Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium graminearum and Their Impact on Wheat Growth and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Effect of SOR Concentration on the Inactivation of F. graminearum
2.3. Spore Suspensions at Different Concentrations
2.4. Effect of Different Treatment Durations
2.5. Methylene Blue Staining
2.6. SEM Observation of Mycelial Morphology of F. graminearum
2.7. Field Trial Design
2.8. Control Efficacy Survey
2.9. Impact of SORs on Wheat Agronomic Traits and Yield
2.10. Data Analysis
3. Results
3.1. Effect of Different SOR Concentrations on the Elimination of F. graminearum Spores
3.2. Effects of SORs on Different Spore Concentrations
3.3. Effects of Different Treatment Times on Spore Inactivation
3.4. Methylene Blue Staining Experiment
3.5. Observations of SEM
3.6. Disease Control Efficacy
3.7. Effect of SORs on Agronomic Traits and Yield of Wheat
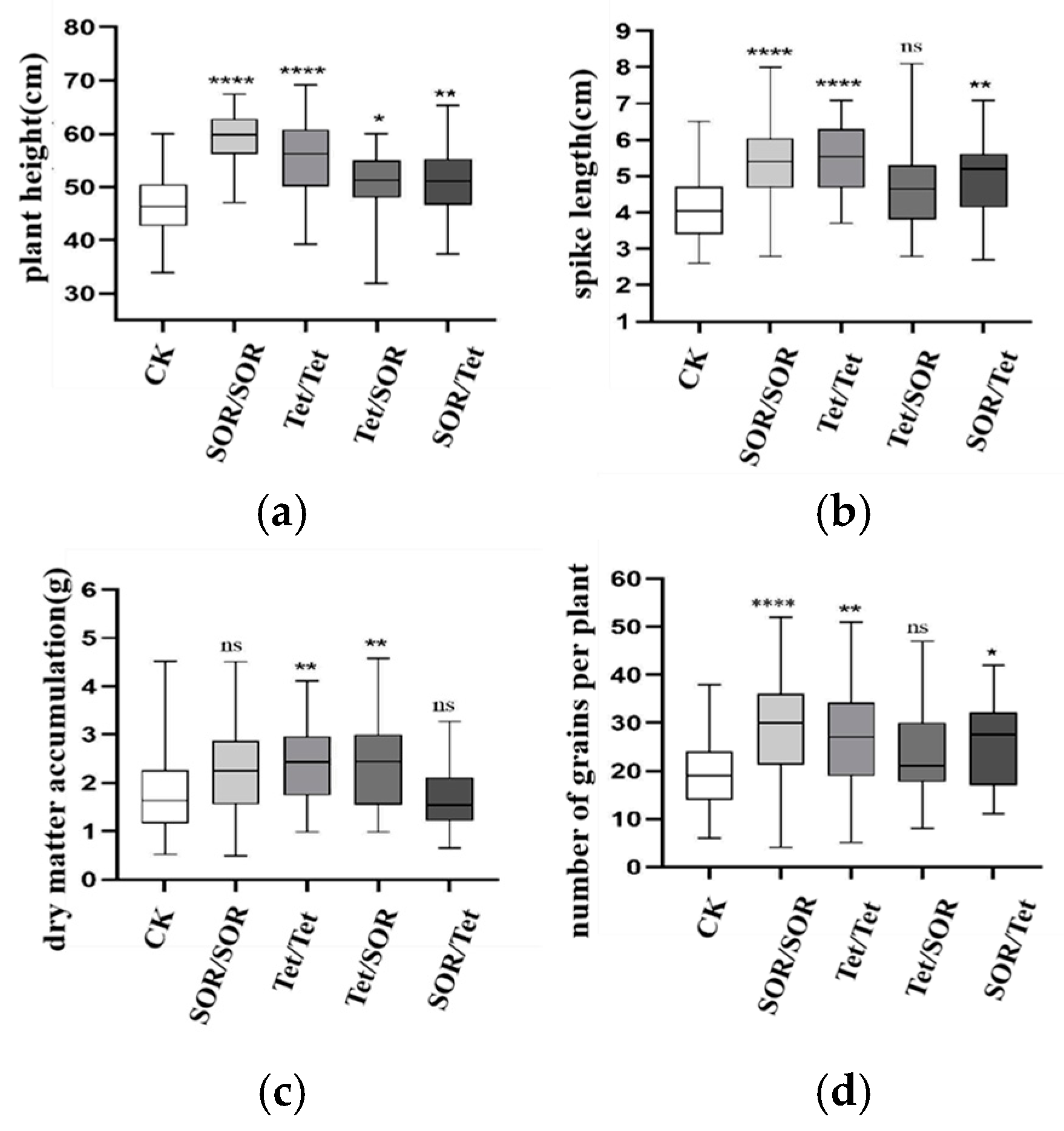
3.7.1. Effects of Different Treatments on Wheat Plant Height, Spike Length, Aboveground Dry Matter, and Kernel Number per Plant
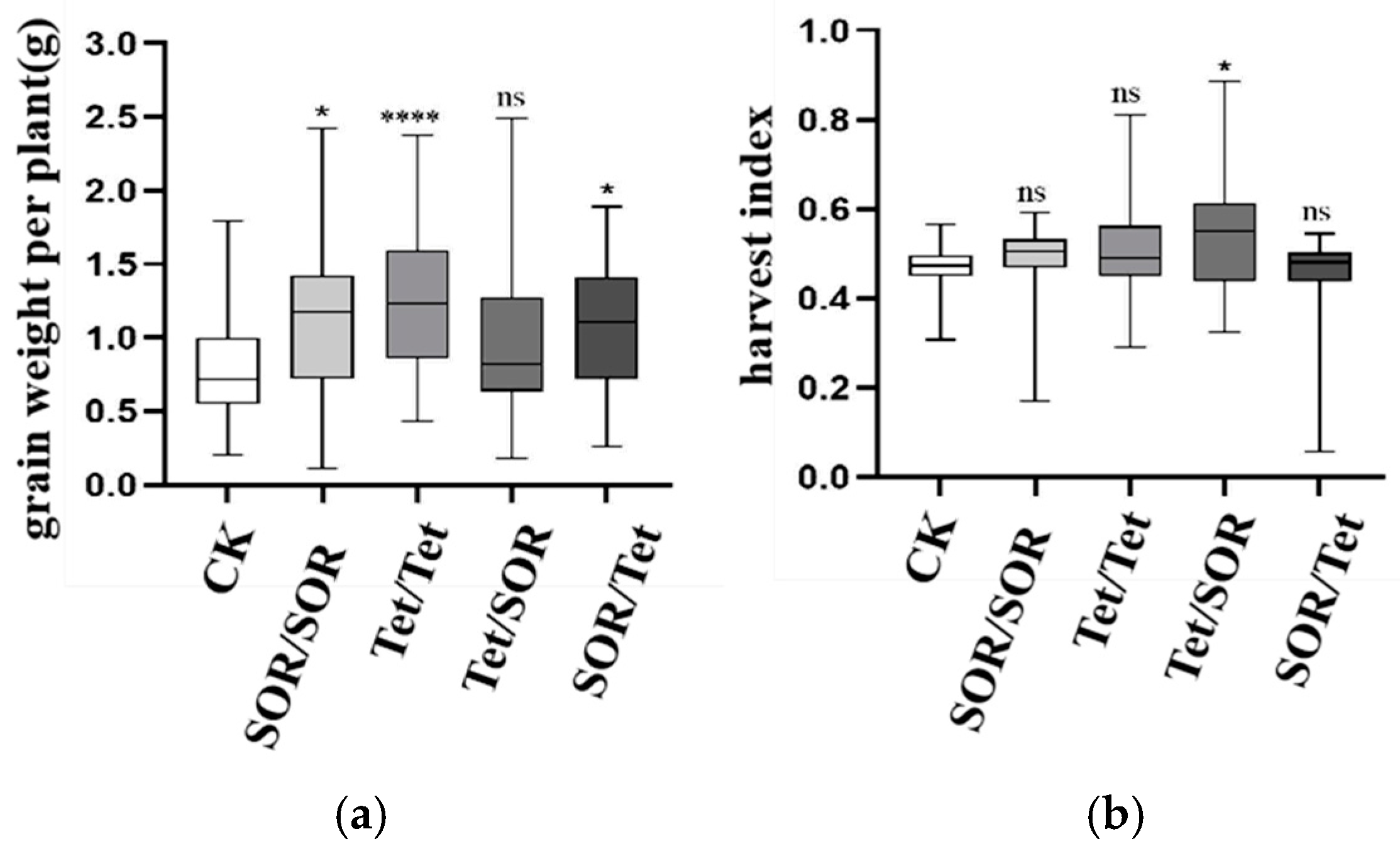
3.7.2. Effects of Different Treatments on Wheat Grain Weight per Plant and Harvest Index
3.7.3. Impact of Different Treatments on Wheat Thousand-Grain Weight and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of lipase and lipoxygenase of wheat germ with temperature-controlled short wave infrared radiation and its effect on storage stability and quality of wheat germ oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Wang, S.; Wang, L.; Xu, B. Study of the interfacial activity of wheat germ lipase. Qual. Assur. Saf. Crops Foods 2019, 11, 491–498. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Talpur, M.A.; Lakhiar, I.A.; Chandio, F.A.; Shaikh, S.A.; Solangi, K.A. Effects of different irrigation frequencies and incorporation of rice straw on yield and water productivity of wheat crop. Int. J. Agric. Biol. Eng. 2020, 13, 138–145. [Google Scholar]
- Xu, S.; Wang, Y.; Hu, J.; Chen, X.; Qiu, Y.; Shi, J.; Wang, G.; Xu, J. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control 2021, 130, 108259. [Google Scholar] [CrossRef]
- Gurung, S.; Hansen, J.M.; Bonman, J.M.; Gironella, A.I.N.; Adhikari, T.B. Multiple Disease Resistance to Four Leaf Spot Diseases in Winter Wheat Accessions from the USDA National Small Grains Collection. Crop Sci. 2012, 52, 1640–1650. [Google Scholar] [CrossRef]
