Abstract
This study aimed to investigate the effects of HDPE and Cd on forms of carbon (C) and nitrogen (N) by measuring the changes in enzymatic activities and physicochemical properties of Spartina alterniflora soil samples from coastal regions of China. Over three incubation periods (14, 28, and 56 days), a soil incubation experiment was conducted to investigate the effects of HDPE (0, 0.5, 1, and 2 g per 100 g of soil) and Cd (0, 2, 4, and 8 mg kg−1) on soil physicochemical properties. The results demonstrated that the sole presence of HDPE had a notable impact on enhancing the C-related physicochemical properties of the soil, particularly by elevating the concentration of Total Organic Carbon (TOC). The sole addition of Cd significantly suppressed enzymic activities in the soil, leading to a considerable reduction in the concentration of NH4+-N and NO3−-N. Under identical Cd treatment conditions, the introduction of 1 g HDPE led to an increase in the concentration of TOC, and the inhibitory effect of Cd on enzymic activities was decreased; thus, an elevated consumption of soluble organic carbon (DOC) was identified. However, upon adding 2 g of HDPE, while the TOC concentration continued to rise, the stimulatory effect on enzymic activities diminished. In conclusion, the addition of HDPE inhibits, to a certain extent, the influence of Cd on the carbon and nitrogen cycling in soil.
1. Introduction
Coastal wetland is the most important ecosystem in the world. It has its unique ecological significance in promoting the formation of silt, reducing coastal damage, mitigating the impact of global warming on the environment, maintaining groundwater level, supplying habitat, and protecting biodiversity. Coastal wetlands have high productivity and a low organic decomposition rate [1], allowing them to store and release most of the carbon in the biosphere, thus restricting global climate change. This is one of the most important roles of coastal wetland. Due to the periodic changes in terrestrial environment and the continuous expansion of human production and living areas, coastal wetlands have been greatly affected. Spartina alterniflora was introduced into China as an “engineering plant” in the last century, but it has become a dominant species in coastal wetlands due to its relatively strong invasive abilities [2].
Due to the extensive utilization and inadequate disposal of plastic products in recent decades, 1.15 to 2.41 million tons per year of plastic waste has been discharged into the aquatic environment directly, or indirectly funneled through surface runoff into terrestrial, coastal, and marine environments through surface water [3]. Due to various factors, including physical abrasion, ultraviolet radiation, aging, and microbial decomposition, these plastics break down into MPs [4]. MPs of different concentration, sizes, and types are discharged into the aquatic environment directly or indirectly through surface runoff, thus causing disturbance all over the planet, and soil has become its main storage site [5]. Since MPs are composed of carbon atoms and hydrogen atoms bound together by polymer chains, microorganisms metabolize the organic matter (OM) in the MPs, and it becomes an organic carbon matrix of microorganisms, thus directly or indirectly affecting the C and N forms in the soil. Common MPs include polyethylene (PE), polypropylene (PP), and polystyrene (PS). Changes their physicochemical properties [6], including the level of TOC, DOC, and pH, and microbial communities in the soil, regulate the SOC cycle by promoting the synthesis of polymer aromatic compounds, which complicates the composition of organic matters in the soil [7]. Although numerous studies have explored the impact of MPs on nitrogen conversion in the soil, there remains a lack of consensus. Some studies indicate that MPs can decrease the concentration of NH4+-N and NO3−-N [8], while others report opposing findings [9]. Additionally, some studies show an increase in NH4+-N with a decrease in NO3−-N under the presence of MP [10,11,12], yet other studies present differing conclusions [13]. Furthermore, some studies suggest that the concentration of NH4+-N and NO3−-N remains virtually the same under the presence of MPs [14].
Simultaneously, among the various mixed pollutants that can interact with MPs, heavy metals become the core pollutants that need special attention due to their inorganic nature and high toxicity [15]. Cd, a non-essential heavy metallic element, has been found to reach an average concentration of 7.79 g kg−1 in the surface soil of certain coastal wetlands [16]. Heavy metals can be diffused through the membranes and pores of MPs [17], and can attach to the surface of MPs through a combination of cation complexation, hydrogen bonding, van der Waals forces, and electrostatic interactions [18]. Different types of MPs have different effects on the solubility and bioavailability of heavy metals, and can enhance or reduce their toxic effects on the soil. The texture, mineral composition, redox state, adsorption and desorption processes, and physical transport processes of the soil influence the distribution and accumulation of MPs and heavy metals [19]. These factors collectively determine the behavioral characteristics and potential risks associated with MPs and heavy metals in the soil.
Understanding how different pollutants influence the forms of essential elements and the adaptive responses of soil in coastal wetlands is critical for assessing ecosystem health. Based on the above studies, we consider how the decomposition and aging of HDPE can affect the carbon content in the soil. Considering the adsorption effect of MPs on Cd, HDPE can indirectly reduce the influence of Cd on the soil’s physicochemical properties and soil enzymatic activity to a certain extent. In this study, soil incubation experiments were conducted for 14, 28, and 56 days to evaluate the effects on the basic physicochemical properties, C and N forms, and enzymic activities of Spartina alterniflora soil in coastal wetland under four different levels of MP content and Cd pollution. The objective of this study was to investigate the effects of varying concentrations of MPs and Cd contamination on enzymatic activities and the forms of C and N in coastal wetland soils under different incubation durations. Additionally, it provides a theoretical foundation for understanding the functions of C and N cycling in coastal wetland soil ecosystems.
2. Materials and Methods
2.1. Experimental Material
Firstly, 6 kg soil samples were collected from a depth of 10–30 cm in the Spartina alterniflora zone at the Liangduo River Estuary, Yancheng City, Jiangsu Province, China (32°52′38.309″ N, 120°55′6.869″ E) (Figure 1). Based on the WRB 2022 classification, the soil in the Liangduo River Estuary is classified as Acrisols (AC), characterized by low-activity clays and a low base status [20]. After air-drying, the soil was sieved through a screen with a diameter of 150 μm to remove the botanic residues, rocks, and other impurities, resulting in uniform original samples with consistent physical properties. The basic physicochemical properties of the soil are set out in Table 1. Since HDPE is the main type of MPs in areas of human activity [21], HDPE powder (particle size 6.5 μm) produced by Dongguan Jingke Polymer Co., Ltd. (Dongguan, China) was chosen as the experimental MP for this study.
Figure 1.
Photograph of sampling sites.

Table 1.
Physicochemical properties of the sample.
2.2. Experimental Design
The experiment started in July 2023. In total, 100 g of treated soil was added to each of 144 beakers and mixed with different amounts of HDPE and different concentrations of Cd. In total, we used the following: 4 doses of HDPE (0, 0.5, 1, and 2 g, expressed as HDPE0, HDPE0.5, HDPE1, and HDPE2, respectively) × 4 levels of Cd (0, 2, 4, and 8 μg g−1, expressed as Cd0, Cd2, Cd4, and Cd8, respectively) × 3 time periods × 3 experiment replications; there were 144 pots in this incubation experiment, and the control groups in the incubation process were, respectively, 14 days for HDPE0Cd0, 28 days for HDPE0Cd0, and 56 days for HDPE0Cd0, as shown in Table 2. During the experiment, we kept the soil water content of the sample at approximately 23%. The beaker was wrapped with tin foil to ensure that the experiment was carried out under dark conditions at 25 °C and 65% humidity. After 7-day pre-incubation to restore the soil biological environment, the samples were incubated for 14, 28, and 56 days (T14, T28, and T56), respectively. During sample collection, part of the samples were dried in the oven at 35 °C for the determination of total carbon, total nitrogen, pH, TOC, DOC, and DON, while the other parts of the fresh samples were frozen in liquid nitrogen and put into a refrigerator at −80 °C for the determination of NO3−-N, NH4+-N, BG, CBH, and NAG. Enzymatic activity was determined within one week of sampling.

