Techniques for In Vitro Fertilisation of Vitrified Cattle Oocytes: Challenges and New Developments
Abstract
1. Introduction
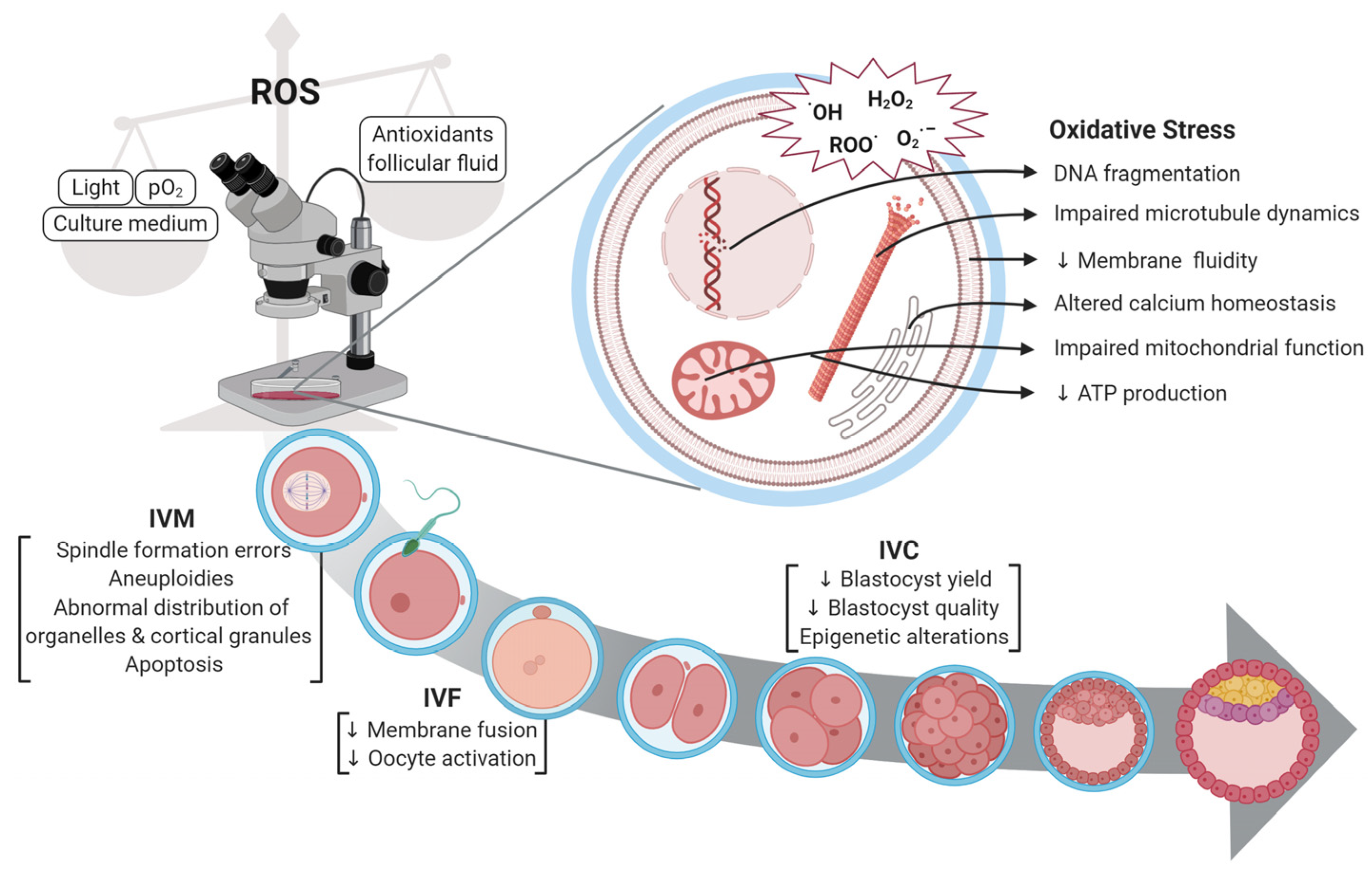
2. Problems Currently Associated with Fertilisation of Vitrified Cattle Oocytes
2.1. Survival Rate of Immature and Matured Oocytes Following Vitrification
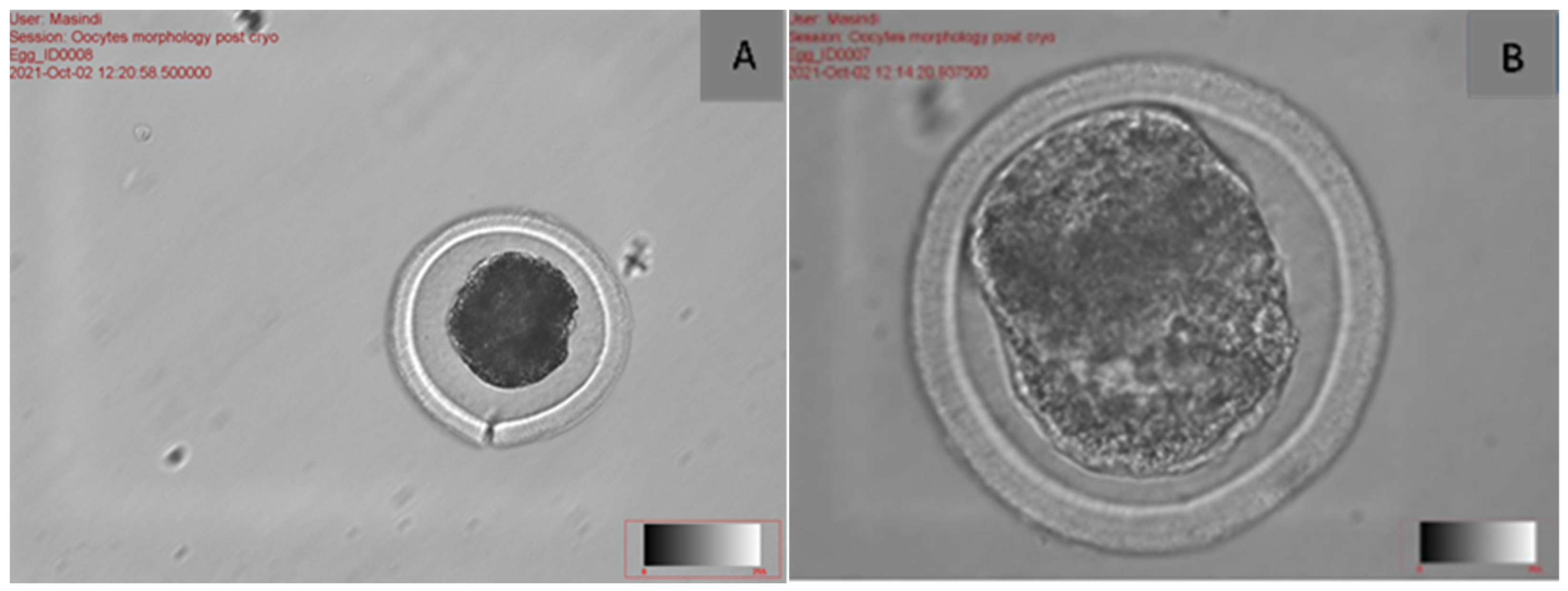
2.2. The Effect of Morphological Structure of Vitrified Oocytes During Fertilisation
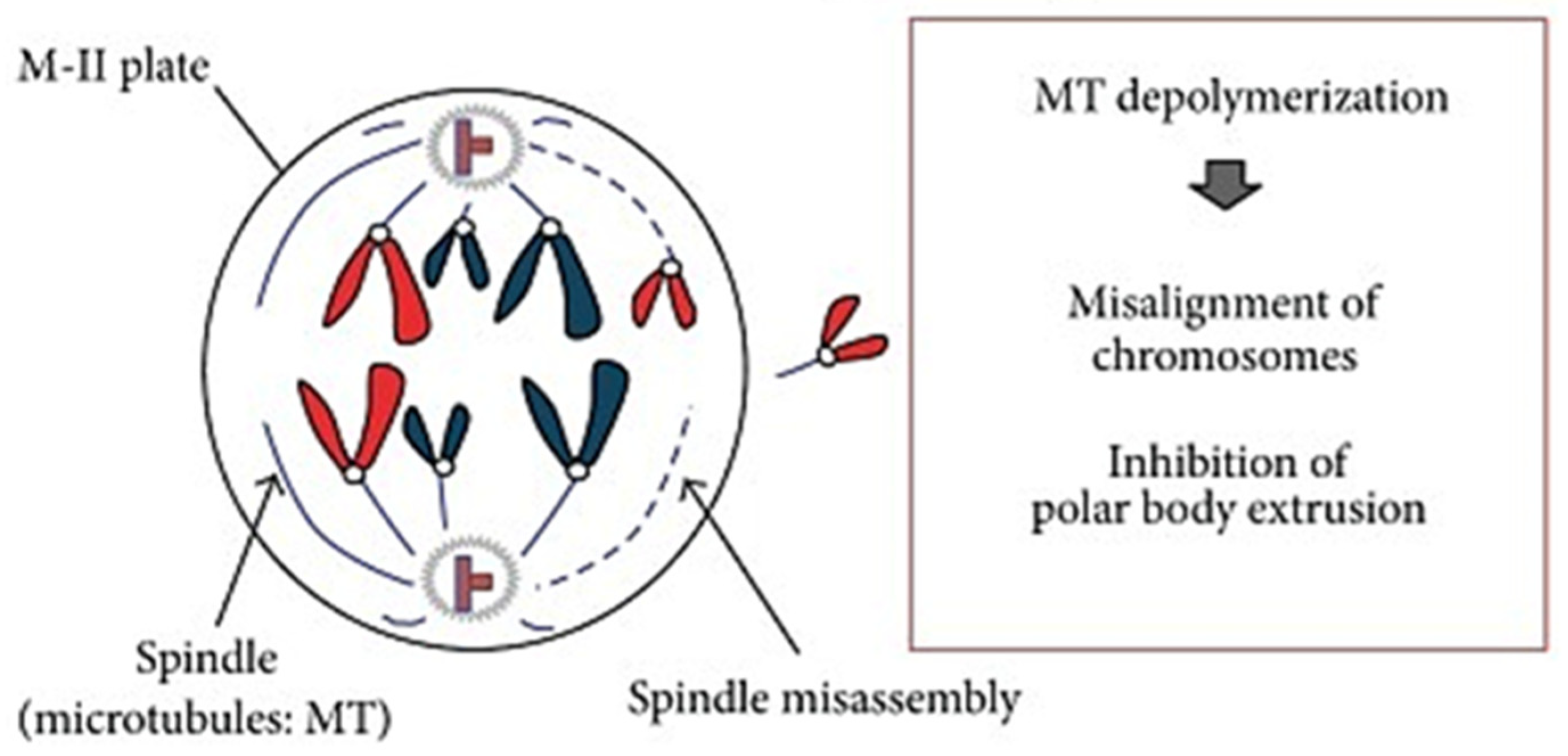
The Effect of Meiotic Spindle on Vitrified Cattle Oocytes
2.3. The Effect of Cumulus Cells During Cryopreservation and Fertilisation
3. How to Improve Fertilisation on Vitrified Cattle Oocytes?
3.1. The Use of Traditional In Vitro Fertilisation on Vitrified Cattle Oocytes
3.2. The Use of Intracytoplasmic Sperm Injection on Vitrified Cattle Oocytes
3.3. The Use of Physiological Intracytoplasmic Sperm Injection on Vitrified Cattle Oocytes
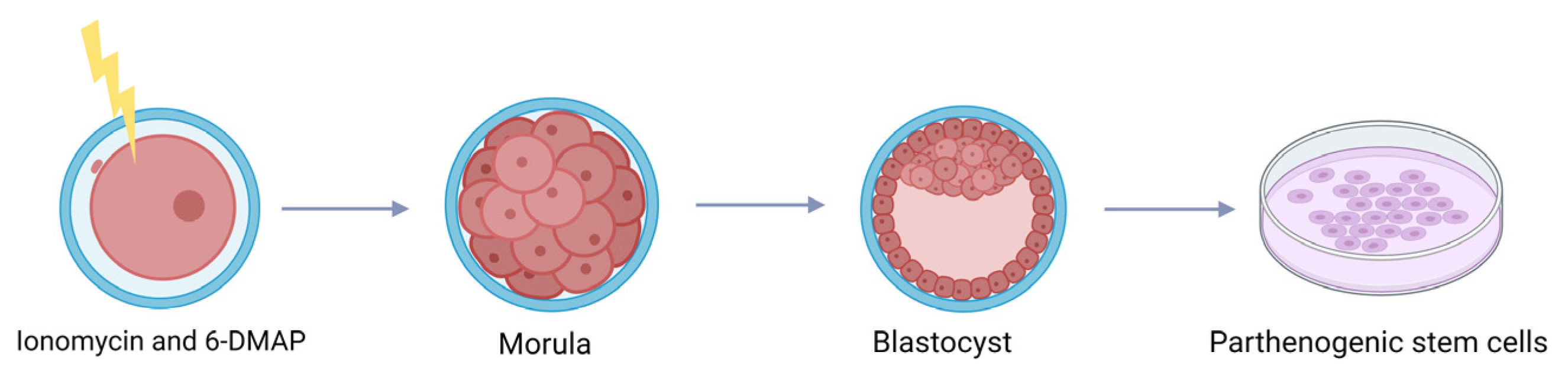
3.4. The Use of Parthenogenetic Activation on Vitrified Cattle Oocytes
4. How to Avoid Cryo Injuries During Vitrification of Cattle Oocytes?
4.1. The Use of Cryoprotectants in Oocytes Cryopreservation
4.2. The Use of Proteins and Antioxidants in Oocyte Cryopreservation
4.3. Cooling and Warming Rates
5. How Can the Cryopreservation Method Be Improved to Enhance the Fertilisation Efficiency?
6. Future Considerations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tharasanit, T.; Thuwanut, P. Oocyte cryopreservation in domestic animals and humans: Principles, techniques and updated outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, A.V.; Olexiková, L.; Főldešiová, M.; Pivko, J.; Kubovičová, E. Ooplasm cryopreservation: Ovarian fragments versus oocytes alone. Slovak J. Anim. Sci. 2018, 51, 156–160. [Google Scholar]
- Díez, C.; Munoz, M.; Caamano, J.N.; Gomez, E. Cryopreservation of the bovine oocyte: Current status and perspectives. Reprod. Domest. Anim. 2012, 47, 76–83. [Google Scholar] [CrossRef]
- Hwang, I.S.; Hochi, S. Recent progress in cryopreservation of bovine oocytes. BioMed Res. Int. 2014, 2014, 570647. [Google Scholar] [CrossRef]
- Dujíčková, L.; Makarevich, A.V.; Olexiková, L.; Kubovičová, E.; Strejček, F. Methodological approaches for vitrification of bovine oocytes. Zygote 2021, 29, 1–11. [Google Scholar] [CrossRef]
- Houda, A.; Jankowski, P.M.; Romeo, M.; Eid, H.M. Female Fertility Preservation: Different Interventions and Procedures. In InCryopreservation-Applications and Challenges; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Gandhi, G.; Ramesh, S.; Khatoon, A. Vitrification of oocytes: General considerations. In Vitrification in Assisted Reproduction: A User’s Manual; Springer: New Delhi, India, 2015; pp. 17–30. [Google Scholar] [CrossRef]
- Menéndez-Blanco, I.; Soto-Heras, S.; Catalá, M.G.; Piras, A.R.; Izquierdo, D.; Paramio, M.T. Effect of vitrification of in vitro matured prepubertal goat oocytes on embryo development after parthenogenic activation and intracytoplasmic sperm injection. Cryobiology 2020, 93, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wiesak, T.; Wasielak, M.; Złotkowska, A.; Milewski, R. Effect of vitrification on the zona pellucida hardening and follistatin and cathepsin B genes expression and developmental competence of in vitro matured bovine oocytes. Cryobiology 2017, 76, 18–23. [Google Scholar] [CrossRef]
- Jung, J.; Shin, H.; Bang, S.; Mok, H.J.; Suh, C.S.; Kim, K.P.; Lim, H.J. Analysis of the phospholipid profile of metaphase II mouse oocytes undergoing vitrification. PLoS ONE 2014, 9, e102620. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, T.; Mogas, T.; Mullen, S.F.; Martínez-Rodero, I.; Gulieva, R.E.; Higgins, A.Z. Effect of cryoprotectant concentration on bovine oocyte permeability and comparison of two membrane permeability modelling approaches. Sci. Rep. 2021, 11, 15387. [Google Scholar] [CrossRef] [PubMed]
