention, a crucial factor for soil microbial communities, especially in tropical climates [8,9]. This process supports the maintenance of fungal hyphae and bacterial colonies in the soil [10,11,12]. Microorganisms represent the living fraction of the soil and play an essential role in the development of sustainable agriculture, significantly contributing to productivity increases. In tropical soils, factors such as temperature fluctuations, drought periods, and characteristics like intense weathering and acidic pH can affect the presence and maintenance of soil OM and microorganisms. Due to their sensitivity to environmental changes, microorganisms serve as key indicators for assessing soil quality and health, acting as effective bioindicators [13,14]. The increase in soil surface residue significantly contributes to organic matter accumulation and, consequently, to nutrient cycling, making them available to cultivated plants and the microbial community. This process enhances the health of sandy soils, which tend to be less fertile due to their granulometric composition.
Agricultural production systems, such as no-tillage systems (NTSs) and integrated crop–livestock systems (ICLSs), have emerged as promising alternatives to enhance agricultural soil health. Understanding how these systems affect soil chemical, microbiological, and enzymatic attributes, as well as crop productivity over time, is essential for developing technical recommendations for more sustainable agriculture. Therefore, this study aimed to evaluate changes in soil chemical attributes and microbial metabolism under NTS, conventional tillage system (CTS—with soil mobilization), and ICLS after four years of implementation. The goal was to compare these systems regarding sandy soil quality and soybean productivity in succession.
2. Materials and Methods
2.1. Location and Characteristics of the Experimental Area
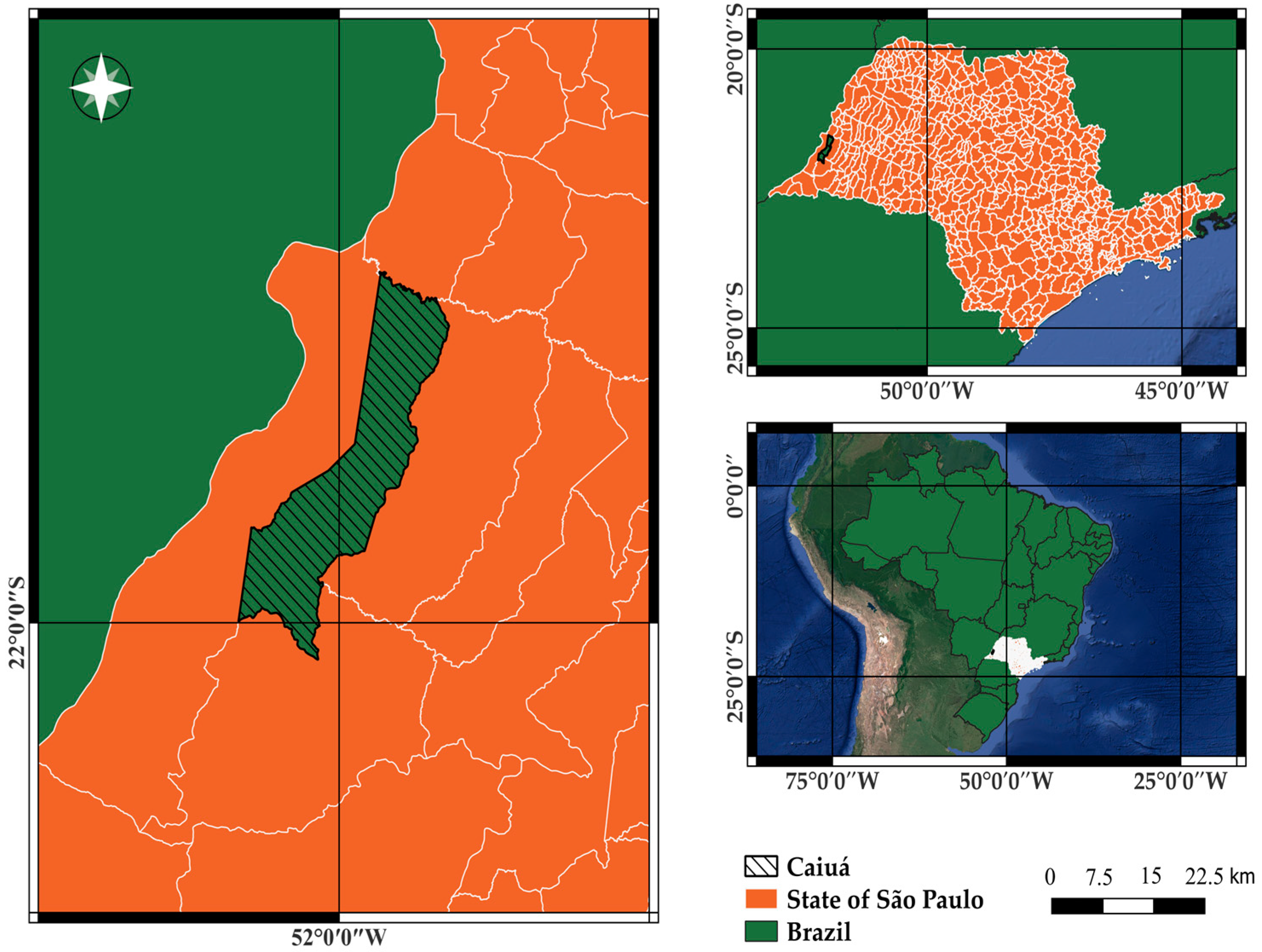
The experiment was conducted at the Vô Altino Research Center of the Facholi Seeds Company, located in the municipality of Caiuá, São Paulo state, Brazil (latitude 21°49′58″ S, longitude 51°59′24″ W), at an altitude of 330 m (Figure 1).
Figure 1.
Experimental location of the Vô Altino Research Center, Caiuá, São Paulo State, Brazil.
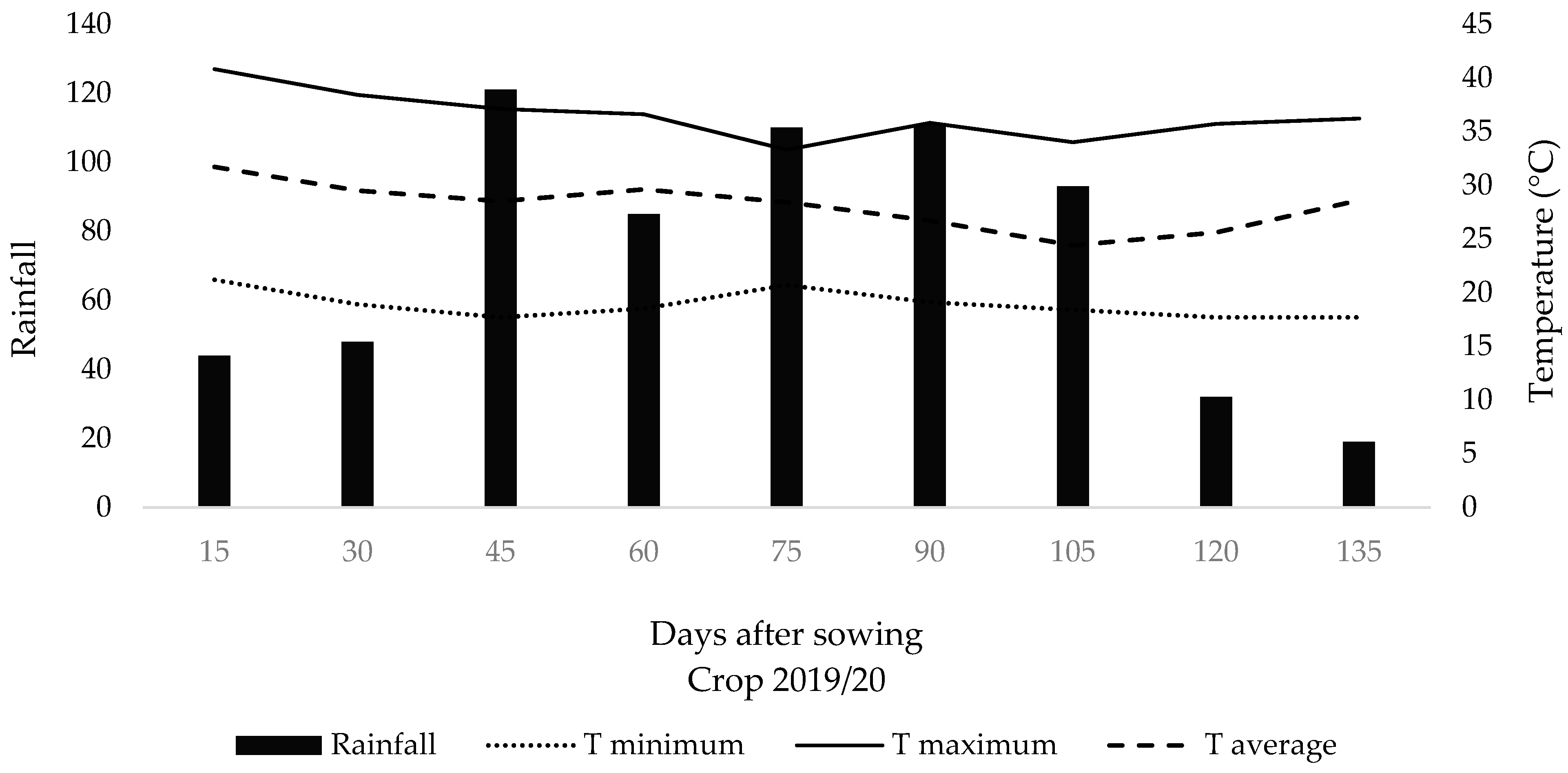
The region’s climate is classified as Aw according to [15], characterized as tropical humid with a rainy season in summer and a dry season in winter. The average annual precipitation is 1353 mm, and the average annual temperature is 24.3 °C. The hottest months are January, February, and December (average of 27 °C), while the coldest months are June and July (average of 21 °C) [16]. Climate data for the experimental period from sowing to soybean harvest were collected at the research center (Figure 2).