Table 2.
Changes in pH in the sample.
2.3. Methods for Analysis of Physicochemical Properties of the Soil
pH value was determined by a potentiometric method with a water-to-soil ratio of 2.5:1. Total carbon (TC, mg g−1) and total nitrogen (TN mg g−1) in the soil were measured using dry combustion with an Elementar Vario Marco Cube (sourced from Germany Element, Frankfurt, Germany). TOC was determined by 10 mL 0.167 mol L−1 K2Cr2O7 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China (SCR)) and 20 mL H2SO4 (SCR) sulfuric acid (external dilution thermo-colorimetric method) with reference to Bao Shidan’s Soil Agrochemical Analysis [22]. DOC and DON were extracted with 25 mL 0.5 M K2SO4 (SCR) at a soil/solution ratio of 1:5 in a mechanical shaker for 40 min at 200 rpm at 25 °C. The extract was filtered through filter paper, and the concentrations of DOC and DON were determined with a TOC-L analyzer (sourced from Shimadzu, Kyoto, Japan). The determination of NH4+-N and NO3−-N was conducted as follows: soil samples were extracted with 2 M KCl (SCR) at a soil-to-solution ratio of 1:5 and shaken on a mechanical shaker at 200 rpm for 40 min at 25 °C. For NH4+-N analysis, 1.5 mL of the filtered soil extract was mixed with 0.5 mL of EDTA masking agent (SCR), 2 mL of color developer (SCR), 7.5 mL of ultrapure water, and 1 mL of buffer solution (SCR) in a 15 mL centrifuge tube. The mixture was heated in a water bath at 40 °C for 30 min, and the absorbance was measured at 667 nm to calculate the NH4+-N content per unit mass of soil. For NO3−-N analysis, 0.06 mL of the filtered soil extract was transferred to a cuvette, mixed with 3 mL of color developer, and incubated in the dark at 25 °C for 15 h. The absorbance was then measured at 540 nm to determine the NO3−-N content per unit mass of soil, referencing the fourth edition of Monitoring and Analysis Methods for Water and Wastewater [23]. Soil samples were extracted with 1 g of fresh soil and 40 mL of phosphate buffer in a mechanical shaker at 200 rpm at 25 °C for 1 h; 10 µM 4-methylumbelliferone was used as the standard; 1 mol L−1 4-MUB-β-D-glucopyranoside (Macklin, Shanghai, China), 4-MUB-β-D-cellobioside (Macklin), and 4-MUB-N-acetyl-b-D-glucosaminide (Macklin) were used as the substrate solution, which was used to determine the enzymatic activity of BG, CBH, and NAG using a Synergy H4 Hybrid Microplate Reader (sourced from BioTek, Shoreline, WA, USA) [24].
2.4. Data Analysis
All of the data were first entered into Microsoft Excel 2021 for processing and expressed as the mean ± standard error. The data were analyzed using IBM SPSS Statistics 27.0 software, with one-way ANOVA employed to assess the differences among various treatments, and Dunkan’s test was used for the results’ evaluation. Pearson’s correlation coefficient analysis was conducted to evaluate the relationship between HDPE, Cd, pH, Total C, TOC, DOC, Total N, NH4+-N, NO3−-N, and enzymatic activities. Origin 2021 software was used to generate figures and tables.
3. Experimental Result
3.1. pH
Under different incubation times, the effects of different doses of HDPE and Cd on the pH value of the soil are set out in Table 2. In general, the pH value decreased with the increase in Cd concentration, but the addition of HDPE inhibited the decrease in pH value in the soil.
3.2. Analysis of Carbon Transformation in the Soil
The total carbon content in the samples was related to the addition of HDPE. The total carbon contents in the treatments of HDPE0, HDPE0.5, HDPE1, and HDPE2 were 14.69 ± 0.45, 18.73 ± 0.59, 23.10 ± 1.04, and 31.37 ± 0.81 g kg−1, respectively.
3.2.1. Changes in TOC and DOC Concentration in the Soil
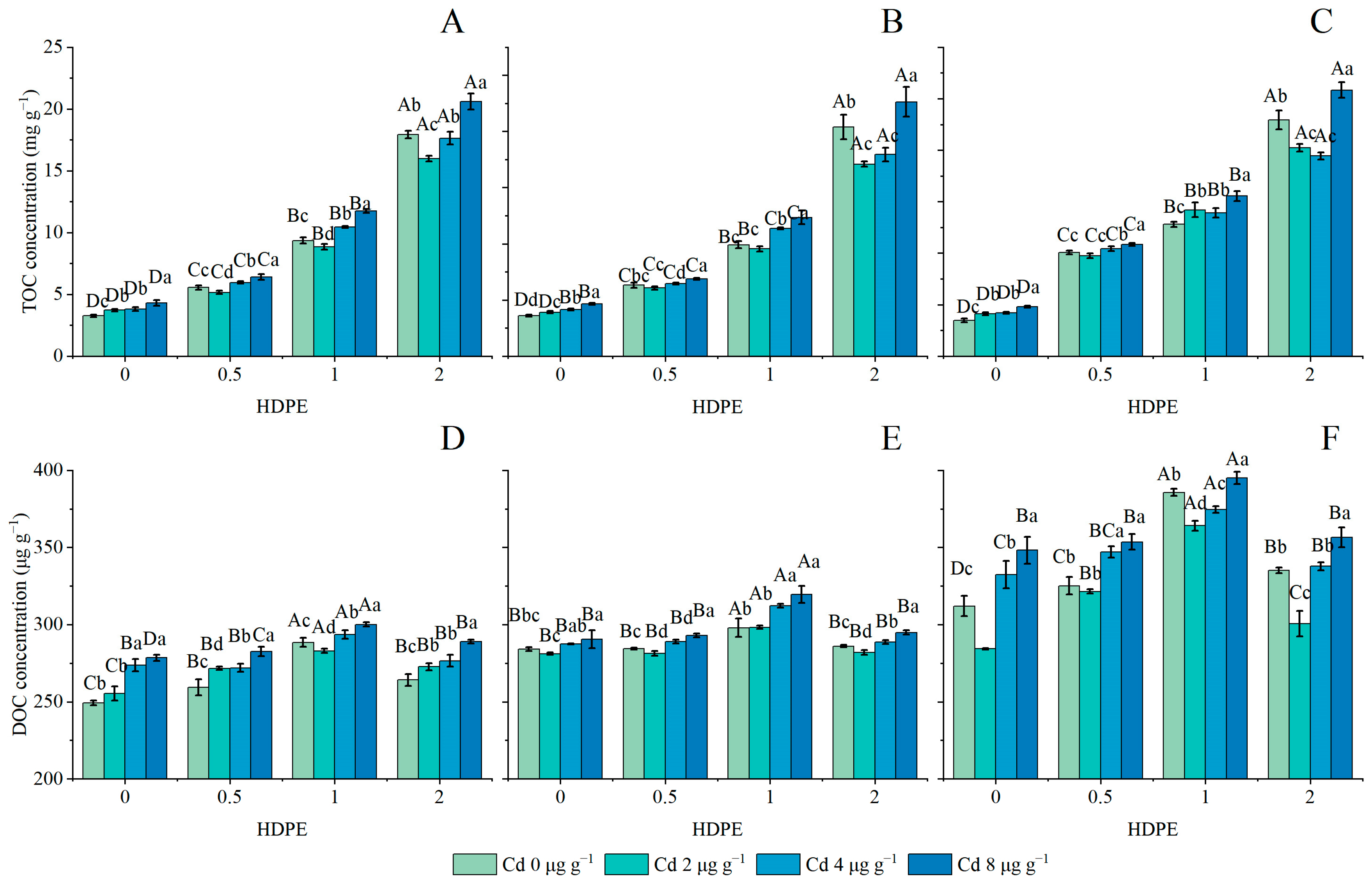
The effects of the addition of HDPE and Cd on TOC concentration in the soil are set out in Figure 2A–C. The addition of HDPE significantly increased the TOC concentration (n = 12, p < 0.001). Under the HDPE0 treatment, the TOC concentration increased with the increase in Cd concentration (n = 12, p < 0.001). Except for the HDPE1 and HDPE2 treatments, the lowest TOC concentrations at T56 were 12.80 ± 0.26 and 19.44 ± 0.35 mg g−1 under Cd0 and Cd4 conditions, respectively (Figure 2C). In other HDPE and incubation time treatment conditions, the TOC concentration decreased first and then increased with the increase in Cd concentration, and the lowest point was identified under Cd2. Under the conditions of high HDPE concentration (HDPE2), the difference caused by the increase in Cd concentration was more significant, and the differences between the maximum value and the minimum value in the three time points were 22.29%, 24.48%, and 24.48%, respectively. Under the same Cd treatment, except for the HDPE0Cd0 group, the TOC concentration mostly showed an increasing trend with the increase in HDPE and the extension of incubation time (n = 9, p < 0.05). Under the condition of HDPE0.5, the TOC concentration increased by the largest proportion over time, and the TOC concentration increased by 44.67%, 47.08%, 42.97%, and 40.92% under different Cd concentrations, respectively.
