Comparative Effects of Sodium Metasilicate and Potassium Silicate in Enhancing Bacillus amyloliquefaciens PMB05 Plant Immune Responses and Control of Bacterial Soft Rot in Cabbage
Abstract
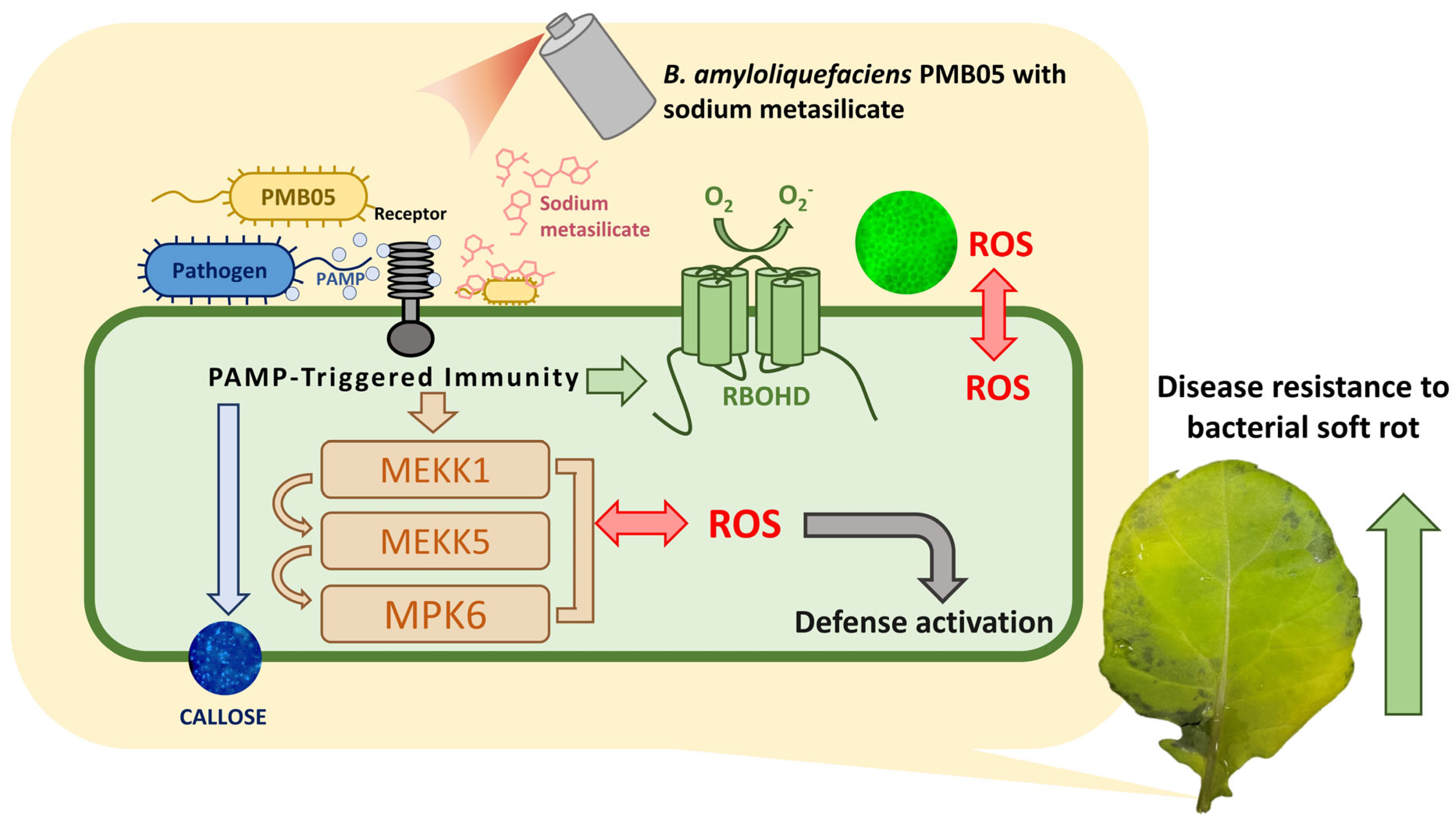
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Growth and Preparations of Microorganisms
2.3. Preparation of Bacillus amyloliquefaciens PMB05 Fermentation Liquid
2.4. HrpN Protein Preparation
2.5. Effects of Bacillus amyloliquefaciens PMB05 on HrpN-Triggered Plant Immune Responses
2.6. Effects of Bacillus amyloliquefaciens PMB05 on the Biocontrol of Bacterial Soft Rot in Cabbage
2.7. Effects of Silicates on HrpN-Triggered Plant Immune Responses
2.8. Effect of Silicates with Bacillus amyloliquefaciens PMB05 on Regulation of Plant Immune Responses
2.9. Effects of Silicates with Bacillus amyloliquefaciens PMB05 Against the Biocontrol of Bacterial Soft Rot Disease in Cabbage
2.10. Data Analysis
3. Results
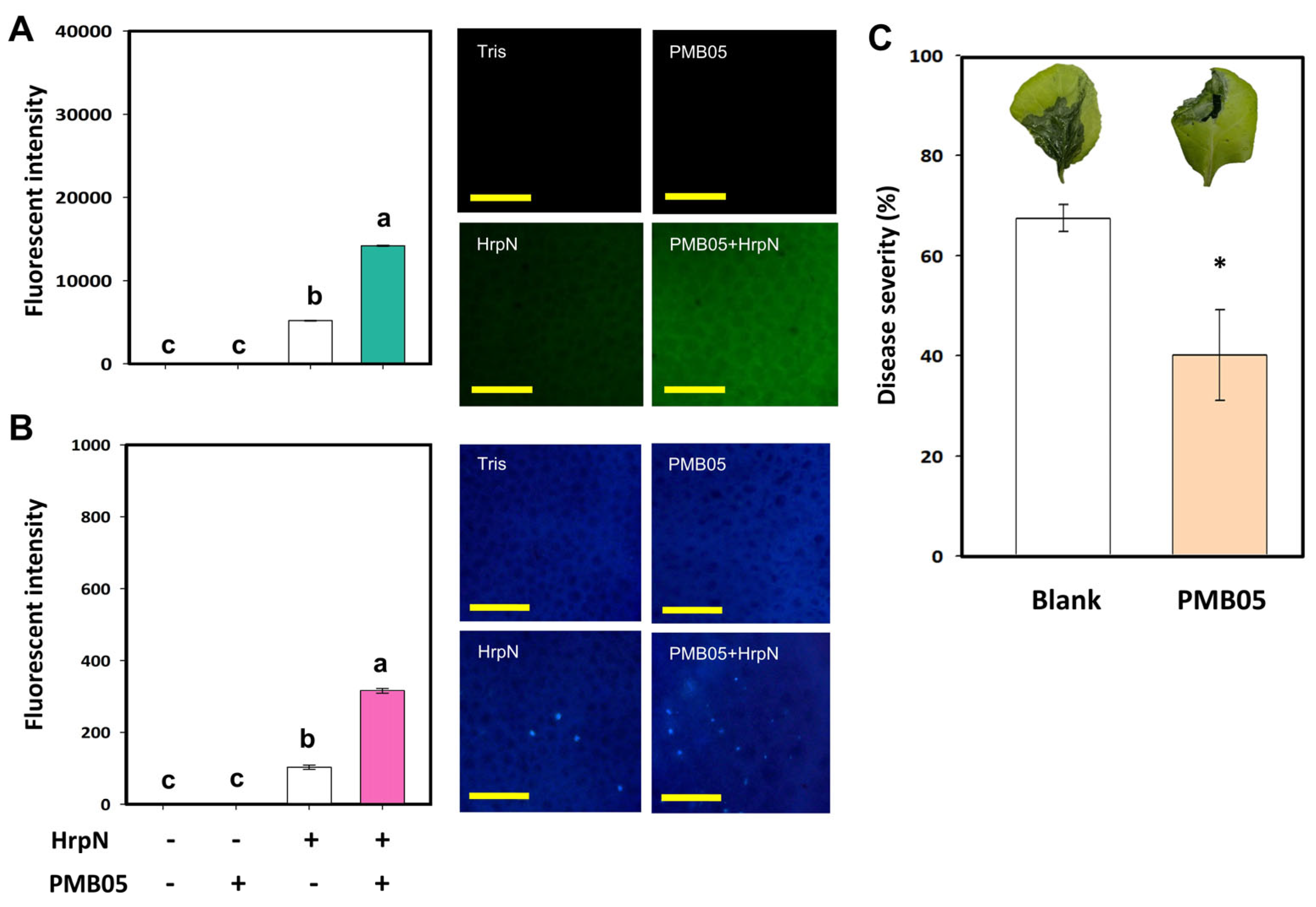
3.1. Effects of Bacillus amyloliquefaciens PMB05 on HrpN-Triggered Plant Immune Responses
3.2. Effects of Bacillus amyloliquefaciens PMB05 on the Biocontrol of Bacterial Soft Rot in Cabbage
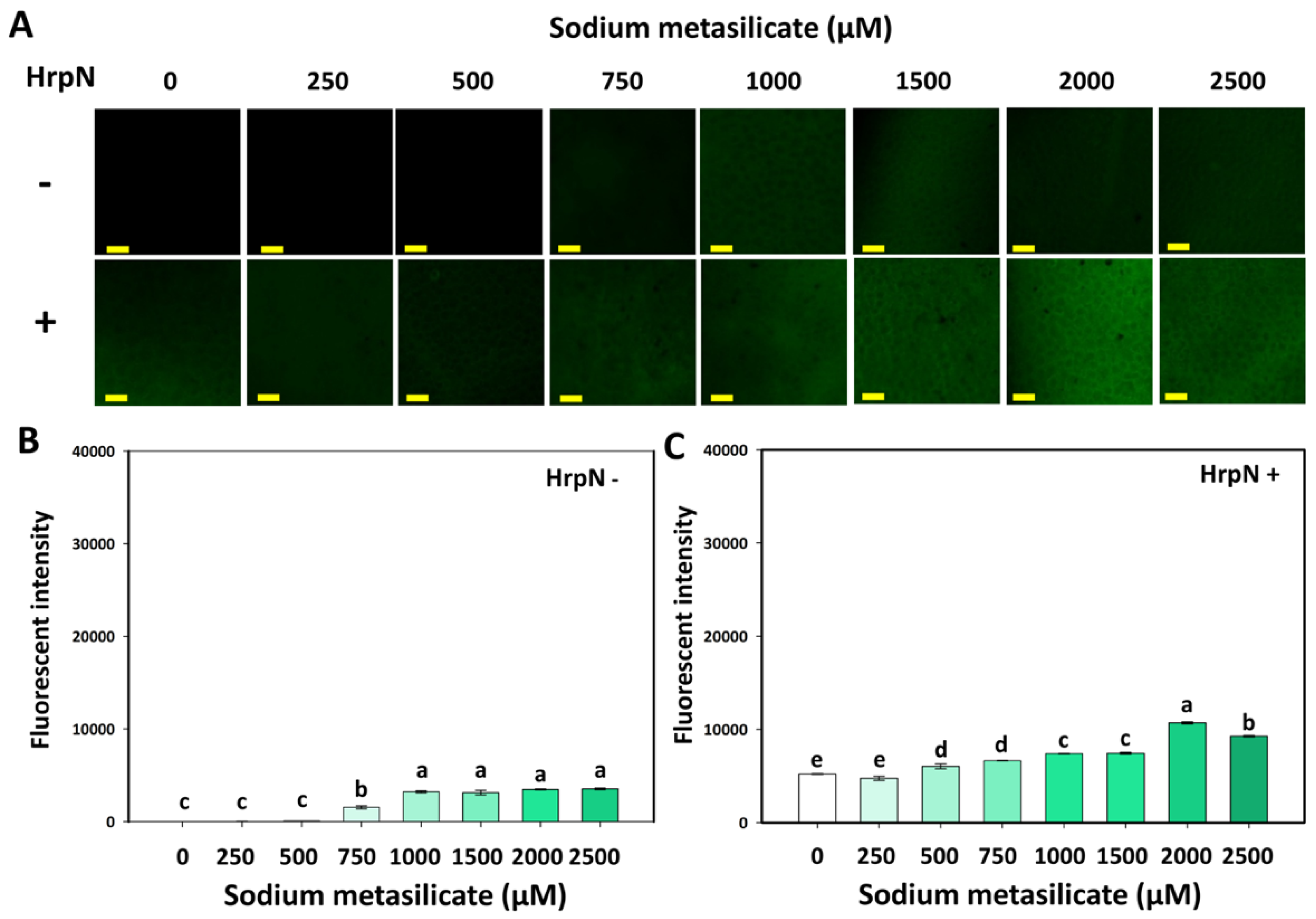
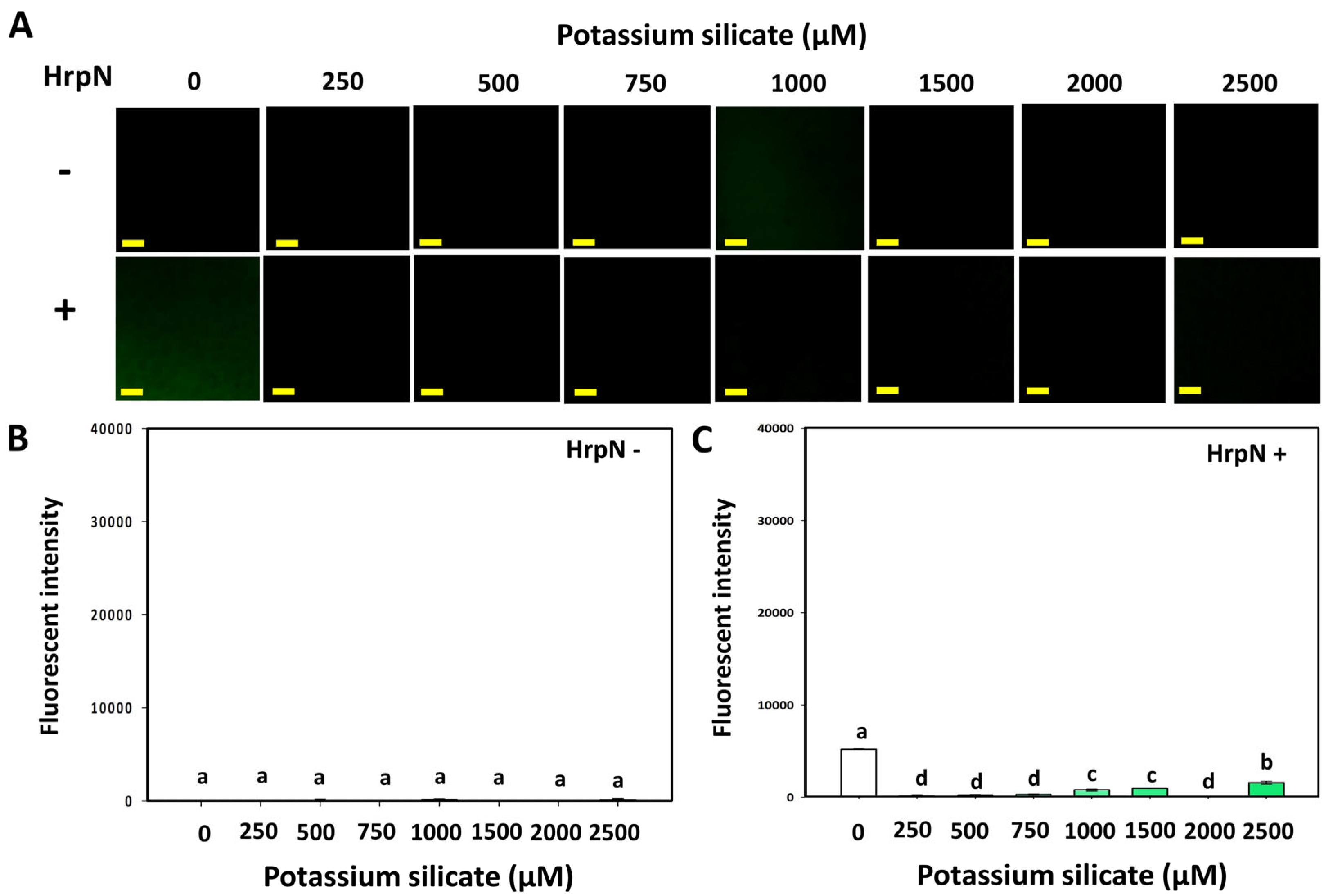
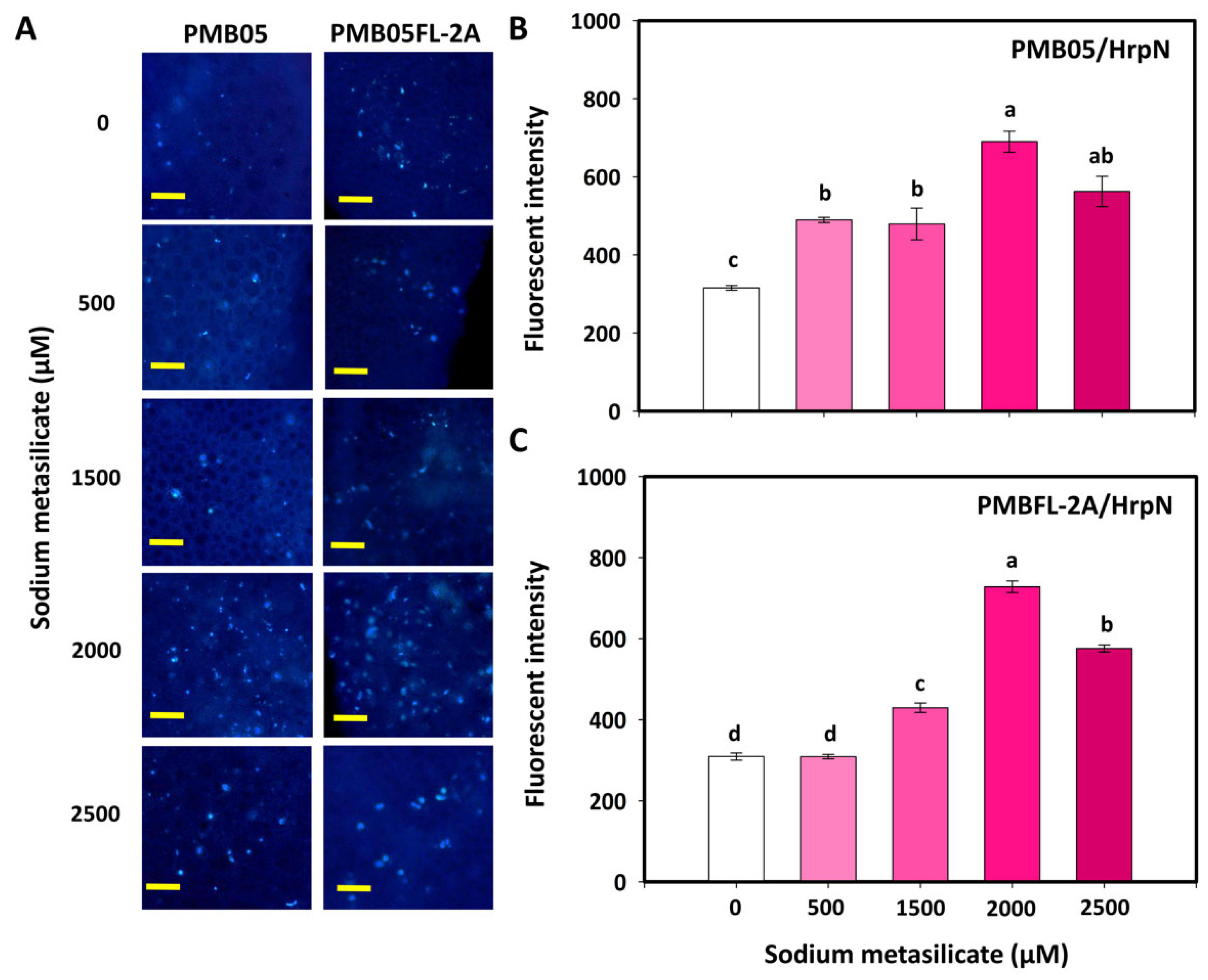
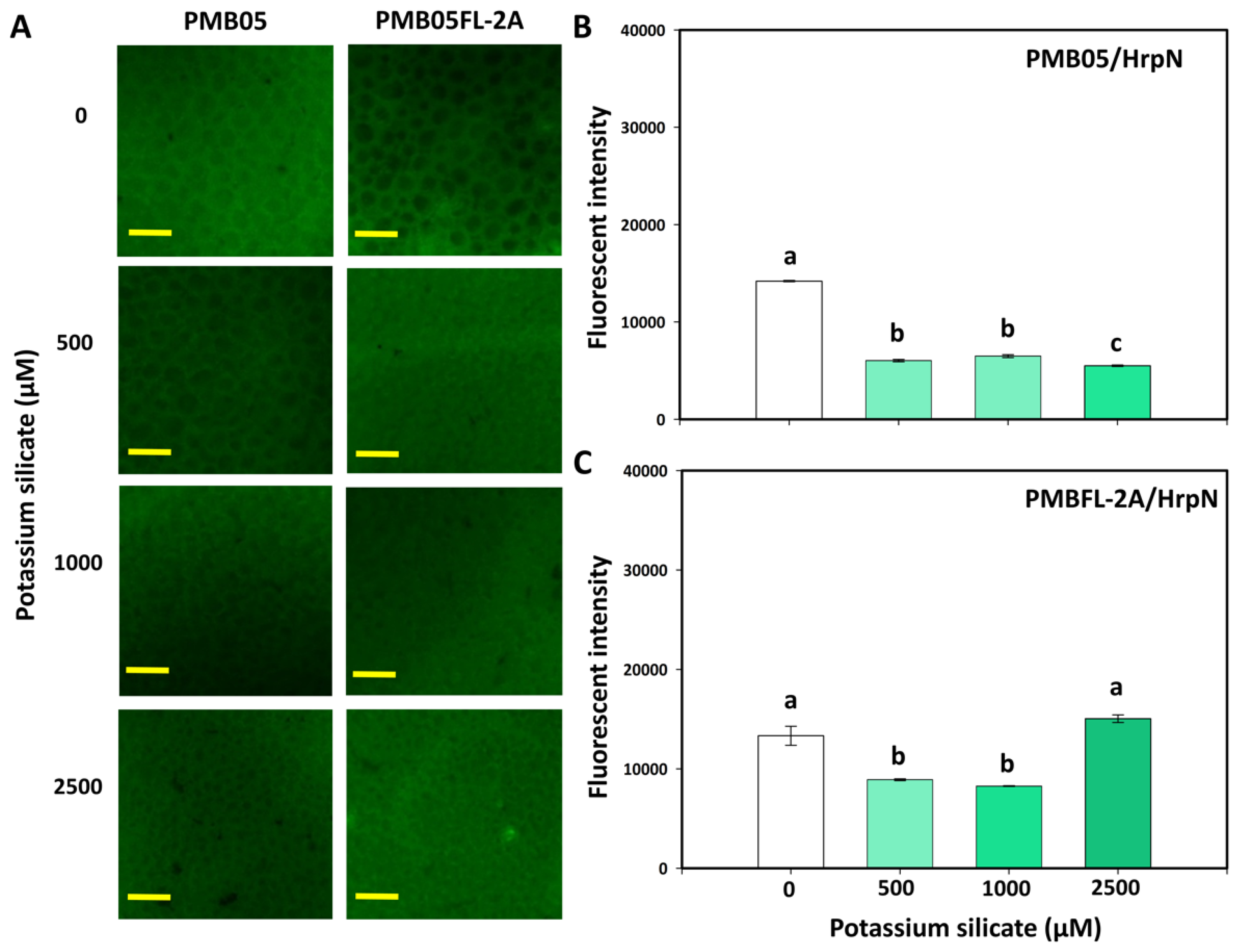
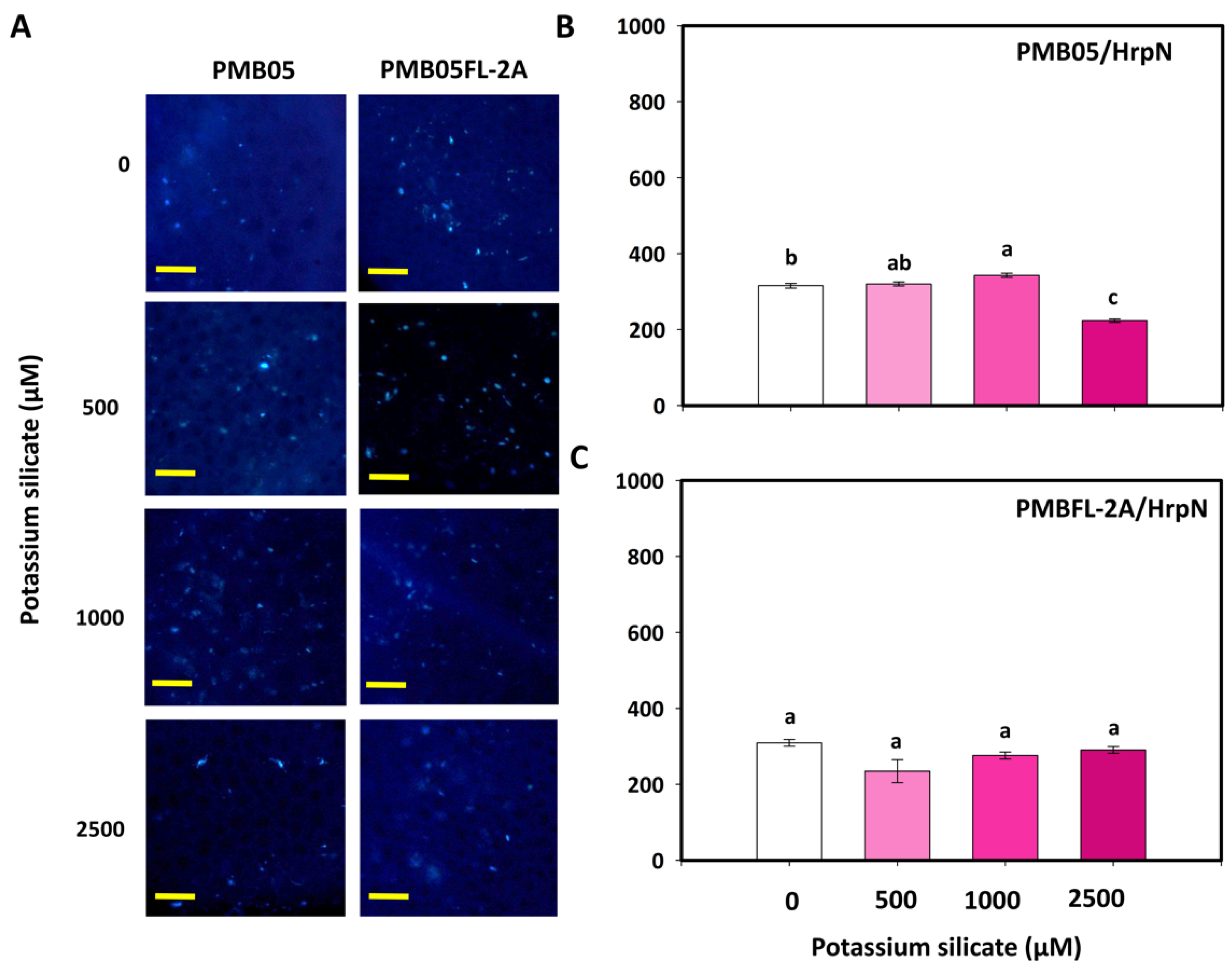
3.3. Effects of Silicates and HrpN on Reactive Oxygen Species Generation
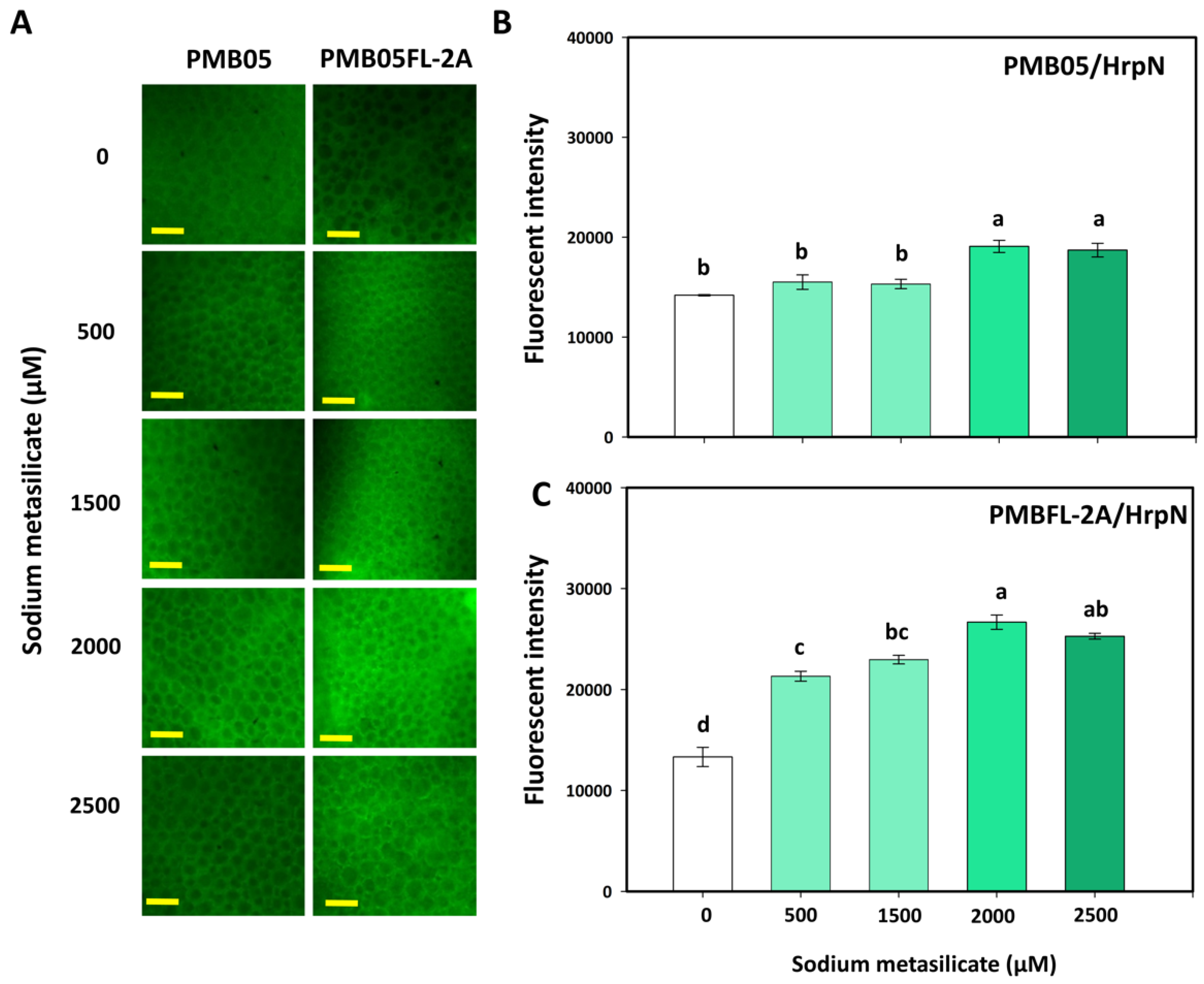
3.4. Effect of Silicates with Bacillus amyloliquefaciens PMB05 on Plant Immune-Triggered Response Assays
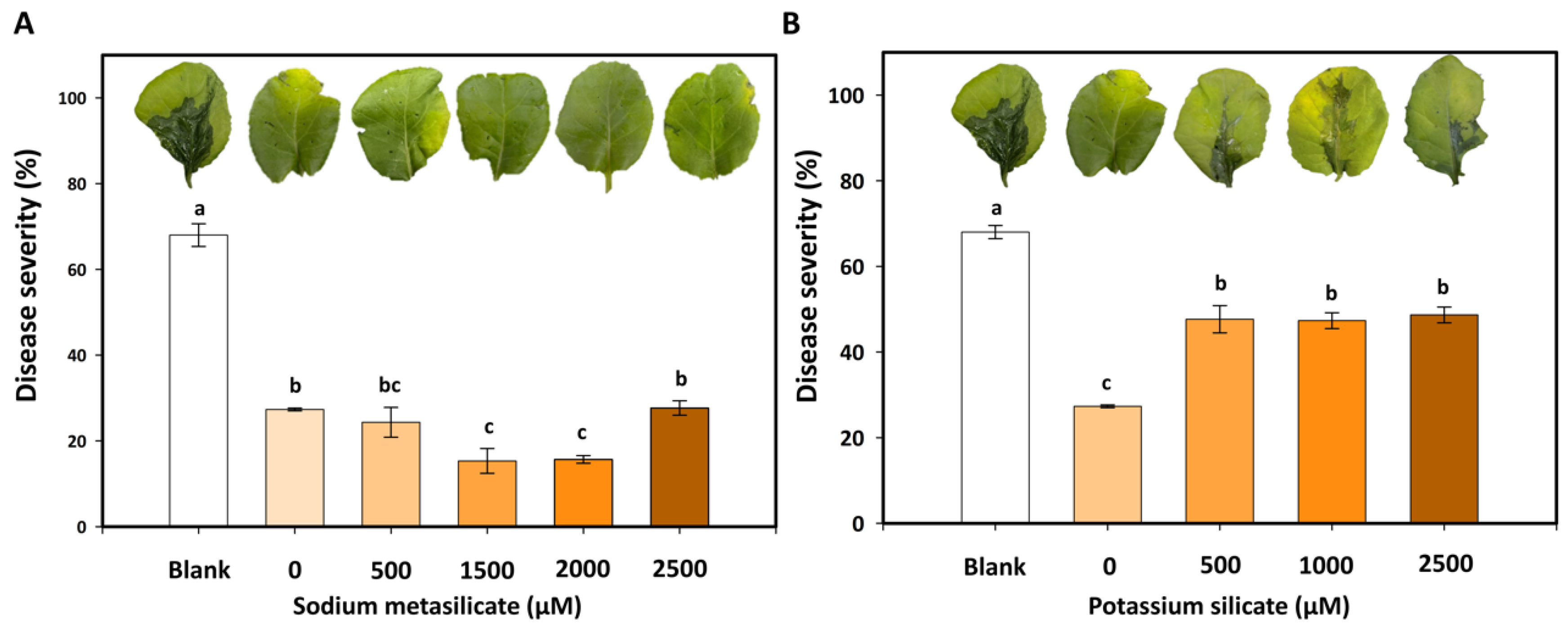
