Impact of Tetranychus urticae Herbivory on Aronia melanocarpa Ecotypes: Physiological, Morphological, and Reproductive Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Free Choice Test
2.3. Mite Rearing and Demographic Measurements
2.4. Surface Structure of A. melanocarpa Leaf
2.5. Physiological Analysis
2.6. Statistical Procedures
3. Results
3.1. Plants Acceptance by T. urticae and Leaf Morphological Characteristics of A. melanocarpa Ecotypes
3.2. Demographic Parameters of T. urticae
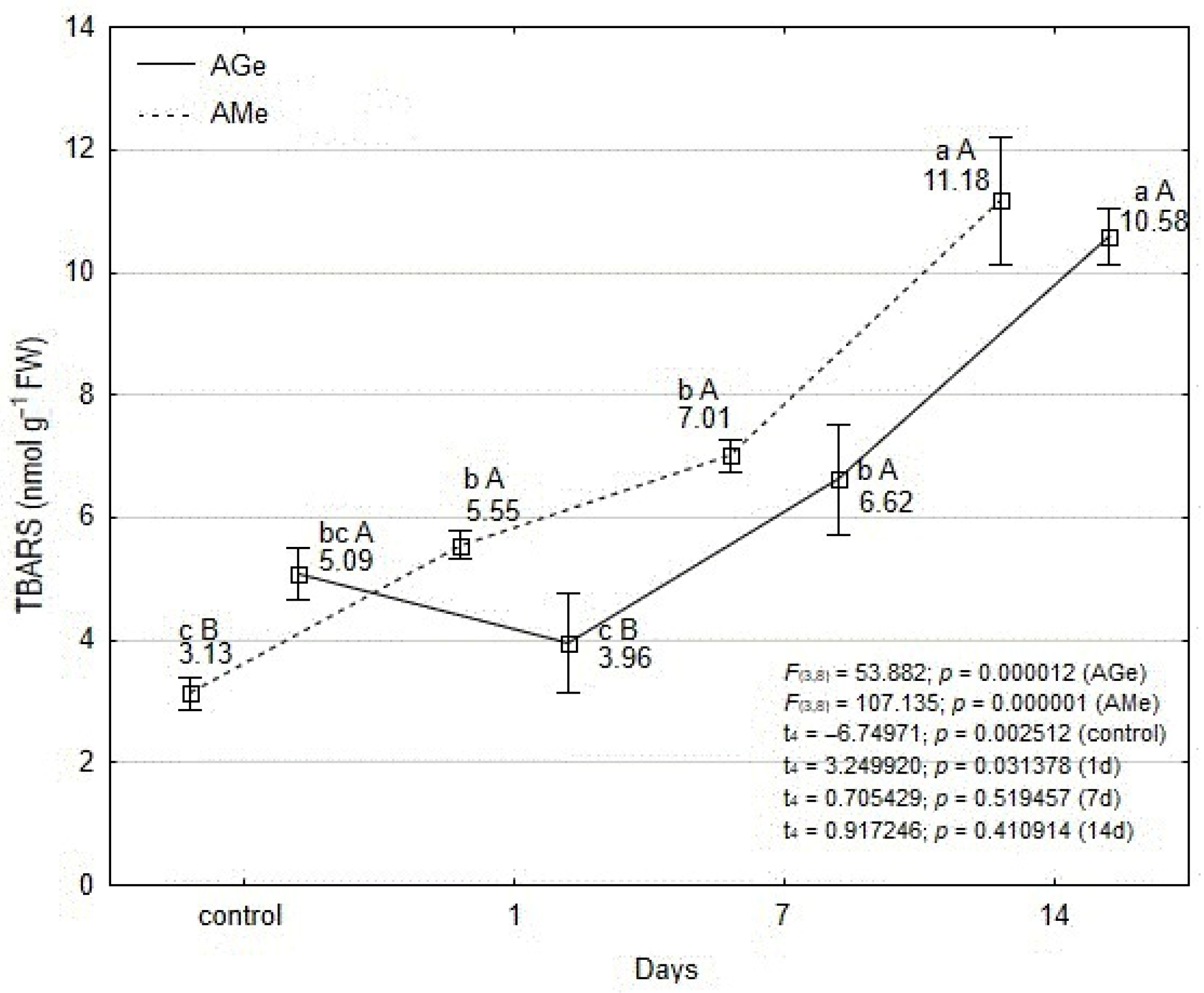
3.3. Changes in Hydrogen Peroxide and Thiobarbituric Acid Reactive Substances
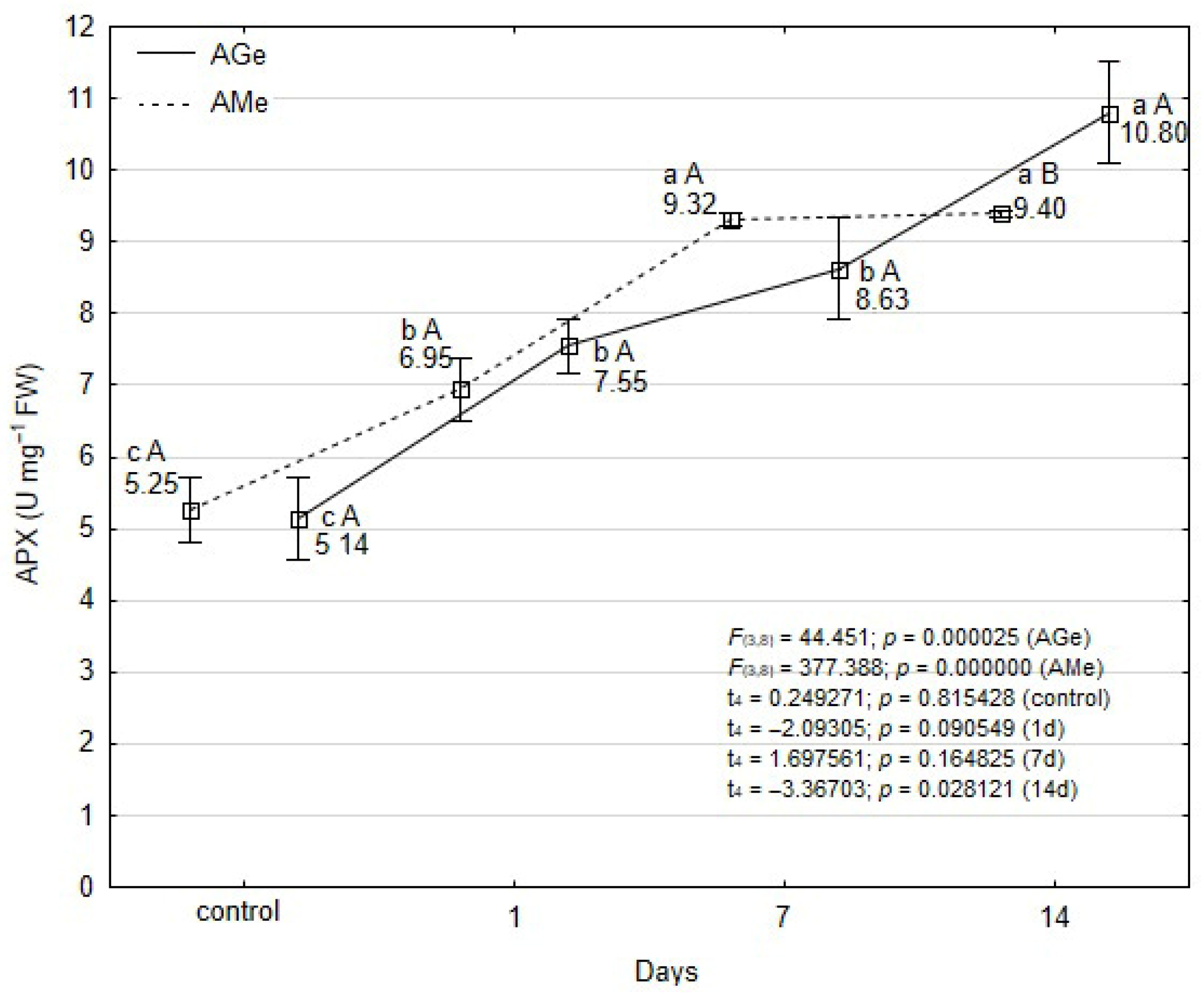
3.4. Changes in Antioxidant Enzymes Activity
4. Discussion
4.1. Host Plant Acceptance: Comparison of A. melanocarpa Ecotypes
4.2. Comparison of Leaf Morphology in A. melanocarpa Ecotypes
4.3. Suitability of A. melanocarpa Ecotypes in Relation to Selected Life History Parameters of T. urticae
4.4. Biochemical Mechanisms of Interactions Between A. melanocarpa and T. urticae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engels, G.; Brinckmann, J. Aronia czarna, Aronia melanocarpa. Am. Bot. Counc. 2014, 101, 1–5. [Google Scholar]
