Effects of Fish Palm Rice and Coconut Palm Rice Oil Mixture on Intestinal Health of Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Sample Collection
2.3. Analysis of the Concentration of Volatile Fatty Acids in Chyme
2.4. Determination of the Digesta pH Values
2.5. Analysis of the Bacterial Community
2.6. Data Statistics and Analysis
3. Results
3.1. Effects of Different Dietary Lipid Sources on Growth Performance and Microbial Metabolites of Weaned Piglets
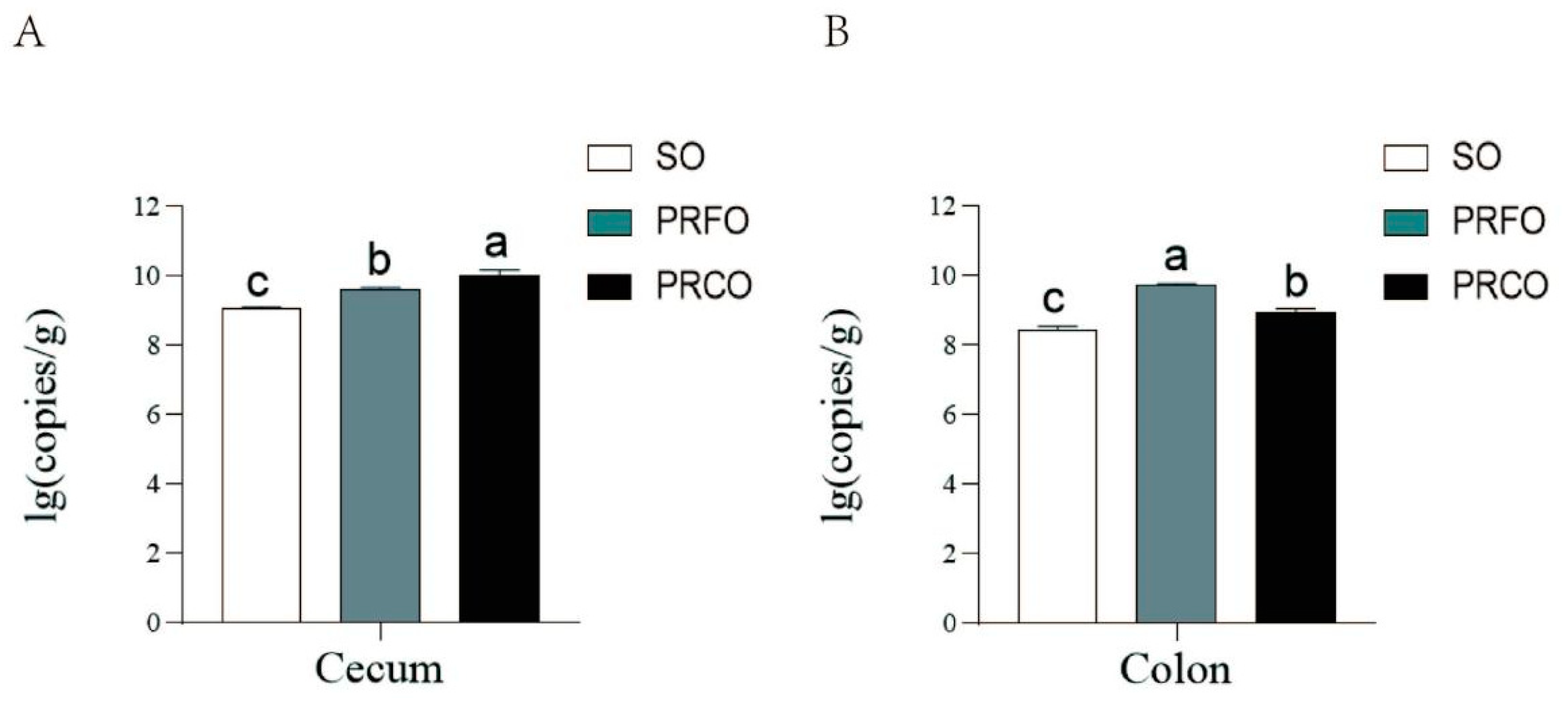
3.2. The Effect of Different Lipid Sources on the Total Bacterial Count of Cecum and Colon Chyme in Weaned Piglets
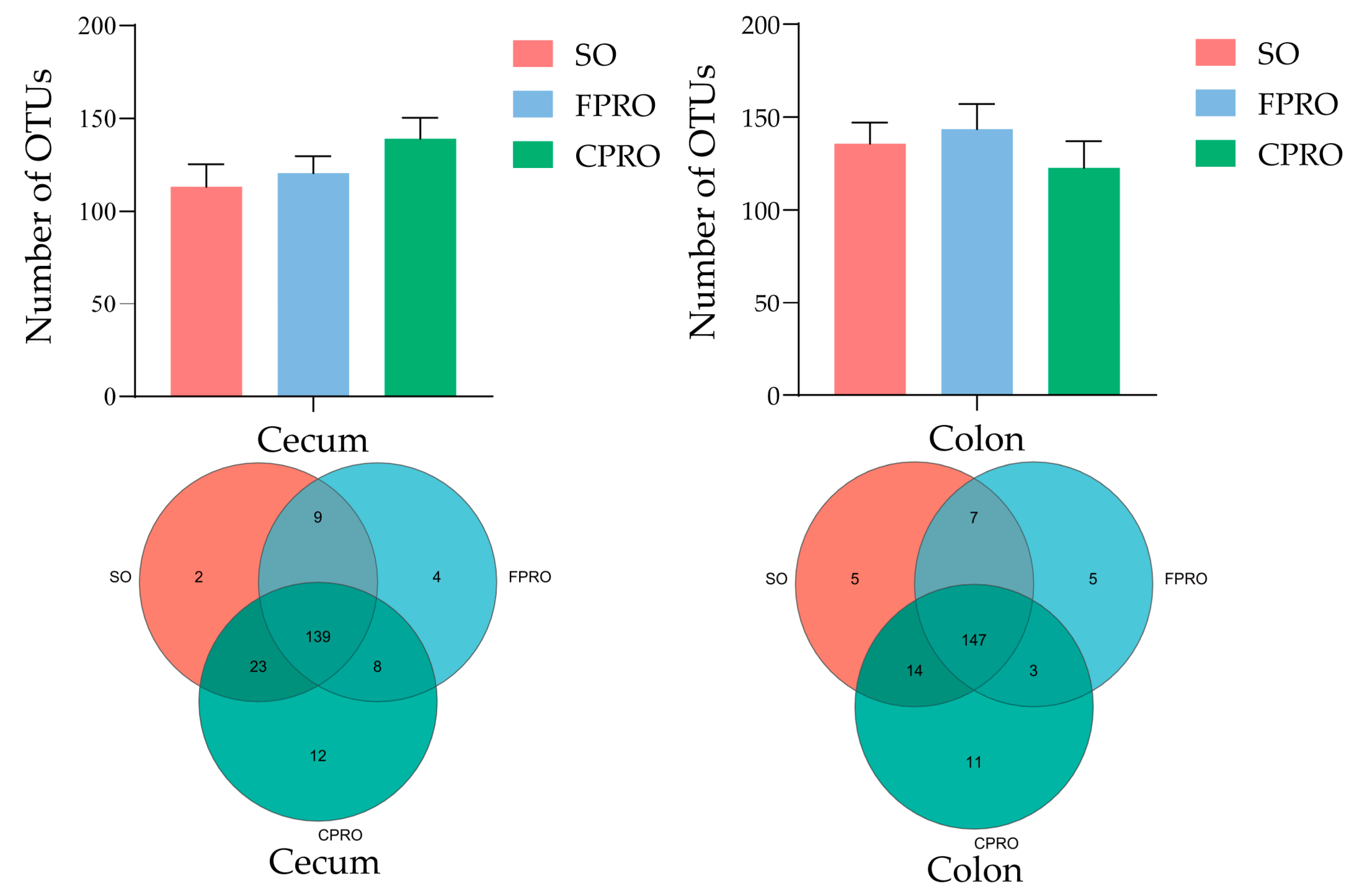
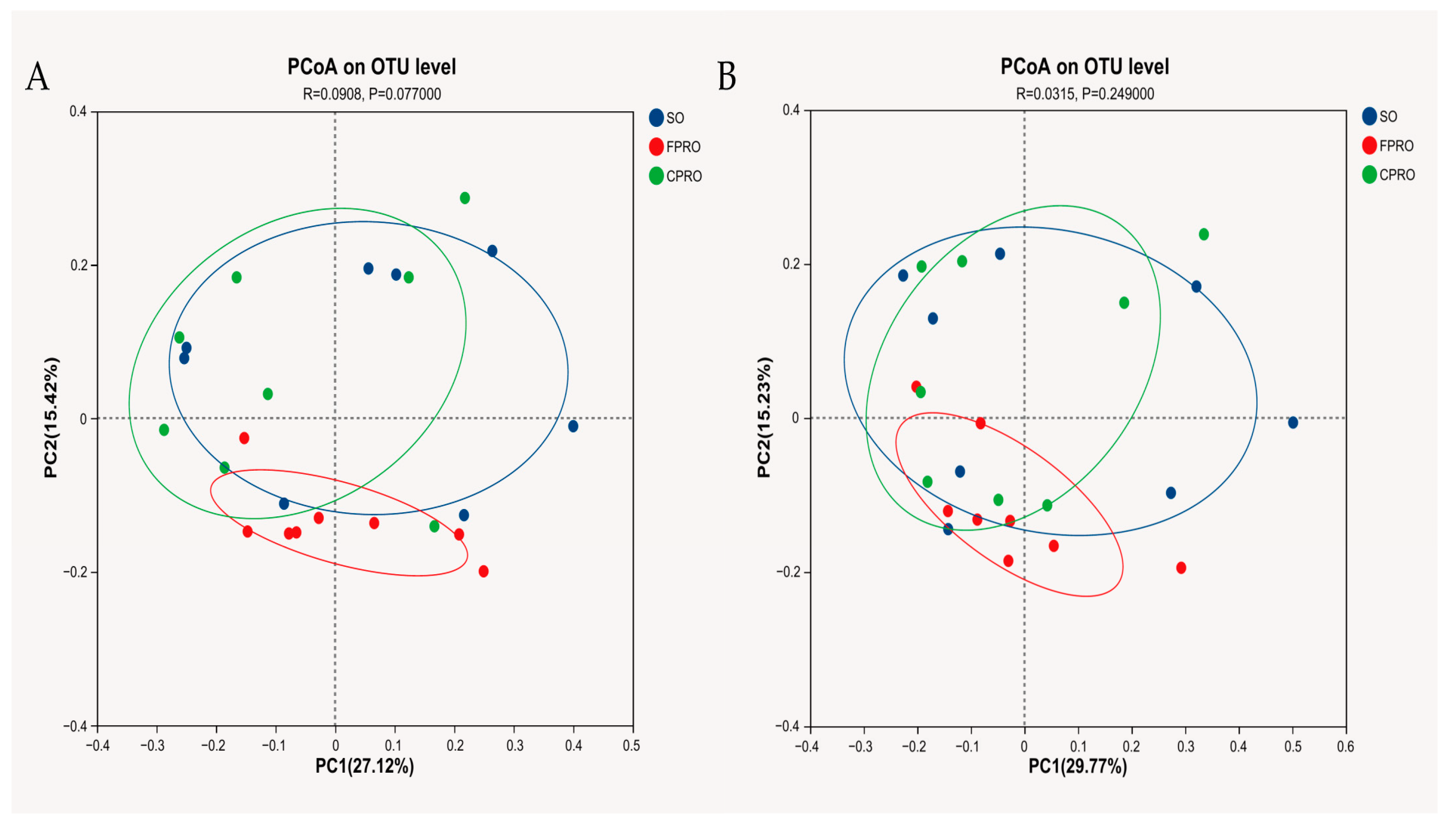
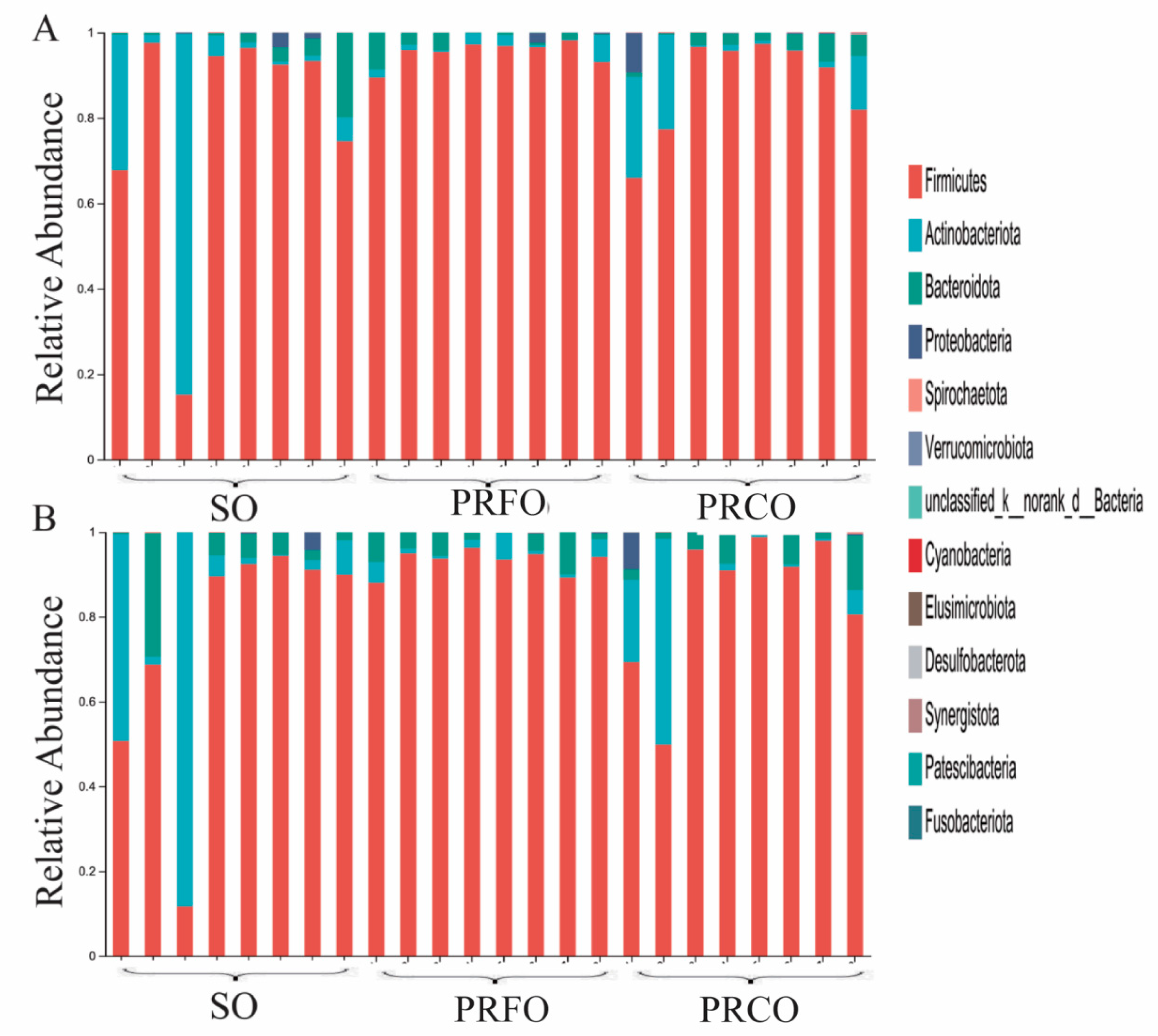
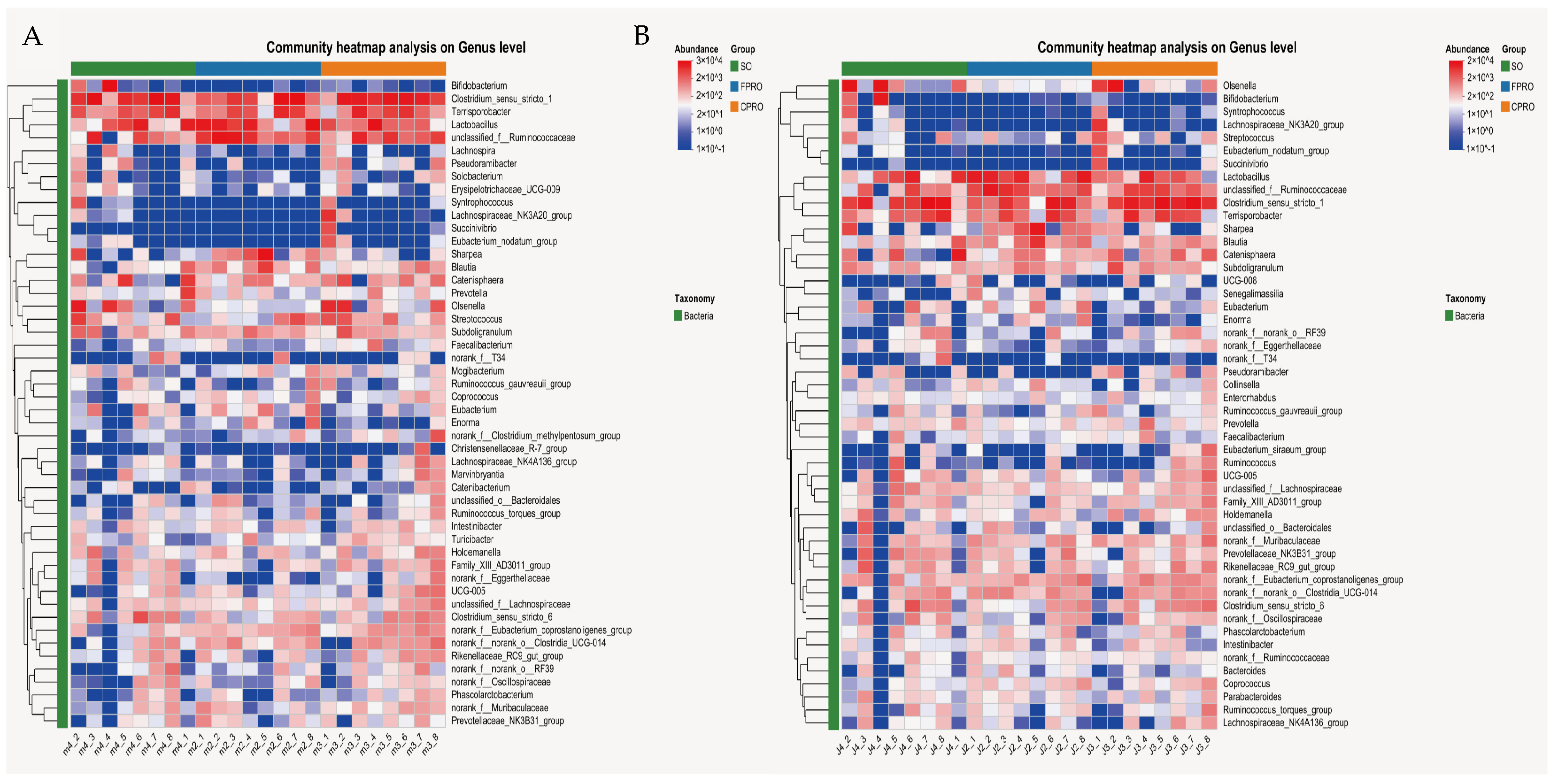
3.3. Effects of Different Dietary Lipid Sources on Bacterial Community Structure of Weaned Piglets
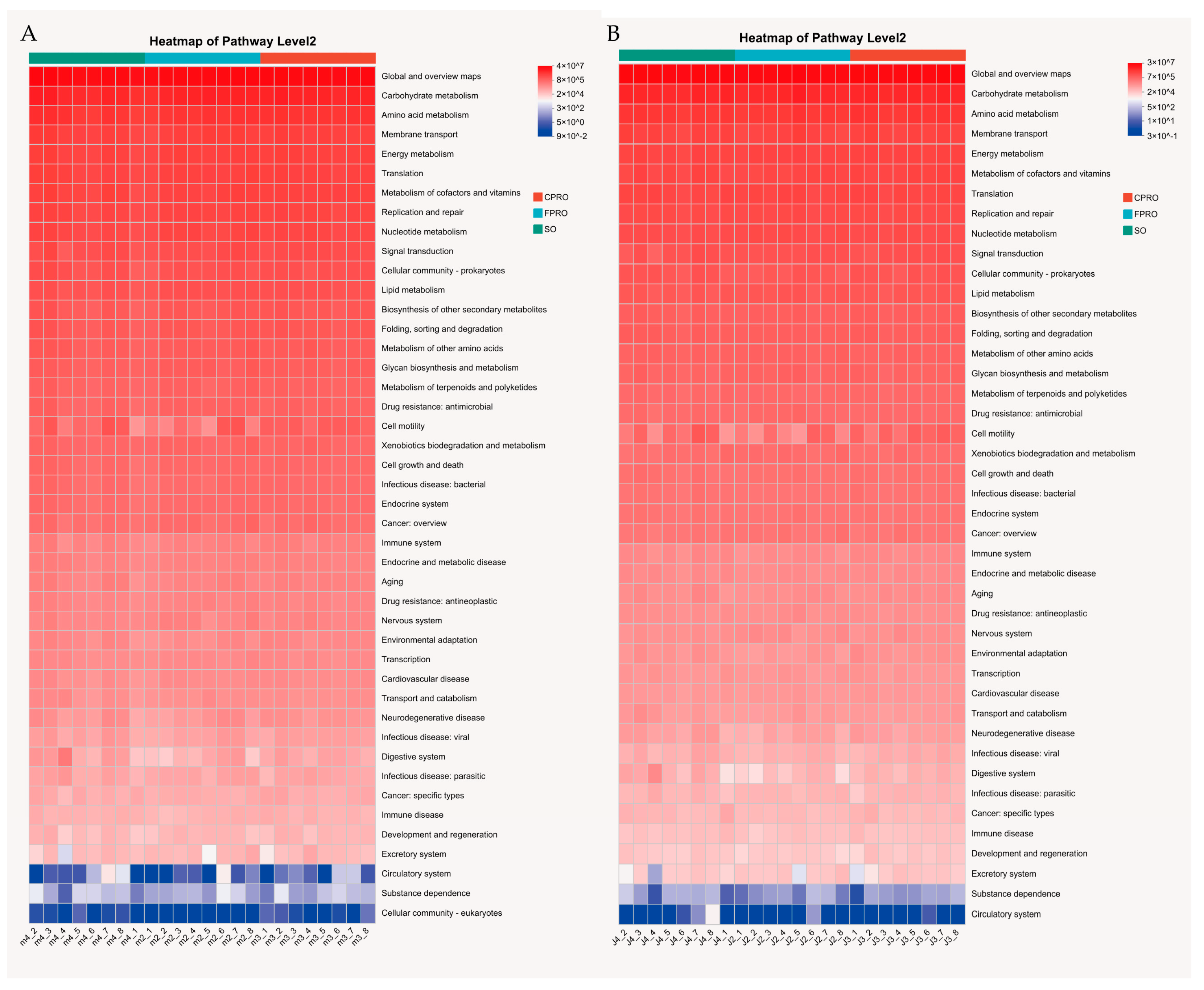
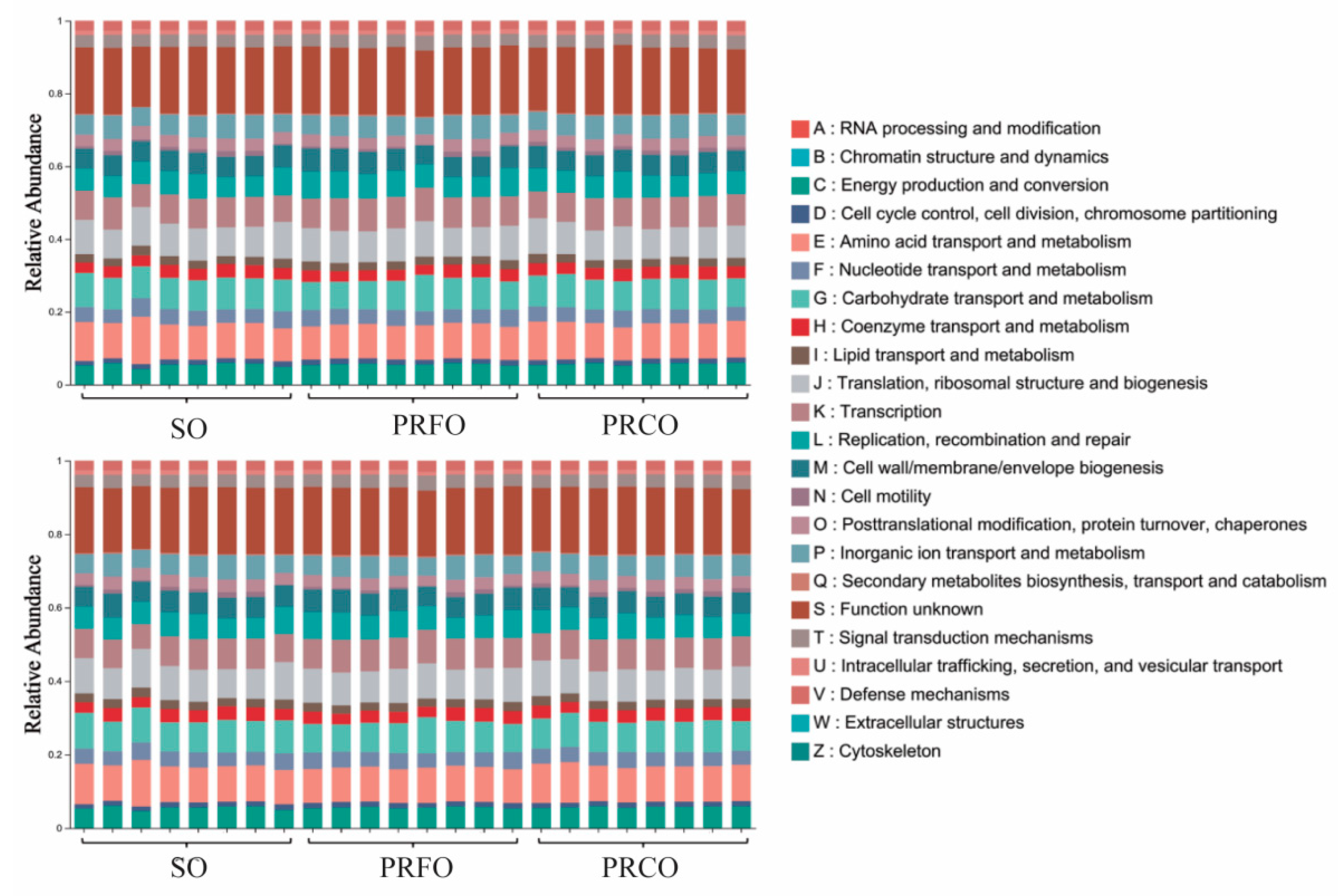
3.4. Functional Profiling of the Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Lallès, J.P.; Sève, B.; Pié, S.; Blazy, F.; Laffitte, J.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE 2015, 10, e0116523. