Early-Maturity Wheat as a Highly Valuable Feed Raw Material with Prebiotic Activity
Abstract
1. Introduction
- − Some studies indicate higher quality indicators of grain at the milk ripeness stage, while other studies provide higher quality indicators at the stage of late wax ripeness;
- − In some studies, only two stages were analyzed—early (milk) and full ripeness. This is a fairly wide range of studies, which does not fully reflect the change in grain at the early stages: the early milk ripeness phase, late milk ripeness phase, early wax ripeness stage, and middle and end of wax ripeness. A more detailed study will allow us to more accurately determine the stage at which the grain has a higher quality and to obtain the maximum benefit from its use;
- − The studies were conducted in different years and regions, and they used different scales to denote the ripeness phases. A large number of authors used the BBCH scale to analyze the changes in the grain composition during ripening [7,8,9,10,11,12,13,14,15]. The use of this scale in the present study will unify the data on changes in the grain composition during ripening and provide a clearer overall picture.
2. Materials and Methods
2.1. Sowing and Harvesting Grain Crops
- -
- The humus content (%) was determined by the method of I.V. Tyurin. The method is based on the oxidation of organic matter with chromic acid to form carbon dioxide;
- -
- The total nitrogen (mg/kg) was determined by the ionometric method. The method involves extracting nitrates from the soil with a 1% solution of potassium alum and measuring the activity of the nitrate ion using an ion-selective electrode;
- -
- To determine the mobile phosphorus and potassium (mg/kg), the Kirsanov method for determining mobile phosphorus and potassium compounds was used. The method is based on the extraction of mobile compounds of phosphorus and potassium from the soil with a solution of hydrochloric acid of a molar concentration of 0.2 mol/dm3, and the subsequent determination of phosphorus on a photoelectrocolorimeter and potassium on a flame photometer;
- -
- The pH of the salt extract. The method is based on obtaining an aqueous extract by extracting cations, nitrates, and mobile sulfur from the soil with a solution of potassium chloride and a potentiometric determination of the pH using a glass electrode.
2.2. Physicochemical Analysis of Harvested Grain Heap
- -
- The mass fraction of moisture (%) was determined by the drying method. The essence of the method lies in drying the sample portion to a constant mass at a temperature of 105 °C;
- -
- The mass fraction of protein (%) was determined by the Kjeldahl method. The protein content was calculated based on the total nitrogen content using a nitrogen-to-protein conversion factor of 6.25;
- -
- The mass fraction of phosphorus (%) was determined by the photometric method. The essence of the method lies in sample mineralization by dry or wet ashing with the formation of orthophosphoric acid salts, and the subsequent photometric determination of phosphorus in the form of a yellow-colored compound—a heteropoly acid formed in an acidic medium in the presence of vanadate and molybdate ions;
- -
- The mass fraction of proteinogenic amino acids (lysine, arginine, tyrosine, phenylalanine, histidine, leucine, isoleucine, methionine, valine, proline, threonine, serine, alanine, cystine, aspartic acid, glutamic acid, and tryptophan) (%) was determined by capillary electrophoresis. The essence of the method lies in the decomposition of the sample for analysis by acid (for all amino acids except tryptophan) or alkaline (for tryptophan) hydrolysis with the conversion of amino acids into free forms, obtaining the phenylthiocarbamyl derivatives of amino acids, their further separation, and quantitative determination by capillary electrophoresis;
- -
- The content of iron and zinc (mg/kg) was determined by the atomic absorption method. The method is based on the mineralization of the product by dry or wet ashing, and the determination of the concentration of the element in the mineralizate solution by flame atomic absorption;
- -
- The selenium content (mg/kg) was determined by the fluorimetric method. The essence of the method lies in the mineralization of the analyzed sample, the conversion of selenium from organic and inorganic forms into selenite ion, the reaction of selenite ion with the reagent 2,3-diaminonaphthalene in an acidic medium to form 4,5-benzopiazoselenol, its extraction with hexane, and measuring the fluorescence intensity of the resulting extract.
- -
- The mass fraction of starch (%) was determined by the Bertrand method. The essence of the method lies in the ability of reducing sugars to reduce divalent copper in an alkaline medium to copper (I) oxide, which is oxidized with ferric ammonium alum followed by titration of the reduced divalent iron with a solution of potassium permanganate;
- -
- The content of vitamin E (mg/kg) was determined by high-performance liquid chromatography with fluorimetric detection. The method is based on the isolation of polycyclic aromatic hydrocarbons from the analyzed sample with their subsequent quantitative determination by high-performance liquid chromatography with fluorimetric detection.