- Rozpara, E.; Morgaś, H.; Filipczak, J.; Meszka, B.; Hołdaj, M.; Łabanowska, B.H.; Sekrecka, M.; Sobiczewski, P.; Lisek, J.; Danelski, W. Metodyka Produkcji Owoców Aronii Metodą Ekologiczną; Inhort: Skierniewice, Poland, 2016; 27p. [Google Scholar]
- Smolik, M.; Ochmian, I.; Smolik, B. RAPD and ISSR methods used for fingerprinting selected, closely related cultivars of Aronia melanocarpa. Not. Bot. Horti Agrobot. Napoca 2011, 39, 276–284. [Google Scholar] [CrossRef]
- Jeppsson, N. The effect of cultivar and cracking on the fruit quality in black chokeberry (Aronia melanocarpa) and the hybrids between chokeberry and rowan (Soubzis). Gartenbauwissenschaft 2000, 65, 93–98. [Google Scholar] [CrossRef]
- Jeppsson, N.; Johansson, R. Changes in fruit quality in black chokeberry (Aronia melanocarpa) during maturation. J. Hortic. Sci. Biot. 2000, 75, 340–350. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Enescu Mazilu, I.C.; Cosmulescu, S. Effect of cultivar, year, and their interaction on nutritional and energy value components in Aronia melanocarpa berries. Not. Bot. Horti. Agrobot. 2023, 51, 13478. [Google Scholar] [CrossRef]