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Neunlist, M.; van Landeghem, L.; Mahé, M.M.; Derkinderen, P.; Des Varannes, S.B.; Rolli-Derkinderen, M. The digestive neuronal–glial–epithelial unit: A new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Li, H.; Xu, C.; Cui, N.; Zhao, X. 16S rRNA genes Illumina sequencing revealed differential cecal microbiome in specific pathogen free chickens infected with different subgroup of avian leukosis viruses. Vet. Microbiol. 2017, 207, 195–204. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre-and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Ryan, C.A.; Hussey, S.; Murphy, B.; Fitzgerald, G.F.; Stanton, C. Role of gut microbiota in early infant development. Clin. Med. Pediatr. 2009, 3, S2008. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post--weaning diarrhoea without using in--feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature. 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Pettigrew, J.E. Fat in swine nutrition. Swine Nutr. 1991, 133–145. [Google Scholar]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary intervention for overweight and obese adults: Comparison of low-carbohydrate and low-fat diets. A meta-analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chartrand, M.S.; Dadar, M.; Bjørklund, G. Is there a relationship between intestinal microbiota, dietary compounds, and obesity? Trends Food Sci. Technol. 2017, 70, 105–113. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Li, M.; Zhao, L.; Ji, C.; Ma, Q. Alterations and structural resilience of the gut microbiota under dietary fat perturbations. J. Nutr. Biochem. 2018, 61, 91–100. [Google Scholar] [CrossRef]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- Messens, W.; Goris, J.; Dierick, N.; Herman, L.; Heyndrickx, M. Inhibition of Salmonella typhimurium by medium-chain fatty acids in an in vitro simulation of the porcine cecum. Vet. Microbiol. 2010, 141, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Boyen, F.; Haesebrouck, F.; Vanparys, A.; Volf, J.; Mahu, M.; van Immerseel, F.; Rychlik, I.; Dewulf, J.; Ducatelle, R.; Pasmans, F. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 2008, 132, 319–327. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Tian, M.; Chen, J.; Chen, F.; Guan, W. Different sources of high fat diet induces marked changes in gut microbiota of nursery pigs. Front. Microbiol. 2020, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Zou Fang, Z.F.; Yu Bing, Y.B.; He Jun, H.J.; Chen DaiWen, C.D. Effects of Dietary Fat Sources on Growth and Postprandial Lipid and Glucose Metabolism in Rats. Chin. J. Anim. Nutr. 2009, 21, 769–776. [Google Scholar]