2.3. Study of Grain Heap of Wheat of Early Stages of Maturity for Prebiotic Activity
2.4. Statistical Analysis
3. Results
3.1. Results of the Study of Changes in the Composition of a Heap of Cereal Crops During the Maturation Process
3.2. Results of the Study of Prebiotic Activity of Grain Heap of Wheat of Early Stages of Maturity
3.2.1. Study of the Effect of High Concentrations of Milk Ripeness Wheat in the Chicken Microbiota Model
3.2.2. Study of Low Concentrations of Milky Ripeness Wheat in the Chicken Microbiota Model
3.2.3. Study of Average Concentrations of Milk-Ripe Wheat in the Chicken Microbiota Model
3.2.4. Study of Waxy Wheat Grain Heap in the Quail Microbiota Model
3.2.5. Study of Dry Grain Heap of Waxy Ripeness Wheat for the Number of Microorganisms of Different Groups Under the Conditions of the Quail Microbiota Model, CFU/mL
3.2.6. Effect of Waxy Wheat Grain Heap on Lactobacilli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ponomarev, S.V.; Grozescu, Y.N.; Bakhareva, A.A. Industrial Fish Farming: Textbook, 2nd ed.; revised. and additional; Publishing House “Lan”: St. Petersburg, Russia, 2022; 448p, Available online: https://e.lanbook.com/book/211118 (accessed on 27 November 2024). (In Russian)
- FAO Global Forum for Animal Feed and Feed Regulators Opening Remarks by Dr QU Dongyu, FAO Director-General 14/11/2023. Available online: https://www.fao.org/director-general/speeches/details/FAO-Global-Forum-for-Animal-Feed-and-Feed-Regulators-Opening-Remarks/en (accessed on 13 January 2025).
- Sandrykin, D.V.; Kondratenko, E.P.; Egushova, E.A.; Pinchuk, L.G. Dynamics of dry matter accumulation and changes in the chemical composition of grain during ripening. Achiev. Sci. Technol. Agro-Ind. Complex 2011, 12, 32–33. (In Russian) [Google Scholar]
- Kraska, P.; Andruszczak, S.; Dziki, D.; Stocki, M.; Stocka, N.; Kwiecińska-Poppe, E.; Różyło, K.; Gierasimiuk, P. Green grain of spelt (Triticum aestivum ssp. spelta) harvested at the stage of milk-dough as a rich source of valuable nutrients. Emir. J. Food Agric. 2019, 31, 263–270. [Google Scholar] [CrossRef]
