Performance and Stress Tolerance of Poppy (Papaver somniferum L.) in Response to Biostimulant Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Open Field Experiment
2.2. Phytotron Experiment
2.3. Analytical Testing Methods
2.4. Statistical Methods
3. Results
3.1. Yield and Active Ingredient Accumulation in the Field Experiment
3.2. Morpho-Phenological Characteristics of Poppy Plants in the Phytotron Experiment
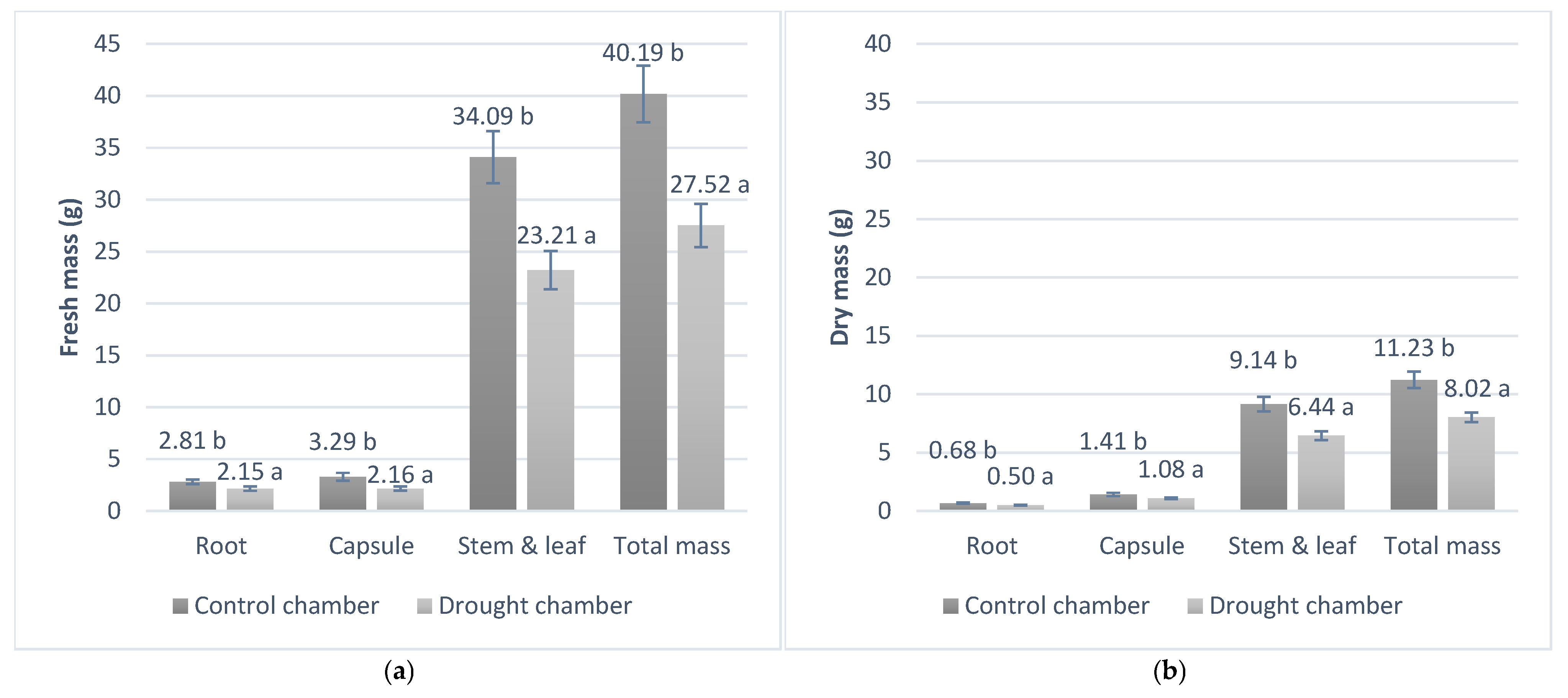
3.3. Development and Plant Mass in the Phytotron Experiment
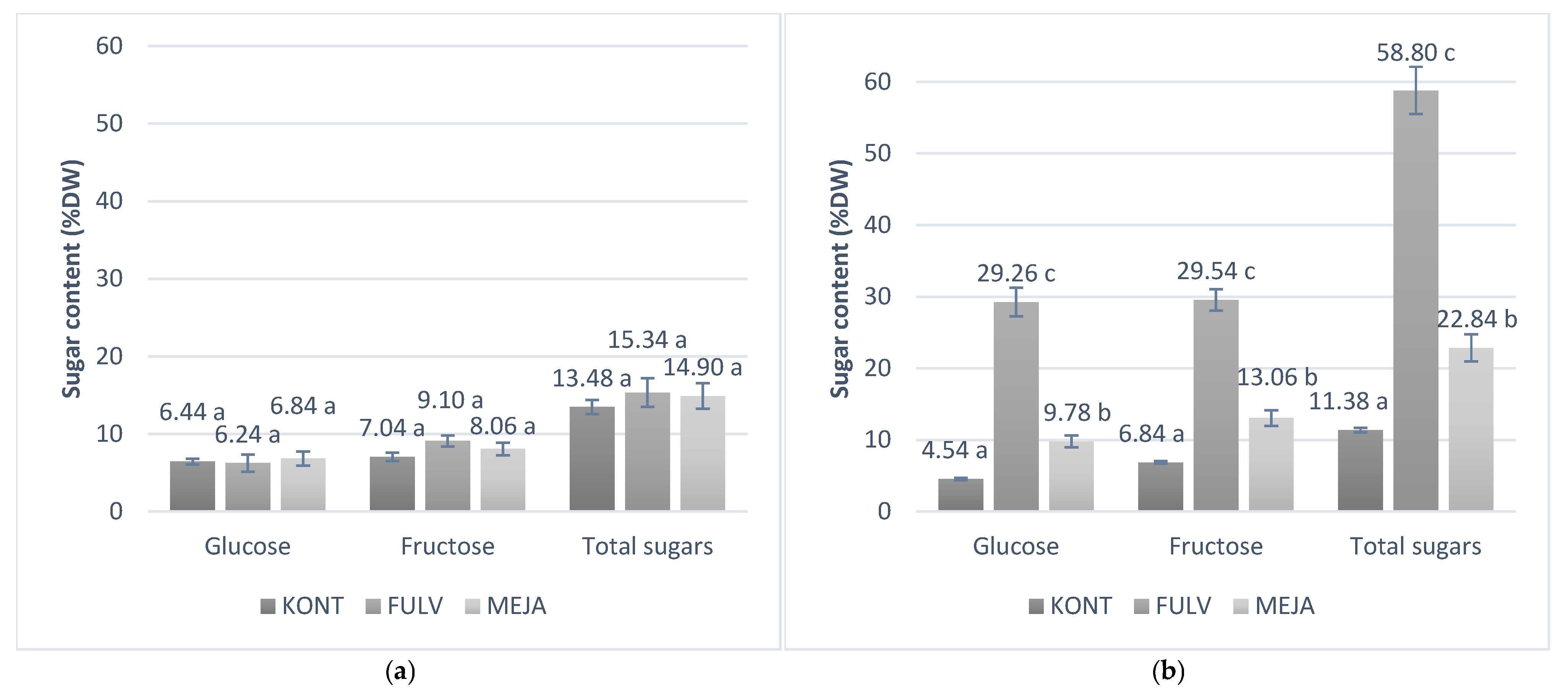
3.4. Stress Markers and Antioxidant Enzymes in Phytotron
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MEJA | methyl jasmonate |
| FULV | fulvic acid |
| KONT | control plants (untreated) |