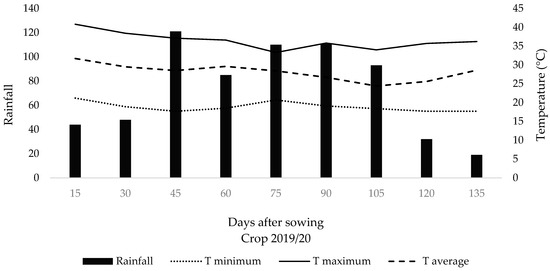
Figure 2.
Biweekly average of precipitation, mean, maximum, and minimum temperatures collected in the experimental area during the experiment period from sowing to soybean harvest.
The soil in the experimental area was classified as Oxisol [17], with 816, 116, and 68 g kg−1 of sand, clay, and silt in the 0 to 0.20 m layer, respectively [18].
2.2. Area History, Experimental Design, and Treatments
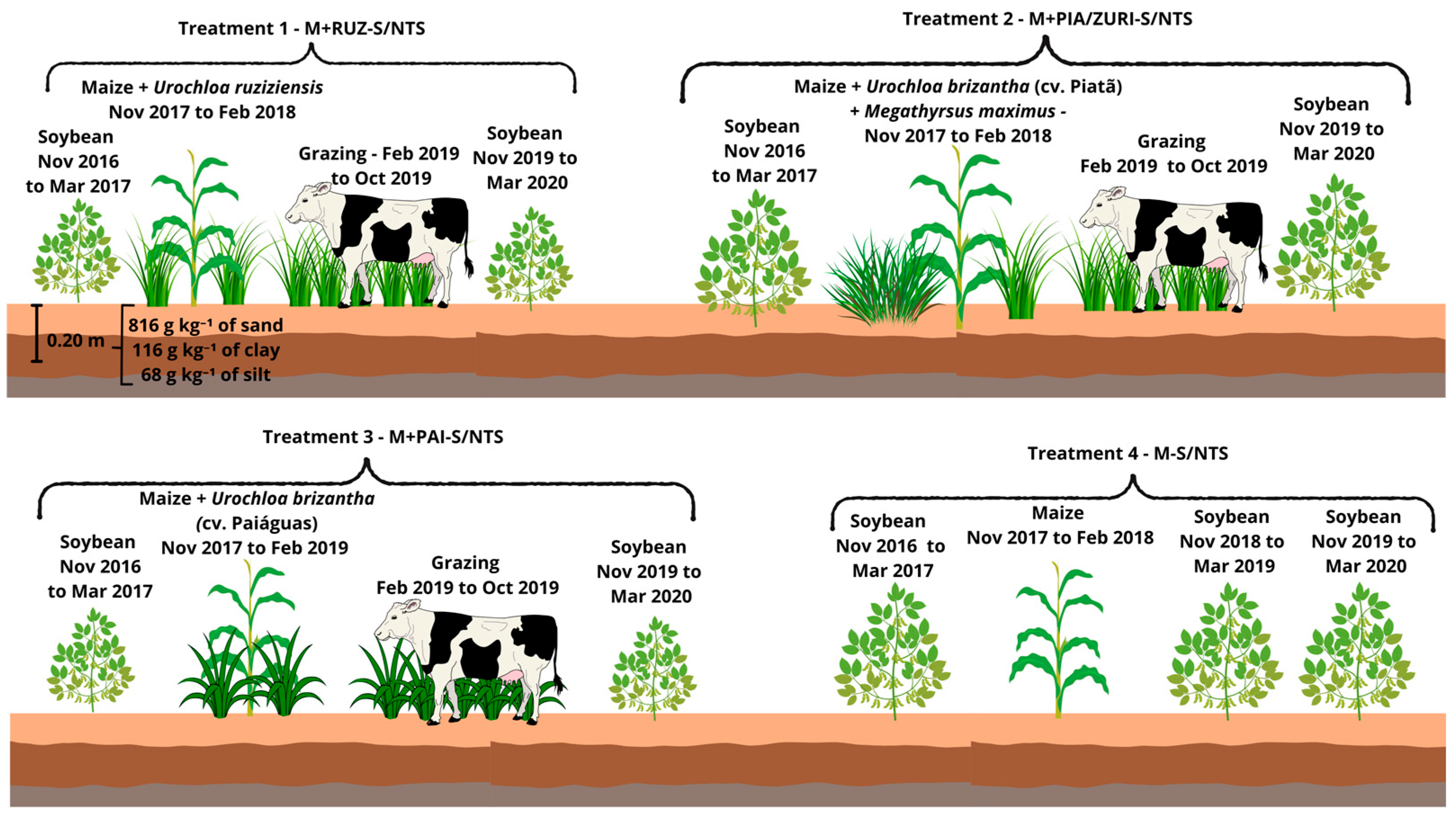
The experimental design was a randomized block design with seven treatments and three replications (Figure 3 and Figure 4), with each plot having an average dimension of 2.0 ha, totaling 42 ha of area. The treatments were as follows:
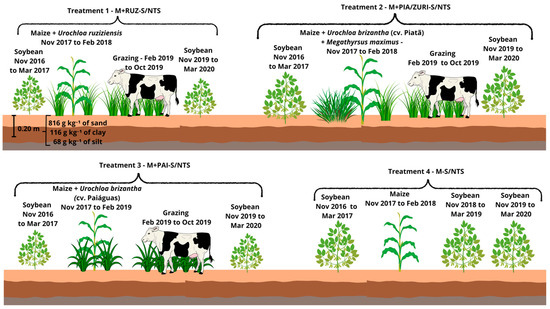
Figure 3.
Description of Treatments 1 to 4.
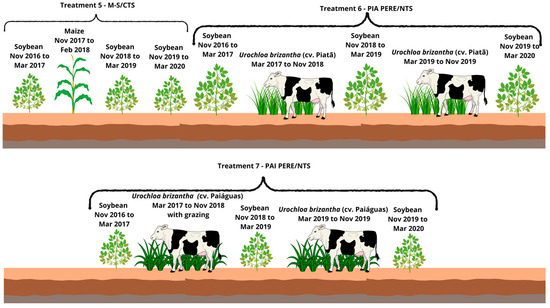
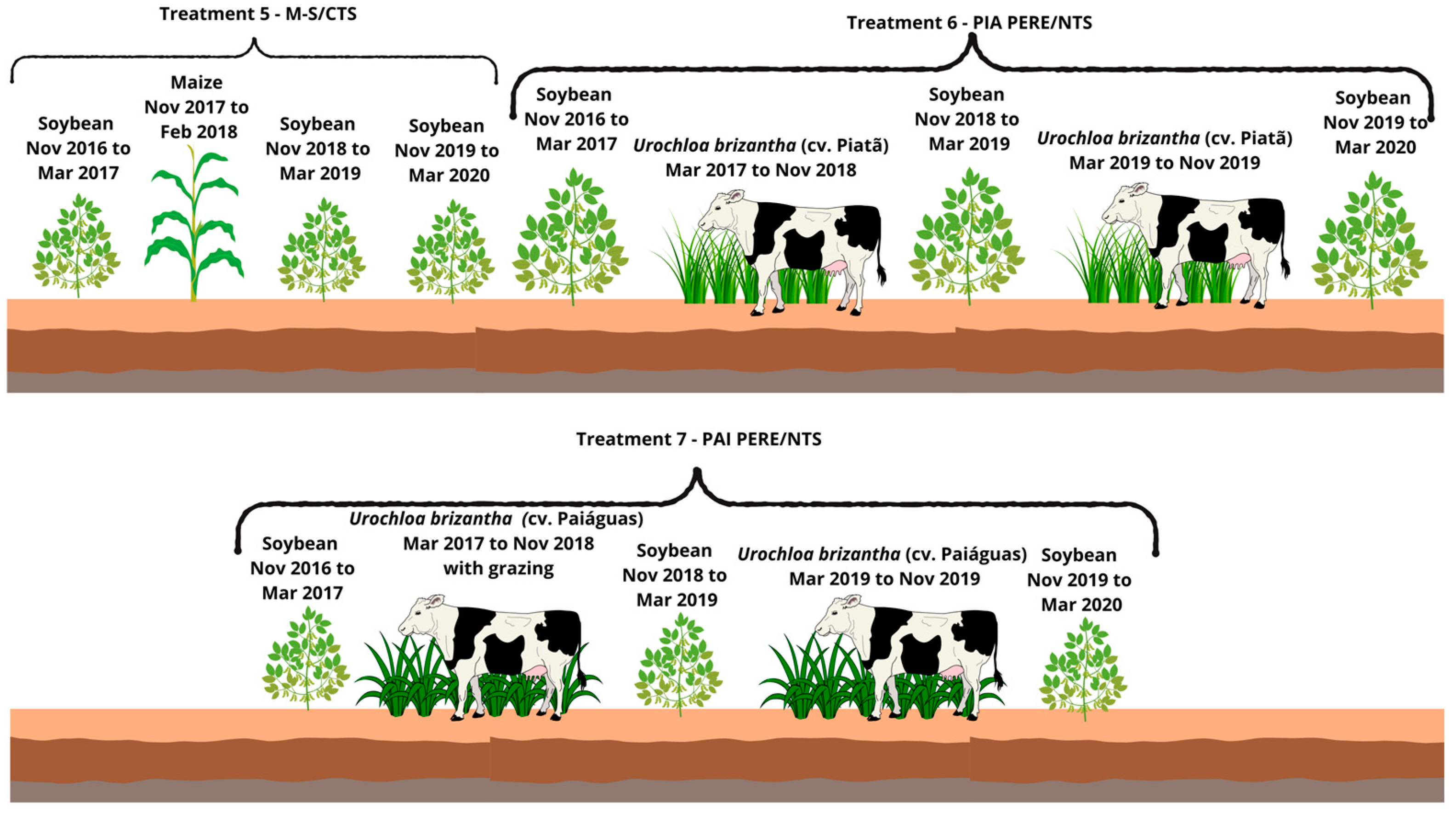
Figure 4.
