Abstract
Global warming has intensified the changes in wetland carbon cycling processes, and the cbbL gene, which plays a key role in carbon fixation, is significantly affected by warming. Therefore, we set up open-top chamber warming and natural controls and used amplicon sequencing to investigate the response of the cbbL carbon-fixing microbial community in the alpine lakeshore wetland to warming. We found that after the warming treatment, the relative abundances of Actinobacteria and Chlorophyta increased, while the relative abundance of Cyanobacteria decreased (p < 0.05). Soil temperature and moisture were the most significant factors influencing the cbbL carbon-fixing microbial community in the lakeshore wetland. Deterministic processes dominated the community assembly of carbon-fixing microbes under warming conditions. Additionally, warming enhanced both cooperative and competitive interactions among carbon-sequestering microorganisms while reducing soil moisture availability and increasing environmental stress, leading to a decrease in the modularity of microbial communities. In summary, warming reduced the carbon sequestration potential of lakeside wetlands but favored the interactions among carbon-sequestering microorganisms.
1. Introduction
Global climate warming has become a major topic of scientific research, especially regarding the impact of rising temperatures on ecosystem functions, which has garnered widespread attention [1]. Temperature, as one of the key ecological factors, significantly affects various biological communities in terrestrial ecosystems and profoundly influences terrestrial carbon (C) dynamics and the atmospheric C exchange of ecosystems [2]. Wetland ecosystems play a critical role in the global carbon cycle. They are not only one of the sources and sinks of carbon but also important sites for greenhouse gas emissions and sequestration [3,4]. Under climate warming, wetland ecosystems are undergoing rapid changes, with temperature profoundly affecting nearly all ecological processes, including the carbon cycle [5,6]. The Qinghai–Tibet Plateau is highly sensitive and vulnerable to climate change, with its warming rate approximately three times that of the global average [7]. Therefore, the Qinghai–Tibet Plateau serves as a typical experimental area for exploring the response and feedback of soil bacterial communities to climate warming [2].
Soil microbes are the most abundant and diverse organisms on Earth [8] and are key drivers in terrestrial biogeochemical processes [9]. Given the high sensitivity of microbial activity to temperature increases, climate warming may significantly alter the community structure and functions of soil microbes [10,11]. It may also influence soil properties, thereby potentially affecting the composition of microbial communities [12]. Guo et al. also found that warming increased the differentiation and succession of soil microbial communities, significantly altering the bacterial community structure [13]. Soil autotrophic bacteria play a crucial role in soil carbon sequestration, and the Calvin–Benson–Bassham cycle dominates due to its high efficiency and widespread presence in prokaryotic microorganisms [14,15]. Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) is the first rate-limiting enzyme in the Calvin–Benson–Bassham cycle. Its activity and the corresponding gene (cbbL, which encodes the large subunit of RubisCO) are commonly used as biomarkers to study autotrophic microorganisms in various environments [16,17,18]. Therefore, exploring the response mechanisms of wetland cbbL carbon-fixing microbial communities under warming conditions is of significant theoretical and practical value for understanding wetland carbon sequestration processes and their environmental regulatory effects.
Lakeshore wetlands, as typical wetland ecosystems, have received increasing attention for their role in the carbon cycle. The present study employed high-throughput sequencing to analyze the cbbL carbon-fixing functional gene of the microbial community in the lakeshore wetlands of the Qinghai Lake Basin and simultaneously assessed the biogeochemical properties of the soil. The main objectives of this research are as follows: (1) to evaluate the response patterns of the cbbL carbon-fixing microbial community under warming conditions; (2) to examine the impact of warming-induced soil property changes on the cbbL carbon-fixing microbial community in the wetlands; and (3) to explore the community assembly process and the evolution of microbial interaction networks under warming conditions in the lakeshore wetlands. By comparing the responses of the carbon-fixing microbial communities under natural control and warming treatments, this study aims to elucidate the mechanisms by which increased temperature affects the diversity, community structure, functional traits, community succession processes, and microbial interaction networks of carbon-fixing microbial communities. This research will provide theoretical insights into the mechanisms of wetland carbon cycling under global warming and offer scientific support for addressing climate change and protecting wetland ecosystems.
2. Materials and Methods
2.1. Study Site and Soil Sampling
Qinghai Lake is located in the northeastern part of the Qinghai–Tibet Plateau, characterized by large diurnal temperature variations. Influenced by climate warming, the region exhibits typical plateau continental climate features. The study area is the Bird Island Internationally Important Wetland of Qinghai Lake (36°57′~37°04′ N, 99°44′~99°54′ E), with an elevation ranging from 3194 to 3226 m. The average annual temperature is −0.7 °C, and the average annual precipitation is 322.7 mm, making it a typical lakeshore wetland. The dominant plant species in the area include Allium przewalskianum, Astragalus adsurgens Pall, and Poa annua L. [19].