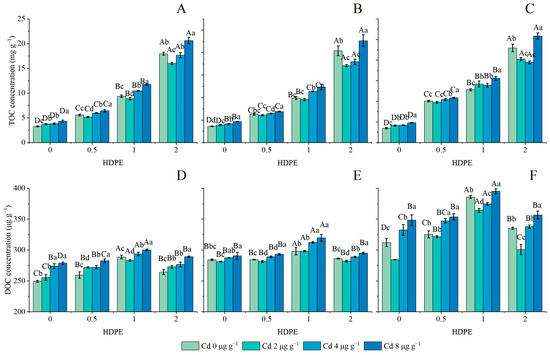
Figure 2.
TOC concentration in samples (mg g−1) (A): 14 days; (B): 28 days; (C): 56 days. DOC concentration in samples (μg g−1) (D): 14 days; (E): 28 days; (F): 56 days. Error bars represent mean ± standard deviation (n = 3). Different capital letters indicate differences in different HDPEs are significant (p < 0.05), and different lowercase letters indicate differences in different Cd are significant (p < 0.05).
The effects of the addition of HDPE and Cd on the DOC concentration in the soil under different incubation times are set out in Figure 2D–F. Under the same HDPE condition, the DOC concentration increased with incubation time (n = 9, p < 0.05). Under the same Cd condition, the DOC concentration increased first and then decreased under the influence of HDPE addition, and the highest point appeared at HDPE1. Under the same incubation time, DOC concentration increased first and then decreased with the addition of HDPE (n = 12, p < 0.05), and the highest point appeared at HDPE1. The DOC concentration in T28 and T56 decreased first and then increased with the addition of Cd (Figure 2E,F), and the lowest point was found in Cd2 (n = 12, p < 0.01). It is worth noting that in T28 and T56, with the same amount of HDPE under different Cd treatments, the difference in DOC concentration is greater. The difference between the maximum and minimum DOC concentration in T28 was 3.24%, 3.96%, 6.64%, and 4.38%, respectively (Figure 2E); in T56, they were 18.33%, 9.90%, 7.63%, and 15.69%, respectively (Figure 2F). Under the same HDPE and Cd conditions, the DOC concentration showed an increasing trend with the increase in incubation time. Among all the conditions, the largest growth was shown in HDPE1Cd0, which was 25.21%, and the least growth was shown in HDPE2Cd2, which was 9.4%.
3.2.2. Changes in Carbon-Related Enzymic Activities
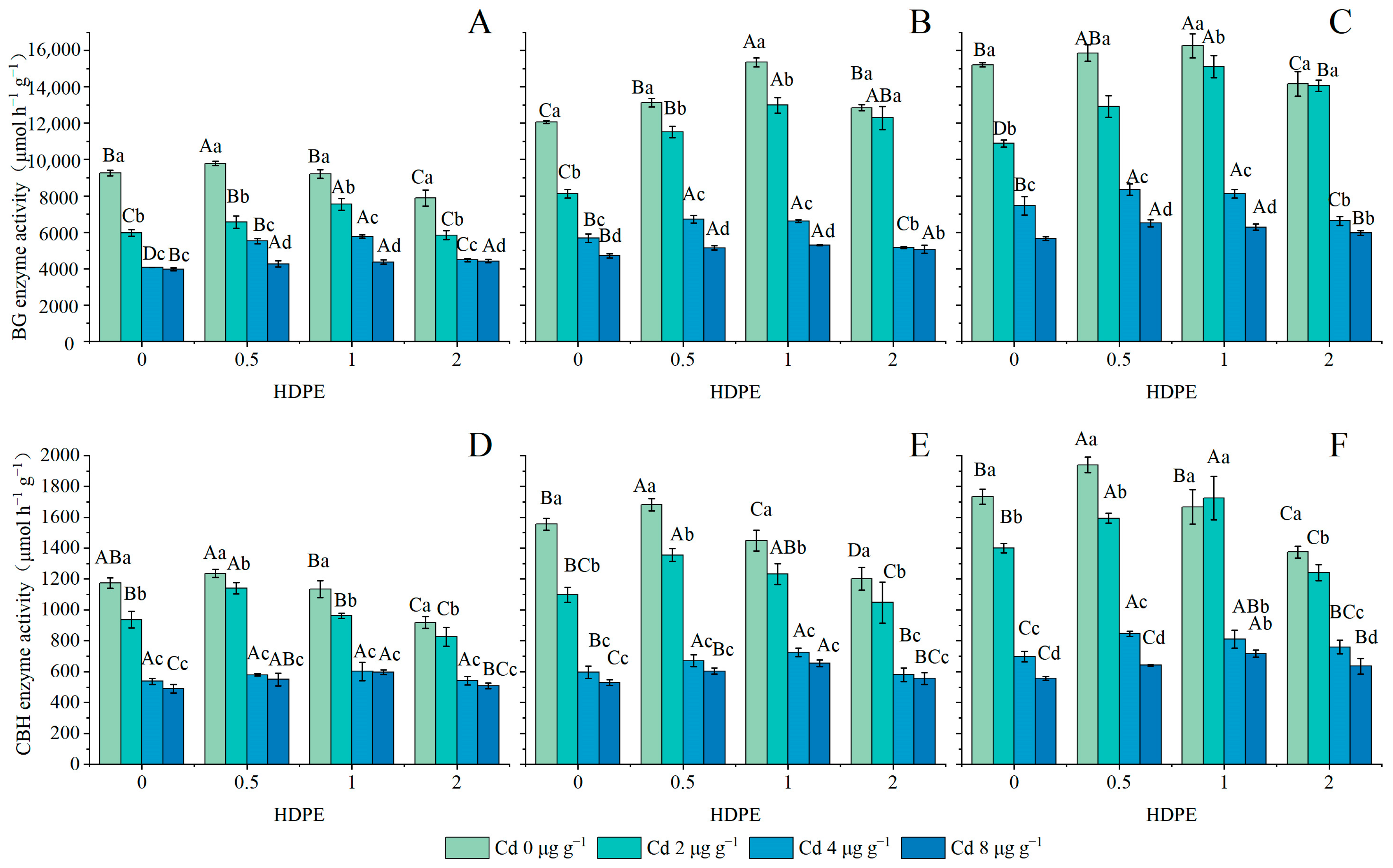
BG enzymic activity is set out in Figure 3A–C, and CBH enzymic activity is set out in Figure 3D–F. The enzymic activities of both BG and CBH increased first and then decreased with the increase in HDPE concentration (n = 12, p < 0.05), and were significantly inhibited with the increase in Cd concentration (n = 12, p < 0.001). With the increase in incubation time, the highest point of enzymic activity gradually shifted from the HDPE0.5 to the HDPE1 treatment. Under the condition of the Cd4 treatment, enzymic activity was significantly increased under the influence of HDPE concentration. However, neither HDPE nor incubation time had a significant effect on enzymic activity under the Cd8 treatment. Under the condition of T56 and HDPE1, the activity of the CBH enzyme at Cd2 was 1723.00 ± 140.87 μmol h−1 g−1, which was even higher than 1667.67 ± 112.93 μmol h−1 g−1 under Cd0. Under the same conditions of HDPE and Cd, BG and CBH enzymatic activities increased with the increase in incubation time (n = 9, p < 0.05). The maximum increase in BG and CBH enzymatic activity was 52.51% and 44.19% under HDPE2Cd2 and HDPE1Cd2, respectively.