Description of Treatments 5 to 7.
- Soybean–Maize + Urochloa ruziziensis (grazing) + Soybean/No-tillage System (NTS) (M+RUZ-S/NTS):2016/17: Soybean cultivation in November 2016.2017/18: Sowing of maize intercropped with U. ruziziensis, without grazing.2019/20: Introduction of animal grazing in 2019 and 2020, with soybean as the summer crop.
- Soybean–Maize + Urochloa brizantha cv. Piatã (grazing)/Megathyrsus maximus cv. BRS Zuri + Soybean/No-tillage System (M+PIA/ZURI-S/NTS):2016/17: Soybean cultivation in November 2016.2017/18: Sowing of maize intercropped with Urochloa brizantha cv. Piatã, without grazing.2018/19: Replacement of Piatã grass with Megathyrsus maximus cv. BRS Zuri due to low dry matter production.2019/20: Introduction of animal grazing in 2019 and 2020, with soybean as the summer crop.
- Soybean–Maize + Urochloa brizantha cv. Paiaguás (grazing) + Soybean/No-tillage System (M+PAI-S/NTS):2016/17: Soybean cultivation in November 2016.2017/18: Sowing of maize intercropped with U. brizantha cv. Paiaguás, without grazing.2019/20: Introduction of animal grazing in 2019 and 2020, with soybean as the summer crop.
- Soybean–Maize/No-tillage system (M-S/NTS):2016/17: Soybean cultivation in November 2016.2017/18: Sowing of maize using no-tillage in the off-season, repeated in 2018.2019/20: Due to low maize productivity, soybean was cultivated followed by fallow in 2019 and 2020.
- Soybean–Maize/Conventional Tillage System—CTS (M-S/CTS):2016/17: Soybean cultivation in November 2016.2017/18: Sowing of maize in the off-season with conventional tillage (one plowing and leveling harrowing), repeated in 2018.2019/20: Due to low maize productivity, soybean was cultivated followed by fallow in 2019 and 2020.
- Soybean–Urochloa brizantha cv. Piatã (grazing for 2 years)—Soybean–Piatã (grazing)—Soybean/No-tillage System (PIA PERE/NTS):2016/17: Soybean cultivation in November 2016.2017/18 and 2018/19: Sowing of U. brizantha cv. Piatã in March 2017, remaining in the area for two years.2019/20: After soybean cultivation in succession, U. brizantha cv. Piatã was resown in March 2019, this time with grazing for one year, followed by soybean as the summer crop.
- Soybean–Urochloa brizantha cv. Paiaguás (grazing for 2 years)—Soybean–Paiaguás (grazing)—Soybean/No-tillage System (PAI PERE/NTS):2016/17: Soybean cultivation in November 2016.2017/18 and 2018/19: Sowing of U. brizantha cv. Paiaguás in March 2017, remaining in the area for two years.2019/20: After soybean cultivation in succession, U. brizantha cv. Paiaguás was resown in March 2019, this time with grazing for one year, followed by soybean as the summer crop.
2.3. Characteristics of the Area and Experimental Conduct
Before the installation of the treatments, the experimental area was cultivated with Megathyrsus maximus (Syn. Panicum maximum) cv. Massai in 2014, intended for seed production by the Facholi Seeds Company, Santo Anastácio, state of São Paulo, Brazil. In the off-season of 2015, maize was cultivated, and in November of the same year, the area was desiccated for soybean cultivation to standardize the land use history. Soybean was sown in November 2015 with a spacing of 0.45 m, a density of 330,000 plants ha−1, and sowing fertilization of 300 kg ha−1 of the 04-30-10 fertilizer. The soybean was harvested on 26 February 2016.
In March 2016, soil fertility was characterized at depths of 0–0.10 m and 0.10–0.20 m. A total of 20 sub-samples for each repetition of the 3 repetitions (plots) to form a composite sample for each treatment were collected to form composite samples at each depth [19]. Undisturbed samples (volumetric rings) were also collected to determine the porosity (macro, micro, and total) and soil density, following the methodology of [18]. The average values for the physical and chemical attributes obtained are presented in Table 1a–c.

Table 1.
(a) Physical attributes, total nitrogen, and C/N ratio of the soil before the installation of the experiment. Caiuá, São Paulo State, Brazil, September 2016. (b) Soil chemical attributes before the installation of the experiment. Caiuá, São Paulo State, Brazil, September 2016. (c) Analysis of soil chemical attributes before the installation of the experiment. Caiuá, São Paulo State, Brazil, September 2016.
2.3.1. Seeding of the Intercropped Forages
The seeding of the different forages took place between the 7th and 10th March 2019, using a row spacing of 0.45 m. An additional box for the forage seeds was attached to the seeder (third box), where the forage seeds were distributed in front of the seeder through ducts spaced 0.40 m apart. The seeding rate for the forages was 5 kg ha−1 of pure and viable seeds. All the forage seeds used in this project were provided by Facholi Seeds Company, Santo Anastácio, state of São Paulo, Brazil.
2.3.2. Desiccation of the Forages in the Intercropping Systems and Soybean Seeding
After desiccating the forages using 2.5 kg ha−1 of Glyphosate herbicide, soybean seeding was carried out in the total area between the 9th and 11th November 2019. The soybean seeds were inoculated with Bradyrhizobium bacteria (Vittea, São Joaquim da Barra, state of São Paulo, Brazil): (solid turf inoculant—strain SEMIA 5079—5 × 10⁹ colony-forming units (CFU)/g, at a dose of 200 g per 50 kg of seeds). The TMG 7063 (TMG, Rio Verde, state of Goias, Brazil), soybean cultivar was used for mechanized seeding, with the aid of a seed and fertilizer drill with a furrow opener mechanism of the knife-type (blade) for no-till planting, with a row spacing of 0.45 m and approximately 14 seeds per meter of furrow, aiming for a population of around 330,000 plants ha−1. The seeding fertilization was 300 kg ha−1 of the 04-30-10 formula.
2.3.3. Harvesting of Soybean Grains
The soybean harvest was carried out on 11 March 2020. Evaluations of the soybean crop were conducted based on randomly selected samples from all experimental plots (around 2 ha paddocks).
The plant stand evaluation was performed at the full physiological maturity stage of the crop. Within each plot, data were collected from an average of four randomly selected points. Each sample consisted of two parallel rows, each 5 m long, totaling a 10 m sample. After counting the plants in these 10 m, all the plants were harvested to measure grain yield. The harvested plants were placed in properly labeled plastic bags for later processing and determination of productive characteristics. To minimize interference within the treatments, the samples were always collected by the same samplers in the block.
The samples collected in the field were processed using a threshing machine for yield determination. The threshed grains were weighed and corrected to 13% moisture, with the final yield results given in kilograms per hectare. This study focused on evaluating the history of treatments and their impact on soil quality and health by assessing chemical and biological factors. Therefore, only soybean grain yield was analyzed, as it was the last crop before soil attribute evaluation. Consequently, maize yield data were not considered in this study, since the main focus was on the impact of treatment history within the agricultural production system.
2.3.4. Soil Sampling for Chemical Analysis
A total of 20 sub-samples for each repetition of the 3 repetitions (plots) to form a composite sample for each treatment, at the depths of 0–0.10, 0.10–0.20, and 0.20–0.40 m, for evaluation of the soil’s chemical attributes, according to the methods described by [16]. The following parameters were analyzed: pH (CaCl₂), P (resin), S-SO₄, K, Ca, Mg, Al3+, H + Al, sum of bases (SB = Ca + Mg + K), cation exchange capacity (CEC = SB + [H + Al]), and base saturation (V% = [100 × SB]/CEC).
2.3.5. Determination of Microbial Respiratory Activity (MRA), Microbial Biomass Carbon (MBC), and Soil Enzymatic Activity
Soil samples were collected from 6 distinct points per plot, with 3 sub-samples taken at each point (between rows–row–between rows), at a depth of 0–0.10 m, and stored in a cooler with ice. The MRA was determined according to the methodology of [20]. For MBC analysis, the procedures described by [20], modified by [21,22], were used. The quantity of MBC was determined using the methodology proposed by [23].
To determine soil enzymatic activity, samples were collected using a soil auger, with 21 samples per plot, at the end of the soybean production cycle (2019/2020 season). The samples were then sieved through a 2 mm mesh and stored in plastic boxes. For each sample, three analytical repetitions were performed in the laboratory, where activities of soil enzymes associated with the carbon cycle (β-glucosidase), phosphorus (acid phosphatase), and sulfur (arylsulfatase) were determined, using the methods described [24].
2.3.6. BioAS Analysis
To assess soil health, the four-quadrant model proposed by [25] was used. This model aims to evaluate soil carbon modifications (loss, gain, or stability) based on the relationship between total organic carbon (SOC) and the average activity of the β-glucosidase (βG) and arylsulfatase (AS) enzymes per unit of SOC.
The model’s threshold lines were defined based on the relationships between the activity levels of βG and AS, as well as SOC content, with the accumulated grain yield (AGY) of soybean and maize obtained in these experiments. The βG, AS, and SOC values correspond to the levels at 50% of the maximum grain yield [26].
2.4. Statistical Analysis
The data were subjected to analysis of variance using the F test, and the means, when significant, were analyzed by the Tukey test at a 5% significance level. The statistical analyses were performed using the R software, version 4.3.2 [27]. Data normality was assessed using the Shapiro–Wilk test. For the analysis of variance and mean comparison tests, the ExpeDes.pt package was used. For the evaluation of soil health, the four-quadrant model proposed by [25,26] was used, which aims to assess modifications in soil carbon (loss, gain, or stability), based on the relationship between total organic carbon (TOC) and the average activity of the enzymes β-glucosidase (βG) and arylsulfatase (AS) per unit of TOC.