In 2018, open-top chamber warming treatments (NW) and natural control plots (Nck) were established in the study site, with a warming amplitude of approximately 1.3 °C (Figure S1). Each had an area of 1 m × 1 m, and five replicates were set for each treatment. Surface soil samples (0–10 cm) were collected from ten plots in June 2020. Each sample was a composite of five soil cores (4.5 cm diameter × 10 cm depth) taken with a soil auger. The mixed soil samples were sieved through a 2 mm mesh to remove stones and plant debris. A portion of the soil samples was air-dried for subsequent physicochemical analysis, while the remaining portion was stored at −80 °C for molecular analysis.
2.2. Soil Physicochemical Properties
An elemental analyzer (Vario EL III, Elemental Analysis System GmbH, Langenselbold, Germany) was used to measure the total carbon and total nitrogen contents of the soil. Soil pH was measured using a pH meter (FE20-FiveEasy pH, Mettler Toledo, Greifensee, Switzerland), with a soil-to-water ratio of 1:2.5. Soil moisture was monitored using a TDR-300 (Spectrum Technologies, Plainfield, IL, USA), and soil temperature was measured with an LI-8100 instrument (LI-COR, Lincoln, NE, USA) [19].
2.3. DNA Extraction and MiSeq Sequencing
Soil DNA was extracted using the standard protocol of the PowerSoil® Total RNA Isolation kit (Mio-bio, Carlsbad, CA, USA). DNA purity was assessed using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The cbbL gene fragment was amplified using the primers (5′-GACTTCACCAAAGACGACGA-3′) and (5′-TCGAACTTGATTTCTTTCCA-3′). The PCR products were pooled at equimolar concentrations, purified using the E.Z.N.A.® Gel Extraction Kit (Omega BioTek, Doraville, GA, USA), and sequenced on the Illumina MiSeq platform (San Diego, CA, USA).
2.4. Statistical Analysis
Alpha diversity and beta diversity were calculated using the MicrobiotaProcess package (v1.18.0). The UpSetR package (v1.4.0) was used to visualize shared and unique OTUs between groups. The microbial community structure refers to the composition and distribution of microorganisms in a specific environment, while microbial diversity refers to the number and distribution of microbial species present in that environment. Therefore, we quantified the relative impact of soil physicochemical factors on the soil carbon-sequestering microbial community structure and diversity using the hierarchical partitioning modeling approach through the rdacca.hp package (v1.1.0). The FAPROTAX database was used to predict ecological functions, such as biogeochemical cycles (carbon, nitrogen, sulfur), to analyze the impact of warming on the functional groups of soil carbon-fixing microbes [20]. A t-test was used to compare significant differences in the soil carbon-fixing microbial community characteristics, microbial functional groups, and soil physicochemical properties between different groups (p < 0.05). The betaNTI index was calculated using the Picante package (v1.8.2) based on a null model, and the Raup–Crick (RCbray) index was calculated using the microeco package (v1.11.0) to analyze the impact of warming on the assembly of carbon-fixing microbial communities. The correlations between data were calculated using the psych package (v2.4.12), and network diagrams were visualized using Gephi 0.9.7 (https://gephi.org/, accessed on 13 May 2024). All R packages were run in R software (v4.1.2) (https://www.r-project.org/, accessed on 15 May 2024).
3. Results
3.1. Soil Physicochemical Properties of the Lakeshore Wetland
After the temperature increase, the soil temperature in the lakeshore wetland rose from 15.5 °C to 16.4 °C (p < 0.05, Figure 1), and soil moisture decreased from 61.9% to 55.0% (p < 0.05, Figure 1). Additionally, the soil pH in the lakeshore wetlands ranged from 8.49 to 9.15, with the total carbon content varying between 9.91 g/kg and 28.32 g/kg and the total nitrogen content ranging from 1.66 g/kg to 2.33 g/kg. However, no statistically significant differences were observed between the groups (p > 0.05, Figure 1).
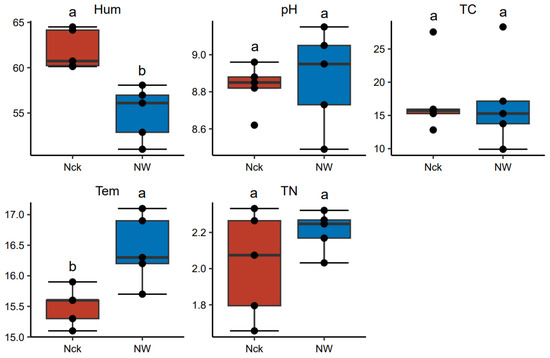
Figure 1.