- Mandawala, A.A.; Harvey, S.C.; Roy, T.K.; Fowler, K.E. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016, 86, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.C.; Wang, Y.; Li, Y.R. Oocyte vitrification: Advances, progress and future goals. J. Assist. Reprod. Genet. 2014, 31, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Qazi, I.H.; Guo, S.; Yang, J.; Qin, J.; Lv, T.; Zang, S.; Zhang, Y.; Zeng, C.; Meng, Q.; et al. Melatonin improves the first cleavage of parthenogenetic embryos from vitrified–warmed mouse oocytes potentially by promoting cell cycle progression. J. Anim. Sci. Biotechnol. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Guo, S.; Yang, J.; Qin, J.; Qazi, I.H.; Pan, B.; Zang, S.; Lv, T.; Deng, S.; Fang, Y.; Zhou, G. Melatonin promotes in vitro maturation of vitrified-warmed mouse germinal vesicle oocytes, potentially by reducing oxidative stress through the Nrf2 pathway. Animals 2021, 11, 2324. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Haraguchi, S.; Dang-Nguyen, T.Q.; Kaneko, H.; Kikuchi, K. Vitrification of porcine immature oocytes and zygotes results in different levels of DNA damage which reflects developmental competence to the blastocyst stage. PLoS ONE 2023, 18, e0282959. [Google Scholar] [CrossRef] [PubMed]
- Berteli, T.S.; Vireque, A.A.; Da Luz, C.M.; Borges, E.D.; Ferreira, C.R.; Navarro, P.A. Equilibration solution composition and extended exposure to equilibration phase affect embryo development and lipid profile of mouse oocytes. Reprod. BioMedicine Online 2022, 44, 961–975. [Google Scholar] [CrossRef]
- Angel-Velez, D.; Meese, T.; Hedia, M.; Fernandez-Montoro, A.; De Coster, T.; Pascottini, O.B.; Van Nieuwerburgh, F.; Govaere, J.; Van Soom, A.; Pavani, K.; et al. Transcriptomics reveal molecular differences in equine oocytes vitrified before and after In Vitro maturation. Int. J. Mol. Sci. 2023, 24, 6915. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Aghaz, F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int. J. Fertil. Steril. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Gutierrez-Castillo, E.; Diaz, F.A.; Talbot, S.A.; Bondioli, K.R. Effect of bovine oocyte vitrification with EGTA and post-warming recovery with resveratrol on meiotic spindle, mitochondrial function, reactive oxygen species, and developmental competence. Theriogenology 2023, 196, 59–67. [Google Scholar] [CrossRef]
- Lemseffer, Y.; Terret, M.; Campillo, C.; Labrune, E. Methods for assessing oocyte quality: A review of literature. Biomedicines 2022, 10, 2184. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y. Updating the markers for oocyte quality evaluation: Intracellular temperature as a new index. Reprod. Med. Biol. 2018, 17, 434–441. [Google Scholar] [CrossRef]
- Ozturk, S. Selection of competent oocytes by morphological criteria for assisted reproductive technologies. Mol. Reprod. Dev. 2020, 87, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.R.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105. [Google Scholar] [CrossRef]
- Gutierrez-Castillo, E.J. Effect of Vitrification on Epigenetic Modifications and the Meiotic Spindle of Bovine Oocytes. Master’s Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2018. [Google Scholar]
- Mo, X.H.; Fu, X.W.; Yuan, D.S.; Wu, G.Q.; Jia, B.Y.; Cheng, K.R.; Du, M.; Zhou, Y.H.; Yue, M.X.; Hou, Y.P.; et al. Effect of meiotic status, cumulus cells and cytoskeleton stabilizer on the developmental competence of ovine oocytes following vitrification. Small Rumin. Res. 2014, 117, 151–157. [Google Scholar] [CrossRef]
- Mphaphathi, M.L. Factors Affecting Cryopreservation of Immature and Matured Cattle Oocytes. Ph.D. Thesis, Department of Animal, Wildlife and Grassland Sciences, Faculty of Natural and Agricultural Sciences, University of the Free State, Bloemfontein, South Africa, 2022. [Google Scholar]
- Purohit, G.N.; Meena, H.; Solanki, K. Effects of vitrification on immature and in vitro matured, denuded and cumulus compact goat oocytes and their subsequent fertilization. J. Reprod. Infertil. 2012, 13, 53. [Google Scholar] [PubMed]