3. Results
3.1. Microbial Respiratory Activity (MRA), Microbial Biomass Carbon (MBC)
No significant effect was observed in the means (p ≤ 0.05) for microbial respiratory activity (MRA) and microbial biomass carbon (MBC) (Table 2). Since the present study evaluates systems with incorporation over four years, it is believed that both MRA and MBC have not yet reached equilibrium within the systems, which is why this result was observed.

Table 2.
Microbial respiratory activity (MRA) and microbial biomass carbon (MBC) in soil under different agricultural production systems and cropping modalities in Caiuá, São Paulo State, Brazil, 2020.
3.2. Soil Enzymatic Activity
Significant differences were observed in the averages (p ≤ 0.05) for the results of acid phosphatase, β-glucosidase, and arylsulfatase (Table 3). For the acid phosphatase enzyme result, the PAI PERENE/NTS treatment stands out, where the highest average was observed. In general, it was noted that the treatments that included forage species in the system presented significantly higher averages due to the high quality of the plant material and soil exploration by the roots. It becomes clear, then, the important role of forage for enzymatic activity and the consequent phosphorus (P) availability in the system. It is also worth noting that the M-S/CTS treatment showed the lowest result for acid phosphatase, which can be explained by the greater soil disturbance, which may destabilize the physical and microbiological aspects, consequently reducing enzymatic activity.

Table 3.
Soil enzymatic activity under different agricultural production systems and crop modalities in Caiuá, São Paulo State, Brazil, 2020.
For the β-glucosidase enzyme activity, it was observed that the cropping system with the BRS Piatã forage resulted in higher values compared to the M-S/NTS and M-S/CTS systems. The use of BRS Piatã resulted in an average increase of 40% in β-glucosidase enzyme activity, indicating good soil quality. For the arylsulfatase enzyme, the results showed significant differences (p ≤ 0.05), with the treatments using grasses again presenting the highest averages. The M+PAI-S/NTS and PIA PERE/NTS treatments had the highest averages among all treatments evaluated, with emphasis on the M+PAI-S/NTS treatment. Once again, the quality of the forages stands out, showing that combining NTS with the use of grasses is essential for improving soil microbiological health.
The treatment M+PIA/ZURI-S/NTS showed a low arylsulfatase value, a fact explained by the characteristics of the forage itself and pronounced variations due to the growing season, highlighting that choosing the right forage is crucial for the implementation and maintenance of the system. In this study, the use of grasses significantly contributed to the increase in acid phosphatase, β-glucosidase, and arylsulfatase activities. For acid phosphatase, the PAI PERE/NTS treatment stood out, confirming the quality of Paiaguás grass. In the case of β-glucosidase and arylsulfatase, the highest activity was observed in the PIA PERE/NTS treatment, most likely due to the high biomass production of Piatã grass, which may have contributed to the positive effect on these two enzymatic parameters.
3.3. Soil Fertility
For the soil fertility results in the 0–0.10 m layer (Table 4), it is noticeable that the treatments showed significant means for Tukey’s test (p ≤ 0.05). The treatment M+RUZ-S/NTS showed significant means for Ca, Mg, S-SO42−, and V%, with emphasis on Al and m%. These results, concerning nutrient levels in the soil, stand out due to the use of grasses, which, after desiccation, release nutrients through the decomposition of the straw left on the soil.

Table 4.
Chemical attributes of the soil under different agricultural production systems and cropping modalities in the 0–0.10 m layer, in the municipality of Caiuá, State of São Paulo, Brazil, 2020.
The treatment M+PIA/ZURI-S/NTS presented significant results for K, Ca, Mg, and SB, with emphasis on Al and m%. Once again, the treatment with grass usage stands out for the nutritional composition of the grass, releasing nutrients into the soil through the decomposition of the straw. Analyzing the results from treatment M+PAI-S/NTS, significant means were observed for P, K, Mg, SB, S-SO42−, and V%, with null levels of Al and m%, highlighting the use of BRS Piatã grass, which is considered of high quality for grazing and biomass production.
In the treatment M-S/NTS, the highest means were observed for P, K, Mg, S-SO42−, and V% levels. It is noteworthy that this treatment also presented zero levels of Al and m%, indicating that the use of NTS is one of the pillars for building fertile soil. For the treatment M-S/CTS, significant means were observed for P and S-SO42− levels, where this treatment showed the highest mean for m%, resulting from soil tillage, which consequently favors faster straw cycling and reduces soil fertility.
The results for the treatment PAI PERE/NTS showed significant means for the levels of K, Ca, Mg, S-SO₄2−, and V%. For the treatment PIA PERE/NTS, significant means were observed for Ca, Mg, SB, and V%, once again highlighting the quality of the material derived from the forage grass.
Table 5 presents the Tukey test results (p ≤ 0.05) for the 0.10–0.20 m layer, where significant means were observed for the test. The treatment M+RUZ-S/NTS showed significant means in pH and SB. In the treatment M+PIA/ZURI-S/NTS, significant means were observed for SB results. This effect is caused by the cycling of the forage grass residues, which typically contain a high amount of nutrients, benefiting soil fertility.

Table 5.
Chemical soil attributes under different livestock production systems and cultivation modalities in the 0.10–0.20 m layer in the municipality of Caiuá, São Paulo State, Brazil, 2020.
The treatment M+PAI-S/NTS showed significant results in pH, K, and SB. The results observed for soil pH, with the addition of grasses, demonstrate that increased OM in consolidated systems can act as a buffer, maintaining pH levels, and also increasing nutrient levels, directly affecting SB and CEC.
The M-S/NTS treatment showed significant means for pH and SB, once again explained by the adoption of a crop rotation system with minimal soil disturbance. In the M-S/CTS treatment, significant means were observed for H + Al, and this treatment also resulted in the highest m% value among all analyzed treatments. It is noteworthy that soils managed under conventional tillage, with greater soil disturbance, tend to shorten the plant residue decomposition cycle and increase base leaching in sandy soils. Additionally, the lack of cover crops significantly affects nutrient cycling and the associated benefits to soil fertility.
In the PAI PERE/NTS treatment, significant means were observed for pH and SB. Meanwhile, in the PIA PERE/NTS treatment, pH, K, and SB values were significantly higher, highlighting once again the importance of grasses, which play a crucial role in improving and/or maintaining soil fertility.
Table 6 presents the soil chemical properties at a depth of 0.20–0.40 m. The M+RUZ-S/NTS treatment showed significant means for P, Mg, S-SO₄2−, and V% (Table 6). The M+PIA/ZURI-S/NTS treatment had significant means for P and S-SO₄2−. Treatments with grasses contributed to soil profile development, as root system exploration facilitated nutrient accumulation at greater depths.

Table 6.
Soil chemical attributes under different agricultural production systems and cropping modalities at a depth of 0.20–0.40 m in Caiuá, São Paulo State, Brazil, 2020.
For the M+PAI-S/NTS treatment, the levels of P, Mg, SB, and V% showed significant means, once again highlighting the impact of incorporating grasses into the treatment. The results for the M-S/NTS treatment showed significant means for P, Mg, SB, S-SO₄2−, and V%. It is noteworthy that crop residues in NTS are essential for maintaining soil fertility through nutrient cycling, which explains the results observed in both treatments.
In the M-S/CTS treatment, the levels of Mg, S-SO₄2−, and V% had significant means according to Tukey’s test (p ≤ 0.05). It is worth mentioning that this treatment presented the highest average m% value among all treatments. For the PAI PERE/NTS treatment, the levels of Mg, SB, S-SO₄2−, and V% were significantly different. Meanwhile, for the PIA PERE/NTS treatment, the levels of Mg, SB, and V% showed significant means, with V% being the highest among all treatments. Once again, the prominence of these treatments is attributed to the use of grasses and the superior nutritional quality of plant material, which enhances nutrient cycling and improves the soil’s nutrient balance for plants.
The treatments that included forage grasses generally showed an increase in soil fertility throughout the profile, mainly evidenced by the increase in V% and SB. When comparing these treatments with the CTS, a considerable increase in soil fertility can be observed, which justifies the use of forage grasses to contribute to the improvement of chemical attributes at depth.