Soil physicochemical properties under different treatments in the lakeshore wetland. “ab” indicates significance where the same letters in the graph refer to no significant difference (p > 0.05), and different letters in the graph stand for significant differences between the data groups (p < 0.05). Tem refers to soil temperature, Hum represents soil humidity, TN indicates total nitrogen, TC denotes total carbon, and pH refers to the soil pH. Nck: natural contrast, NW: warming treatment.
3.2. cbbL Carbon-Fixing Microbial Community Structure
We compared the effects of warming on the richness and diversity of the cbbL carbon-fixing microbial community in the lakeshore wetland by calculating the ACE, Chao1, Shannon, and Simpson indices. The results showed that warming significantly increased the community richness indices (ACE, Chao1, p < 0.05, Figure 2a). Additionally, the values of the community diversity indices tended to stabilize (Shannon, Simpson, p > 0.05, Figure 2a). The total explained variance of the PCA reached 79.23%, and the results showed that the warming treatment strongly altered the cbbL carbon-fixing microbial community, forming a distinct group compared to the control soil (Figure 2b). Hierarchical partitioning analysis indicated that soil temperature and moisture were the most important explanatory factors for the changes in the microbial community structure (Figure 2c), accounting for 92.1% of the variation in community structure. Soil temperature and moisture were also the most important factors explaining the changes in microbial community diversity, followed by the carbon and nitrogen contents, both of which showed a negative impact on community diversity (Figure 2d).
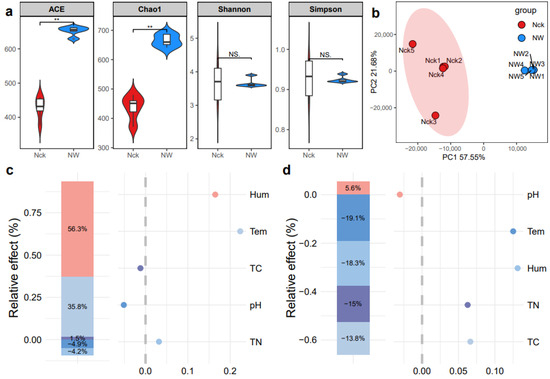
Figure 2.
Diversity and community-influencing factors of carbon-fixing microorganisms in the lakeshore wetland. (a) Alpha diversity index; (b) PCA (Principal Component Analysis); (c) Hierarchical partitioning analysis of factors influencing the structure of the carbon-fixing microbial community; (d) Hierarchical partitioning analysis of factors influencing the diversity of the carbon-fixing microbial community. Tem refers to soil temperature, Hum represents soil humidity, TN indicates total nitrogen, TC denotes total carbon, and pH refers to the soil pH. Nck: natural contrast, NW: warming treatment. ** indicates p < 0.01, NS indicates p > 0.05.
The overlap between cbbL microbial communities was measured using an OTU-based upset plot analysis (Figure S2). The number of shared OTUs across all groups was 432, and warming increased the number of group-specific OTUs from 237 to 370. Figure S3 shows the community composition of cbbL carbon-fixing microbes at the phylum level in the lakeshore wetland, with the dominant phyla including Proteobacteria (10.95–68.46%), Actinobacteria (0.02–15.64%), Chlorophyta (0.02–8.47%), and Cyanobacteria (6.59–8.78%). The warming treatment increased the relative abundances of Actinobacteria and Chlorophyta while decreasing the relative abundance of Cyanobacteria (Figure 3). The cbbL carbon-fixing microorganisms of the lakeshore wetland included 17 dominant genera, with relatively high abundances of Thioflexothrix (9.73%), Nitrosomonas (9.44%), and Ferrithrix (7.32%) (Figure S4). The results from the t-test indicated that warming significantly increased the relative abundances of seven genera, namely, Thioflexothrix, Ferrithrix, Rhodospirillum, Bradyrhizobium, Thiohalomonas, Thiobacillus, and Sulfuricaulis, but significantly decreased the relative abundances of five genera: Tolypothrix, Thermoleptolyngbya, Pycnacronema, Phormidium, and Nostoc (p < 0.05, Figure 4).
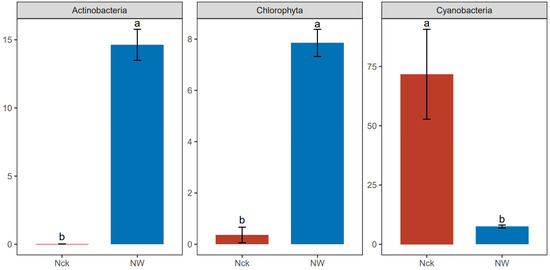
Figure 3.