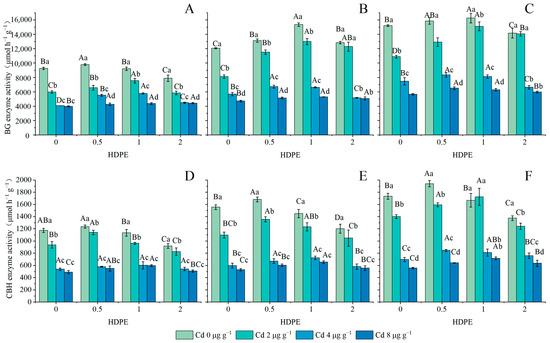
Figure 3.
BG enzymatic activity in samples (μmol h−1 g−1) (A): 14 days; (B): 28 days; (C): 56 days. CBH enzymatic activity in samples (μmol h−1 g−1) (D): 14 days; (E): 28 days; (F): 56 days. Error bars represent the mean ± standard deviation (n = 3). Different capital letters indicate differences in different HDPE are significant (p < 0.05), and different lowercase letters indicate differences in different Cd are significant (p < 0.05).
3.3. Analysis of Nitrogen Transformation in the Soil
In this experiment, the total nitrogen content of the samples was 0.51 ± 0.06 g kg−1.
3.3.1. Changes in DON, NH4+-N, and NO3−-N Concentration in the Soil
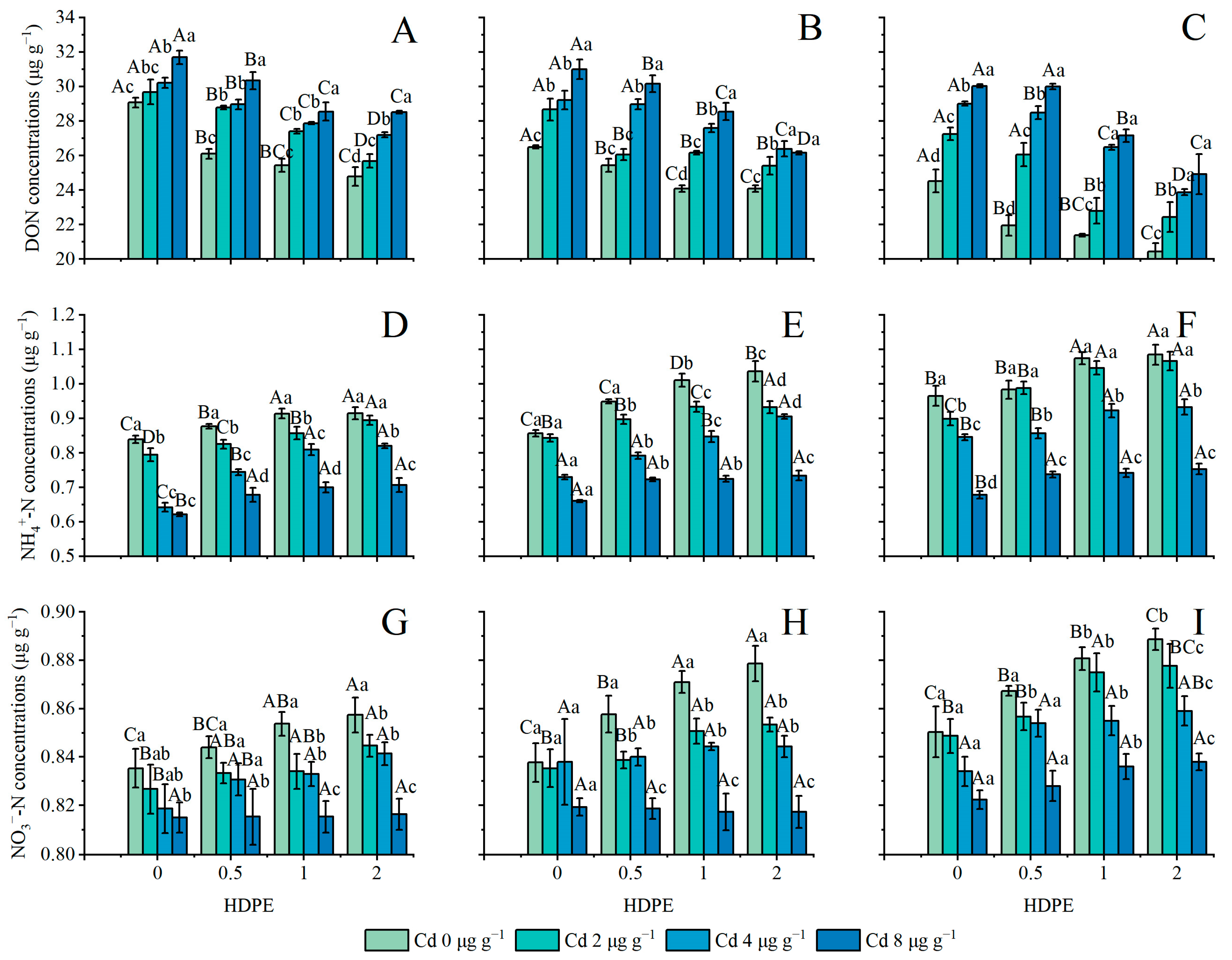
The concentration of DON in the soil is shown in Figure 4A–C. The application of only HDPE and Cd inhibited the concentration of DON in the soil (n = 12, p < 0.05), but Cd had a stronger effect on the concentration of DON than HDPE did. In particular, under the condition of T56 (Figure 4C), the greatest change in DON caused by the change in HDPE occurred under the treatment of Cd4, which was 17.66%. The maximum difference in DON caused by the change in Cd was 26.82% under HDPE0.5T56 treatment. With the presence of HDPE alone, the maximum reduction in DON was 7.92% under Cd8T28 (Figure 4B). The increase in incubation time had an inhibitory effect on the concentration of DOC (n = 9, p < 0.05). With the extension of incubation time, under the treatment of HDPE0Cd4, the concentration of DON decreased the least, to 3.99%. The HDPE2Cd0 group showed the highest decrease in DON concentration, which was 17.55%.