- Chaves, D.F.; Corbin, E.; Almanana, C.; Locatelli, Y.; Souza-Fabjan, M.G.; Bhat, M.H.; Freitas, V.J.F.; Mermillod, P. Vitrification of immature and in vitro matured bovine cumulus–oocyte complexes: Effects on oocyte structure and embryo development. Livest. Sci. 2017, 199, 50–60. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.Y.; Cai, M.D.; Li, X.X.; Li, Y.H.; Lei, Y.; Yu, X.L. Effect of vitrification temperature and cryoprotectant concentrations on the mRNA transcriptome of bovine mature oocytes after vitrifying at immature stage. Theriogenology 2020, 148, 225–235. [Google Scholar] [CrossRef]
- Canesin, H.S.; Brom-de-Luna, J.G.; Choi, Y.H.; Ortiz, I.; Diaw, M.; Hinrichs, K. Blastocyst development after intracytoplasmic sperm injection of equine oocytes vitrified at the germinal-vesicle stage. Cryobiology 2017, 75, 52–59. [Google Scholar] [CrossRef]
- De Munck, N.; Vajta, G.; Rienzi, L. Oocyte cryopreservation technique. In Preventing Age Related Fertility Loss; Springer: Cham, Switzerland; Gewerbestrasse, Switzerland, 2018; pp. 87–101. [Google Scholar] [CrossRef]
- Baust, J.M.; Corwin, W.; Snyder, K.K.; Van Buskirk, R.; Baust, J.G. Cryopreservation: Evolution of molecular based strategies. In Biobanking and Cryopreservation of Stem Cells; Springer: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar] [CrossRef]
- Gebel, J.; Tuppi, M.; Sänger, N.; Schumacher, B.; Dötsch, V. DNA damaged induced cell death in oocytes. Molecules 2020, 25, 5714. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Deng, T.; Zou, P.; Wang, Y.; Quan, F.; Zhang, Y. Effects of oocyte vitrification on epigenetic status in early bovine embryos. Theriogenology 2016, 86, 868–878. [Google Scholar] [CrossRef]
- Coello, A.; Sanchez, E.; Vallejo, B.; Meseguer, M.; Remohí, J.; Cobo, A. Effect of oocyte morphology on post-warming survival and embryo development in vitrified autologous oocytes. Reprod. BioMedicine Online 2019, 38, 313–320. [Google Scholar] [CrossRef]
- Tejera, A.; Herrero, J.; de Los Santos, M.J.; Garrido, N.; Ramsing, N.; Meseguer, M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil. Steril. 2011, 96, 618–623.e2. [Google Scholar] [CrossRef]
- Minasi, M.G.; Anagnostopoulou, C.; Boitrelle, F.; Vogiatzi, P.; Sallam, H.; Saleh, R.; Colpi, G.; Agarwal, A. Oocytes evaluation and in-vitro fertilization/intra cytoplasmic sperm injection outcomes. Panminerva Medica 2023, 27, 179–187. [Google Scholar] [CrossRef]
- Hassa, H.; Aydın, Y.; Taplamacıoğlu, F. The role of perivitelline space abnormalities of oocytes in the developmental potential of embryos. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 161. [Google Scholar] [CrossRef] [PubMed]
- Gutnisky, C.; Morado, S.; Gadze, T.; Donato, A.; Alvarez, G.; Dalvit, G.; Cetica, P. Morphological, biochemical and functional studies to evaluate bovine oocyte vitrification. Theriogenology 2020, 143, 18–26. [Google Scholar] [CrossRef]
- Rusciano, G.; De Canditiis, C.; Zito, G.; Rubessa, M.; Roca, M.S.; Carotenuto, R.; Sasso, A.; Gasparrini, B. Raman-microscopy investigation of vitrification-induced structural damages in mature bovine oocytes. PLoS ONE 2017, 12, e0177677. [Google Scholar] [CrossRef]
- Arav, A.; Natan, Y. The near future of vitrification of oocytes and embryos: Looking into past experience and planning into the future. Transfus. Med. Hemotherapy 2019, 46, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Reyez, F.; Ducolomb, Y.; Romo, S.; Casas, E.; Salazar, Z.; Betancourt, M. Viability, maturation and embryo development in vitro of immature porcine and ovine oocytes vitrified in different devices. Cryobiology 2012, 64, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Casillas, F.; Teteltitla-Silvestre, M.; Ducolomb, Y.; Lemus, A.E.; Salazar, Z.; Casas, E.; Betancourt, M. Co-culture with granulosa cells improve the in vitro maturation ability of porcine immature oocytes vitrified with cryolock. Cryobiology 2014, 69, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Casillas, F.; Ducolomb, Y.; Lemus, A.E.; Cuello, C.; Betancourt, M. Porcine embryo production following in vitro fertilization and intracytoplasmic sperm injection from vitrified immature oocytes matured with a granulosa cell co-culture system. Cryobiology 2015, 71, 299–305. [Google Scholar] [CrossRef]