- Strigl, A.W.; Leitner, E.; Pfannhauser, W. Die schwarze Apfelbeere (Aronia melanocarpa) als natürliche Farbstoffquelle. Dtsch. Lebensmitt. Rundsch. 1995, 91, 177–180. [Google Scholar]
- Ochmian, I.; Grajkowski, J.; Smolik, M. Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia melanocarpa). Not. Bot. Horti. Agrobo. 2012, 40, 253–260. [Google Scholar] [CrossRef]
- Górska-Drabik, E. Trachycera advenella (Zinck.) (Lepidoptera, Pyralidae)—A new pest on black chokeberry (Aronia melanocarpa). Prog. Plant Prot. 2009, 49, 531–534. [Google Scholar]
- Górska-Drabik, E. Owady i roztocza zagrażające aronii czarnoowocowej. In Materiały IX Konferencji Sadowniczej “Trendy w uprawie gatunków jagodowych i pestkowych”; Związku Sadowników RP: Grójec, Poland, 2013; pp. 30–32. [Google Scholar]
- Migeon, A.; Nouguier, E.; Dorkeld, F. Spider mites web: A comprehensive database for the Tetranychidae. In Trends in Acarology; Sabelis, M., Bruin, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 557–560. [Google Scholar]
- Spider Mites Web. Available online: https://www1.montpellier.inrae.fr/CBGP/spmweb/public/ (accessed on 16 September 2025).