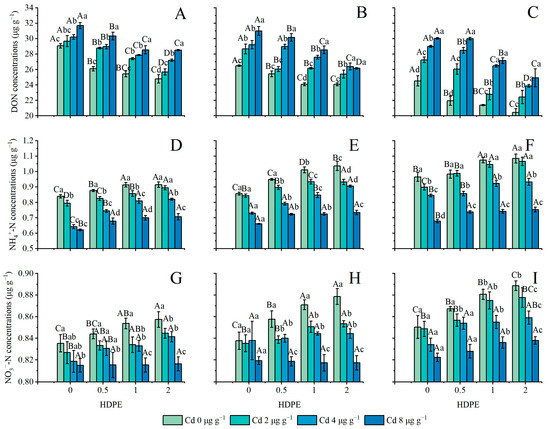
Figure 4.
DON concentration in samples (μg g−1) (A): 14 days; (B): 28 days; (C): 56 days. NH4+-N concentration in samples (μg g−1) (D): 14 days; (E): 28 days; (F): 56 days. NO3−-N concentration in samples (μg g−1) (G): 14 days; (H): 28 days; (I): 56 days. Error bars represent the mean ± standard deviation (n = 3). Different capital letters indicate differences in different HDPEs are significant (p < 0.05), and different lowercase letters indicate differences in different Cd are significant (p < 0.05).
The changes in the concentration of NH4+-N are set out in Figure 4D–F, and the changes in the concentration of NO3−-N are set out in Figure 4G–I. The addition of HDPE increased the concentration of NH4+-N and NO3−-N (n = 12, p < 0.05), Cd significantly inhibited the concentration of NH4+-N and NO3−-N (n = 12, p < 0.05), and the incubation time promoted the concentration of N in both forms (n = 9, p < 0.05). Under Cd8 conditions, neither the presence of HDPE nor the incubation time significantly affected the concentration of NH4+-N (Figure 4D–F); under the conditions of Cd0, Cd2, and Cd4, the concentration of NH4+-N increased greatly under the influence of HDPE0, HDPE0.5, and HDPE1. Under the conditions of T28 and HDPE2, NO3−-N demonstrated the maximum difference under the presence of Cd, which was 0.061 ± 0.001 μg g−1 (Figure 4H). Under the Cd8 condition, NO3−-N concentration did not increase by 0.075 ± 0.005 μg g−1 until T56 with the addition of HDPE (Figure 4I).
3.3.2. Analysis of Nitrogen-Related Enzymic Activities
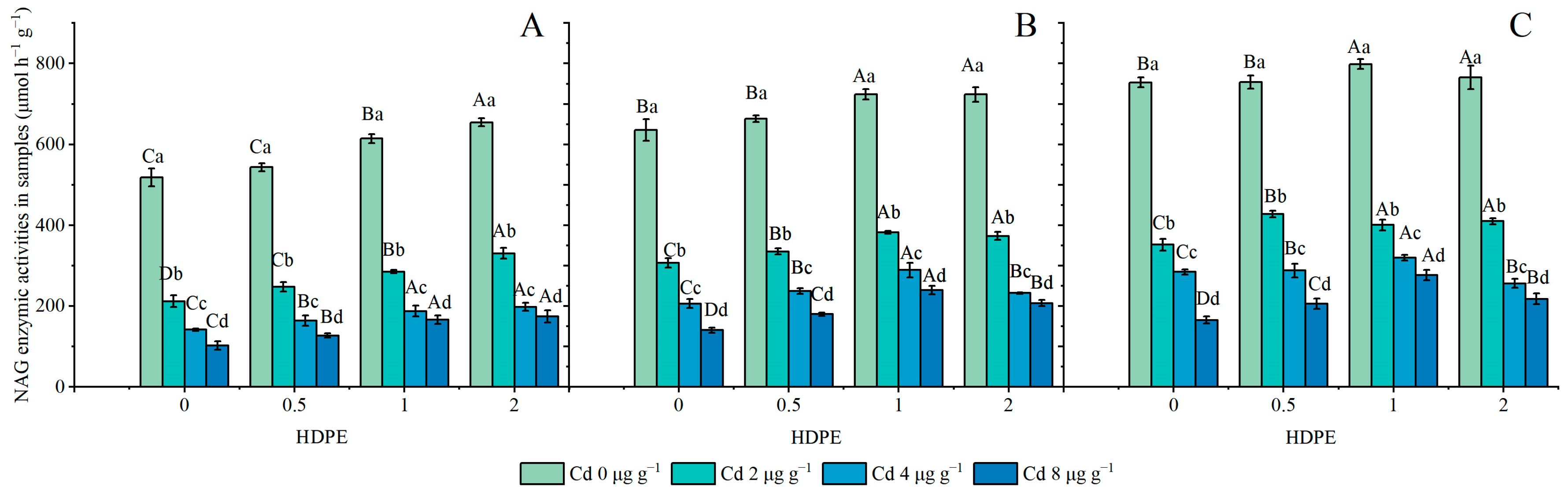
The NAG enzymic activities are set out in Figure 5. HDPE promoted an increase in NAG enzymic activity (n = 12, p < 0.05), while Cd significantly inhibited it (n = 12, p < 0.001), and the incubation time significantly enhanced NAG enzymic activity (n = 9, p < 0.05). Under different HDPE conditions, NAG enzymic activity decreased with the increase in Cd concentration, and the maximum reduction in NAG enzymatic activity was 41.36% at Cd8T28 (Figure 5B). The NAG enzymatic activity decreased with the increase in Cd concentration, and the maximum decreased degree was 80.26% in HDPE0T14 (Figure 5A). Under the same HDPE and Cd conditions, NBG enzymatic activity increased with the increase in incubation time. The treatment conditions with the smallest and largest increases were HDPE2Cd0 and HDPE0Cd4, at 14.50% and 50.41%, respectively.
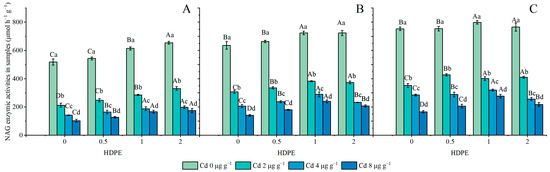
Figure 5.
NAG enzymic activities in samples (μmol h−1 g−1) (A): 14 days; (B): 28 days; (C): 56 days. Error bar represent the mean ± standard deviation (n = 3). Different capital letters indicate differences in different HDPEs are significant (p < 0.05), and different lowercase letters indicate differences in different Cd are significant (p < 0.05).