3.5. Effects of Silicates with Bacillus amyloliquefaciens PMB05 on the Biocontrol of Bacterial Soft Rot Disease in Cabbage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Juventia, S.D.; Rossing, W.A.; Ditzler, L.; Van Apeldoorn, D.F. Spatial and genetic crop diversity support ecosystem service delivery: A case of yield and biocontrol in Dutch organic cabbage production. Field Crops Res. 2021, 261, 108015. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Blanco, S.D.; Peng, A.-L.; Fu, J.-Y.; Chen, B.-W.; Luo, M.-C.; Xie, X.-Y.; Lin, Y.-H. Seed treatment with calcium carbonate containing Bacillus amyloliquefaciens PMB05 powder is an efficient way to control black rot disease of cabbage. Agriculture 2023, 13, 926. [Google Scholar] [CrossRef]
- Cui, W.; He, P.; Munir, S.; He, P.; He, Y.; Li, X.; Yang, L.; Wang, B.; Wu, Y.; He, P. Biocontrol of soft rot of Chinese cabbage using an endophytic bacterial strain. Front. Microbiol. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Li’aini, A.S.; Lin, Y.-H.; Huang, T.-C.; Sulistyowati, L. Application of Bacillus amyloliquefaciens to control black rot disease on cabbage caused by Xanthomonas campestris pv. campestris. Plant Med. 2017, 59, 39–44. [Google Scholar]
- Kalaivanan, R.; Eraivan, K.; Thiruvudainambi, S.; Senthil, N.; Beaulah, A.; Harish, S. Chemical inducers in priming the induction of defense enzymes and phenols in banana and resistance to soft rot disease caused by Pectobacterium carotovorum subsp. carotovorum. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2806–2817. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, C.; Sun, W.; Tian, Y.; Xie, H. Characteristics and rapid diagnosis of Pectobacterium carotovorum ssp. associated with bacterial soft rot of vegetables in China. Plant Dis. 2020, 104, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Tsuji, G.; Higashiyama, M.; Ogiyama, H.; Umemura, K.; Mitomi, M.; Kubo, Y.; Kosaka, Y. Biological control of bacterial soft rot in Chinese cabbage by Lactobacillus plantarum strain BY under field conditions. Biol. Control 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Lin, S.-T.; Li, A.-T.; Li, S.-H.; Hsiao, C.-Y.; Lin, Y.-H. Bacillus amyloliquefaciens PMB05 increases resistance to bacterial wilt by activating MAPK and ROS pathway crosstalk in Arabidopsis thaliana. Phytopathology 2022, 112, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Chuang, C.Y.; Zheng, J.L.; Chen, H.H.; Liang, Y.S.; Huang, T.P.; Lin, Y.H. Bacillus amyloliquefaciens strain PMB05 intensifies plant immune responses to confer resistance against bacterial wilt of tomato. Phytopathology 2020, 110, 1877–1885. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Fu, J.-Y.; Chao, S.-H.; Tzean, Y.; Hsiao, C.-Y.; Yang, Y.-Y.; Chen, Y.-K.; Lin, Y.-H. Postharvest application of Bacillus amyloliquefaciens PMB04 fermentation broth reduces anthracnose occurrence in mango fruit. Agriculture 2022, 12, 1646. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Chen, X.; Wang, F.; Hsiao, C.-Y.; Yang, C.-Y.; Lin, S.-T.; Wu, L.-H.; Chen, Y.-K.; Liang, Y.-S.; Lin, Y.-H. Bacillus amyloliquefaciens strains control strawberry anthracnose through antagonistic activity and plant immune response intensification. Biol. Control 2021, 157, 104592. [Google Scholar] [CrossRef]
- Li, A.-T.; Liu, S.-K.; Li, J.-R.; Blanco, S.D.; Tsai, H.-W.; Xie, J.-X.; Tsai, Y.-C.; Tzean, Y.; Lin, Y.-H. A mitogen-activated protein kinase pathway is required for Bacillus amyloliquefaciens PMB05 to enhance disease resistance to bacterial soft rot in Arabidopsis thaliana. Plants 2024, 13, 2591. [Google Scholar] [CrossRef]