- Salgado, J.D.; Madden, L.V.; Paul, P.A. Quantifying the Effects of Fusarium Head Blight on Grain Yield and Test Weight in Soft Red Winter Wheat. Phytopathology 2015, 105, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hao, Y.; Mergoum, M.; Bai, G.; Humphreys, G.; Cloutier, S.; Xia, X.; He, Z. Breeding wheat for resistance to Fusarium head blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar] [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects. Toxins 2017, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Dweba, C.C.; Figlan, S.; Shimelis, H.; Motaung, T.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Qiu, J.; Gu, H.; Wang, S.; Ji, F.; He, C.; Jiang, C.; Shi, J.; Liu, X.; Shen, G.; Lee, Y.-W.; et al. A diverse Fusarium community is responsible for contamination of rice with a variety of Fusarium toxins. Food Res. Int. 2024, 195, 114987. [Google Scholar] [CrossRef]
- Yi, X.; Cheng, J.; Jiang, Z.; Hu, W.; Bie, T.; Gao, D.; Li, D.; Wu, R.; Li, Y.; Chen, S.; et al. Genetic Analysis of Fusarium Head Blight Resistance in CIMMYT Bread Wheat Line C615 Using Traditional and Conditional QTL Mapping. Front. Plant Sci. 2018, 9, 573. [Google Scholar] [CrossRef]
- Zheng, Z.; Hoogenboom, G.; Cai, H.; Wang, Z. Winter wheat production on the Guanzhong Plain of Northwest China under projected future climate with SimCLIM. Agric. Water Manag. 2020, 239, 106233. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation–Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Lipps, S.; Bohn, M.; Rutkoski, J.; Butts-Wilmsmeyer, C.; Mideros, S.; Jamann, T. Comparative Review of Fusarium graminearum Infection in Maize and Wheat: Similarities in Resistance Mechanisms and Future Directions. Mol. Plant-Microbe Interact. 2024. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Heick, T.M.; Havis, N.; Holdgate, S.; Clark, B.; Blake, J.; Glazek, M.; Korbas, M.; Danielewicz, J.; et al. Decreasing azole sensitivity of Z. tritici in Europe contributes to reduced and varying field efficacy. J. Plant Dis. Prot. 2020, 128, 287–301. [Google Scholar] [CrossRef]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Contemporary status and molecular approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- Boyles, R.E.; Ballén-Taborda, C.; Brown-Guedira, G.; Costa, J.; Cowger, C.; DeWitt, N.; Griffey, C.A.; Harrison, S.A.; Ibrahim, A.; Johnson, J.; et al. Approaching 25 years of progress towards Fusarium head blight resistance in southern soft red winter wheat (Triticum aestivum L.). Plant Breed. 2023, 143, 66–81. [Google Scholar] [CrossRef]