3.4. Correlation Analysis
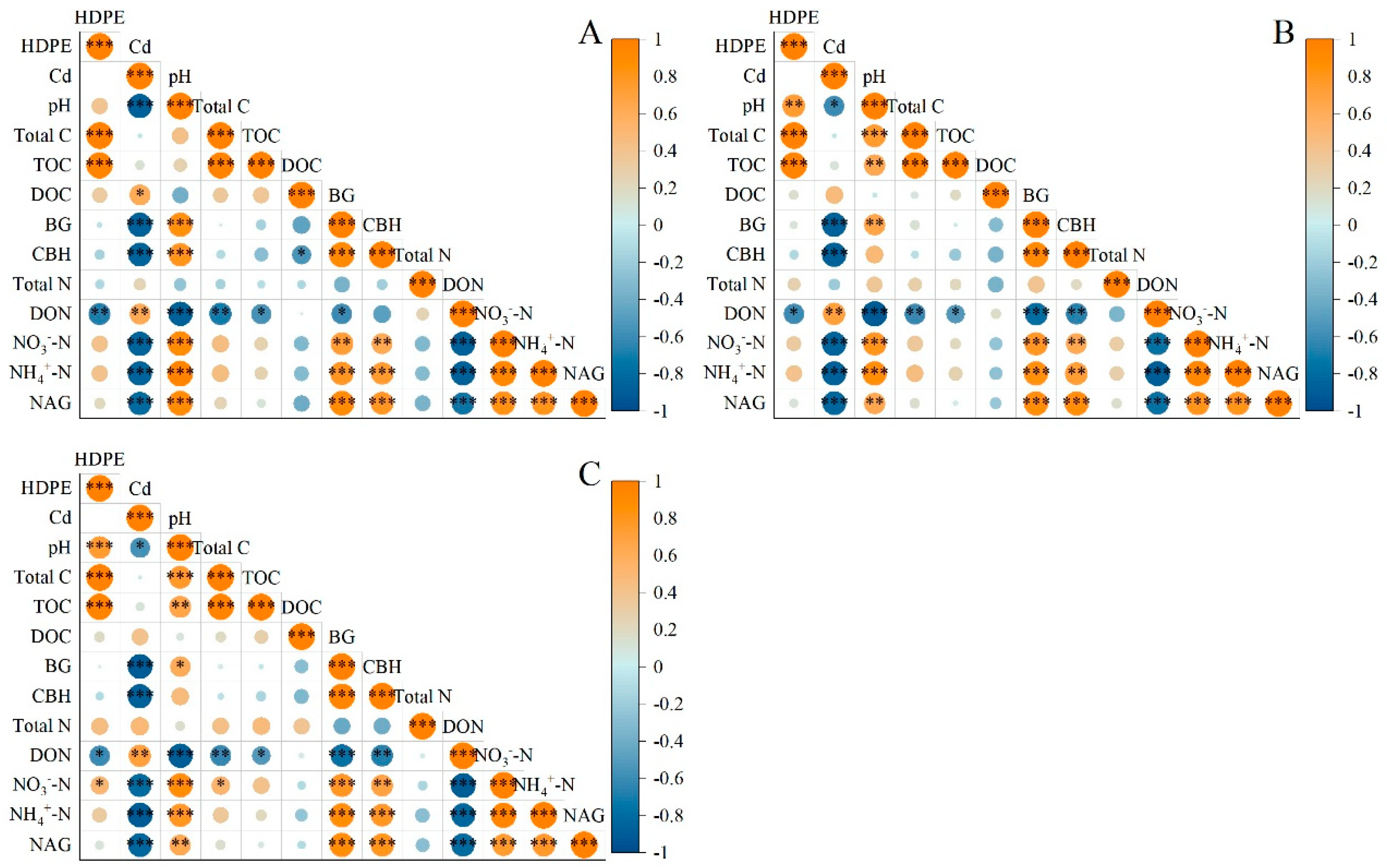
As shown in Figure 6, there was a positive correlation between pH and HDPE in Spartina alterniflora soil samples, and it became more and more significant with the increase in the incubation period. The correlation between pH and HDPE was not significant when the incubation time was 14 days, but p ≤ 0.01 and p ≤ 0.001 were detected when the incubation time was 28 days and 56 days, respectively. There was no significant correlation between DOC, BG, CBH, TN, NH4+-N, NO3−-N, and NAG and HDPE, while there was a significant negative correlation between DON and HDPE (p ≤ 0.01), which was consistent under the three incubation times. There was a negative correlation between pH and Cd, which decreased with the incubation time: p ≤ 0.001 under 14-day incubation, and p ≤ 0.05 under 28-day and 56-day incubation. There was a positive correlation between Cd and DOC concentration, but it was significant only under 14-day incubation, at p ≤ 0.05. Under the three incubation conditions, Cd had a significant negative correlation with BG, CBH, NH4+-N, NO3−-N, and NAG (p ≤ 0.001), a positive correlation with DON (p ≤ 0.01), and no significant correlation with TC, TON, or TN.
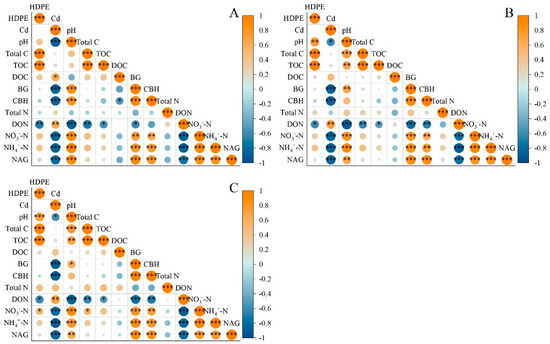
Figure 6.
Correlation between different treatments and physicochemical properties of samples. (A): 14 days (B): 28 days (C): 56 days. *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001.
4. Discussion
4.1. Analysis of the Change in the pH Value of the Soil
The pH value of the soil is an important characteristic, affecting nutrient absorption and microbial heavy metal availability and absorption in the soil [25], and pH can affect the surface charge of the adsorbent [26] and the form of Cd, so pH is an important factor that can change MPs’ performance in adsorbing Cd [27]. Microplastics will release some harmful chemicals into the soil environment, and these substances will cause the pH of the soil to decrease by reacting with other substances; meanwhile, Cd has an effect on pH by changing its form and solubility profile in the soil. In this study, the decrease of the pH value of the soil, and this was proportional to the concentration of HDPE and Cd (Table 2 and Figure 6), which was consistent with the research results on PE [28]. This is possibly because OH− is an important buffering ion in the soil, playing a crucial role in maintaining the acid–base balance of the soil. The hydroxyl groups on the surface of MPs can consume OH− in the soil through deprotonation, resulting in a decrease in the pH value of the soil. Moreover, the MPs in the soil could decompose into smaller particles under the influence of water molecules, or cause the release of H+ ions on the surface of MPs under the influence of microbial degradation, which further reduces the pH value of the soil.
4.2. Effects of Dual Contamination of HDPE and Cd on C Conversion in the Soil
The soil on the coastal wetland plays a vital role in carbon sequestration and storage, which can mitigate climate change to a great extent [29]. Previous studies have found that MPs can stimulate microbial activities, cause an “excitation effect” [30] upon entering the soil, and promote the mineralization of organic carbon SOC in the soil [31,32], and the concentration of TOC and DOC would change [33,34] accordingly. This phenomenon is more prominent when the MP’s aging time is approximately 60 days [6]. Similarly, the concentration of TOC increased significantly under T56 treatment (Figure 2C) in this experiment. The degradation of MPs produces a large amount of TOC (Figure 2A–C), which is consistent with the decrease in the pH value [35] and the aging of MPs with the passage of incubation time. Moreover, the presence of MPs provides a large number of ecological sites for microorganisms in the soil, which can increase enzymic activities (Figure 3) and promote the decomposition of organic matters in the soil. Hence, the degradation rate of HDPE was affected, and the concentration of TOC increased. Therefore, the addition of HDPE contributes to the increase in TOC concentration (Figure 6). The addition of a low concentration of HDPE (HDPE0 or HDPE1) can promote the generation and accumulation of DOC [36]. In this experiment, there is a similar trend under the same Cd and incubation time conditions (Figure 2D–F). Since MPs had a certain adsorption effect on DOC [37], the concentration of DOC under the HDPE2 condition decreases. Therefore, the increase in the concentration of HDPE had no significant antagonistic effect on the DOC concentration in the soil [38] (Figure 6).