- Wasielak-Politowska, M.; Kordowitzki, P. Chromosome segregation in the oocyte: What goes wrong during aging. Int. J. Mol. Sci. 2022, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef]
- Girka, E.; Gatenby, L.; Gutierrez, E.J.; Bondioli, K.R. The effects of microtubule stabilizing and recovery agents on vitrified bovine oocytes. Theriogenology 2022, 182, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, J.N.; Díez, C.; Trigal, B.; Muñoz, M.; Morató, R.; Martín, D.; Carrocera, S.; Mogas, T.; Gomez, E. Assessment of Meiotic Spindle Configuration and Post-Warming Bovine Oocyte Viability Using Polarized Light Microscopy. Reprod. Domest. Anim. 2013, 48, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Swearman, H.; Koustas, G.; Knight, E.; Liperis, G.; Grupen, C.; Sjoblom, C. pH: The silent variable significantly impacting meiotic spindle assembly in mouse oocytes. Reprod Biomed Online 2018, 37, 279–290. [Google Scholar] [CrossRef]
- Serra, E.; Gadau, S.D.; Berlinguer, F.; Naitana, S.; Succu, S. Morphological features and microtubular changes in vitrified ovine oocytes. Theriogenology 2020, 148, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tilia, L.; Venetis, C.; Kilani, S.; Cooke, S.; Chapman, M. Is oocyte meiotic spindle morphology associated with embryo ploidy? A prospective cohort study. Fertil. Steril. 2016, 105, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Castillo, E.; Diaz, F.A.; Talbot, S.A.; Bondioli, K.R. Recovery of spindle morphology and mitochondrial function through extended culture after vitrification-warming of bovine oocytes. Theriogenology 2022, 189, 192–198. [Google Scholar] [CrossRef]
- Yurchuk, T.; Likszo, P.; Witek, K.; Petrushko, M.; Skarzynski, D.J. New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model. Int. J. Mol. Sci. 2024, 25, 6074. [Google Scholar] [CrossRef]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Bauer, C.; Ståhlberg, A.; Kubista, M.; Skutella, T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online 2018, 36, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.Y.; Xiang, D.C.; Zhang, B.; Quan, G.B.; Shao, Q.Y.; Hong, Q.H.; Wu, G.Q. Quality of vitrified porcine immature oocytes is improved by coculture with fresh oocytes during in vitro maturation. Mol. Reprod. Dev. 2019, 86, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Escribano, N.; Smits, K.; Piepers, S.; Van den Abbeel, E.; Woelders, H.; Van Soom, A. Role of cumulus cells during vitrification and fertilization of mature bovine oocytes: Effects on survival, fertilization, and blastocyst development. Theriogenology 2016, 86, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014, 55, 24–37. [Google Scholar] [CrossRef]
- Hamze, J.G.; Canha-Gouveia, A.; Algarra, B.; Gómez-Torres, M.J.; Olivares, M.C.; Romar, R.; Jiménez-Movilla, M. Mammalian spermatozoa and cumulus cells bind to a 3D model generated by recombinant zona pellucida protein-coated beads. Sci. Rep. 2019, 9, 17989. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Wang, J.; Luo, G.; Xi, Q.; Zhou, X.; Li, Z.; Yang, X.; Duan, J.; Jin, L.; et al. Novel homozygous mutations in PATL2 lead to female infertility with oocyte maturation arrest. J. Assist. Reprod. Genet. 2020, 37, 841–847. [Google Scholar] [CrossRef]
- Wiltshire, A.; Schaal, R.; Wang, F.; Tsou, T.; McKerrow, W.; Keefe, D. Vitrification with dimethyl sulfoxide induces transcriptomic alteration of gene and transposable element expression in immature human oocytes. Genes 2023, 14, 1232. [Google Scholar] [CrossRef]
- Sithole, S.M.; Mphaphathi, M.L.; Sebopela, M.D.; Nedambale, T.L. Comparison of in vitro Maturation Media on Cattle Oocytes after in vitro Embryo Production. Am. J. Anim. Vet. Sci. 2023, 18, 27–39. [Google Scholar] [CrossRef]