- Berihuete-Azorín, M.; Stika, H.-P.; Hallama, M.; Valamoti, S.M. Distinguishing ripe spelt from processed green spelt (Grünkern) grains: Methodological aspects and the case of early La Tène Hochdorf (Vaihingen a.d. Enz, Germany). J. Archaeol. Sci. 2020, 118, 105143. [Google Scholar] [CrossRef]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Baumgartner, B.; Özkeser, İ.; Köksel, H. Changes in the functional constituents and phytic acid contents of firiks produced from wheats at different maturation stages. Food Chem. 2018, 246, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wollmer, A.C.; Pitann, B.; Mühling, K.H. Grain storage protein concentration and composition of winter wheat (Triticum aestivum L.) as affected by waterlogging events during stem elongation or ear emergence. J. Cereal Sci. 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Martínez-Núñez, M.; Ruiz-Rivas, M.; Vera-Hernández, P.F.; Bernal-Muñoz, R.; Luna-Suárez, S.; Rosas-Cárdenas, F.F. The phenological growth stages of different amaranth species grown in restricted spaces based in BBCH code. S. Afr. J. Bot. 2019, 124, 436–443. [Google Scholar] [CrossRef]
- Brandán, J.P.; Curti, R.N.; Acreche, M.M. Phenological growth stages in chia (Salvia hispanica L.) according to the BBCH scale. Sci. Hortic. 2019, 255, 292–297. [Google Scholar] [CrossRef]
- Hernández, D.P.M.; Aranguren, M.; Reig, C.; Galván, D.F.; Mesejo, C.; Fuentes, A.M.; Saúco, V.G.; Agustí, M. Phenological growth stages of mango (Mangifera indica L.) according to the BBCH scale. Sci. Hortic. 2011, 130, 536–540. [Google Scholar] [CrossRef]
- Miedaner, T.; Haffke, S.; Siekmann, D.; Fromme, F.J.; Roux, S.R.; Hackauf, B. Dynamic quantitative trait loci (QTL) for plant height predict biomass yield in hybrid rye (Secale cereale L.). Biomass Bioenergy 2018, 115, 10–18. [Google Scholar] [CrossRef]
- Cutignano, A.; Mamone, G.; Boscaino, F.; Ceriotti, A.; Maccaferri, M.; Picariello, G. Monitoring changes of lipid composition in durum wheat during grain development. J. Cereal Sci. 2021, 97, 103131. [Google Scholar] [CrossRef]
- Li, G.; Ren, X.; Pang, S.; Feng, C.; Niu, Y.; Qu, Y.; Liu, C.; Lin, X.; Wang, D. Nitrogen redistribution during the grain-filling stage and its correlation with senescence and TaATG8 expression in leaves of winter wheat. J. Integr. Agric. 2024; In Press, Journal Pre-proof. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Zheng, B.; Xu, Y.; He, Y. Early estimation of glutelin to gliadin ratio in wheat grain using high-dimensional and hyperspectral reflectance. Comput. Electron. Agric. 2024, 227, 109542. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, M.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van den Boom, T.; et al. The BBCH system to coding the phenological growth stages of plants-history and publications. J. Kult. 2009, 61, 41–52. [Google Scholar] [CrossRef]