- Liu ZhongChen, L.Z.; Chen DaiWen, C.D.; Yu Bing, Y.B.; Lv Mei, L.M.; Mao XiangBing, M.X.; Han GuoQuan, H.G.; Chen Hong, C.H.; Mao Qian, M.Q. Effect of Different Fat Sources on Growth Performance and Lipid Metabolism in Weaned Piglets. Chin. J. Anim. Nutr. 2011, 23, 1466–1474. [Google Scholar]
- Yang, W.; Jiang, F.; Yu, B.; Huang, Z.; Luo, Y.; Wu, A.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Effect of different dietary lipid sources on growth performance, nutrient digestibility, and intestinal health in weaned pigs. Animals 2023, 13, 3006. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Aarnink, A.J.; Verstegen, M.W.; Gerrits, W.J.; Heetkamp, M.J.; Kemp, B.; Canh, T.T. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 2005, 83, 1385–1396. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, H.; Wang, L.; Hu, J.; Wang, L.; Zhang, S. Effect of weaning at 21 days of age on the content of bile acids in chyme of cecum. Animals 2022, 12, 2138. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.A.; Mathew, A.G.; Vickers, J.R.; Clift, R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J. Anim. Sci. 2002, 80, 2904–2910. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Mahaffee, W.F.; Kloepper, J.W. Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb. Ecol. 1997, 34, 210–223. [Google Scholar] [CrossRef]