- Fuentes, F.; Munoz, E.; Contreras, M.J.; Arias, M.E.; Felmer, R. Bovine ICSI: Limiting factors, strategies to improve its efficiency and alternative approaches. Zygote 2022, 30, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Mezzalira, J.C.; Ohlweiler, L.U.; Klein, N.; dos Santos Brum, D.; Leivas, F.G.; Mezzalira, A. Intracytoplasmic Sperm Injection after Vitrification of Immature Oocytes in Follicular Fluid Increases Bovine Embryo Production. Acta Sci. Vet. 2017, 45, 1–7. [Google Scholar] [CrossRef][Green Version]
- Unnikrishnan, V.; Kastelic, J.; Thundathil, J. Intracytoplasmic sperm injection in cattle. Genes 2021, 12, 198. [Google Scholar] [CrossRef]
- Ozcan, P.; Varli, B.; Findikli, N.; Basar, M.; Oral, E. Oocyte quality evaluation and cryopreservation. In Management of Infertility; Academic Press: New York, NY, USA, 2023; pp. 211–222. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; López, A.; Juárez-Rojas, L.; Retana-Márquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health Manag. 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salamone, D.F.; Canel, N.G.; Rodríguez, M.B. Intracytoplasmic sperm injection in domestic and wild mammals. Reproduction 2017, 154, F111–F124. [Google Scholar] [CrossRef]
- Avalos-Durán, G.; Cañedo-Del Ángel, A.M.; Rivero-Murillo, J.; Zambrano-Guerrero, J.E.; Carballo-Mondragón, E.; Checa-Vizcaíno, M.Á. Physiological ICSI (PICSI) vs. conventional ICSI in couples with male factor: A systematic review. JBRA Assist. Reprod. 2018, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Baso, J.; García-Villafaña, G. Routine Sperm Selection Using the Physiological ICSI Technique. Ph.D. Thesis, Subspecialty: Autonomous University of Baja California, Mexicali, Mexico, 2012. [Google Scholar]
- Mokánszki, A.; Tóthné, E.V.; Bodnár, B.; Tándor, Z.; Molnár, Z.; Jakab, A.; Ujfalusi, A.; Oláh, É. Is sperm hyaluronic acid binding ability predictive for clinical success of intracytoplasmic sperm injection: PICSI vs. ICSI? Syst. Biol. Reprod. Med. 2014, 60, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Novoselsky Persky, M.; Hershko-Klement, A.; Solnica, A.; Bdolah, Y.; Hurwitz, A.; Ketzin, E.l.; Gilad, M.; Nefesh, I.; Esh-Broder, E. Conventional ICSI vs. physiological selection of spermatozoa for ICSI (Picsi) Sibling Oocytes. Andrology 2021, 9, 873–877. [Google Scholar] [CrossRef]
- Miller, D.; Pavitt, S.; Sharma, V.; Forbes, G.; Hooper, R.; Bhattacharya, S.; Kirkman-Brown, J.; Coomarasamy, A.; Lewis, S.; Cutting, R.; et al. Physiological, hyaluronan-selected intracytoplasmic sperm injection for infertility treatment (HABSelect): A parallel, two-group, randomised trial. Lancet 2019, 393, 416–422. [Google Scholar] [CrossRef]
- Worrilow, K.C.; Eid, S.; Woodhouse, D.; Perloe, M.; Smith, S.; Witmyer, J.; Ivani, K.; Khoury, C.; Ball, G.D.; Elliot, T.; et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): Significant improvement in clinical outcomes-multicenter, double-blinded and randomized controlled trial. Hum. Reprod. 2013, 28, 306–314. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, H.T.T.; Van Nguyen, T.; Nguyen, T.T.T.; Dang, H.N.T.; Dang, T.C.; Nguyen, Q.H.V. Physiological intracytoplasmic sperm injection does not improve the quality of embryos: A cross-sectional investigation on sibling oocytes. Clin. Exp. Reprod. Med. 2023, 50, 123. [Google Scholar] [CrossRef] [PubMed]
- Blanca Paraíso, M.D.; Ferrer, C.M.; Ramírez, J.M.; Espinel, L.Q.; Gómez, M.B. What Is PICSI or Physiological ICSI in IVF? 2024. Available online: https://www.invitra.com/en/picsi-physiological-icsi/#picsi-procedure (accessed on 17 September 2024).
- Liu, Y.; Feenan, K.; Chapple, V.; Roberts, P.; Matson, P. Intracytoplasmic sperm injection using hyaluronic acid or polyvinylpyrrolidone: A time-lapse sibling oocyte study. Hum. Fertil. 2019, 22, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rao, S. PICSI—Physiological Intracytoplasmic Sperm Injection. 2023. Available online: https://www.esco-medical.com/news/picsi-physiological-intracytoplasmic-sperm-injection (accessed on 9 December 2024).