3.4. Soil Health
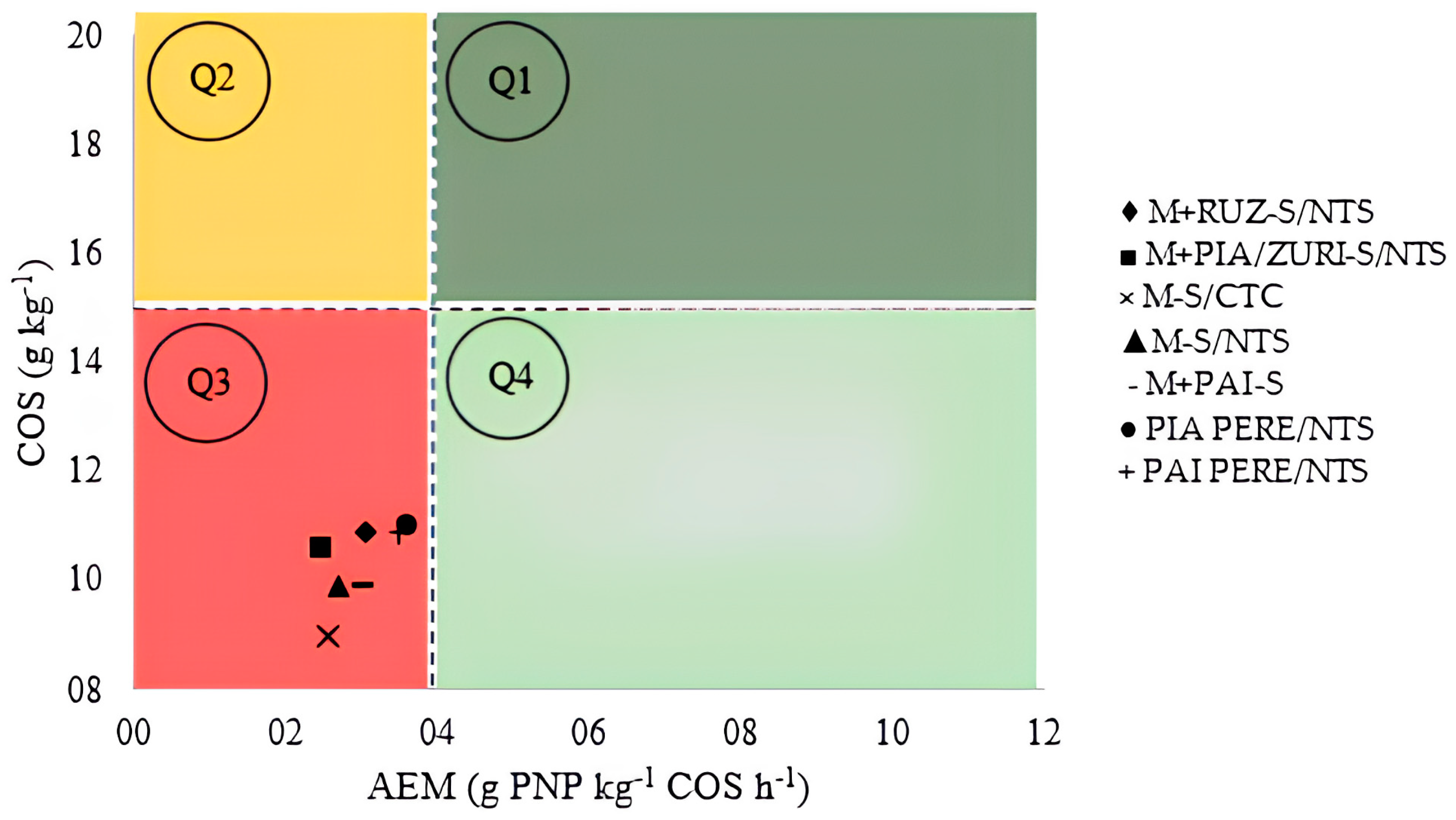
Via the BioAS analysis (Figure 5), it was possible to confirm the results of the one-way statistical analysis. The quadrant method confirmed that all studied treatments fall into Q3 (third quadrant), indicating that the soil is in a state of negative equilibrium, with low enzymatic activity, low soil organic carbon (SOC), and stable carbon. When ICLS practices are implemented in soils in Q3 equilibrium, the biological component of the soil improves considerably and enzymatic activity (β-glucosidase and arylsulfatase) increases linearly, while SOC does not show a significant variation at the initial stages.
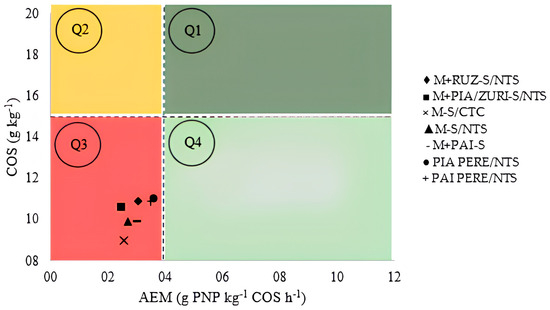
Figure 5.
Four-quadrant analysis using BioAS technology to determine soil health after four years of ICLS implementation in Caiuá, São Paulo State, Brazil, 2020. Note: TOC: total organic carbon, AEA: mean specific enzymatic activity, Q1: healthy soil, Q2: deteriorating soil, Q3: degraded soil, Q4: recovering soil. AEA = ((βG/TOC) + (AS/TOC))/2, where βG: β-glucosidase; AS: arylsulfatase; SOC: soil organic carbon; AEA: average specific enzymatic activity.
In this case, after four years of system adoption, the changes have not yet been significant enough to improve soil health. However, with BioAS, it was possible to confirm that the treatments are in constant progress, indicating a potential improvement over the years. Therefore, it is necessary to continue the implementation, as the benefits will become increasingly evident over time, especially in sandy soils with low carbon accumulation.
3.5. Soybean Grain Productivity
Regarding soybean grain productivity (Table 7), there were differences between treatments, showing that integration systems influence soybean yield. In the treatments with off-season grazing (M+RUZ-S/NTS, M+PIA/ZURI-S/NTS, M+PAI-S/NTS), soybean yields were similar across forages, indicating that regardless of cultivar/species, productivity was not affected. Furthermore, treatments with off-season grazing also showed similar results to the conventional system (M-S/CTS) and no-tillage soybean cultivation (M-S/NTS).

Table 7.
Final plant stand and grain yield of soybean cultivated in different integrated agricultural production systems, in the municipality of Caiuá, State of São Paulo, Brazil, 2019/2020 crop season.
Even with a lower plant population, soybean productivity in the cultivation after fallow under conventional soil preparation was similar to the treatments with off-season grazing followed by no-tillage soybean cultivation, as well as the treatment with cultivation after fallow under no-tillage (M-S/NTS), due to favorable climatic conditions throughout the crop development (Figure 1).
In treatments with grazing (PAI PERE/NTS and PIA PERE/NTS), the highest soybean productivity in succession was observed; however, the results were similar across forage cultivars. Thus, the choice between Paiaguás or Piatã as a perennial pasture in an integrated system depends on other factors, not just soybean productivity in succession, such as animal performance, ease of management, longevity, nutritional value, and others. The influence between cultivars on the productivity of the succeeding crop, in this case, soybean, was statistically the same in the system with three years of pasture.
Compared to the system with soybean cultivation after fallow with conventional soil preparation (M-S/CTS), the systems with pasture showed significantly higher productivity. As a result, the increase in productivity was 826 kg ha−1, which corresponds to approximately 14 additional sacks of soybeans in the treatments with Piatã or Paiaguás pasture.
4. Discussion
The MRA is defined by the absorption of O2 or the release of CO2 by microorganisms, where CO2 release measures all metabolic activities in order to monitor ecosystems, which is a direct indicator of soil microbiological quality [28,29]. Integrated agricultural production systems (SOC) are characterized by the use of cover crops and no-till practices, aiming to maintain an adequate organic matter (OM) content and prevent OM oxidation (pillars of NTS), which can positively influence microbiological activity [30,31]. Thus, when the system reaches equilibrium, it is capable of maintaining its fertility and microbiology equilibrium, being responsible for nourishing any plant present (pillar of fertilization in systems) [32,33]. In light of this, the research shows that after four years of implementing SOC, the systems have not yet reached a positive equilibrium regarding microbiological conditions (Table 2; Figure 5), which indicates that changes in soil microbiology are quite slow, especially in sandy soils with a higher likelihood of oxidation.
It is important to highlight that studies on sandy soils are extremely important for the development of productive agriculture, given the need for food production and the complexity of managing soils with such texture [34]. Therefore, though microbial C is an excellent indicator, this attribute alone is not sufficient to provide a complete indication of soil microbial biomass activity. It is essential to evaluate other attributes, such as microbial respiratory activity or basal respiration, biomass nitrogen, the microbial quotient, and the respiration rate, which is known as the microbial quotient and is considered the most traditional method for measuring metabolic activity [35,36,37,38,39,40]. A thorough investigation of different attributes over the long term is necessary for this type of soil due to the slow rate of changes.
Soil enzymatic activity is another important attribute for assessing the quality of agricultural soils. Sandy-textured soils tend to be less fertile than clayey soils due to their granulometry and few charges for nutrient retention [34]. Treatments incorporating grasses resulted in higher enzyme activity (Table 3). According to studies in the field, the adoption of forage grasses in soil conservation methods is essential to help balance the system. Additionally, the greater accumulation of nutrients in the leaves, the production of plant material, and the extensive soil exploration by the roots are favorable factors that contribute to soil fertility [41,42,43,44].