- Pitta, D.W.; Parmar, N.; Patel, A.K.; Indugu, N.; Kumar, S.; Prajapathi, K.B.; Patel, A.B.; Reddy, B.; Joshi, C. Bacterial diversity dynamics associated with different diets and different primer pairs in the rumen of Kankrej cattle. PLoS ONE 2014, 9, e111710. [Google Scholar] [CrossRef] [PubMed]
- Oberauner, L.; Zachow, C.; Lackner, S.; Högenauer, C.; Smolle, K.-H.; Berg, G. The ignored diversity: Complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci. Rep. 2013, 3, 1413. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, T.; Kang, J.; Li, Y.; Li, Y.; Xi, L. Effects of weaning modes on the intestinal ph, activity of digestive enzymes, and intestinal morphology of piglets. Animals 2022, 12, 2200. [Google Scholar] [CrossRef]
- Wan, F.; Wen, X.; Zhao, H.; Tang, S.; Wang, M.; Yi, B.; Cheng, L.; Lu, Y.; Zhong, R.; Zhang, H. Chemically protected sodium butyrate supplementation improves anti-inflammatory and antioxidant capacities potentially through modulating gut microbiota and short-chain fatty acids levels in piglets. J. Funct. Foods 2024, 121, 106434. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef]
- Evans, S.; Martiny, J.B.H.; Allison, S.D. Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 2017, 11, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Havenaar, R. Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gong, J.; Wang, Q.; Cui, S.; Yu, H.; Huang, B. In-vitro assessment of the effects of dietary fibers on microbial fermentation and communities from large intestinal digesta of pigs. Food Hydrocoll. 