- Valencia, C.; Pérez-García, F.; Aguila, L.; Felmer, R.; Arias, M.E. Combined Exogenous Activation of Bovine Oocytes: Effects on Maturation-Promoting Factor, Mitogen-Activated Protein Kinases, and Embryonic Competence. Int. J. Mol. Sci. 2023, 24, 15794. [Google Scholar] [CrossRef]
- Kharche, S.D.; Birade, H.S. Parthenogenesis and activation of mammalian oocytes for in vitro embryo production: A review. Adv. Biosci. Biotechnol. 2013, 2, 28406. [Google Scholar]
- Ailia, M.J.; Jin, Y.K.; Kim, H.K.; Jang, G. Development of in-vitro maturation protocol for rat oocytes; under simple culture vs co-culture with cumulus cell monolayer and its developmental potential via Parthenogenetic/artificial activation. BMC Vet. Res. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Piñeiro-Silva, C.; Gadea, J. Optimizing oocyte electroporation for genetic modification of porcine embryos: Evaluation of the parthenogenetic activation. Theriogenology 2024, 218, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.G.; Hur, C.G.; Park, M.R.; Park, J.Y.; Hwang, K.C.; Kim, J.H.; Kim, J.H.; Cho, S.K. Electrical activation enhances pre-implantation embryo development following sperm injection into in vitro matured pig oocytes. J. Vet. Med. Sci. 2012, 74, 429–434. [Google Scholar] [CrossRef]
- Taşkın, A.; Coşkun, N.; Kocabay, A. Effects of different parthenogenetic activation periods on mouse embryo development and quality. Kocatepe Vet. J. 2020, 13, 383–387. [Google Scholar] [CrossRef]
- He, W.; Chen, J.; Gao, S. Mammalian haploid stem cells: Establishment, engineering and applications. Cell. Mol. Life Sci. 2019, 76, 2349–2367. [Google Scholar] [CrossRef] [PubMed]
- Felmer, R.; Arias, M.E. Activation treatment of recipient oocytes affects the subsequent development and ploidy of bovine parthenogenetic and somatic cell nuclear transfer (SCNT) embryos. Mol. Reprod. Dev. 2015, 82, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Sánchez, R.; Felmer, R. Effect of anisomycin, a protein synthesis inhibitor, on the in vitro developmental potential, ploidy and embryo quality of bovine ICSI embryos. Zygote 2016, 24, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Pérez, F.A.; Matus, C.; Felmer, R.; Arias, M.E. Activation of bovine oocytes by protein synthesis inhibitors: New findings on the role of MPF/MAPKs. Biol. Reprod. 2021, 104, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Suvá, M.; Canel, N.G.; Salamone, D.F. Effect of single and combined treatments with MPF or MAPK inhibitors on parthenogenetic haploid activation of bovine oocytes. Reprod. Biol. 2019, 19, 386–393. [Google Scholar] [CrossRef]
- Teng, J. Improving Cryopreservation of Bovine Oocytes and Embryos. Bachelor’s Thesis, College of Agriculture and Life Sciences, Animal Science of Cornell University, Ithaca, NY, USA, 2020. [Google Scholar]
- Sprícigo, J.F.; Morais, K.; Ferreira, A.R.; Machado, G.M.; Gomes, A.C.; Rumpf, R.; Franco, M.M.; Dode, M.A. Vitrification of bovine oocytes at different meiotic stages using the Cryotop method: Assessment of morphological, molecular and functional patterns. Cryobiology 2014, 69, 256–265. [Google Scholar] [CrossRef]
- Gutnisky, C.; Morado, S.; Dalvit, G.C.; Thompson, J.G.; Cetica, P.D. Glycolytic pathway activity: Effect on IVM and oxidative metabolism of bovine oocytes. Reprod. Fertil. Dev. 2013, 25, 1026–1035. [Google Scholar] [CrossRef]