- Wang, F.; Chao, S.-H.; Tsai, C.-H.; Blanco, S.D.; Yang, Y.-Y.; Lin, Y.-H. Developing fermentation liquid of Bacillus amyloliquefaciens PMB04 to control bacterial leaf spot of sweet pepper. Agriculture 2023, 13, 1456. [Google Scholar] [CrossRef]
- Ahsan, T.; Zang, C.; Yu, S.; Pei, X.; Xie, J.; Lin, Y.; Liu, X.; Liang, C. Screening, and optimization of fermentation medium to produce secondary metabolites from Bacillus amyloliquefaciens, for the biocontrol of early leaf spot disease, and growth promoting effects on Peanut (Arachis hypogaea L.). J. Fungi 2022, 8, 1223. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-K.; Liang, Y.-S.; Hsiao, C.-Y.; Wang, F.; Huang, T.-P.; Lin, Y.-H. Application of fermentation broth of Bacillus amyloliquefaciens PMB05 to control bacterial canker disease on lemon. J. Plant Med. 2021, 63, 17–26. [Google Scholar]
- Etesami, H. Enhancing crop disease management through integrating biocontrol bacteria and silicon fertilizers: Challenges and opportunities. J. Environ. Manag. 2024, 371, 123102. [Google Scholar] [CrossRef]
- Lyu, L.; Bi, Y.; Li, S.; Xue, H.; Li, Y.; Prusky, D.B. Sodium silicate prime defense responses in harvested muskmelon by regulating mitochondrial energy metabolism and reactive oxygen species production. Food Chem. 2019, 289, 369–376. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Jiang, N.; Yan, J.; Lin, W.; Cai, K. Silicon controls bacterial wilt disease in tomato plants and inhibits the virulence-related gene expression of Ralstonia solanacearum. Int. J. Mol. Sci. 2022, 23, 6965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.-H.; Zhang, S.-H. Effects of combined application of potassium silicate and salicylic acid on the defense response of hydroponically grown tomato plants to Ralstonia solanacearum infection. Sustainability 2021, 13, 3750. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Si, J.; Römheld, V. Effects of foliar-and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, X.; Li, C.; Ayaz, M.; Abdulsalam, S.; Peng, D.; Qi, R.; Peng, H.; Kong, L.; Jia, J. Silicon mediated plant immunity against nematodes: Summarizing the underline defence mechanisms in plant nematodes interaction. Int. J. Mol. Sci. 2022, 23, 14026. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculik, M.; Corpas, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2021, 408, 124820. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701–715. [Google Scholar] [CrossRef]
- Zakaria, M.A.T.; Sakimin, S.Z.; Ismail, M.R.; Ahmad, K.; Kasim, S.; Baghdadi, A. Biostimulant activity of silicate compounds and antagonistic bacteria on physiological growth enhancement and resistance of banana to Fusarium wilt disease. Plants 2023, 12, 1124. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Alippi, A.M.; López, A.C. First Report of Pectobacterium carotovorum subsp. carotovorum on Spathiphyllum wallisii in Argentina. Plant Dis. 2009, 93, 842. [Google Scholar]
- Mavrič Čermelj, A.; Fideršek, E.; Golob, A.; Kacjan Maršić, N.; Vogel Mikuš, K.; Germ, M. Different concentrations of potassium silicate in nutrient solution affects selected growth characteristics and mineral composition of barley (Hordeum vulgare L.). Plants 2022, 11, 1405. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Hase, T.; Shimizu, M.; Hyakumachi, M. Suppressive effects of a polymer sodium silicate solution on powdery mildew and root rot diseases of miniature rose. Afr. J. Biotechnol. 2015, 14, 2917–2927. [Google Scholar] [CrossRef]