2011, 25, 180–188. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Kim, S.H.; Choi, Y.J.; Soriano, A.P.; Cho, K.K.; Lee, K.; Bae, G.S.; Lee, S.S. Increased propionate concentration in Lactobacillus mucosae–fermented wet brewers grains and during in vitro rumen fermentation. J. Appl. Microbiol. 2017, 123, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Han, Y.; Wang, K.; Guo, S.; Wu, D.; Huang, X.; Li, Z.; Zhu, L. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017, 16, 62. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, M.K. Fatty acid composition of rice bran oil and growth-promoting effect of rice bran extract and rice bran oil on Bifidobacterium and Lactobacillus. Appli Biol Chem. 2002, 45, 121–124. [Google Scholar]
- Rolinec, M.; Medo, J.; Gábor, M.; Miluchová, M.; Bíro, D.; Šimko, M.; Juráček, M.; Hanušovský, O.; Schubertová, Z.; Gálik, B. The effect of coconut oil addition to feed of pigs on rectal microbial diversity and bacterial abundance. Animals 2020, 10, 1764. [Google Scholar] [CrossRef]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Bao, W.; Yu, J.; He, Y.; Liu, M.; Yang, X. The diversity analysis and gene function prediction of intestinal bacteria in three equine species. Front. Microbiol. 