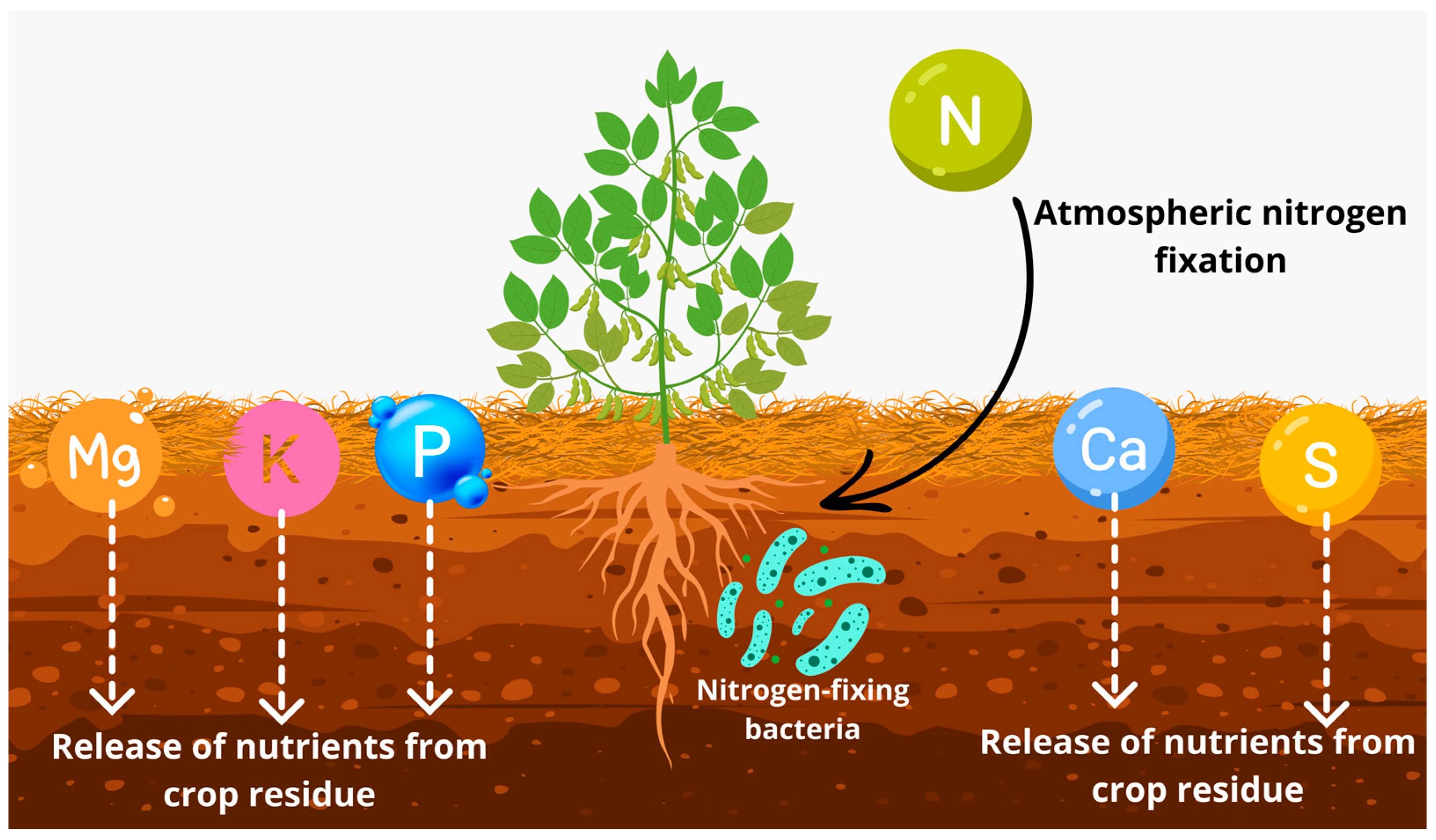
The soil OM content is another key aspect to be addressed, as soils with higher organic matter content tend to increase CEC and V%, enhance nutrient cycling, and alter pH, especially in the surface layer [45,46]. What is found in the research is that the treatments that incorporated forage grasses once again contributed to the chemical attributes of the soil. In general, it was found that the treatments incorporating grasses presented higher levels of P, K, Mg, and S-SO42−, with considerable improvements in CEC and V%, which is extremely important for sandy soils (Figure 6).
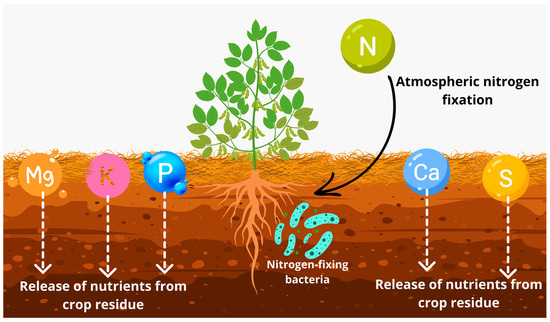
Figure 6.
Nutrient cycling dynamics in soil through the effect of cover crop residue and atmospheric nitrogen fixation by bacteria.
The improvement in soil fertility results from the addition of plant material, which significantly enhances nutrient cycling due to its high aerial biomass production and deep root system [47,48,49]. It is important to emphasize that the adoption of ICLS is a vital factor in developing regenerative agriculture systems, as the economically relevant crop begins its cycle with greater nutrient availability [50,51], significantly reducing the dependence on commercial fertilizers and helping to lower production costs [51,52].
The research shows that, even with slow changes in sandy soils, adopting ICLS is crucial for developing sustainable agriculture. As soil health improvement is a gradual process and depends on living factors, the adaptation of microorganisms to changes tends to be slow. Since this is a four-year study, the BioAS analysis (Figure 5) indicates that the soil microbiota is still adapting to these changes. Moreover, this trend will depend on the specific microorganisms present in the soil, as well as climatic conditions. However, despite the slow changes in soil biological attributes, a faster improvement in chemical attributes has been observed, increasing nutrient availability and reflecting positively on crop productivity. This compensates for the slower shifts in biological characteristics, demonstrating the benefits of adopting ICLS.
With regard to soybean productivity, it is possible to verify that the treatments that provided the highest yields were, once again, those that included forage grasses in their rotation (Table 7). Both Paiaguás grass and Piatã grass resulted in the highest grain yield averages. Similar studies determined that forage grasses are extremely important for increasing soil fertility, contributing to soybean grain production, as well as significantly improving grain quality. Additionally, they provide quick responses to soil management in sandy soils, increase the amount of crop residue, and enhance soil microbiological activity over time [53,54,55].
It is observed that the choice between Paiaguás or Piatã grass for integration should take into account other aspects, such as the initial soil conditions, material adaptability, ease of acquisition, and seed cost, in addition to its impact on productivity. Paiaguás grass is known for being more tolerant to water deficits, capable of adapting to soils with medium to low fertility, and is easily available due to its more competitive seed price [56]. On the other hand, Piatã grass produces high-quality biomass in large volumes, with extensive root system exploration of the soil; however, it requires a greater water supply [57]. Farmers must assess their local conditions to select the most suitable species or cultivar for their edaphoclimatic conditions. Those who adopt integrated crop–livestock systems (ICLS) will benefit significantly from incorporating cover crops into the soil, as improvements in physical, chemical, and microbiological properties become increasingly favorable over the years, leading to better land use with reduced dependence on synthetic commercial inputs [58,59,60,61,62,63,64].
In this study, the treatment that resulted in the lowest soybean yield was the conventional system (M-S/CTS). This occurs because systems with lower biomass input tend to have reduced nutrient availability for the soil. Additionally, the increased soil disturbance in conventional systems decreases organic matter content, leading to lower moisture retention (faster drying), higher soil temperature indices, reduced microbial activity, and greater resistance to root penetration, which directly impacts soybean productivity [65,66].
Therefore, when selecting crops and evaluating expected outcomes, the duration of soil management systems plays a crucial role. Long-term experiments, such as this study, are essential for characterizing and selecting management systems that aim to conserve soil and maintain crop productivity [67]. Thus, despite the slow changes observed in sandy soils over time, the positive results obtained, even in the initial period, support the viability of adopting ICLS.
As sandy soils tend to be more challenging in terms of management, practices aimed at improving soil health, as evaluated in this study, are extremely important for maintaining agricultural sustainability and enhancing crop production in this type of soil. This is crucial for food production, as the positive effects of changes in soil chemical and biological attributes can be observed over the years. Thus, the introduction of ICLS with the use of forage species significantly contributes to soil organic matter input and, consequently, to nutrient cycling, which is reflected in crop productivity.
5. Conclusions
The integrated crop–livestock systems (ICLS) that incorporated forages in rotation with soybean improved the chemical and biological quality of sandy soil, justifying their implementation. The chemical attributes of sandy soil showed little change within four years after the establishment of ICLS compared to the no-till system (NTS). This suggests that the benefits related to enzymatic activity and nutrient cycling may become more pronounced in the medium and long term when compared to conventional systems or NTSs in the soybean–maize succession.
Overall, systems with pastures had a more significant impact on soil quality improvement, particularly regarding base saturation. According to the BioAS analysis, the soil is still in quadrant Q3, in a state of negative equilibrium, indicating that the changes have not yet resulted in a transition to sustainable systems. However, based on unidirectional analyses and production results, a gradual improvement in soil health over the four-year period is evident, supporting the viability of adopting ICLSs with forage grasses in sandy soils. Over the years, the soil tends to reach a physical, chemical, and microbiological balance, which will contribute to soil health.
Author Contributions
Conceptualization, L.d.L.F., E.A.P.P., M.V.G.G. and D.d.A.S.; methodology, L.d.L.F., E.A.P.P., M.V.G.G., D.d.A.S. and B.M.S.S.; software, V.C.M. and N.C.d.S.J.; validation, V.C.M. and M.A.; formal analysis, L.d.L.F., E.A.P.P., M.V.G.G., D.d.A.S., V.C.M. and M.A.; investigation, L.d.L.F., D.d.A.S., V.C.M. and M.A.; resources, G.C.L. and M.A.; data curation, V.C.M. and N.C.d.S.J.; writing—original draft preparation, L.d.L.F., D.d.A.S. and V.C.M.; writing—review and editing, N.C.d.S.J., V.A.M.G., N.A.A.R., A.M.S.M. and M.A.; visualization, N.C.d.S.J., V.A.M.G., N.A.A.R., A.M.S.M. and M.A.; supervision, V.C.M., G.C.L. and M.A.; project administration, L.d.L.F., D.d.A.S., V.C.M. and M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, for the first author’s master’s studies, with grant number 88887.463837/2019-00, and by the last author’s CNPq research productivity grant (award number 309307/2023-6) of the corresponding author.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data from this study are contained in the article itself and are derived from the master’s thesis of the first author. The master’s thesis of the first author is available at the following link: https://repositorio.unesp.br/entities/publication/7b01c2c7-2d0e-4fdc-9785-638a67a2234c (accessed on 14 November 2024).