Phylum-level differential microbial communities under different treatments in the lakeshore wetland. “ab” indicates significance where the same letters in the graph refer to no significant difference (p > 0.05), and different letters in the graph stand for significant differences between the data groups (p < 0.05). Nck: natural contrast, NW: warming treatment.
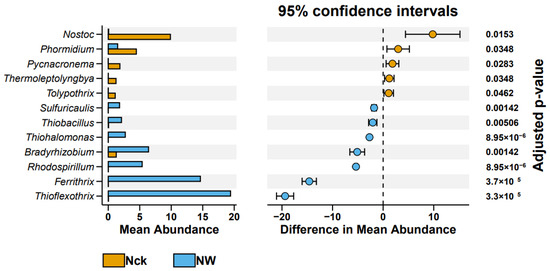
Figure 4.
Genus-level differential microbial communities under different treatments in the lakeshore wetland. Nck: natural contrast, NW: warming treatment.
3.3. Community Assembly Mechanisms and the Functional Groups
Warming significantly influenced the assembly process of cbbL carbon-fixing microorganisms in the lakeshore wetland (p < 0.001). In the natural control, both deterministic (|βNTI| > 2) and stochastic processes (|βNTI| < 2) contributed to the community assembly, whereas under the warming treatment, the process was predominantly driven by deterministic factors (Figure 5a). To further explore the relative contributions of various factors to community variation, the RCbray was calculated, distinguishing between dispersal limitation, drift, homogeneous dispersal, and selection. The findings revealed that warming shifted the balance between deterministic and stochastic processes in the assembly of the cbbL carbon-fixing microbial community. Heterogeneous selection played a dominant role, accounting for 100% of the assembly process under warming conditions, as compared to 50% under natural conditions. Furthermore, under natural control conditions, dispersal limitation and drift contributed collectively to 50% of the community dynamics (Figure 5b).
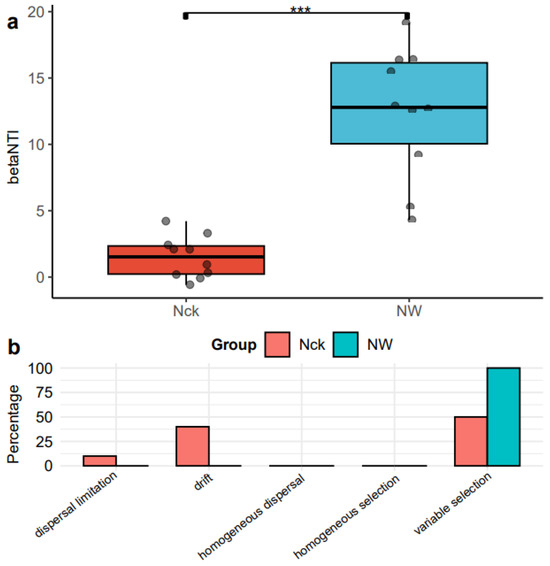
Figure 5.
Community assembly of cbbL carbon-fixing microorganisms in the lakeshore wetland. (a) Distribution of the βNTI index of the carbon-fixing microbial community; (b) Distribution of the community assembly processes of the carbon-fixing microorganisms. *** indicates p < 0.001. Nck: natural contrast, NW: warming treatment.
The predicted functional groups of carbon-fixing microorganisms (Figure S5) showed that their functional categories were primarily concentrated in phototrophy (18.02%), photoautotrophy (16.80%), photosynthetic cyanobacteria (15.93%), oxygenic photoautotrophy (15.93%), nitrification (4.21%), aerobic ammonia oxidation (4.19%), nitrogen fixation (3.72%), dark iron oxidation (3.10%), chemoheterotrophy (2.95%), and aerobic chemoheterotrophy (2.95%), with a total proportion exceeding 87%. Warming significantly elevated the relative abundances of functional groups associated with dark iron oxidation, chemoheterotrophy, and aerobic chemoheterotrophy. In contrast, it notably reduced the relative abundances of functional groups associated with phototrophy, photoautotrophy, photosynthetic cyanobacteria, and oxygenic photoautotrophy (p < 0.05, Figure 6).
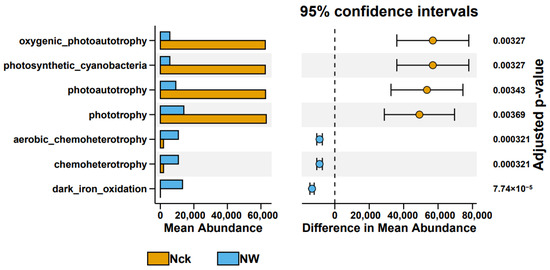
Figure 6.