2022, 13, 973828. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Ma, M.; Ma, S.; Zeng, D.; Huang, X.; Zeng, Y.; Zhu, G.; Chen, L. Temperature-dependent microbial mechanism and accumulation of volatile fatty acids in primary sludge pretreated with peroxymonosulfate. Bioresour. Technol. 2024, 408, 131201. [Google Scholar] [CrossRef] [PubMed]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]


| ITEM | Treatments | SEM | p-Value 4 | ||
|---|---|---|---|---|---|
| SO | PRFO | PRCO | |||
| ADG, g/d | 362.96 | 378.27 | 380.19 | 9.47 | 0.736 |
| ADFI, g/d | 611.43 | 604.52 | 633.15 | 11.92 | 0.736 |
| FCR | 1.69 | 1.60 | 1.66 | 0.02 | 0.436 |
| Cecum(mmol/L) | |||||
| AA | 1500.94 | 1926.48 | 1815.39 | 86.04 | 0.108 |
| PA | 626.32 b | 895.49 a | 970.01 a | 54.66 | 0.023 |
| IBA | 35.75 | 28.27 | 39.18 | 4.78 | 0.671 |
| BA | 507.17 b | 697.27 ab | 877.14 a | 58.08 | 0.028 |
| IVA | 51.41 | 60.46 | 74.85 | 6.48 | 0.347 |
| VA | 40.31 b | 82.19 ab | 153.16 a | 17.21 | 0.021 |
| pH value | 6.35 a | 6.27 b | 6.28 ab | 0.015 | 0.042 |
| Colon(mmol/L) | |||||
| AA | 1452.85 | 1247.52 | 1168.24 | 63.73 | 0.153 |
| PA | 800.75 | 902.13 | 842.98 | 83.32 | 0.866 |
| IBA | 50.84 | 45.66 | 40.89 | 4.39 | 0.561 |
| BA | 650.45 | 652.58 | 586.34 | 38.77 | 0.775 |
| IVA | 93.94 | 99.38 | 85.88 | 7.69 | 0.775 |