Acknowledgments
The authors acknowledge the São Paulo State University (UNESP) “Júlio de Mesquita Filho”—Ilha Solteira campus—São Paulo state, Brazil, the Coordination for the Improvement of Higher Education Personnel (CAPES), the Vô Altino Research Center of Sementes Facholi Company, and all members of the Andreotti team. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) for funding the publication of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Oliveira, L.; Carvalho, M.L.M.; Silva, T.T.A.; Borges, D.I. Temperatura e regime de luz na germinação de sementes de Tabebuia impetiginosa (Martius ex AP de Candolle) Standley e T. serratifolia Vahl Nich.-Bignoniaceae. Ciência e Agrotecnologia. Ciên. Agrotec. 2005, 29, 642–648. [Google Scholar] [CrossRef]
- Rodrigues, L.N.F.; Borges, W.L.B.; Modesto, V.C.; Ribeiro, N.A.A.; de Souza, N.C., Jr.; Girardi, V.A.M.; Matos, A.M.S.; Silva, B.P.C.; Galindo, F.S.; Andreotti, M. Use of Soil Remineralizer to Replace Conventional Fertilizers: Effects on Soil Fertility, Enzymatic Parameters, and Soybean and Sorghum Productivity. Agriculture 2024, 14, 2153. [Google Scholar] [CrossRef]
- Macedo, M.C.M. Integração Lavoura e Pecuária: O estado da arte e inovações tecnológicas. Rev. Bras. Zootec. 2009, 38, 133–146. Available online: http://www.fcav.unesp.br/Home/departamentos/zootecnia/anaclaudiaruggieri/10.-ilp-inovacoes.pdf (accessed on 6 February 2025). [CrossRef]
- Ceccon, G.; Fonseca, I.C.; Luiz Neto, A.; Sereia, R.C.; Leite, L.F. Estabelecimento de Brachiaria Ruziziensis Consorciada com Milho Resistente à Spodoptera Frugiperda; Anais FertBio—XXIX Reunião Brasileira de Fertilidade do Solo e Nutrição de Plantas: Guarapari, ES, Brazil, 13–17 September 2010. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/23349/1/329281583.pdf (accessed on 6 February 2025).
- Pacheco, L.P.; Pires, F.R.; Monteiro, F.P. Profundidade de semeadura e crescimento inicial de espécies forrageiras utilizadas para cobertura do solo. Rev. Ciênc. Agrotec. 2010, 34, 1211–1218. Available online: https://www.researchgate.net/profile/Fernando_Monteiro10/publication/262432070_Depth_of_sowing_and_initial_growth_of_forage_species_used_for_soil_coverage/links/554b9c5c0cf29f836c971bf1.pdf> (accessed on 6 February 2025). [CrossRef]
- Gregory, A.S.; Joynes, A.; Dixon, E.R.; Beaumont, D.A.; Murray, P.J.; Humphreys, M.W.; Richter, G.M.; Dungait, J.A.J. High-Yielding Forage Grass Cultivars Increase Root Biomass and Soil Organic Carbon Stocks Compared with Mixed-Species Permanent Pasture in Temperate Soil. Eur. J. Soil Sci. 2021, 73, e13160. [Google Scholar] [CrossRef]
- Salomão, P.E.A.; Kriebel, W.; Santos, A.A.; Martins, A.C.E. A importância do sistema de plantio direto na palha para reestruturação do solo e restauração da matéria orgânica. Res. Soc. Dev. 2020, 9, e154911870. [Google Scholar] [CrossRef]
- Devkota, P.; Singh, R.K.; Smith, N.G.; Slaughter, L.C.; van Gestel, N. Residue Addition Can Mitigate Soil Health Challenges with Climate Change in Drylands: Insights from a Field Warming Experiment in Semi-Arid Texas. Soil Syst. 2024, 8, 102. [Google Scholar] [CrossRef]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the Complexity of Soil Microbiomes in a Large-Scale Study Subjected to Different Agricultural Management in Styria. Front. Microbiol. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Bettiol, W.; Silva, C.A.; Cerri, C.E.P.; Martin-Neto, L.; Andrade, C.A. Entendendo a Matéria Orgânica do Solo em Ambientes Tropical e Subtropical; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2023; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1153147/entendendo-a-materia-organica-do-solo-em-ambientes-tropical-e-subtropical (accessed on 7 February 2025).
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Impact of Various Grass Species on Soil Bacteriobiome. Diversity 2020, 12, 212. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Cons. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2019, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Köppen, W.; Geiger, R. Klimate der Erde; Verlag Justus Perthes: Gotha, Germany, 1928; pp. 91–102. [Google Scholar]
- Inmet. Instituto Nacional de Meteorologia. Climatologia e Histórico de Previsão do Tempo em Caiuá, São Paulo, Brasil. Brasília. Available online: https://www.climatempo.com.br/climatologia/2248/caiua-sp (accessed on 7 February 2025).
- Soil Survey Staff. Natural Resources Conservation Service. In Keys to Soil Taxonomy, 12th ed.; USDA: Washington, DC, USA, 2014. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo. In Empresa Brasileira de Pesquisa Agropecuária Embrapa Solos Ministério da Agricultura, 3rd ed.; Pecuária e Abastecimento—EMBRAPA: Brasília, Brazil, 2017; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1087262/1/Pt1Cap10Analisegranulometrica.pdf (accessed on 18 December 2024).
- van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; p. 284. [Google Scholar]
- Rezende, L.A.; Assis, L.C.; Nahas, E. Carbon, nitrogen and phosphorus mineralization in two soils amended with distillery yeast. Bioresour. Technol. 2004, 94, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. In Soil Biology and Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1976; Volume 5, pp. 415–471. [Google Scholar]
- Oliveira, J.R.A.; Mendes, I.d.C.; Vivaldi, L.J. Carbono da biomassa microbiana em solos de cerrado: Comparação dos métodos fumigação-incubação e fumigação-extração. In Empresa Brasileira de Pesquisa Agropecuária Embrapa Solos Ministério da Agricultura; Embrapa Cerrados: Planaltina, Goiás, Brazil, 2001; Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/567076 (accessed on 18 December 2024).
- Anderson, T.H.; Domsch, K.H. The metabolic quocient for CO2 (q CO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Bilo. Biochem. 1978, 25, 393–395. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Scott, A., Bottomeley, P.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 778–835, (Special Publication 5). [Google Scholar]
- Chaer, G.M.; Mendes, I.C.; Dantas, O.D.; Malaquias, J.V.; Reis Junior, F.B.; Oliveira, M.I.L. Evaluating C trends in clayey Cerrado oxisols using a four-quadrant model based on specific arylsulfatase and β-glucosidase activities. Appl. Soil Ecol. 2023, 183, 104–742. [Google Scholar] [CrossRef]
- Mendes, I.C.; Chaer, G.M.; Reis, F.B., Jr.; Oliveira, M.I.L.; Dantas, O.D.; Malaquias, J.V. Fazendas de Referência para a Implementação do Modelo de Quatro Quadrantes na Avaliação de Tendências do Carbono do Solo Pela Tecnologia BioAS Tendências de Ganho ou Perda de C no Solo. Documentos 403—EMBRAPA, Planaltina—DF, 2023. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1154741 (accessed on 18 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- dos Santos, T.E.B.; de Melo, M.A.; Ramos, T.V.; Souza, A.G.V.; Brandão, T.P. Comportamento da comunidade microbiana no sistema silviagrícola na região de cerrado. Rev. Agrotec. 2018, 9, 18–27. [Google Scholar] [CrossRef]
- Siqueira, M.G.; Santos, W.P.; Dias, C.B.M.; Vieira, A.S.; Santos, W.P.; Ferreira, A.G.; Souza, S.P. Respiração do solo em sistemas de manejo no sudoeste da Amazônia. In Elementos da Natureza e Propriedades do Solo; Steiner, F.A., Zuffo, M., Eds.; Atena: Pontagrossa, PA, Brazil, 2018; Volume 6, pp. 95–106. [Google Scholar]
- Silva, M.A.; Nascente, A.S.; de Mello Frasca, L.L.; Rezende, C.C.; Ferreira, E.A.S.; de Filippi, M.C.C.; Lanna, A.C.; de Brito Ferreira, E.P.; Lacerda, M.C. Plantas de cobertura isoladas e em mix para a melhoria da qualidade do solo e das culturas comerciais no Cerrado. Res. Soc. Dev. 2021, 10, 11101220008. [Google Scholar] [CrossRef]
- Calegari, A.; de Araujo, A.G.; Tiecher, T.; Bartz, M.L.C.; Lanillo, R.F.; dos Santos, D.R.; Capandeguy, F.; Zamora, J.H.; Jump, J.R.B.; Moriya, K. No-till farming systems for sustainable agriculture in South America. In No-Till Farming Systems for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2021; pp. 533–565. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; Arroja, L.; Dias, A.C. Environmental performance of different end-of-life alternatives of wood fly ash by a consequential perspective. SM&T 2022, 32, 1–13. [Google Scholar] [CrossRef]
- Rosa, D.M.; Nóbrega, L.H.P.; Mauli, M.M.; Lima, G.P.; Pacheco, F.P. Humic substances in soil cultivated with cover crops rotated with maize and soybean. Rev. Cienc. Agron. 2017, 48, 221–230. [Google Scholar] [CrossRef]
- Demattê, J.L.I.; Demattê, J.A.M. Manejo de Solos Arenosos: Fundamentos e Aplicações; Portal de Livros Abertos da USP; Universidade de São Paulo (USP). Escola Superior de Agricultura Luiz de Queiroz (ESALQ): São Paulo, Brazil, 2024; pp. 1–352. [Google Scholar] [CrossRef]
- Araújo, F.C.; Nascente, A.S.; Guimarães, J.L.N.; Sousa, V.S.; Silva, M.A. Cultivo de Plantas de Cobertura na Produção de Biomassa de Plantas Daninhas. In Inovação e Desenvolvimento na Orizicultura: Anais Eletrônico. Embrapa Arroz e Feijão-Artigo em Anais de Congresso. Congresso Brasileiro de Arroz Irrigado, 11, 2019, Balneário Camboriú, SC, Brasil. Epagri: Sosbai. Available online: https://www.alice.cnptia.embrapa.br/alice/bitstream/doc/1111824/1/CNPAF2019cbaiasn2.pdf (accessed on 18 December 2024).