Differential functional groups under different treatments in the lakeshore wetland. Nck: natural contrast, NW: warming treatment.
3.4. Co-Occurrence Network
To gain a deeper insight into the interactions among cbbL carbon-fixing microorganisms in the lakeshore wetland, symbiotic networks were constructed for both the control and warming treatments. The findings revealed that warming significantly impacted the topological characteristics of the cbbL carbon-fixing microbial network, leading to increased network complexity and enhanced connectivity among species (Figure 7, Table 1). Under warming conditions, the number of both positive and negative correlations doubled, and the average degree of the network exhibited a similar increase (Figure 7, Table 1). Furthermore, warming resulted in a reduction in the modularity of the carbon-fixing microbial network (Figure 7, Table 1).
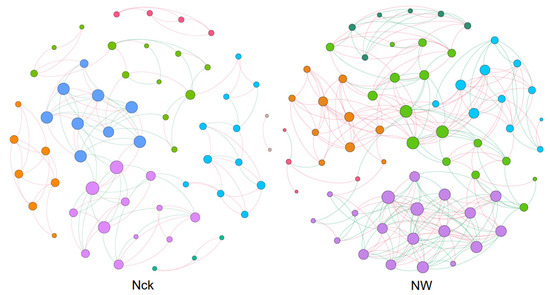
Figure 7.
Network patterns of cbbL carbon-fixing microorganisms in the lakeshore wetland under different treatments. The size of the nodes represents the degree. The node color represents different modules. Edge colors indicate positive or negative correlations, with red representing positive correlations and green representing negative correlations. Nck: natural contrast, NW: warming treatment.

Table 1.
Topological parameters of the cbbL carbon-fixing microbial network in the lakeshore wetland under different treatments.
4. Discussion
4.1. Response of cbbL Carbon-Fixing Microbial Communities in the Lakeshore Wetland to Warming
Warming-induced alterations in water availability have influenced the response strategies of soil microbial community diversity and composition [21,22]. The findings of this study demonstrated that warming led to an increase in the diversity index of cbbL carbon-fixing microbial communities in the lakeshore wetland (Figure 2a). Similarly, Yu et al. observed that warming significantly enhanced the alpha diversity of soil bacterial communities [23]. However, other studies have reported that warming-induced changes in water availability resulted in a decline in soil bacterial diversity [21,24]. This inconsistency may be due to variations in soil microenvironments and nutrient availability [25]. Additionally, warming significantly decreased soil moisture in the lakeshore wetland (Figure 1), with moderate moisture levels being found to promote the alpha diversity of cbbL carbon-fixing microbial communities [26].
Temperature changes did not induce a shift in the dominant microbial communities in the cbbL carbon-fixing community. The dominant phylum was Proteobacteria, followed by Actinobacteria, Chlorophyta, and Cyanobacteria (Figure S3). This is typically attributed to the soil’s buffering capacity to environmental changes and the rapid evolutionary adaptation of microbial communities [27]. The dominance of Proteobacteria in the lakeshore wetland can be attributed to its strong environmental adaptability, rapid growth and reproduction, and high substrate absorption capacity [28,29]. A recent study by Li et al. further supported these findings, demonstrating that Proteobacteria is the most abundant phylum in carbon-fixing microbial communities at the phylum level, making up the largest proportion in the soil [30].
The dominant genera in the lakeshore wetland were Thioflexothrix, Nitrosomonas, and Ferrithrix, which had relatively high abundances (Figure S4). Previous studies have shown that the dominant genera of carbon-fixing microorganisms in karst wetlands were Rubrivivax, Cyanobium, and Methyllium, while among estuarine cbbL carbon-fixing bacteria, the dominant genus was Endothiovibrio [31,32]. This finding diverges from the results of the present study, which may be attributed to the significantly higher nitrogen content in karst wetlands compared to lakeside wetlands, whereas the carbon content is higher in the latter. Differences in soil properties and genetic characteristics across study regions have led to variations in dominant microbial genera [16]. We also found that the warming treatment increased the relative abundances of Actinobacteria, Chlorophyta, Thioflexothrix, and Ferrithrix, while it decreased the relative abundance of Cyanobacteria (Figure 3 and Figure 4). This is likely due to changes in the soil temperature that limit soil moisture and nutrient availability, which directly or indirectly kill microorganisms and reduce their abundance; however, the surviving tolerant/adapted species increased their dominance [33,34]. Actinobacteria, a typical oligotrophic phylum, are capable of degrading more recalcitrant carbon sources, such as chitin [35,36,37]. Under warming treatment, the relative abundance of this phylum increased (Figure 3), which may have facilitated the decomposition of recalcitrant carbon in the lakeshore wetland.