- Ma, Z.; Xie, Q.; Li, G.; Jia, H.; Zhou, J.; Kong, Z.; Li, N.; Yuan, Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, M.; von Tiedemann, A.; Winter, M. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J. Plant Dis. Prot. 2016, 124, 121–130. [Google Scholar] [CrossRef]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, E.R.; Fernando, W.G.D. Weather-based models for forecasting Fusarium head blight risks in wheat and barley: A review. Plant Pathol. 2023, 73, 492–505. [Google Scholar] [CrossRef]
- Maulenbay, A.; Rsaliyev, A. Fungal Disease Tolerance with a Focus on Wheat: A Review. J. Fungi 2024, 10, 482. [Google Scholar] [CrossRef]
- Mastanaiah, N.; Banerjee, P.; Johnson, J.A.; Roy, S. Examining the Role of Ozone in Surface Plasma Sterilization Using Dielectric Barrier Discharge (DBD) Plasma. Plasma Process. Polym. 2013, 10, 1120–1133. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, B.; Zhu, W.; Tian, K.; Zhang, H. A Review on Ultrasonic Catalytic Microbubbles Ozonation Processes: Properties, Hydroxyl Radicals Generation Pathway and Potential in Application. Catalysts 2018, 9, 10. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2017, 58, 2176–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-Z. Metal-independent Hydroxyl Radical Production and DNA Damage by Sunlight-Irradiation of Hydroxamic Acid Carcinogens. Free Radic. Biol. Med. 2017, 112, 67. [Google Scholar] [CrossRef]
- Gebicki, J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016, 595, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cheng, Z.; Liang, Y.; Hu, X.; Shen, T.; Li, Y.; Han, Z.; Zhang, X.; Zou, X. A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis. Foods 2023, 12, 2206. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Zhou, C. Novel assisted/unassisted ultrasound treatment: Effect on respiration rate, ethylene production, enzymes activity, volatile composition, and odor of cherry tomato. LWT 2021, 149, 111779. [Google Scholar] [CrossRef]
- Agostini, F.; Faccini, M.; Fitarelli, F.; Ortiz, M.A.L.; Salmeron, S.; Oliveira, R.C.G.; Valarelli, F.P.; Oliveira, R.C.; Freitas, K.M.S. In Vitro Comparison of Antibacterial Effect of Ozonated Water and Ozonated Gas. Ozone Sci. Eng. 2020, 43, 394–400. [Google Scholar] [CrossRef]