Cd is a highly toxic heavy metallic element that can significantly inhibit microbial activities in a short period of time. Under the same condition of the HDPE concentration and incubation time, with the increase in Cd concentration, more TOC was retained because the toxicity of Cd inhibited the decomposition rate of SOC in soil by microorganisms. At the same incubation time, a high concentration of HDPE can adsorb part of Cd to a certain extent [39], weakening its transfer ability and reducing the toxic effect of Cd on microorganisms. However, with regards to microorganisms and other factors in Spartina alterniflora soil, Cd4 may have reached a level that can seriously affect their activities or physicochemical properties. Therefore, in this experiment, under the conditions of Cd4 and Cd8, and especially Cd8, the enzymic activities were not significantly promoted due to the increase in HDPE and the duration of the incubation time. Consequently, under the condition of the same Cd concentration and incubation time, with the increase in HDPE, the TOC concentration (Figure 2A–C) and the enzymic activities increased (Figure 3), the consumption of SOC by microorganisms increased, and the DOC concentration increased first and then decreased due to the transformation of SOC and the aging of MPs (Figure 2D–F). Cd’s toxic effects on microorganisms were positively correlated with the concentration of Cd (Figure 6). Under the same HDPE condition, with the increase in Cd concentration, microorganisms’ capacity to utilize the DOC decreased. Under the influence of relatively higher toxic effect, their decomposition rate of HDPE decreased and the concentration of TOC decreased. With the extension of incubation time, the concentration of TOC and DOC decreased due to the restriction of decomposition rate and the consumption of microorganisms. However, when incubation time was 28 days and 56 days, and HDPE content was the same, and the DOC concentration in Cd2 was lower (Figure 2D–F), because MPs’ adsorption effect on the DOC is more prominent in the Spartina alterniflora soil (Figure 2E,F), and microorganisms consume more SOC at a lower concentration of Cd. Under this circumstance, however, the activities of BG, CBH, and NAG enzymes were enhanced (Figure 3 and Figure 5). During the 14-day incubation period, enzymes and microorganisms may not have fully adapted to the experimental conditions, leading to fluctuations in DOC concentration, as shown in Figure 2D.
4.3. Impact of Dual Contamination of HDPE and Cd on Nitrogen Transformation in Soil
Nitrogen is an indispensable element to all living organisms, and nitrogen formations is a microbial-mediated redox process involving various kinds of nitrogen, which plays an important role in the biosphere cycle. In this experiment, the total concentration of N in all samples did not change significantly, possibly because the closed incubation conditions in the laboratory hindered the exchange between the soil and the outside environment. In this experiment, although the pH value of the soil showed a downward trend, it still maintained an alkaline state, so the decrease in pH value did not have a significant impact on the concentration of NH4+-N or NO3−-N [35]. Notably, in this experiment, under the conditions of the same incubation time and Cd concentration, the increase in the HDPE concentration had a weak influence on the increase of NH4+-N and NO3−-N concentration (Figure 4 and Figure 6). This is similar to the research results that found no significant changes in the concentration of NH4+-N and NO3−-N in the soil after 30 days of incubation with 1% and 5% PE-MPs added to the soil [36]. This is possibly because MPs contain a large amount of C, a growth substrate that could be easily utilized by microorganisms, and which can cause a great change in the ratio of C:N in the soil [40], thus promoting relevant enzymic activities and the consumption of TON in the soil, and increasing the conversion of NH4+-N and NO3−-N [41]. The concentration of NH4+-N increased slightly with the increase in incubation time, indicating that the mineralization process of OM was active under the influence of incubation time, but the nitrification was not enough to offset the resulting large amount of NH4+-N. However, this study did not find that a large increase in DOC in soil would limit microorganisms’ access to nitrogen-related nutrients, thus affecting microbial growth [42]. This was possibly because Cd’s toxic effects had a more prominent inhibitory effect on the activities of NAG, which is an N-related enzyme (Figure 5). Therefore, the toxic effects’ influence on the enzymic activities under Cd8 conditions were not significantly improved during the experimental period, so there were not significant changes in the concentration of NH4+-N or NO3−-N under Cd8 conditions (Figure 4). With the increase in incubation time, the stress resistance of the enzyme in the soil gradually manifested, and the enzymatic activity increased, which changed the physicochemical properties of the sample, and thus the concentration of NH4+-N and NO3−-N increased.
5. Conclusions
Enzyme activities exhibited a relatively consistent trend due to the combined effects of multiple factors. Specifically, higher concentrations of HDPE increased SOC content in the soil through aging and decomposition, while the adsorption of Cd was enhanced and the toxicity of Cd to microorganisms was reduced. As incubation time progressed, microbial growth and reproduction gradually recovered, contributing to an upward trend in enzyme activity. However, high concentrations of HDPE significantly enhanced the adsorption of DOC, thereby reducing the concentration of SOC for microbial utilization. Additionally, elevated Cd concentrations further suppressed enzyme activity. These combined effects ultimately influenced the concentrations of TOC, DOC, DON, NH4+-N, and NO3−-N. With the extension of incubation time, under the conditions of Cd2 and Cd4, when the concentration of HDPE exceeded 1 g, the enzymatic activity increased. In addition, the inhibitory effect of HDPE on the toxic effect of Cd was more significant when the concentration of Cd was low, which further enhanced the fixation effect of soil on C and N. In summary, this study revealed the complex mechanism of MPs and Cd accumulation on C and N forms in Spartina alterniflora soil, which is expected to provide some important references for the study of the invasive behavior of Spartina alterniflora and further understanding of the ecological effects of environmental pollutants. In the future, we hope to study the changes in Cd forms and the effects on plants under the dual pollution of HDPE and Cd.