4.2. Factors Influencing cbbL Carbon-Fixing Microbial Communities and Their Interrelationships in the Lakeshore Wetland
Functional genes associated with the carbon cycle exhibit significant correlations with soil temperature and moisture [38]. In this study, soil temperature and moisture were identified as the primary factors influencing changes in the diversity and community structure of cbbL carbon-fixing microorganisms in the lakeshore wetland (Figure 2c,d). Similarly, Liao et al. reported a strong correlation between soil cbbL bacterial communities and soil temperature and moisture, also highlighting the importance of pH as a contributing factor [39]. However, the relatively narrow local soil pH range observed in this study (Figure 1) likely had minimal impact on altering the overall soil autotrophic bacterial community [40]. Changes in the soil functional community structure were often associated with a reduction in stochastic processes during community assembly, leading to a shift toward deterministic processes (Figure 5) [41]. The results here indicate that warming significantly affected the community assembly of cbbL carbon-fixing microorganisms in the lakeshore wetland, with deterministic processes playing a more dominant role under warming conditions [24]. Nonetheless, some studies suggest that selective pressures induced by warming are the key drivers of microbial community turnover [22,42]. These divergent findings may be attributed to differences in ecosystem vulnerability and sensitivity [43].
Climate warming can promote the connectivity and complexity of microbial networks [44,45]. Our results similarly showed that warming increased network complexity and connectivity between species (Figure 7, Table 1). Previous studies on warming in the Qinghai–Tibet Plateau have also indicated this trend [46]. This change may enhance the community’s ability to withstand warming [47,48] while also reducing community composition turnover [49,50]. Under the warming treatment, both positive and negative correlations among carbon-fixing microorganisms increased (Figure 7, Table 1), likely due to nutrient changes induced by warming, which promoted microbial cooperation and competition [51,52]. Additionally, warming reduced the modularity of the carbon-fixing microbial network (Figure 7, Table 1), which may be a result of the high environmental pressure caused by warming, leading to a reduction in the modularity of microbial communities [53]. Microbial interactions are considered important drivers of ecosystem functions [54]. Warming significantly increased the heterotrophic functions of carbon-fixing microorganisms in the lakeshore wetland but significantly decreased microbial photosynthesis (Figure 6).
5. Conclusions
We found that warming shifted the community assembly of carbon-fixing microorganisms towards deterministic processes and promoted both cooperative and competitive interactions among these microorganisms. Warming reduced the effectiveness of soil moisture, increased environmental stress, and further led to a decrease in the modularity of the carbon-fixing microbial community. In addition, soil temperature and moisture were found to be the most important explanatory factors for changes in the diversity and community structure of cbbL carbon-fixing microorganisms in the lakeshore wetland. These findings enhance our understanding of the carbon sequestration mechanisms and functional potential of wetlands, thereby significantly improving the predictability of ecological responses to climate change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15060580/s1, Figure S1. Sampling plot layout diagram; Figure S2. Distribution of OTUs; Figure S3. Dominant phyla; Figure S4. Dominant genera; Figure S5. Functional groups.
Author Contributions
Conceptualization, N.Z. and K.C.; methodology and experiments, N.Z., D.Q., S.Z. and S.W.; data acquisition and formal analysis, N.Z.; Investigation, D.Q., S.Z. and L.F.; writing—original draft preparation, N.Z.; writing—review and editing, N.Z. and K.C.; visualization, N.Z., S.W., L.F. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Second Comprehensive Scientific Expedition to the Qinghai-Tibet Plateau (2019QZKK0405), the Qinghai Province Science and Technology Plan (2023-ZJ-905T), and the Ningxia Natural Science Foundation (2023AAC03345).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in NCBI at https://www.ncbi.nlm.nih.gov/, reference number [PRJNA1210475].
Conflicts of Interest
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work.