- Rudoy, D.; Pakhomov, V.; Olshevskaya, A.; Maltseva, T.; Ugrekhelidze, N.; Zhuravleva, A.; Babajanyan, A. Review and analysis of perennial cereal crops at different maturity stages. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 022111. [Google Scholar] [CrossRef]
- Zhang, C.; Pi, X.; Li, X.; Huo, J.; Wang, W. Edible herbal source-derived polysaccharides as potential prebiotics: Composition, structure, gut microbiota regulation, and its related health effects. Food Chem. 2024, 458, 140267. [Google Scholar] [CrossRef]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as functional ingredients: Focus on Mediterranean fish aquaculture. Rev. Aquac. 2018, 10, 800–832. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mozanzadeh, M.T.; Gisbert, E.; Hoseinifar, S.H. Probiotics, prebiotics, and synbiotics in shrimp aquaculture: Their effects on growth performance, immune responses, and gut microbiome. Aquac. Rep. 2024, 38, 102362. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; de Carvalho, N.M.; Antunes, F.; Mota, I.F.; Pintado, M.E.; Madureira, A.R.; Costa, P.S. Lignin from sugarcane bagasse as a prebiotic additive for poultry feed. Int. J. Biol. Macromol. 2023, 239, 124262. [Google Scholar] [CrossRef]
- Serradell, A.; Torrecillas, S.; Soares, F.; Silva, T.; Montero, D. Modelling the effect of prebiotics, probiotics and other functional additives on the growth, feed intake and feed conversion of European sea bass (Dicentrarchus labrax) juveniles. Aquac. Rep. 2023, 32, 101729. [Google Scholar] [CrossRef]
- Mazanko, M.; Prazdnova, E.; Statsenko, V.; Bren, A.; Rudoy, D.; Maltseva, T.; Chistyakov, V.; Chikindas, M. Oil Cakes of Essential Oil Plants as a Source of Prebiotics for Poultry Production. Agriculture 2023, 13, 591. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Chen, L.; Qu, D.; Li, Z.; Gao, K.; Chen, J.; Sun, Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Ke, Y.; Li, C.; Zhang, Z.; Wu, Y.; Hu, B.; Liu, A.; Luo, Q.; Wu, W. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata olysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr. Polym. 2021, 251, 117041. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, X.; Jia, A.; Zhang, M.; Cui, T.; Bai, X.; Liu, C. A fucoidan from Sargassum fusiforme with novel structure and its regulatory effects on intestinal microbiota in high-fat diet-fed mice. Food Chem. 2021, 358, 129908. [Google Scholar] [CrossRef] [PubMed]