- Kaschuk, G.; Alberton, O.; Hungria, M. Quantifying effects of different agricultural land uses on soil microbial biomass and activity in Brazilian biomes: Inferences to improve soil quality. Plant Soil 2010, 338, 467–481. [Google Scholar] [CrossRef]
- Rangel-Vasconcelos, L.G.T.; Zarin, D.J.; de Assis Oliveira, F.; Vasconcelos, S.S.; de Carvalho, C.J.R.; de Lourdes Silva Santos, M.M. Effect of water availability on soil microbial biomass in secondary forest in Eastern Amazonia. Rev. Bras. Ciênc. Solo 2015, 39, 377–384. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, H.; Sarkar, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.; et al. Animal carcass and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Clayton, J.; Lemanski, K.; Bonkowski, M. Shifts in Soil Microbial Stoichiometry and Metabolic Quotient Provide Evidence for a Critical Tipping Point at 1% Soil Organic Carbon in an Agricultural Post-Mining Chronosequence. Biol. Fertil. Soils 2021, 57, 435–446. [Google Scholar] [CrossRef]
- Pereira, L.F.; Ferreira, C.F.C.; Guimarães, R.M.F. Manejo, qualidade e dinâmica da degradação de pastagens na Mata Atlântica de Minas Gerais—Brasil. Nativa 2018, 6, 370–379. [Google Scholar] [CrossRef]
- Cruz, N.T.; Dias, L.S.D.; Fries, D.D.; Jardim, R.R.; De Lana Sousa, B.M.; Pires, A.J.V.; Ramos, B.L.P. Alternativas para recuperação e renovação de pastagens degradadas. Pesqui. Agropecuária Gaúcha 2022, 28, 15–35. [Google Scholar] [CrossRef]
- Dhakal, D.; Islam, M.A. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability 2018, 10, 2718. [Google Scholar] [CrossRef]
- Meza, K.; Vanek, S.J.; Sueldo, Y.; Olivera, E.; Ccanto, R.; Scurrah, M.; Fonte, S.J. Grass–Legume Mixtures Show Potential to Increase Above- and Belowground Biomass Production for Andean Forage-Based Fallows. Agronomy 2022, 12, 142. [Google Scholar] [CrossRef]
- Silva, A.C.; Barbosa, M.S.; Barral, U.M.; Silva, B.P.C.; Fernandes, J.S.C.; Viana, A.J.S.; Mendonça Filho, C.V.; Bispo, D.F.A.; Christófaro, C.; Ragonezi, C.; et al. Organic matter composition and paleoclimatic changes in tropical mountain peatlands currently under grasslands and forest clusters. Catena 2019, 180, 69–82. [Google Scholar] [CrossRef]
- Favarato, L.F.; de Souza, J.L.; Galvão, J.C.C.; de Souza, C.M.; Guarçoni, R.C. Atributos químicos do solo sobre diferentes plantas de cobertura no sistema plantio direto orgânico. Rev. Bras. Agrop. Sust. 2015, 5, 19–28. [Google Scholar] [CrossRef]
- Nascente, A.S.; Crusciol, C.A.C.; Cobucci, T. The no-tillage system and cover crops—Alternatives to increase upland rice yields. Eur. J. Agron. 2013, 45, 124–131. [Google Scholar] [CrossRef]
- Cordeiro Júnior, P.S.; Finoto, E.L.; Bárbaro-Torneli, I.M.; Martins, M.H.; Soares, M.B.B.; Bolonhezi, D.; Martins, A.L.M. Agronomic performance of soybean cultivars for the central north region of São Paulo, crop year 2016/17. Nucleus 2017, 34, 59–66. [Google Scholar] [CrossRef][Green Version]
- Oligini, K.F.; Batista, V.V.; Barhy, C.A.; Conceição, P.C.; Sartor, L.R.; Adami, P.F. Cover crops biomass yield grown as a 2nd summer crop in relation to sowing periods. Aust. J. Crop. Sci. 2022, 16, 982–989. [Google Scholar] [CrossRef]
- Adeux, G.; Cordeau, S.; Antichi, D.; Carlesi, S.; Mazzoncini, M.; Munier-Jolain, N.; Bàrberi, P. over crops promote crop productivity but do not enhance weed management in tillage-based cropping systems. Eur. J. Agron. 2021, 123, 126221. [Google Scholar] [CrossRef]
- Koudahe, K.; Allen, S.C.; Djaman, K. Critical review of the impact of cover crops on soil properties. Int. Soil Water Conserv. Res. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Lima, C.L.R.; Silva, A.P.; Imhoff, S.; Lima, H.V.; Leão, T.P. Heterogeneidade da compactação de um Latossolo Vermelho-Amarelo sob pomar de laranja. Rev. Bras. Cienc. Solo 2004, 28, 409–414. [Google Scholar] [CrossRef]
- Machado, L.V.; Rangel, O.J.P.; Mendonça, E.S.; Machado, R.V.; Ferrari, J.L. Fertilidade e compartimentos da matéria orgânica do solo sob diferentes sistemas de manejo. Coffee Sci. 2014, 9, 289–299. [Google Scholar]
- Morgan, B.S.T.; Tian, G.; Oladeji, O.O.; Cox, A.E.; Granato, T.C.; Zhang, H.; Podczewinski, E.W. Analysis of effects and factors linked to soil microbial populations and nitrogen cycling under long-term biosolids application. Sci. Total Environ. 2024, 934, 173216. [Google Scholar] [CrossRef]
- Santos, M.E.R.; de Moraes, L.S.; de Oliveira Fernandes, F.H.; Carvalho, B.H.R.; de Oliveira Rocha, G.; de Andrade, C.M.S. Herbage accumulation and canopy structure during stockpiling of Marandu, Piatã, Xaraés, and Paiaguás brachiariagrass cultivars. Pesqui. Agropecuária Bras. 2021, 56, e02207. [Google Scholar] [CrossRef]
- Valle, C.B.; Euclides, V.P.B.; Valério, J.R.; Macedo, M.C.M.; Fernandes, C.D.; Dias Filho, M.B. Brachiaria brizantha cv. Piatã: Uma forrageira para diversificação de pastagens tropicais. Seed News 2007, 11, 28–30. [Google Scholar]
- Souza, L.S.; Ambrosano, E.J.; Rossi, F.; Carlos, J.A.D. Adubação verde na física do solo. In Adubação Verde e Plantas de Cobertura no Brasil: Fundamentos e Prática, 1st ed.; Filho, O.F.L., Ambrosano, E.J., Rossi, F., Carlos, J.A.D., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 337–369. [Google Scholar]
- Balbinot Junior, A.A.; dos Santos, J.C.F.; Debiasi, H.; Yokoyama, A.H. Contribution of roots and shoots of Brachiaria species to soybean performance in succession. Pesqui. Agropecuária Bras. 2017, 52, 592–598. [Google Scholar] [CrossRef]
- Coser, T.R.; Ramos, M.L.G.; de Figueiredo, C.C.; deCarvalho, A.M.; Cavalcante, E.; dos Reis Moreira, M.K.; Araújo, P.S.M.; de Oliveira, S.A. Soil microbiological properties and available nitrogen for corn in monoculture and intercropped with forage. Pesqui. Agropecuária Bras. 2016, 51, 1660–1667. [Google Scholar] [CrossRef]
- Moraes, M.T.D.; Debiasi, H.; Franchini, J.; Rodrigues da Silva, V. Benefícios das plantas de cobertura sobre as propriedades físicas do solo. In Manejo e Conservação do Solo e da Água em Pequenas Propriedades Rurais no sul do Brasil: Práticas Alternativas de Manejo Visando a Conservação do Solo e da Água; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2016; Volume 1, p. 34. Available online: https://www.researchgate.net/publication/309285857_BENEFICIOS_DAS_PLANTAS_DE_COBERTURA_SOBRE_AS_PROPRIEDADES_FISICAS_DO_SOLO (accessed on 18 December 2024).
- Planisich, A.; Utsumi, S.A.; Larripa, M.; Galli, J.R. Grazing of Cover Crops in Integrated Crop-Livestock Systems. Animals 2021, 15, 100054. [Google Scholar] [CrossRef] [PubMed]
- de Faccio Carvalho, P.C.; Peterson, C.A.; de Albuquerque Nunes, P.A.; Martins, A.P.; de Souza Filho, W.; Bertolazi, V.T.; Kunrath, T.R.; de Moraes, A.; Anghinoni, I. Animal production and soil characteristics from integrated crop-livestock systems: Toward sustainable intensification. J. Anim. Sci. 2018, 96, 4923. [Google Scholar] [CrossRef]
- Tobin, C.; Singh, S.; Kumar, S.; Wang, T.; Sexton, P. Demonstrating Short-Term Impacts of Grazing and Cover Crops on Soil Health and Economic Benefits in an Integrated Crop-Livestock System in South Dakota. Open J. Soil Sci. 2020, 10, 109–136. [Google Scholar] [CrossRef]
- Rosa Filho, G.; de Passos Carvalho, M.; Andreotti, M.; Montanari, R.; da Silva Binotti, F.F.; Gioia, M.T. Variabilidade da produtividade da soja em função de atributos físicos de um latossolo vermelho distroférrico sob plantio direto. Rev. Bras. Cienc. Solo 2009, 33, 283–293. [Google Scholar] [CrossRef]
- Rossi, C.Q.; Pereira, M.G.; Giacomo, S.G.; Betta, M.; Polidoro, J.C. Frações húmicas da matéria orgânica do solo cultivado com soja sobre palhada de braquiária e sorgo. Bragantia 2011, 70, 622–630. [Google Scholar] [CrossRef]
- Brown, V.; Barbosa, F.T.; Bertol, I.; Mafra, Á.L.; Muzeka, L.M. Efeitos no solo e nas culturas após vinte anos de cultivo convencional e semeadura direta. Rev. Bras. Ciências Agrárias 2018, 13, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).