References
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; p. 896. [Google Scholar]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bloom, A.A.; Bowman, K.W.; Lee, M. A global wetland methane emissions and uncertainty dataset for atmospheric chemical transport models (WetCHARTs version 1.0). Geosci. Model. Dev. 2017, 10, 2141–2156. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Y.; Jiang, M.; Han, G.X.; Zhang, L.W.; Zhou, J.; Bian, C.Y.; Du, Y.; Yan, L.M.; Xia, J.Y. Experimental warming reduces ecosystem resistance and resilience to severe flooding in a wetland. Sci. Adv. 2022, 8, eabl9526. [Google Scholar] [CrossRef]
- Qiu, J. China: The third pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Romero-Olivares, A.; Allison, S.; Treseder, K. Soil microbes and their response to experimental warming over time: A meta analysis of field studies. Soil. Biol. Biochem. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Che, R.; Wang, S.; Wang, Y.; Xu, Z.; Cui, X. Total and active soil fungal community profiles were significantly altered by six years of warming but not by grazing. Soil. Biol. Biochem. 2019, 139, 107611. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Pold, G.; Topçuoğlu, B.D.; van Diepen, L.T.; Varney, R.M.; Blanchard, J.L.; Melillo, J.; Frey, S.D. Long-term forest soil warming alters microbial communities in temperate forest soils. Front. Microbiol. 2015, 6, 104. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Lauren, H.; Yuan, T.; Wang, J.; Qin, Y. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Kelly, D.P.; Wood, A.P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Berg, I.A. Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl. Environ. Microbiol. 2011, 77, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Tolli, J.; King, G.M. Diversity and structure of bacterial chemolithotrophic communities in pine forest and agroecosystem soils. Appl. Environ. Microbiol. 2005, 71, 8411–8418. [Google Scholar] [CrossRef] [PubMed]
- Selesi, D.; Pattis, I.; Schmid, M.; Kandeler, E.; Hartmann, A. Quantification of bacterial RubisCO genes in soils by cbbL targeted real-time PCR. J. Microbiol. Methods 2007, 69, 497–503. [Google Scholar] [CrossRef]
- Xiao, K.Q.; Bao, P.; Bao, Q.L.; Jia, Y.; Huang, F.Y.; Su, J.Q.; Zhu, Y.G. Quantitative analyses of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RubisCO) large-subunit genes (cbbL) in typical paddy soils. FEMS Microbiol. Ecol. 2014, 87, 89–101. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, K.; Wang, S.; Qi, D.; Zhou, Z.; Xie, C.; Liu, X. Dynamic Response of the cbbL Carbon Sequestration Microbial Community to Wetland Type in Qinghai Lake. Biology 2023, 12, 1503. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Jiang, Y.; Wu, S.; Zhou, Y.; Zhu, W. Functional distribution of bacterial community under different land use patterns based on faprotax function prediction. Pol. J. Env. Stud. 2020, 29, 1245–1261. [Google Scholar] [CrossRef]
- Wu, L.W.; Zhang, Y.; Guo, X.; Ning, D.L.; Zhou, X.S.; Feng, J.J.; Yuan, M.M.; Liu, S.; Guo, J.J.; Gao, Z.P.; et al. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 2022, 7, 1054. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, F.; Zhang, Z.; Fang, J.; Xing, L.; Zeng, J.; Zhang, Q.; Liu, H.; Liu, W.; et al. Deterministic assembly of grassland soil microbial communities driven by climate warming amplifies soil carbon loss. Sci. Total Environ. 2024, 923, 171418. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Wang, J.; Zhang, Y.; Xiao, C. Effects of warming on the bacterial community and its function in a temperate steppe. Sci. Total Environ. 2021, 792, 148409. [Google Scholar] [CrossRef]
- Feng, J.J.; Wang, C.; Lei, J.S.; Yang, Y.F.; Yan, Q.Y.; Zhou, X.S.; Tao, X.Y.; Ning, D.L.; Yuan, M.T.M.; Qin, Y.J.; et al. Warming-induced permafrost thaw exacerbates tundra soil carbon decomposition mediated by microbial community. Microbiome 2020, 8, 3. [Google Scholar] [CrossRef]
- Qin, J.; Li, M.; Zhang, H.; Liu, H.; Zhao, J.; Yang, D. Nitrogen Deposition Reduces the Diversity and Abundance of cbbL Gene-Containing CO2-Fixing Microorganisms in the Soil of the Stipa baicalensis Steppe. Front. Microbiol. 2021, 12, 570908. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, W.; Liu, T.; Yan, W.; Wu, X.; Lei, J.; Xu, Y.; Chen, Y.; Yao, Y.; Jiang, W.; et al. Midseason drying increases soil dissolved organic carbon and rice yield via soil cbbL bacteria. J. Environ. Manag. 2024, 371, 123131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ding, J.; Li, T.; Zhang, X. Plant communities are more sensitive than soil microbial communities to multiple environmental changes in the Eurasian steppe. Glob. Ecol. Conserv. 2020, 21, e00779. [Google Scholar] [CrossRef]