| VA | 42.23 b | 58.59 b | 141.99 a | 16.10 | 0.012 |
| pH value | 6.51 | 6.50 | 6.51 | 0.007 | 0.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Yang, W.; Jiang, F.; Yu, B.; Huang, Z.; Luo, Y.; Wu, A.; Zheng, P.; Mao, X.; Yu, J.; et al. Effects of Fish Palm Rice and Coconut Palm Rice Oil Mixture on Intestinal Health of Weaned Piglets. Agriculture 2025, 15, 384. https://doi.org/10.3390/agriculture15040384
Yang K, Yang W, Jiang F, Yu B, Huang Z, Luo Y, Wu A, Zheng P, Mao X, Yu J, et al. Effects of Fish Palm Rice and Coconut Palm Rice Oil Mixture on Intestinal Health of Weaned Piglets. Agriculture. 2025; 15(4):384. https://doi.org/10.3390/agriculture15040384
Chicago/Turabian StyleYang, Kaiyun, Wenjuan Yang, Fei Jiang, Bing Yu, Zhiqing Huang, Yuheng Luo, Aimin Wu, Ping Zheng, Xiangbing Mao, Jie Yu, and et al. 2025. "Effects of Fish Palm Rice and Coconut Palm Rice Oil Mixture on Intestinal Health of Weaned Piglets" Agriculture 15, no. 4: 384. https://doi.org/10.3390/agriculture15040384
APA StyleYang, K., Yang, W., Jiang, F., Yu, B., Huang, Z., Luo, Y., Wu, A., Zheng, P., Mao, X., Yu, J., Luo, J., Yan, H., & He, J. (2025). Effects of Fish Palm Rice and Coconut Palm Rice Oil Mixture on Intestinal Health of Weaned Piglets. Agriculture, 15(4), 384. https://doi.org/10.3390/agriculture15040384