- Li, X.; Ren, X.; Ibrahim, E.; Kong, H.; Wang, M.; Xia, J.; Wang, H.; Shou, L.; Zhou, T.; Li, B. Response of Chinese cabbage (Brassica rapa subsp. pekinensis) to bacterial soft rot infection by change of soil microbial community in root zone. Front. Microbiol. 2024, 15, 1401896. [Google Scholar]
- Turan, M.; Ekinci, M.; Yildirim, E.; Güneş, A.; Karagöz, K.; Kotan, R.; Dursun, A. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk. J. Agric. For. 2014, 38, 327–333. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Ekinci, M.; Dursun, A.; Gunes, A.; Donmez, M. Growth and mineral content of cabbage seedlings in response to nitrogen fixing rhizobacteria treatment. Rom. Biotechnol. Lett. 2015, 20, 10929–10935. [Google Scholar]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Risoli, S.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, A. Trichoderma-induced resistance to Botrytis cinerea in Solanum species: A meta-analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef]
- Risoli, S.; Petrucci, A.; Vicente, I.; Sarrocco, S. Trichoderma gamsii T6085, a biocontrol agent of Fusarium head blight, modulates biocontrol-relevant defence genes expression in wheat. Plant Pathol. 2023, 72, 1442–1452. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y.; Fu, X.; Wu, F. Application of sodium silicate enhances cucumber resistance to Fusarium wilt and alters soil microbial communities. Front. Plant Sci. 2018, 9, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Tian, S.; Guo, Y.; Ge, Y.; Qin, G. Sodium silicate reduces postharvest decay on Hami melons: Induced resistance and fungistatic effects. Plant Dis. 2006, 90, 279–283. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Saleh, M.A.; Atala, S.A.; Bardisi, E.A. Effect of foliar application with potassium silicate and seaweed extract on plant growth, productivity, quality attributes and storability of potato. Sci. J. Agric. Sci. 2024, 6, 78–96. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.-S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.-K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Riedelsberger, J.; Miller, J.K.; Valdebenito-Maturana, B.; Piñeros, M.A.; González, W.; Dreyer, I. Plant HKT channels: An updated view on structure, function and gene regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef]
- Yoshioka, H.; Hino, Y.; Iwata, K.; Ogawa, T.; Yoshioka, M.; Ishihama, N.; Adachi, H. Dynamics of plant immune MAPK activity and ROS signaling in response to invaders. Physiol. Mol. Plant Pathol. 2023, 125, 102000. [Google Scholar] [CrossRef]
- Markelova, N.; Chumak, A. Antimicrobial activity of Bacillus cyclic lipopeptides and their role in the host adaptive response to changes in environmental conditions. Int. J. Mol. Sci. 2025, 26, 336. [Google Scholar] [CrossRef]
- Nasser, A.; Bhai, S.R. Inhibition of Pythium myriotylum by silicates and its impact on soft rot disease of ginger (Zingiber officinale Rosc.). Arch. Phytopathol. Plant Prot. 2021, 54, 702–721. [Google Scholar] [CrossRef]
- Abd-El-Kareem, F.; Elshahawy, I.E.; Abd-Elgawad, M.M. Management of strawberry leaf blight disease caused by Phomopsis obscurans using silicate salts under field conditions. Bull. Natl. Res. Cent. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Carré-Missio, V.; Rodrigues, F.; Schurt, D.; Resende, R.; Souza, N.; Rezende, D.; Moreira, W.; Zambolim, L. Effect of foliar-applied potassium silicate on coffee leaf infection by Hemileia vastatrix. Ann. Appl. Biol. 2014, 164, 396–403. [Google Scholar] [CrossRef]