- Salcher, M.M.; Pernthaler, J.; Zeder, M.; Psenner, R.; Posch, T. Spatio-temporal niche separation of planktonic Betaproteobacteria in an oligo mesotrophic lake. Environ. Microbiol. 2010, 10, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Simek, K.; Kasalicky, V.; Zapomelova, E.; Hornak, K. Alga-derived substrates select for distinct Betaproteobacterial lineages and contribute to niche separation in Limnohabitans strains. Appl. Environ. Microbiol. 2011, 77, 7307–7315. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Shi, Y.; Yang, Y.; Li, H. Effects of Nitrogen Addition on Soil Carbon-Fixing Microbial Diversity on Different Slopes in a Degraded Alpine Meadow. Front. Plant Sci. 2022, 13, 921278. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Cheng, A.; Shen, T.; Xiao, Y.; Zhu, M.; Pan, X.; Yu, L. Community characteristics of autotrophic CO2-fixing bacteria in karst wetland groundwaters with different nitrogen levels. Front. Microbiol. 2022, 13, 949208. [Google Scholar] [CrossRef]
- Xu, J.; Ming, H.; Ren, K.; Li, D.; Huang, H.; Li, J.; Shao, K.; Li, H.; Fan, J. Spatial heterogeneity plays a vital role in shaping the structure and function of estuarine carbon-fixing bacterial communities. Mar. Environ. Res. 2024, 198, 106544. [Google Scholar] [CrossRef]
- Walker, V.K.; Palmer, G.R.; Voordouw, G. Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl. Environ. Microbiol. 2006, 72, 1784–1792. [Google Scholar] [CrossRef]
- Wilson, S.L.; Walker, V.K. Selection of low-temperature resistance in bacteria and potential applications. Environ. Technol. 2010, 31, 943–956. [Google Scholar] [CrossRef]
- Wang, K.; Pan, R.; Fei, H. Changes in soil prokaryotic communities and nitrogen cycling functions along a groundwater table drawdown gradient in desert wetlands. Sci. Total Environ. 2022, 842, 156868. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. Available online: http://www.jstor.org/stable/27651243 (accessed on 8 January 2025). [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Yu, H.; Deng, Y.; He, Z.; Van Nostrand, J.D.; Wang, S.; Jin, D.; Wang, A.; Wu, L.; Wang, D.; Tai, X.; et al. Elevated CO2 and Warming Altered Grassland Microbial Communities in Soil Top-Layers. Front. Microbiol. 2018, 9, 1790. [Google Scholar] [CrossRef]
- Liao, H.; Hao, X.; Qin, F.; Delgado-Baquerizo, M.; Liu, Y.; Zhou, J. Microbial autotrophy explains large-scale soil CO2 fixation. Glob. Chang. Biol. 2022, 29, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ning, D.; Lu, Z.; Zhang, N.; Hale, L.; Wu, L. Century long fertilization reduces stochasticity controlling grassland microbial community succession. Soil. Biol. Biochem. 2020, 151, 108023. [Google Scholar] [CrossRef]
- Ning, D.L.; Yuan, M.T.; Wu, L.W.; Zhang, Y.; Guo, X.; Zhou, X.S.; Yang, Y.F.; Arkin, A.P.; Firestone, M.K.; Zhou, J.Z. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, L.Y.; Hu, J.P.; Khan, A.; Yang, X.X.; Dong, Q.M. Different grazers and grazing practices alter the growth, soil properties, and rhizosphere soil bacterial communities of Medicago ruthenica in the Qinghai-Tibetan plateau grassland. Agric. Ecosyst. Environ. 2023, 352, 108522. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Chen, W.J.; Zhou, H.K.; Wu, Y.; Li, Y.Z.; Qiao, L.L.; Wang, J.; Zhai, J.Y.; Song, Y.H.; Zhao, Z.W.; Zhang, Z.H.; et al. Plant-mediated effects of long-term warming on soil microorganisms on the Qinghai-Tibet Plateau. Catena 2021, 204, 105391. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Yuan, M.; He, Z.; Deng, Y.; Xue, K.; Wu, L.; Hobbie, S.E.; Reich, P.B.; Zhou, J. Fungal communities respond to long-term CO2 elevation by community reassembly. Appl. Environ. Microbiol. 2015, 81, 2445–2454. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Cohesion: A method for quantifying the connectivity of microbial communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nature reviews. Microbiology 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.J.; Bufford, J.L.; Barnes, A.D.; Barratt, B.I.P.; Deslippe, J.R.; Dickie, I.A.; Goldson, S.L.; Howlett, B.G.; Hulme, P.E.; Lavorel, S.; et al. A network perspective for sustainable agroecosystems. Trends Plant Sci. 2022, 27, 769–780. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).