- Lachuga, Y.F.; Meskhi, B.C.; Pakhomov, V.I.; Rudoy, D.V. Harvesting Machine. Patent of the Russian Federation No 206314 U1, 6 September 2021. [Google Scholar]
- Pakhomov, V.; Rudoy, D.; Kambulov, S.; Maltseva, T. Research on Energy Intensity of Wheat Harvesting at Different Ripeness Phases with a New Stripping–Threshing Unit. AgriEngineering 2024, 6, 3159–3173. [Google Scholar] [CrossRef]
- Mazanko, M.S.; Prazdnova, E.V.; Kulikov, M.P.; Maltseva, T.A.; Rudoy, D.V.; Chikindas, M.L. Antioxidant and Antimutagenic Properties of Probiotic Lactobacilli Determined Using LUX-Biosensors. Enzym. Microb. Technol. 2022, 155, 109980. [Google Scholar] [CrossRef]
- Kolmakov, V.I.; Kolmakova, A.A. Amino acids in promising feeds for fish aquaculture: A review of experimental data. J. Sib. Fed. University. Biol. 2020, 13, 424–442. [Google Scholar] [CrossRef]
- Abrosimova, N.A.; Abrosimov, S.S.; Saenko, E.M. Feed Raw Materials and Additives for Aquaculture Objects; Publishing House “Media-Polis”: Rostov-on-Don, Russia, 2006; 147p, Available online: http://dspace.vniro.ru/handle/123456789/1645?show=full (accessed on 27 November 2024). (In Russian)
- Aleshin, M.A.; Mikhailova, L.A. Changes in the yield and biochemical composition of grain of field crops in mixed crops when using mineral fertilizers. Fertility 2020, 2, 9–13. (In Russian) [Google Scholar] [CrossRef]
- Gao, L.; Haesaert, G.; Bockstaele, F.V.; Vermeir, P.; Skirtach, A.; Eeckhout, M. Combined effects of nitrogen and sulfur fertilizers on chemical composition, structure and physicochemical properties of buckwheat starch. Food Chem. 2024, 459, 140351. [Google Scholar] [CrossRef]
- Lu, T.; Shi, J.; Lu, Z.; Wu, Z.; Wang, Y.; Luo, P.; Han, X. Appropriate application of organic fertilizer enhanced yield, microelement content, and quality of maize grain under a rotation system. Ann. Agric. Sci. 2024, 69, 19–32. [Google Scholar] [CrossRef]

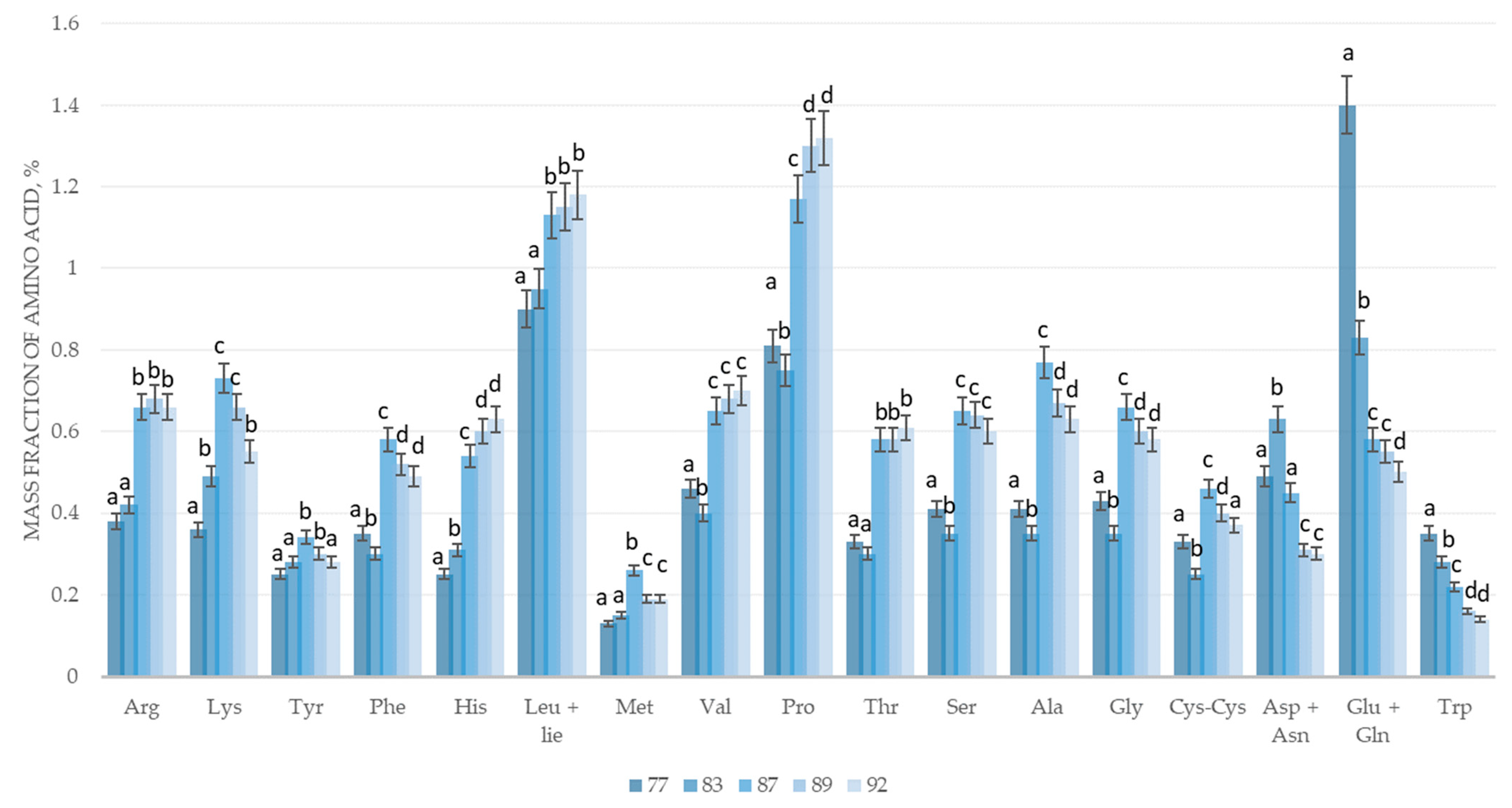
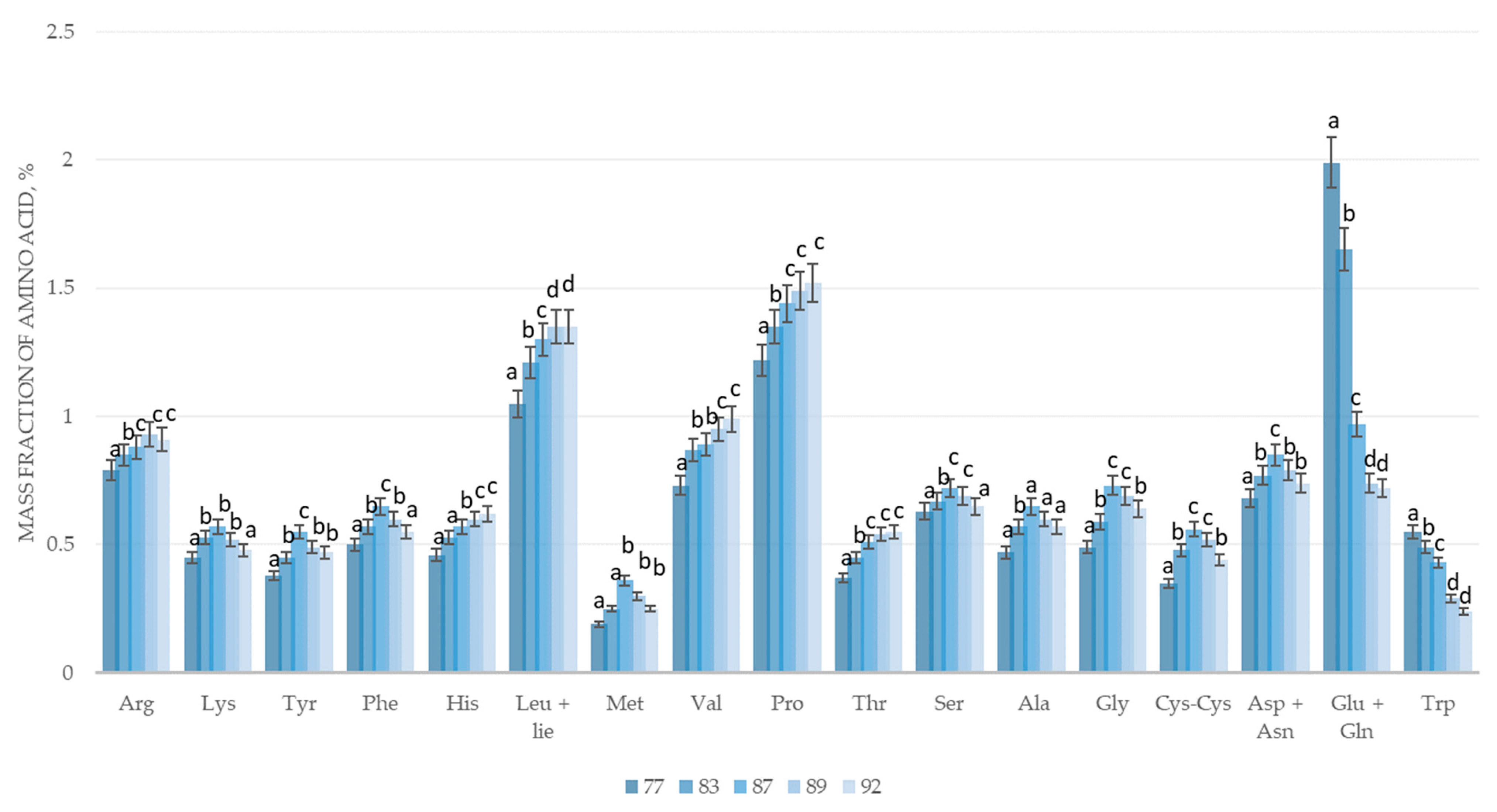
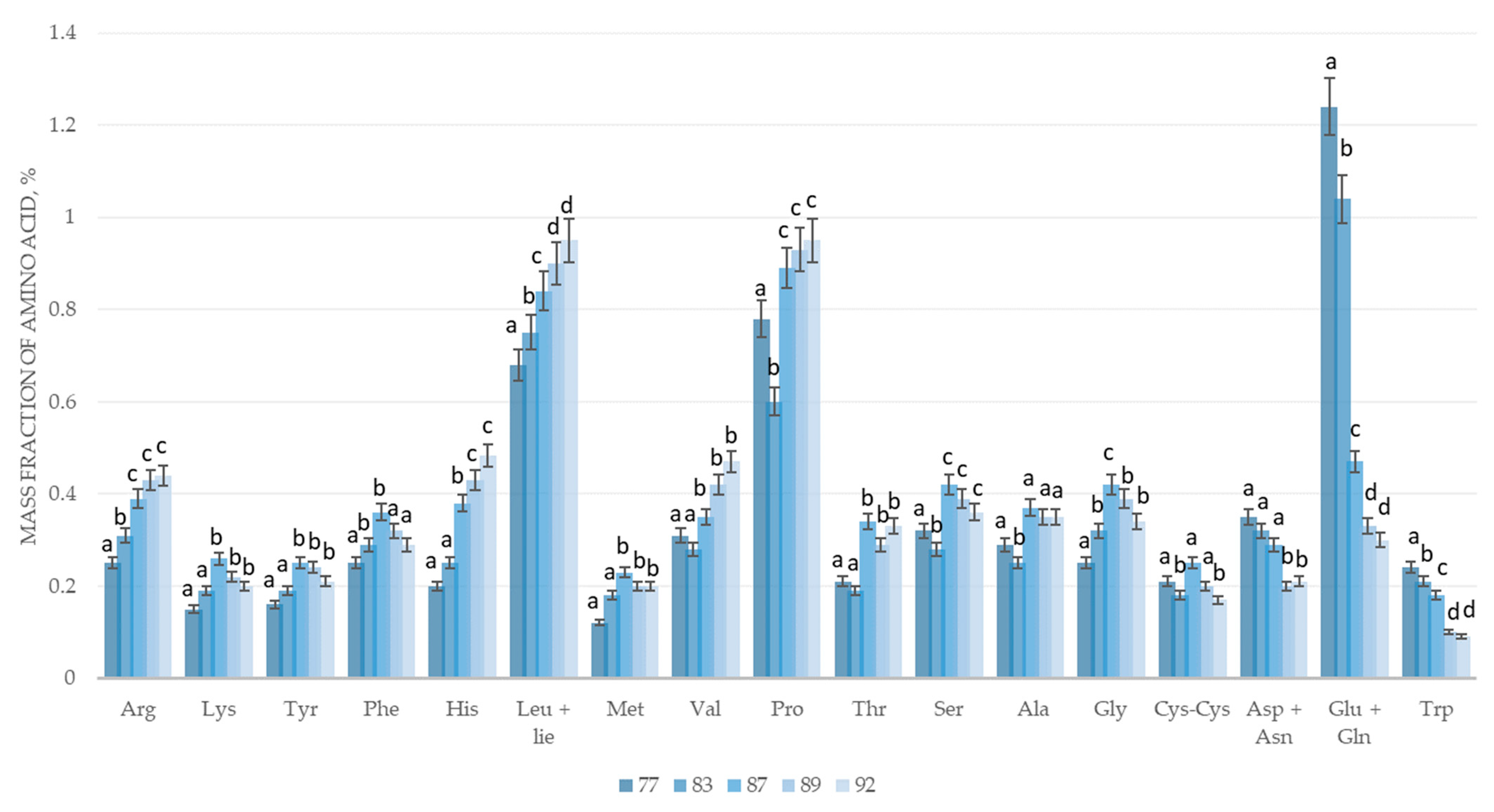
| Sample Name, % | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the “Admiral” variety | 12.65 ± 0.41 a | 12.32 ± 0.40 a | 13.49 ± 0.43 b | 13.17 ± 0.42 b | 12.87 ± 0.41 a |
| Grain heap of perennial winter wheat (Trititrigia) of the “Pamyati Lyubimovoy” variety | 12.93 ± 0.41 a | 15.01 ± 0.47 b | 16.11 ± 0.50 c | 15.76 ± 0.49 b | 15.41 ± 0.48 b |