- Khalifa, M.M.A.; Fetyan, N.A.H.; Magid, M.S.A.; El-Sheery, N.I. Effectiveness of potassium silicate in suppression white rot disease and enhancement physiological resistance of onion plants, and its role on the soil microbial community. Middle East. J. Agric. Res. 2017, 6, 376–394. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, S.D.; Li, J.-R.; Yan, J.-C.; Yen, T.-B.; Huang, T.-P.; Lin, Y.-H. Comparative Effects of Sodium Metasilicate and Potassium Silicate in Enhancing Bacillus amyloliquefaciens PMB05 Plant Immune Responses and Control of Bacterial Soft Rot in Cabbage. Agriculture 2025, 15, 2436. https://doi.org/10.3390/agriculture15232436
Blanco SD, Li J-R, Yan J-C, Yen T-B, Huang T-P, Lin Y-H. Comparative Effects of Sodium Metasilicate and Potassium Silicate in Enhancing Bacillus amyloliquefaciens PMB05 Plant Immune Responses and Control of Bacterial Soft Rot in Cabbage. Agriculture. 2025; 15(23):2436. https://doi.org/10.3390/agriculture15232436
Chicago/Turabian StyleBlanco, Sabrina Diana, Jia-Rong Li, Jo-Ching Yan, Tsair-Bor Yen, Tzu-Pi Huang, and Yi-Hsien Lin. 2025. "Comparative Effects of Sodium Metasilicate and Potassium Silicate in Enhancing Bacillus amyloliquefaciens PMB05 Plant Immune Responses and Control of Bacterial Soft Rot in Cabbage" Agriculture 15, no. 23: 2436. https://doi.org/10.3390/agriculture15232436
APA StyleBlanco, S. D., Li, J.-R., Yan, J.-C., Yen, T.-B., Huang, T.-P., & Lin, Y.-H. (2025). Comparative Effects of Sodium Metasilicate and Potassium Silicate in Enhancing Bacillus amyloliquefaciens PMB05 Plant Immune Responses and Control of Bacterial Soft Rot in Cabbage. Agriculture, 15(23), 2436. https://doi.org/10.3390/agriculture15232436