- Chaidez, C.; Lopez, J.; Vidales, J.; Campo, N.C.-D. Efficacy of chlorinated and ozonated water in reducingSalmonella typhimuriumattached to tomato surfaces. Int. J. Environ. Health Res. 2007, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, F.; Li, P.-W.; Zhang, W.; Ding, X.-X.; Zhang, Q.; Li, M.; Wang, Y.-R.; Xu, B.-C. Effect of ozone on aflatoxins detoxification and nutritional quality of peanuts. Food Chem. 2014, 146, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Contigiani, E.V.; Kronberg, M.F.; Sánchez, G.J.; Gómez, P.L.; García-Loredo, A.B.; Munarriz, E.; Alzamora, S.M. Ozone washing decreases strawberry susceptibility to Botrytis cinerea while maintaining antioxidant, optical and sensory quality. Heliyon 2020, 6, e05416. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Feye, K.M.; Peyton, B.; Worlie, D.; Draper, M.J.; Ricke, S.C. The Addition of Viriditec™ Aqueous Ozone to Peracetic Acid as an Antimicrobial Spray Increases Air Quality While Maintaining Salmonella Typhimurium, Non-pathogenic Escherichia coli, and Campylobacter jejuni Reduction on Whole Carcasses. Front. Microbiol. 2018, 9, 3180. [Google Scholar]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Castillo, A. Reduction of Salmonella and Shiga toxin-producing Escherichia coli on alfalfa seeds and sprouts using an ozone generating system. Int. J. Food Microbiol. 2019, 289, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.P.; Zhou, H.M. Impact of aqueous ozone mixing on microbiological, quality and physicochemical characteristics of semi-dried buckwheat noodles. Food Chem. 2021, 336, 127709. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Białucha, A.; Wiktorczyk, N.; Bartkowska, A.; Kowalska, M.; Kruszewski, S.; Gospodarek-Komkowska, E. Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains. Microorganisms 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Bittencourt, K.O.; Scussel, V.M. Ozone treatment efficiency on Fusarium graminearum and deoxynivalenol degradation and its effects on whole wheat grains (Triticum aestivum L.) quality and germination. J. Stored Prod. Res. 2014, 59, 245–253. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Zhang, N.; Tian, Y.; Chen, C.; Meng, X. Treatment of 250 t/h Ballast Water in Oceanic Ships Using ·OH Radicals Based on Strong Electric-Field Discharge. Plasma Chem. Plasma Process. 2012, 32, 693–702. [Google Scholar] [CrossRef]
- Piskarev, I.M. The Formation of Ozone-Hydroxyl Mixture in Corona Discharge and Lifetime of Hydroxyl Radicals. IEEE Trans. Plasma Sci. 2021, 49, 1363–1372. [Google Scholar] [CrossRef]
- NY/T 1464.15-2007; Guidelines for the Field Efficacy Trials of Pesticides, Part 15: Fungicides for Controlling Wheat Fusarium Head Blight. China Agriculture Press: Beijing, China, 2007.