| Grain heap of the blue wheatgrass variety “Sova” | 8.74 ± 0.30 a | 8.78 ± 0.30 a | 9.12 ± 0.31 b | 9.0 ± 0.30 b | 8.45 ± 0.29 a |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 66.58 ± 1.88 b | 46.40 ± 2.03 a | 41.20 ± 2.00 a | 15.80 ± 1.37 c | 11.90 ± 0.92 c |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 65.78 ± 1.90 b | 44.32 ± 2.02 a | 40.31 ± 1.99 a | 14.92 ± 1.34 c | 10.60 ± 0.92 c |
| Grain heap of the Sova variety of wheatgrass | 55.12 ± 2.02 b | 39.78 ± 1.98 a | 35.22 ± 1.92 a | 13.78 ± 1.29 c | 10.40 ± 0.95 c |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 43.65 ± 4.37 a | 46.25 ± 4.63 a | 51.39 ± 5.14 b | 34.30 ± 3.43 c | 33.23 ± 3.32 c |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 44.08 ± 4.41 a | 46.17 ± 4.62 a | 51.97 ± 5.20 b | 36.19 ± 3.62 c | 35.78 ± 3.58 c |
| Grain heap of the Sova variety of wheatgrass | 46.45 ± 4.65 a | 49.21 ± 4.92 a | 53.14 ± 5.31 b | 55.68 ± 5.57 b | 54.12 ± 5.41 b |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 0.12 ± 0.03 a | 0.15 ± 0.03 a | 0.17 ± 0.04 a | 0.18 ± 0.04 a | 0.37 ± 0.05 b |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 0.17 ± 0.04 a | 0.21 ± 0.04 b | 0.23 ± 0.04 b | 0.27 ± 0.05 b | 0.4 ± 0.05 c |
| Grain heap of the Sova variety of wheatgrass | 0.22 ± 0.04 a | 0.27 ± 0.05 a | 0.30 ± 0.05 b | 0.36 ± 0.05 b | 0.49 ± 0.05 c |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 0.23 ± 0.06 a | 0.34 ± 0.07 b | 0.51 ± 0.08 c | 0.36 ± 0.07 b | 0.29 ± 0.07 a |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 0.20 ± 0.04 a | 0.31 ± 0.07 b | 0.45 ± 0.08 c | 0.31 ± 0.07 b | 0.25 ± 0.07 b |
| Grain heap of the Sova variety of wheatgrass | 0.32 ± 0.07 a | 0.40 ± 0.08 b | 0.55 ± 0.08 c | 0.49 ± 0.08 b | 0.44 ± 0.08b b |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 34.16 ± 3.42 a | 31.09 ± 3.11 a | 35.61 ± 3.56 a | 26.34 ± 2.63 b | 23.07 ± 2.31 b |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 38.47 ± 3.85 a | 35.49 ± 3.55 a | 37.98 ± 3.80 a | 32.74 ± 3.27 b | 30.55 ± 3.06 b |
| Grain heap of the Sova variety of wheatgrass | 40.12 ± 4.01 a | 38.22 ± 3.82 b | 41.03 ± 4.10 b | 37.12 ± 3.71 b | 35.54 ± 3.55 b |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 59.65 ± 9.34 a | 61.22 ± 9.57 a | 63.71 ± 9.95 a | 67.83 ± 10.56 b | 67.23 ± 10.47 b |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 55.17 ± 8.67 a | 59.74 ± 9.35 a | 62.95 ± 9.83 b | 65.12 ± 10.16 b | 64.75 ± 10.10 b |