- Arav, A. Cryopreservation of oocytes and embryos. Theriogenology 2014, 81, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.C.; Santos-Silva, C.; Rodrigues, C.; Matos, J.E.; Moura, T.; Baptista, M.C.; Horta, A.E.; Bessa, R.J.; Alves, S.P.; Soveral, G.; et al. Bovine oocyte membrane permeability and cryosurvival: Effects of different cryoprotectants and calcium in the vitrification media. Cryobiology 2018, 81, 4–11. [Google Scholar] [CrossRef]
- Kojayan, G.; Whaley, D.; Alexander, M.; Rodriguez, S.; Lee, S.; Lakey, J.R. Improved cryopreservation yield of pancreatic islets using combination of lower dose permeable cryoprotective agents. Cryobiology 2019, 88, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chinen, S.; Yamanaka, T.; Hirabayashi, M.; Hochi, S. Rescue of vitrified-warmed bovine mature oocytes by short-term recovery culture with resveratrol. Cryobiology 2020, 97, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, I.; Tagiri, M.; Ogawa, H.; Tashima, K.; Takashima, S.; Hara, H.; Hirabayashi, M.; Hochi, S. High Revivability of Vitrified-Warmed Bovine Mature Oocytes After Recovery Culture with Alpha-Tocopherol. Ph.D. Thesis, Shinshu University Library, Matsumoto, Japan, 2015. [Google Scholar]
- Somfai, T.; Hirao, Y. Vitrification of immature bovine oocytes in protein-free media: The impact of the cryoprotectant treatment protocol, base medium, and ovary storage. Theriogenology 2021, 172, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.M.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal Res. 2021, 70, e12707. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, C.; Cheng, W.; Zhang, T.; Tao, R.; Ma, Y.; Si, L.; Xu, Y.; Li, J. The Effect of L-Carnitine Additive During In Vitro Maturation on the Vitrification of Pig Oocytes. Cell. Reprogr. 2020, 22, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, S.; Pan, B.; Qazi, I.H.; Qin, J.; Zang, S.; Han, H.; Meng, Q.; Zhou, G. Melatonin promotes in vitro maturation of vitrified-warmed mouse GV oocytes potentially by modulating MAD2 protein expression of SAC component through MTRs. Cryobiology 2021, 102, 82–91. [Google Scholar] [CrossRef]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative stress and oocyte cryopreservation: Recent advances in mitigation strategies involving antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione Ethyl Ester Protects In Vitro-Maturing Bovine Oocytes against Oxidative Stress Induced by Subsequent Vitrification/Warming. Int. J. Mol. Sci 2020, 21, 7547. [Google Scholar] [CrossRef] [PubMed]
- Iussig, B.; Maggiulli, R.; Fabozzi, G.; Bertelle, S.; Vaiarelli, A.; Cimadomo, D.; Ubaldi, F.M.; Rienzi, L. A brief history of oocyte cryopreservation: Arguments and facts. Acta Obstet. Et Gynecol. Scand. 2019, 98, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An overview of principles and cell-specific considerations. Cell Transplant. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; De Vos, M.; Humaidan, P. The future of cryopreservation in assisted reproductive technologies. Front. Endocrinol. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Banker, M.; Kotdawala, A.; Gupta, R. The impact of vitrification in artificial reproductive technology programmes. Eur. Med. J. 2017, 2, 82–89. [Google Scholar] [CrossRef]