- Monteiro, L.B.; Kuhn, T.M.; Mogor, A.F.; da Silva, E.D. Biology of the Two-Spotted Spider Mite on Strawberry Plants. Neotrop. Entomol. 2014, 43, 183–1888. [Google Scholar] [CrossRef]
- Bounfour, M.; Tanigoshi, L.K.; Chen, C.; Cameron, S.; Klauer, S. Chlorophyll Content and Chlorophyll Fluorescence in Red Raspberry Leaves Infested with Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae). Environ. Entomol. 2002, 31, 215–220. [Google Scholar] [CrossRef]
- Warabieda, W. Effect of two-spotted spider mite population (Tetranychus urticae Koch) on growth parameters and yield of the summer apple cv. Katja. Hort. Sci. 2015, 42, 167–175. [Google Scholar] [CrossRef]
- Skorupska, A. Resistance of apple cultivars to two-spotted spider mite, Tetranychus urticae Koch (Acarina, Tetranychidae). Part I. Bionomy of two-spotted spider mite on selected cultivars of apple trees. J. Plant Prot. Res. 2004, 44, 75–80. [Google Scholar]
- Leus, L.; Minguely, C.; Van Poucke, C.; Audenaert, J.; Witters, J. Cultivar susceptibility and stress hormone response in raspberry (Rubus ideaus) to two-spotted spider mite (Tetranychus urticae). Acta Hortic. 2020, 1277, 417–424. [Google Scholar] [CrossRef]
- Tehri, K.; Gulami, R.; Geroh, M. Host plant responses, biotic stressors and management strategies for the control of Tetranychus utricae Koch (Acarina: Tetranychidae). Agric. Rev. 2014, 35, 250–260. [Google Scholar] [CrossRef]
- Azadi Dana, E.; Sadeghi, A.; Maroufpoor, M.; Khanjani, M.; Babolhavaeji, H.; Ullah, M.S. Comparison of the life table and reproduction parameters of the Tetranychus urticae (Acari: Tetranychidea) on five strawberry varieties. Int. J. Acarol. 2018, 44, 254–261. [Google Scholar] [CrossRef]
- Ansari-Shiri, H.; Hajiqanbar, H.; Zalucki, M.P.; Fathipour, Y. The role of host plant resistance as a critical factor for the management of Tetranychus urticae (Acari: Tetranychidae) in strawberry. Persian J. Acarol. 2025, 14, 253–262. [Google Scholar] [CrossRef]
- Meyer, M.K.P.S.; Craemer, C. Mites (Arachnida: Acari) as crop pests in southern Africa: An overview. Afr. Plant Prot. 1999, 5, 37–51. [Google Scholar]
- Bensoussan, N.; Santamaria, M.E.; Zhurov, V.; Diaz, I.; Grbić, M.; Grbić, V. Plant-herbivore interaction: Dissection of the cellular pattern of Tetranychus urticae feeding on the host plant. Front. Plant Sci. 2016, 7, 1105. [Google Scholar] [CrossRef]
- Estrella Santamaria, M.; Arnaiz, A.; Rosa-Diaz, I.; González-Melendi, P.; Romero-Hernandez, G.; Ojeda-Martinez, D.A.; Garcia, A.; Contreras, E.; Martinez, M.; Diaz, I. Plant Defenses against Tetranychus urticae: Mind the Gaps. Plants 2020, 9, 464. [Google Scholar] [CrossRef]
- Jakubowska, M.; Fiedler, Ż.; Bocianowski, J.; Torzyński, K. The effect of spider mites (Acari: Tetranychidae) occurrence on sugar beet yield depending on the variety. Agron. Sci. 2018, 73, 41–50. [Google Scholar] [CrossRef]
- Rotem, K.A.; Agrawal, A.A. Density dependent population growth of the two-spotted spider mite, Tetranychus urticae, on the host plant Leonurus cardiaca. Oikos 2003, 103, 559–565. [Google Scholar] [CrossRef]
- Marinosci, C.; Magalhães, S.; Macke, E.; Navajas, M.; Carbonell, D.; Devaux, C.; Olivieri, I. Effects of host plant on life-history traits in the polyphagous spider mite Tetranychus urticae. Ecol. Evol. 2015, 5, 3151–3158. [Google Scholar] [CrossRef] [PubMed]
- Puspitarini, R.D.; Fernando, I.; Rachmawati, R.; Hadi, M.S.; Rizali, A. Host plant variability affects the development and reproduction of Tetranychus urticae. Int. J. Acarol. 2021, 47, 381–386. [Google Scholar] [CrossRef]