Author Contributions
Conceptualization, Z.N.; Methodology, Z.N., S.X. and X.Z.; Validation, Z.N., S.X. and X.Z.; Formal Analysis, Z.N.; Investigation, S.X. and X.Z.; Resources, Z.N., S.X. and X.Z.; Data Curation, Z.N., S.X. and X.Z.; Writing—Original Draft Preparation, Z.N.; Writing—Review and Editing, Z.N. and J.L.; Visualization, Z.N.; Supervision, S.X., J.L. and D.D.; Project Administration, J.L. and D.D.; Funding Acquisition, H.L., Z.W., S.H. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key technologies demonstration for the ecological restoration of degraded coastal wetlands in Wenzhou Bay, grant number 2023YFE0101700-02; the Yellow Sea wetland project, grant number HHSDKT202417; the Open Research Fund of State Key Laboratory of Estuarine and Coastal Research, grant number SKLECKF202306; and the Carbon Peak and Carbon Neutrality Technology Innovation Special Foundation of Jiangsu Province, grant number BK20220030.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, China.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, C.; Wang, H.; Liao, X.; Xiao, R.; Liu, K.; Bai, J.; Li, B.; He, Q. Heavy Metal Pollution in Coastal Wetlands: A Systematic Review of Studies Globally over the Past Three Decades. J. Hazard. Mater. 2022, 424, 127312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Schwarz, C.; Lin, W.; Naing, H.; Cai, H.; Zhu, Z. A New Perspective on the Impacts of Spartina Alterniflora Invasion on Chinese Wetlands in the Context of Climate Change: A Case Study of the Jiuduansha Shoals, Yangtze Estuary. Sci. Total Environ. 2023, 868, 161477. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in Surface Waters and Sediments of the Wei River, in the Northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, D.; Rong, H.; Li, M.; Tong, M.; Kim, H. Influence of Nano- and Microplastic Particles on the Transport and Deposition Behaviors of Bacteria in Quartz Sand. Environ. Sci. Technol. 2018, 52, 11555–11563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Jiang, W.; Chen, J.; Chen, Y.; Zhang, X.; Wang, G. Microplastics with Cadmium Inhibit the Growth of Vallisneria Natans (Lour.) Hara Rather than Reduce Cadmium Toxicity. Chemosphere 2021, 266, 128979. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of Microplastics on Soil Properties: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of Soil Dissolved Organic Matter to Microplastic Addition in Chinese Loess Soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Sun, X.; Tao, R.; Xu, D.; Qu, M.; Zheng, M.; Zhang, M.; Mei, Y. Role of Polyamide Microplastic in Altering Microbial Consortium and Carbon and Nitrogen Cycles in a Simulated Agricultural Soil Microcosm. Chemosphere 2023, 312, 137155. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of Plastic Pollution in Soil Properties and Growth of Grass Species in Semi-Arid Regions: A Laboratory Experiment. Environ. Sci. Pollut. Res. 2022, 29, 59118–59126. [Google Scholar] [CrossRef]
- Shen, H.; Sun, Y.; Duan, H.; Ye, J.; Zhou, A.; Meng, H.; Zhu, F.; He, H.; Gu, C. Effect of PVC Microplastics on Soil Microbial Community and Nitrogen Availability under Laboratory-Controlled and Field-Relevant Temperatures. Appl. Soil Ecol. 2023, 184, 104794. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Wang, X. Microplastic Presence Significantly Alters Soil Nitrogen Transformation and Decreases Nitrogen Bioavailability under Contrasting Temperatures. J. Environ. Manag. 2022, 317, 115473. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic Addition Alters the Microbial Community Structure and Stimulates Soil Carbon Dioxide Emissions in Vegetable-Growing Soil. Environ. Toxic Chem. 2021, 40, 352–365. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Zang, H.; Graf, M.; Liu, X.; Wang, K.; Greenfield, L.M.; Jones, D.L. Bioplastic (PHBV) Addition to Soil Alters Microbial Community Structure and Negatively Affects Plant-Microbial Metabolic Functioning in Maize. J. Hazard. Mater. 2023, 441, 129959. [Google Scholar] [CrossRef]
- Su, P.; Gao, C.; Zhang, X.; Zhang, D.; Liu, X.; Xiang, T.; Luo, Y.; Chu, K.; Zhang, G.; Bu, N.; et al. Microplastics Stimulated Nitrous Oxide Emissions Primarily through Denitrification: A Meta-Analysis. J. Hazard. Mater. 2023, 445, 130500. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Polyethylene Microplastics Increase Cadmium Uptake in Lettuce (Lactuca Sativa L.) by Altering the Soil Microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Suzaki, P.Y.R.; Munaro, M.T.; Triques, C.C.; Kleinübing, S.J.; Klen, M.R.F.; De Matos Jorge, L.M.; Bergamasco, R. Biosorption of Binary Heavy Metal Systems: Phenomenological Mathematical Modeling. Chem. Eng. J. 2017, 313, 364–373. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil Contamination with Cadmium, Consequences and Remediation Using Organic Amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Buccolieri, A.; Buccolieri, G.; Cardellicchio, N.; Dell’Atti, A.; Di Leo, A.; Maci, A. Heavy Metals in Marine Sediments of Taranto Gulf (Ionian Sea, Southern Italy). Mar. Chem. 2006, 99, 227–235. [Google Scholar] [CrossRef]
- Schad, P. World Reference Base for Soil Resources—Its Fourth Edition and Its History. J. Plant Nutr. Soil Sci. 2023, 186, 151–163. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Dai, Y.; Ren, J.; Li, Y.; Wang, X.; Zhang, P.; Peng, C. Effects of Co-Loading of Polyethylene Microplastics and Ciprofloxacin on the Antibiotic Degradation Efficiency and Microbial Community Structure in Soil. Sci. Total Environ. 2020, 741, 140463. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Agrochemical Analysis of Soils, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- State Environmental Protection Administration of China. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 210–213. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer Saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of Microplastics and Cadmium on Plant Growth and Arbuscular Mycorrhizal Fungal Communities in an Agricultural Soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The Adsorption Behavior of Metals in Aqueous Solution by Microplastics Effected by UV Radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.Q.; Neto, V.O.S.; Oliveira, J.T.; Barros, A.L.; Gomes, E.C.C.; Raulino, G.S.C.; Longuinotti, E.; Nascimento, R.F. Adsorption Equilibria of Cu2+, Zn2+, and Cd2+ on EDTA-Functionalized Silica Spheres. J. Chem. Eng. Data 2013, 58, 798–806. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar Alters Chemical and Microbial Properties of Microplastic-Contaminated Soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine Microplastic-Associated Biofilms—A Review. Environ. Chem. 2015, 12, 551. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, D.; Lin, J.; Kumar, A.; Jia, K.; Tian, X.; Yu, Z.; Zhu, B. Priming Effects Induced by Degradable Microplastics in Agricultural Soils. Soil Biol. Biochem. 2023, 180, 109006. [Google Scholar] [CrossRef]
- Wang, F.; Wang, T.; Gustave, W.; Wang, J.; Zhou, Y.; Chen, J. Spatial-Temporal Patterns of Organic Carbon Sequestration Capacity after Long-Term Coastal Wetland Reclamation. Agric. Ecosyst. Environ. 2023, 341, 108209. [Google Scholar] [CrossRef]
- Lee, Y.K.; Murphy, K.R.; Hur, J. Fluorescence Signatures of Dissolved Organic Matter Leached from Microplastics: Polymers and Additives. Environ. Sci. Technol. 2020, 54, 11905–11914. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yang, H.; Wang, G.; Ma, J.; Feng, L.; Liu, J. Response of Soil Carbon Fractions and Enzyme Activities to Mowing Management on in a Coastal Wetland of the Yellow River Delta. Front. Mar. Sci. 2022, 9, 993181. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of Microplastics and Carbon Nanotubes on Soil Geochemical Properties and Bacterial Communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, X.; Wu, D.; Peng, L.; Fan, C.; Zhang, W.; Li, Q.; Ge, C. Addition of Biodegradable Microplastics Alters the Quantity and Chemodiversity of Dissolved Organic Matter in Latosol. Sci. Total Environ. 2022, 816, 151960. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The Microplastisphere: Biodegradable Microplastics Addition Alters Soil Microbial Community Structure and Function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Chen, C.; Pan, J.; Xiao, S.; Wang, J.; Gong, X.; Yin, G.; Hou, L.; Liu, M.; Zheng, Y. Microplastics Alter Nitrous Oxide Production and Pathways through Affecting Microbiome in Estuarine Sediments. Water Res. 2022, 221, 118733. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X. Variations in Aggregate-Associated Organic Carbon and Polyester Microfibers Resulting from Polyester Microfibers Addition in a Clayey Soil. Environ. Pollut. 2020, 258, 113716. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, J.; Chang, S.X.; Wang, S. The Quality and Quantity of Exogenous Organic Carbon Input Control Microbial NO3− Immobilization: A Meta-Analysis. Soil Biol. Biochem. 2017, 115, 357–363. [Google Scholar] [CrossRef]
- Qi, Y.; Beriot, N.; Gort, G.; Huerta Lwanga, E.; Gooren, H.; Yang, X.; Geissen, V. Impact of Plastic Mulch Film Debris on Soil Physicochemical and Hydrological Properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, N.; Ma, X.; Wang, F.; Peng, J.; Yang, S.; Cao, W. Microplastics Strengthen Nitrogen Retention by Intensifying Nitrogen Limitation in Mangrove Ecosystem Sediments. Environ. Int. 2024, 185, 108546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).