| Grain heap of the Sova variety of wheatgrass | 30.17 ± 4.92 a | 32.23 ± 5.22 a | 38.47 ± 6.16 a | 38.95 ± 6.23 b | 40.70 ± 6.50 b |
| Sample Name | Growth Phase According to BBCH Scale | ||||
|---|---|---|---|---|---|
| 77 | 83 | 87 | 89 | 92 | |
| Grain heap of one-year winter wheat of the Admiral variety | 6.63 ± 1.14 a | 3.35 ± 0.58 b | 4.71 ± 0.81 c | 30.22 ± 5.21 d | 38.98 ± 6.72 d |
| Grain heap of perennial winter wheat (Trititrigia) of the Pamyati Lyubimovoy variety | 6.78 ± 1.17 a | 3.55 ± 0.61 b | 5.02 ± 0.87 c | 35.77 ± 6.17 d | 41.44 ± 7.14 f |
| Grain heap of the Sova variety of wheatgrass | 6.25 ± 1.08 a | 2.91 ± 0.50 b | 3.76 ± 0.65 c | 25.84 ± 4.46 d | 33.72 ± 5.81 f |
| Name of Bacteria | Control | 1% | 2% | 5% |
|---|---|---|---|---|
| Lactic acid bacteria | 1.2 ± 0.2·107 | 4.7 ± 0.2·108 * | 4.4 ± 0.3·108 * | 2.0 ± 0.3·108 * |
| Bifidobacterium | 106 | 104 * | 104 * | 104 * |
| Enterococcus | 4.0 ± 0.4·106 | 9.4 ± 0.1·104 * | 1.3 ± 0.2·104 * | 1.1 ± 0.2·103 * |
| E. coli | 5.7 ± 0.5·106 | - | - | - |
| Staphylococcus | 6.1 ± 0.2·106 | 9.6 ± 0.3·104 * | 3.3 ± 0.4·104 * | 3.0 ± 0.3·103 * |
| Bacillus | 3.2 ± 0.3·106 | 6.0 ± 0.3·103 * | 7.3 ± 0.3·102 * | - |
| pH | 7.1 | 6.2 * | 5.9 * | 5.2 * |
| Name of Bacteria | Control | 0.1% | 0.25% | 0.5% | Sugar |
|---|---|---|---|---|---|
| Lactic acid bacteria | 1.8 ± 0.2·107 | 1.0 ± 0.4·107 | 7.0 ± 0.2·107 | 1.4 ± 0.5·108 * | 3.3 ± 0.3·107 |
| Bifidobacterium | 108 | 108 | 108 | 108 | 108 |
| Enterococcus | 1.2 ± 0.3·108 | 1.2 ± 0.3·108 | 1.3 ± 0.3·108 | 6.0 ± 0.3·107 * | 9.1 ± 0.4·107 |
| E. coli | 1.1 ± 0.2·108 | 6.0 ± 0.3·107 | 1.0 ± 0.4·108 | 1.0 ± 0.5·108 | 1.6 ± 0.4·108 |
| Staphylococcus | 5.5 ± 0.2·107 | 6.0 ± 0.2·107 | 9.7 ± 0.3·107 | 3.2 ± 0.3·107 | 5.2 ± 0.2·107 |
| Bacillus | 1.3 ± 0.3·107 | 9.5 ± 0.5·106 | 8.0 ± 0.5·106 | 2.3 ± 0.4·107 | 1.7 ± 0.3·107 |
| pH | 7.0 | 6.9 | 7.2 | 6.7 * | 7.1 |
| Name of Bacteria | Control | 0.5% | 0.75% | 1% |
|---|---|---|---|---|
| Lactic acid bacteria | 4.5 ± 0.2·107 | 1.3 ± 0.4·108 * | 1.5 ± 0.3·108 * | 7.6 ± 0.2·108 * |
| Bifidobacterium | 108 | 108 | 108 | 108 |
| Enterococcus | 3.5 ± 0.3·107 | 2.7 ± 0.4·107 | 7.4 ± 0.3·106 * | 8.0 ± 0.3·106 * |
| E. coli | 2.5 ± 0.4·108 | 1.2 ± 0.2·107 * | 2.0 ± 0.3·106 * | 4.0 ± 0.4·106 * |
| Staphylococcus | 9.4 ± 0.3·106 | 2.2 ± 0.3·106 | 4.0 ± 0.4·106 | 6.6 ± 0.3·106 |
| Bacillus | 3.1 ± 0.2·107 | 4.0 ± 0.3·106 * | 3.4 ± 0.5·106 * | 1.0 ± 0.3·106 * |
| pH | 6.9 | 6.6 | 6.4 * | 6.0 * |