- Kropczyńska-Linkiewicz, D.; Garnis, J.; Jaworski, S.; Sagan, A.; Krężlewicz, M. Drapieżne roztocza (Acari: Phytoseiidae) występujące na roślinach w otoczeniu plantacji krzewów jagodowych. Pog. Plant Prot. 2009, 49, 1048–1056. [Google Scholar]
- Łabanowska, B.H.; Gajek, D. Control of the twospottedspider mite Tetranychus Urticae Koch. on black currant. Acta Hortic. 1993, 352, 583–586. [Google Scholar] [CrossRef]
- Gajek, D. Species Composition Of Tetranychid Mites (Tetranychidae) And Predatory Mites (Phytoseiidae) Occurring On Raspberry Plantations In Poland. J. Plant Prot. Res. 2003, 43, 353–360. [Google Scholar]
- Łabanowska, B.H.; Tartanus, M.; Piotrowski, W.; Sobieszek, B. Usefulness of products with mechanical action to control of two-spotted spider mite (Tetranychus urticae Koch.) on strawberry. Zesz. Nauk. Instyt. Ogrodn. 2017, 25, 31–38. [Google Scholar]
- Jonathan, F.T.; Mahendranathan, C. Impact of climate change on plant diseases. Agrieast J. Agric. Sci. 2024, 18, 1–17. [Google Scholar] [CrossRef]
- Ma, C.S.; Wang, B.X.; Wang, X.J.; Lin, Q.C.; Zhang, W.; Yang, X.F.; van Baaren, J.; Bebber, D.P.; Eigenbrode, S.D.; Zalucki, M.P.; et al. Crop pest responses to global changes in climate and land management. Nat. Rev. Earth Environ. 2025, 6, 264–283. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Recognition of Herbivory-Associated Molecular Patterns. Plant Physiol. 2008, 146, 825. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Dent, D. Host plant resistance. In Insect Pest Management; Dent, D., Ed.; CABI Publishing: Oxfordshire, UK, 2000; pp. 123–179. [Google Scholar]
- Sarfraz, M.; Dosdall, L.M.; Keddie, B.A. Diamondback moth-host plant interactions: Implications for pest management. Crop Prot. 2006, 25, 625–636. [Google Scholar] [CrossRef]
- Painter, R.H. Insect resistance in crop plants. Soil Sci. 1951, 72, 481. [Google Scholar]
- Dąbrowski, Z.T. Mechanizmy odporności roślin na szkodniki. In Podstawy Odporności Roślin na Szkodniki; PWRiL: Warszawa, Poland, 1976; pp. 15–17. [Google Scholar]
- Kogan, M.; Ortman, E.F. Antixenosis—A new term proposed to define Painter’s “non-preference” modality of resistance. Bull. Entomol. Soc. Am. 1978, 24, 175–176. [Google Scholar] [CrossRef]
- Dąbrowski, Z.T. Podstawy Odporności Roślin Uprawnych na Szkodniki; PWRiL: Warszawa, Poland, 1988; 260p. [Google Scholar]
- Smith, C.M. Plant Resistance to Insects. A Fundamental Approach; Joan Willey & Sons: New York, NY, USA, 1989; 286p. [Google Scholar]
- Kiełkiewicz-Szaniawska, M. Strategie Obronne Roślin Pomidorów (Lycopersicon Esculentum Miller) Wobec Przędziorka Szklarniowca (Tetranychus Cinnabarinus Boisduval, Acari: Tetranychidae); Wydawnictwo SGGW: Warszawa, Poland, 2003; Volume 264, 140p. [Google Scholar]
- Boczek, J. Niechemiczne Metody Zwalczania Szkodników Roślin; Wydawnictwo SGGW: Warszawa, Poland, 1992; 242p. [Google Scholar]
- Ross, C.; Santiago-Vazquez, L.; Paul, V. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aqu. Toxicol. 2006, 78, 66–73. [Google Scholar] [CrossRef]
- Minibayeva, F.; Beckett, R.P.; Kranner, I. Roles of Apoplastic Peroxidases in Plant Response to Wounding. Phytochemistry 2015, 112, 122–129. [Google Scholar] [CrossRef]