- Marques, L.S.; Fossati, A.A.; Rodrigues, R.B.; Da Rosa, H.T.; Izaguirry, A.P.; Ramalho, J.B.; Moreira, J.C.; Santos, F.W.; Zhang, T.; Streit, D.P., Jr. Slow freezing versus vitrification for the cryopreservation of zebrafish (Danio rerio) ovarian tissue. Sci. Rep. 2019, 9, 15353. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Meng, L.; Liang, L.; Zhang, C. Advantages of vitrification preservation in assisted reproduction and potential influences on imprinted genes. Clin. Epigenetics 2022, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Zeqiraj, A.; Beadini, S.; Beadini, N.; Aliu, H.; Gashi, Z.; Elezaj, S.; Bexheti, S.; Shabani, A. Male infertility and sperm DNA fragmentation. Open Access Maced. J. Med. Sci. 2018, 6, 1342. [Google Scholar] [CrossRef] [PubMed]
- Zini, A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med. 2011, 57, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, M.; Conatti, M.; Gomes, A.; Bonetti, T.C.; Monteleone, P.A. Effectivity of conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) when male factor is absent: A perspective point of view. JBRA Assist. Reprod. 2022, 26, 123. [Google Scholar] [CrossRef]

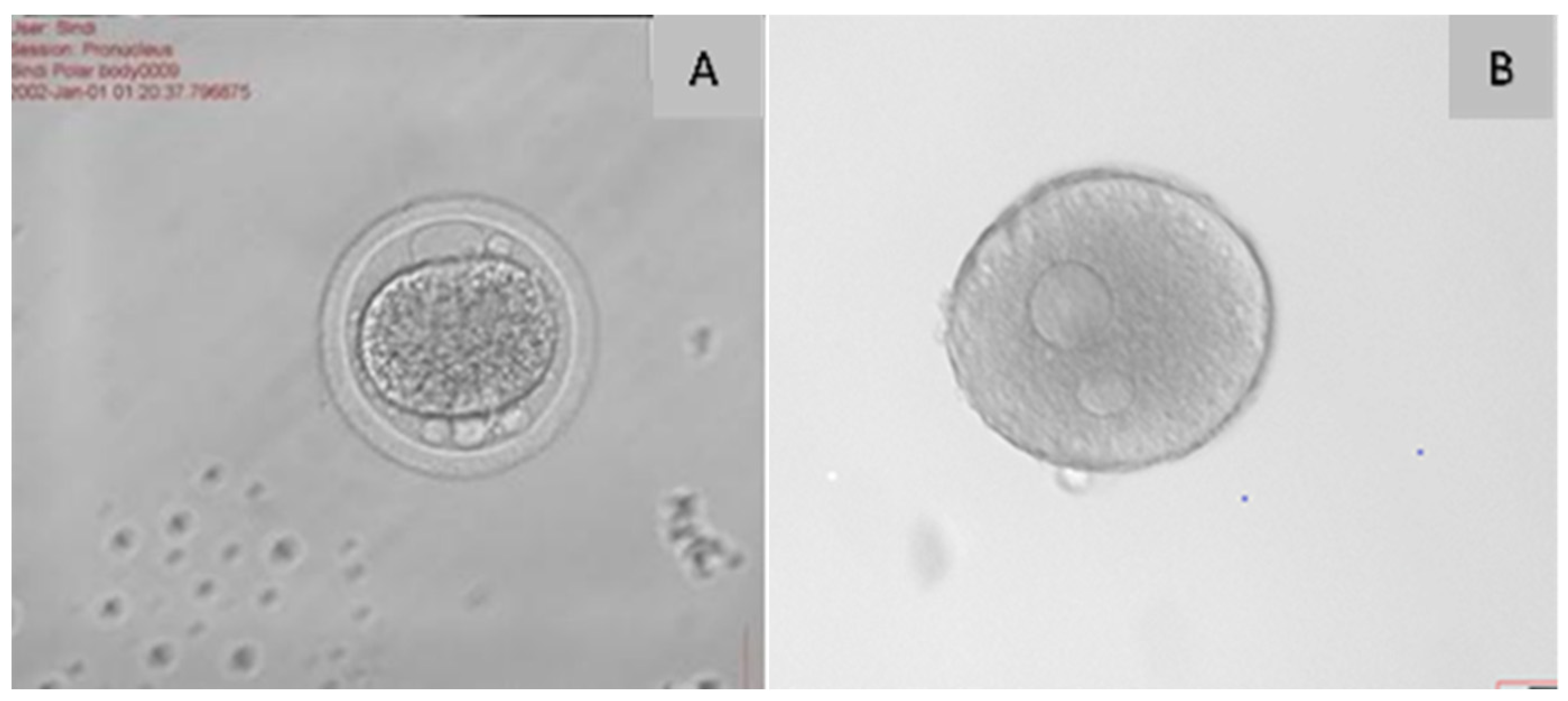

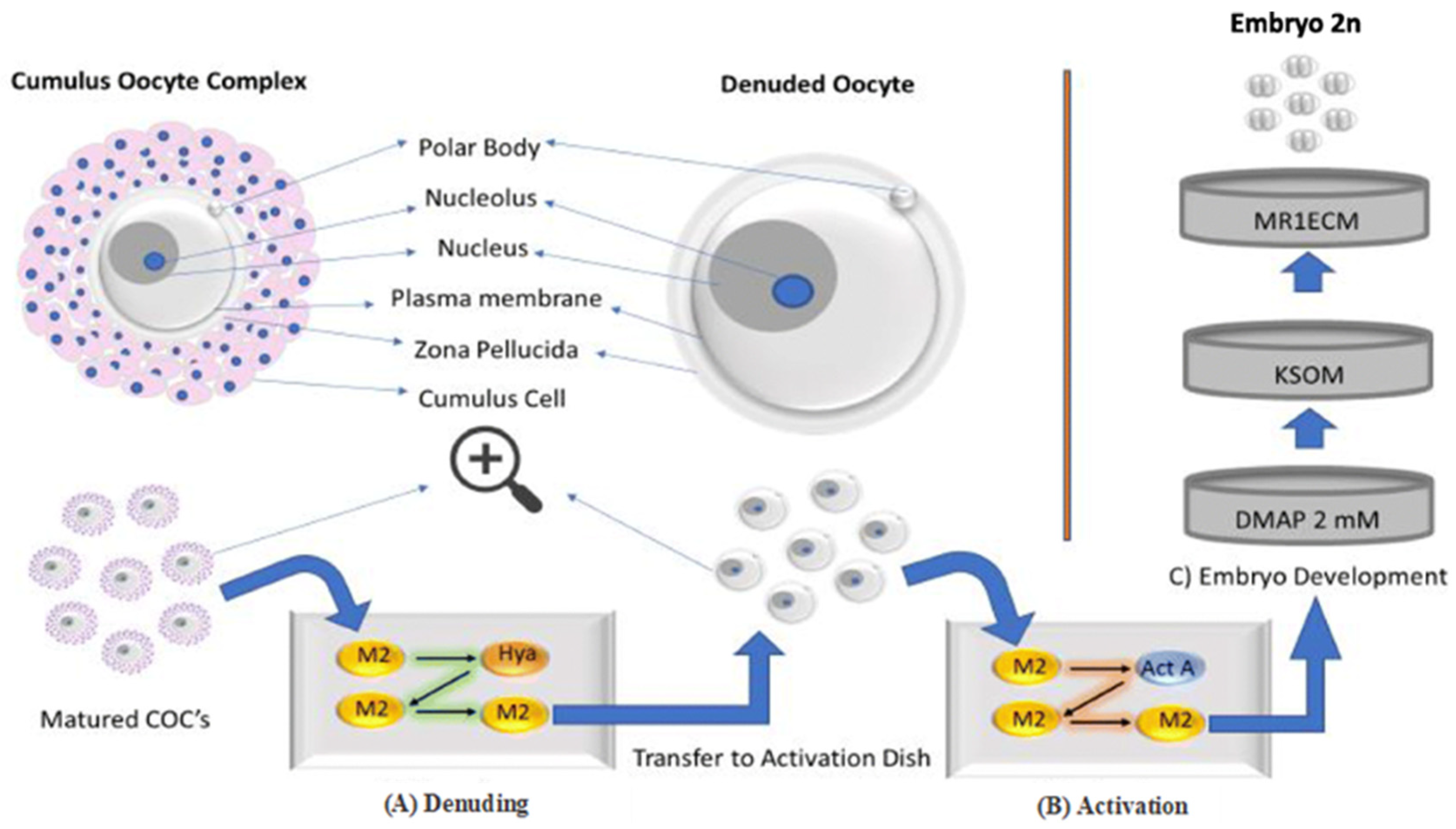
| Criterion | Function | Reference |
|---|---|---|
| Cumulus–oocyte complex | Cumulus cells are essential for oocyte quality; they sustain energy production in the cumulus–oocyte complex and may safeguard the oocyte against ROS during cryopreservation. | [25] |
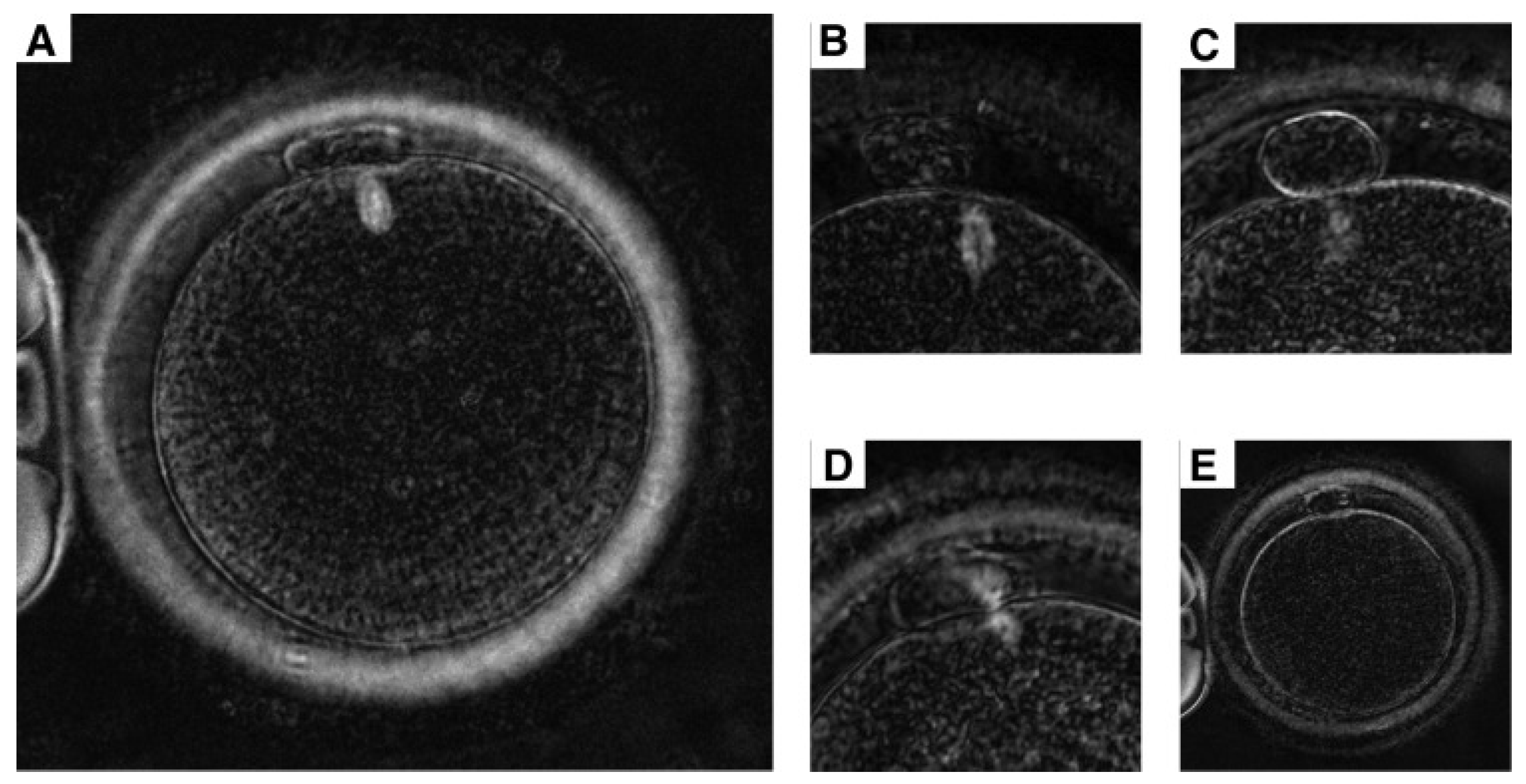
| Polar body | The polar globule is a tiny cell with 23 chromosomes that is released during the oocyte’s maturation. The intact, properly shaped, and smooth surface of the polar body increases the rate of fertilisation and the quality of the embryo. | [24,26] |
| Zona pellucida | The zona pellucida is a glycoprotein layer that covers oocytes and their surrounding cells, serving to safeguard the mammalian ovum. A robust zona pellucida or a substantial inner layer of the zona pellucida correlates with enhanced oocyte development and embryo quality. | [26,27] |
| Perivitelline space | The perivitelline space (PVS) reveals that the acellular region between the plasma membrane of the oocyte (oolema) and the zona pellucida (ZP) has a hyaluronan-rich extracellular matrix that is not discernible under optical microscopy. | [27] |
| Cytoplasm | Cytoplasmic and nuclear factors are critical determinants of oocyte quality, directly affecting the developmental competence of embryos in ART. Nuclear maturity signifies the continuation of meiosis and the advancement to metaphase II, the stage at which it is interrupted during ovulation. | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwaba, M.R.; O’Neill, H.A.; Thema, M.A.; Maqhashu, A.; Mphaphathi, M.L. Techniques for In Vitro Fertilisation of Vitrified Cattle Oocytes: Challenges and New Developments. Agriculture 2025, 15, 363. https://doi.org/10.3390/agriculture15040363
Ledwaba MR, O’Neill HA, Thema MA, Maqhashu A, Mphaphathi ML. Techniques for In Vitro Fertilisation of Vitrified Cattle Oocytes: Challenges and New Developments. Agriculture. 2025; 15(4):363. https://doi.org/10.3390/agriculture15040363
Chicago/Turabian StyleLedwaba, Mahlatsana Ramaesela, Hester Adri O’Neill, Mamonene Angelinah Thema, Ayanda Maqhashu, and Masindi Lottus Mphaphathi. 2025. "Techniques for In Vitro Fertilisation of Vitrified Cattle Oocytes: Challenges and New Developments" Agriculture 15, no. 4: 363. https://doi.org/10.3390/agriculture15040363
APA StyleLedwaba, M. R., O’Neill, H. A., Thema, M. A., Maqhashu, A., & Mphaphathi, M. L. (2025). Techniques for In Vitro Fertilisation of Vitrified Cattle Oocytes: Challenges and New Developments. Agriculture, 15(4), 363. https://doi.org/10.3390/agriculture15040363