| Name of Bacteria | Control | 0.5% | 0.75% | 1% |
|---|---|---|---|---|
| Lactic acid bacteria | 1.3 ± 0.3·105 | 2.2 ± 0.4·107 * | 2.9 ± 0.3·107 * | 7.4 ± 0.3·107 * |
| Bifidobacterium | 106 | 106 | 106 | 106 |
| Enterococcus | 6.5 ± 0.2·108 | 6.9 ± 0.3·108 | 6.7 ± 0.2·108 | 4.5 ± 0.4·108 |
| E. coli | 6.5 ± 0.4·108 | 5.2 ± 0.3·108 | 6.1 ± 0.4·108 | 8.9 ± 0.3·108 |
| Staphylococcus | 1.2 ± 0.2·108 | 1.8 ± 0.2·108 | 2.7 ± 0.3·108 | 2.9 ± 0.5·108 |
| Bacillus | 1.3 ± 0.3·107 | 1.3 ± 0.4·107 | 2.3 ± 0.5·107 | 1.4 ± 0.3·107 |
| pH | 7.2 | 6.8 | 6.5 * | 6.4 * |
| Name of Bacteria | Control | 0.5% | 0.75% | 1% |
|---|---|---|---|---|
| Lactic acid bacteria | 1.3 ± 0.2·105 | 1.0 ± 0.3·107 * | 1.4 ± 0.2·107 * | 1.5 ± 0.3·107 * |
| Bifidobacterium | 106 | 106 | 106 | 106 |
| Enterococcus | 6.5 ± 0.4·108 | 5.0 ± 0.3·108 | 8.2 ± 0.3·108 | 7.4 ± 0.4·108 |
| E.coli | 6.5 ± 0.3·108 | 2.3 ± 0.2·108 | 7.1 ± 0.4·108 | 6.2 ± 0.2·108 |
| Staphylococcus | 1.2 ± 0.2·108 | 3.4 ± 0.4·108 | 4.5 ± 0.3·108 | 3.5 ± 0.3·108 |
| Bacillus | 1.3 ± 0.3·107 | 1.0 ± 0.3·107 | 2.4 ± 0.4·107 | 6.3 ± 0.2·107 |
| pH | 7.2 | 6.7 | 6.4 * | 6.3 * |
| Nutrient Medium | pH |
|---|---|
| MRS | 3.1 |
| AIM | 6.9 |
| AIM + 0.5% of wet wheat | 6.7 |
| AIM + 0.75% of wet wheat | 6.2 |
| AIM + 1% of wet wheat | 5.9 |
| AIM + 0.5% of dry wheat | 6.6 |
| AIM + 0.75% of dry wheat | 6.3 |
| AIM + 1% of dry wheat | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meskhi, B.; Pakhomov, V.; Rudoy, D.; Maltseva, T.; Olshevskaya, A.; Mazanko, M. Early-Maturity Wheat as a Highly Valuable Feed Raw Material with Prebiotic Activity. Agriculture 2025, 15, 317. https://doi.org/10.3390/agriculture15030317
Meskhi B, Pakhomov V, Rudoy D, Maltseva T, Olshevskaya A, Mazanko M. Early-Maturity Wheat as a Highly Valuable Feed Raw Material with Prebiotic Activity. Agriculture. 2025; 15(3):317. https://doi.org/10.3390/agriculture15030317
Chicago/Turabian StyleMeskhi, Besarion, Viktor Pakhomov, Dmitry Rudoy, Tatyana Maltseva, Anastasiya Olshevskaya, and Maria Mazanko. 2025. "Early-Maturity Wheat as a Highly Valuable Feed Raw Material with Prebiotic Activity" Agriculture 15, no. 3: 317. https://doi.org/10.3390/agriculture15030317
APA StyleMeskhi, B., Pakhomov, V., Rudoy, D., Maltseva, T., Olshevskaya, A., & Mazanko, M. (2025). Early-Maturity Wheat as a Highly Valuable Feed Raw Material with Prebiotic Activity. Agriculture, 15(3), 317. https://doi.org/10.3390/agriculture15030317