- Peng, M.; Kuc, J. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 1992, 82, 696–699. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Rani, P.U.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Bhattacharjee, S. The language of reactive oxygen species signaling in plants. J. Bot. 2012, 2012, 341–362. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant Machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Uniyal, N.; Panneerselvam, P.; Senapati, A.; Ganeshamurthy, A.N. Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life Sci. Appl. Sci. 2019, 1, 1–10. [Google Scholar]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Jaiti, F.; Verdeil, J.L.; El Hadrami, I. Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol. Mol. Plant Pathol. 2009, 74, 84–90. [Google Scholar] [CrossRef]
- Ranger, C.M.; Singh, A.P.; Frantz, J.M.; Cañas, L.; Locke, J.C.; Reding, M.E.; Vorsa, N. Influence of silicon on resistance of Zinnia elegans to Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 2009, 38, 129–136. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 2, 239–247. [Google Scholar] [CrossRef]
- Marchi-Werle, L.; Heng-Moss, T.M.; Hunt, T.E.; Baldin, E.L.L.; Baird, L.M. Characterization of Peroxidase Changes in Tolerant and Susceptible Soybeans Challenged by Soybean Aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2014, 107, 1985–1991. [Google Scholar] [CrossRef]
- Gulsen, O.; Eickhoff, T.; Heng-Moss, T.; Shearman, R.; Baxendale, F.; Sarath, G.; Lee, D. Characterization of peroxidase changes in resistant and susceptible warm-season turfgrasses challenged by Blissus occiduus. Arthropod. Plant Interact. 2010, 4, 45–55. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Tran, N.T.; Nguyen, D.S. The involvement of peroxidases in soybean seedlings’ defense against infestation of cowpea aphid. Arthropod-Plant Int. 2016, 10, 283–292. [Google Scholar] [CrossRef]
- Druciarek, T.; Lewandowski, M.; Kozak, M. Demographic parameters of Phyllocoptes adalius (Acari: Eriophyoidea) and influence of insemination on female fecundity and longevity. Exp. Appl. Acarol. 2014, 63, 349–360. [Google Scholar] [CrossRef]
- Jena, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1981, 12, 345–354. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Effect of light on lipid peroxidation in chloroplasts. Biochem. Biophys. Res. Commun. 1968, 19, 716–720. [Google Scholar] [CrossRef]
- Małolepsza, A.; Urbanek, H.; Polit, J. Some biochemical of strawberry plants to infection with Botrytis cinerea and salicylic acid treatment. Acta Agrobot. 1994, 47, 73–81. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Golizadeh, A.; Ghavidel, S.; Razmjou, J.; Fathi, S.A.A.; Hassanpour, M. Comparative life table analysis of Tetranychus urticae Koch (Acari: Tetranychidae) on ten rose cultivars. Acarologia 2017, 57, 607–616. [Google Scholar] [CrossRef]
- Skorupska, A. Food acceptance of spider mite (Schizotetranychus schizopus Zacher) and two-spotted spider mite (Tetranychus urticae Koch.) in the selection basket willow varieties (Salix viminalis L.). Prog. Plant Prot. 2012, 52, 456–460. [Google Scholar]
- van den Boom, C.E.M.; van Beek, T.A.; Dicke, M. Differences among plant species in acceptance by the spider mite Tetranychus urticae Koch. J. Appl. Entomol. 2003, 127, 177–183. [Google Scholar] [CrossRef]
- Skorupska, A.; Rogalińska, M. Food preference of two-spotted spider mite (Tetranychus urticae Koch) to choosing annual weed species in cereals. Prog. Plant Prot. 2012, 52, 461–466. [Google Scholar]