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Chen, X.; Zhao, Y.; Xie, J. Insights into the antibacterial mechanism of ozone water combined with tea polyphenols against Shewanella putrefaciens: Membrane disruption and oxidative stress. Int. J. Food Sci. Technol. 2022, 57, 7423–7433. [Google Scholar] [CrossRef]
- Abreu, M.R.; Delalibera, I.; Pereira, N.R.C.; Camargo-Mathias, M.I. Morphophysiological analysis of the salivary glands of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) exposed to ozonated water: A control strategy. Med. Vet. Entomol. 2021, 35, 88–96. [Google Scholar] [CrossRef]
- Xu, H.; Yi, L.; Li, C.; Sun, Y.; Hou, L.; Bai, J.; Kong, F.; Han, X.; Lan, Y. Design and Experiment of Ecological Plant Protection UAV Based on Ozonated Water Spraying. Drones 2023, 7, 291. [Google Scholar] [CrossRef]
- Girgin Ersoy, Z.; Barisci, S.; Dinc, O. Mechanisms of the Escherichia coli and Enterococcus faecalis inactivation by ozone. LWT 2019, 100, 306–313. [Google Scholar] [CrossRef]
- Young, J.C.; Zhu, H.; Zhou, T. Degradation of trichothecene mycotoxins by aqueous ozone. Food Chem. Toxicol. 2006, 44, 417–424. [Google Scholar] [CrossRef]
- Sun, C.; Ji, J.; Wu, S.; Sun, C.; Pi, F.; Zhang, Y.; Tang, L.; Sun, X. Saturated aqueous ozone degradation of deoxynivalenol and its application in contaminated grains. Food Control 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Sun, X.; Ji, J.; Gao, Y.; Zhang, Y.; Zhao, G.; Sun, C. Fate of deoxynivalenol and degradation products degraded by aqueous ozone in contaminated wheat. Food Res. Int. 2020, 137, 109357. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, M.M.; Campayo, A.; Moratalla-López, N.; de la Hoz, K.S.; Alonso, G.L.; Salinas, M.R. Ozonated water applied in grapevines is a new agronomic practice that affects the chemical quality of wines. Eur. Food Res. Technol. 2021, 247, 1869–1882. [Google Scholar] [CrossRef]
- Terao, D.; Nechet, K.d.L.; Frighetto, R.T.S.; Sasaki, F.F.C. Ozonated water combined with heat treatment to control the stem-end rot of papaya. Sci. Hortic. 2019, 257, 108722. [Google Scholar] [CrossRef]




| Treatment | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | Application Timing |
|---|---|---|---|---|---|---|
| The first round of application | Tetramycin | CK | SORs | Tetramycin | SORs | BBCH 61 |
| The second round of application | SORs | CK | Tetramycin | Tetramycin | SORs | BBCH 65 |
| Treatment | The Disease Index Under Medication with 14 Days | The Control Effect Under Medication with 14 Days (%) |
|---|---|---|
| CK | 28.29 | - |
| SORs/SORs | 3.43 | 87.87 |
| Tetramycin/tetramycin | 2.57 | 90.91 |
| Tetramycin/SORs | 1.43 | 94.95 |
| SORs/tetramycin | 1.71 | 93.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, B.; He, H.; Zhang, L.; Hu, X.; Wu, C. Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium graminearum and Their Impact on Wheat Growth and Yield. Agriculture 2025, 15, 404. https://doi.org/10.3390/agriculture15040404
Zhang H, Zhang B, He H, Zhang L, Hu X, Wu C. Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium graminearum and Their Impact on Wheat Growth and Yield. Agriculture. 2025; 15(4):404. https://doi.org/10.3390/agriculture15040404
Chicago/Turabian StyleZhang, Huanhuan, Bo Zhang, Huagang He, Lulu Zhang, Xinkang Hu, and Chundu Wu. 2025. "Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium graminearum and Their Impact on Wheat Growth and Yield" Agriculture 15, no. 4: 404. https://doi.org/10.3390/agriculture15040404
APA StyleZhang, H., Zhang, B., He, H., Zhang, L., Hu, X., & Wu, C. (2025). Fungicidal Effect of Strong Oxidative Free Radicals Against Fusarium graminearum and Their Impact on Wheat Growth and Yield. Agriculture, 15(4), 404. https://doi.org/10.3390/agriculture15040404