- Luczynski, A.; Isman, M.B.; Raworth, D.A.; Chan, C.K. Chemical and morphological factors of resistance against the twospotted spider mite in beach strawberry. J. Econ. Entomol. 1990, 83, 564–569. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant Defense against Herbivorous Pests: Exploiting Resistance and Tolerance Traits for Sustainable Crop Protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Rakha, M.; Bouba, N.; Ramasamy, S.; Regnard, J.-L.; Hanson, P. Evaluation of wild tomato accessions (Solanum spp.) for resistance to two-spotted spider mite (Tetranychus urticae Koch) based on trichome type and acylsugar content. Genet. Resour. Crop Evol. 2017, 64, 1011–1022. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Shelepova, O.; Vergun, O.; Grygorieva, O.; Kuklina, A.; Brindza, J. Differences between Aronia Medik. taxa on the morphological and biochemical characters. Environ. Res. Engin. Manag. 2018, 74, 43–52. [Google Scholar] [CrossRef]
- Sempruch, C. Interakcje między mszycami a roślinami we wstępnych etapach wyboru żywiciela. Kosmos 2012, 61, 573–586. [Google Scholar]
- Alba, J.M.; Montserrat, M.; Fernández-Muñoz, R. Resistance to the two-spotted spider mite (Tetranychus urticae) by acylsucroses of wild tomato (Solanum pimpinellifolium) trichomes studied in a recombinant inbred line population. Exp. Appl. Acarol. 2009, 47, 35–47. [Google Scholar] [CrossRef]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Li, D.; Tian, J.; Shen, Z. Assessment of sublethal effects of clofentezine on life-table parameters in hawthorn spider mite (Tetranychus viennensis). Exp. Appl. Acarol. 2006, 38, 255–273. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Martinez, M.; Arnaiz, A.; Ortego, F.; Grbic, V.; Diaz, I. MATI, a novel protein involved in the regulation of herbivore-associated signaling pathways. Front. Plant Sci. 2017, 8, 975. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S.; Wojcicka, A.; Goławski, A. Effect of host plants on antioxidant system of pea aphid Acyrthosiphon pisum. Bull. Insect. 2011, 64, 153–158. [Google Scholar]
- Leitner, M.; Boland, W.; Mithöfer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef]


| Host Plant | Number of Mite Mean (SD) (%) | Morphological Characteristics of Plant Leaves Mean (SD) | |
|---|---|---|---|
| Number of Trichome Per cm2 | Trichome Length (mm) | ||
| AMe | 66.52 (5.56) * | 73.00 (22.83) | 0.96 (0.28) |
| AGe | 33.48 (5.56) | 108.70 (21.75) * | 1.02 (0.11) |
| T8 = 8.395 p = 0.000 | T18 = 3.06 p = 0.007 | T18 = 0.60 p = 0.556 | |
| Host Plant | Daily Facundity (Egg/Female/Day) Mean (SD) | Development Time Mean (SD, Days) | |
|---|---|---|---|
| Egg | Larva | ||
| AMe | 2.15 (0.74) | 4.79 (1.49) | 2.86 (1.26) |
| AGe | 2.34 (1.22) | 3.83 (0.86) * | 2.66 (1.49) |
| U = 220.00 p = 1.000 | U = 108.00 p = 0.022 | U = 101.00 p = 0.330 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska-Drabik, E.; Golan, K.; Rubinowska, K.; Sempruch, C. Impact of Tetranychus urticae Herbivory on Aronia melanocarpa Ecotypes: Physiological, Morphological, and Reproductive Responses. Agriculture 2025, 15, 2617. https://doi.org/10.3390/agriculture15242617
Górska-Drabik E, Golan K, Rubinowska K, Sempruch C. Impact of Tetranychus urticae Herbivory on Aronia melanocarpa Ecotypes: Physiological, Morphological, and Reproductive Responses. Agriculture. 2025; 15(24):2617. https://doi.org/10.3390/agriculture15242617
Chicago/Turabian StyleGórska-Drabik, Edyta, Katarzyna Golan, Katarzyna Rubinowska, and Cezary Sempruch. 2025. "Impact of Tetranychus urticae Herbivory on Aronia melanocarpa Ecotypes: Physiological, Morphological, and Reproductive Responses" Agriculture 15, no. 24: 2617. https://doi.org/10.3390/agriculture15242617
APA StyleGórska-Drabik, E., Golan, K., Rubinowska, K., & Sempruch, C. (2025). Impact of Tetranychus urticae Herbivory on Aronia melanocarpa Ecotypes: Physiological, Morphological, and Reproductive Responses. Agriculture, 15(24), 2617. https://doi.org/10.3390/agriculture15242617