| SE | standard error |
| AC | antioxidant capacity |
| AAE | ascorbic acid equivalent |
| TPC | total polyphenol content |
| GAE | gallic acid equivalent |
| GR | glutathione reductase |
| GST | glutathione S-transferase |
| APX | ascorbate peroxidase |
| CAT | catalase |
| GPX | guaiacol peroxidase |
| SOD | superoxide dismutase |
| POD | peroxidase |
Appendix A

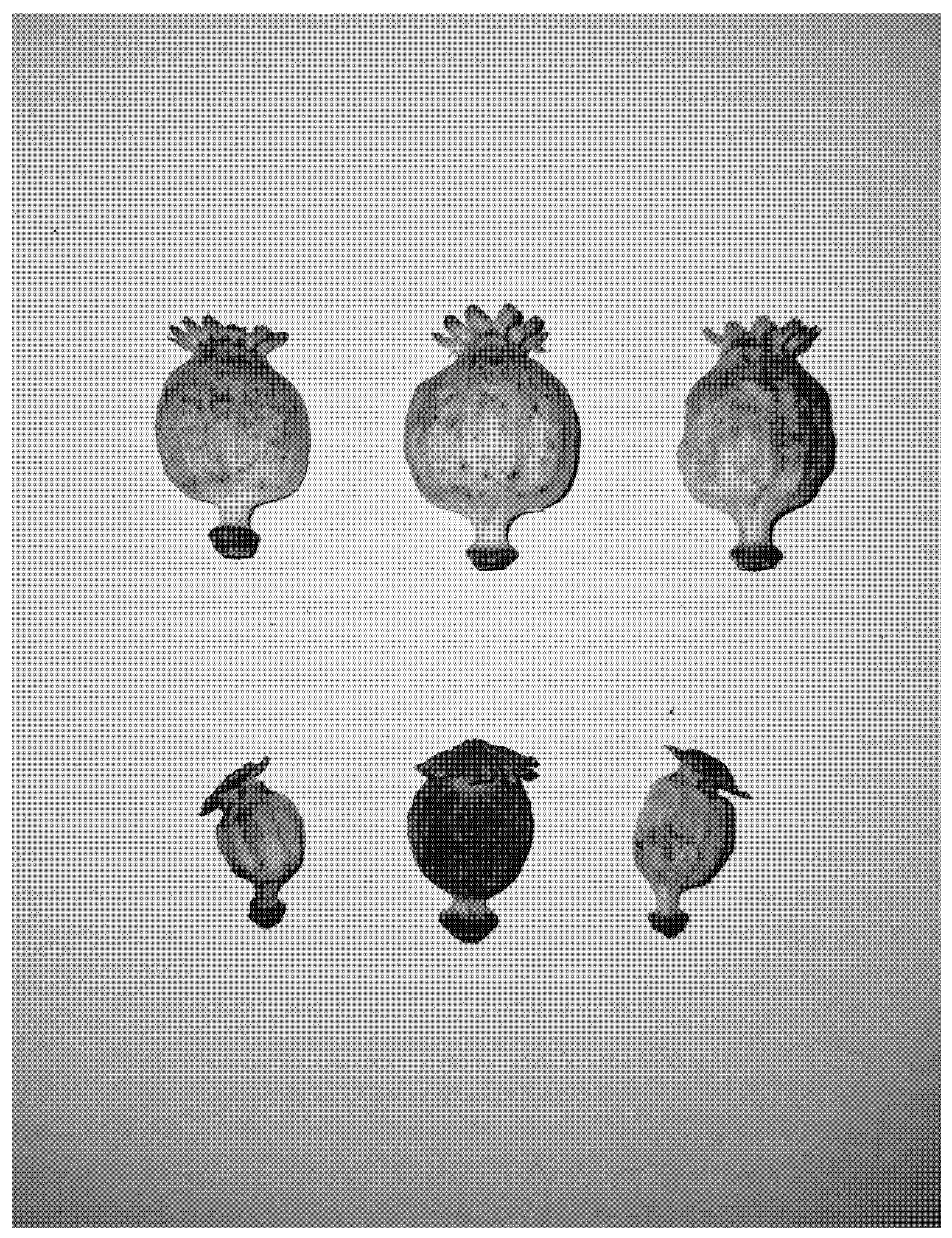
References
- Carod-Artal, F.J. Psychoactive plants in ancient Greece. Neurosci. Hist. 2013, 1, 28–38. [Google Scholar]
- Luqman, S. The saga of opium poppy: Journey from traditional medicine to modern drugs and nutraceuticals. Acta Hortic. 2011, 1036, 91–100. [Google Scholar] [CrossRef]
- Fejér, J.; Salamon, I. Agro-technology of the poppy: Large scale cultivation in Slovakia. Acta Hortic. 2014, 1036, 181–185. [Google Scholar] [CrossRef]
- Stranska, I.; Skalicky, M.; Novak, J.; Matyasova, E.; Hejnak, V. Analysis of selected poppy (Papaver somniferum L.) cultivars: Pharmaceutically important alkaloids. Ind. Crops Prod. 2013, 41, 120–126. [Google Scholar] [CrossRef]
- Schiff, P.L. Opium and its alkaloids. Am. J. Pharm. Educ. 2002, 66, 188–196. [Google Scholar]
- INCB. Report of the International Narcotics Control Board; United Nations: Vienna, Austria, 2025; pp. 3–4. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Arslan, N. Opium Poppy (Papaver somniferum). In Medicinal and Aromatic Plants of the Middle-East; Yaniv, Z., Dudai, N., Eds.; Medicinal and Aromatic Plants of the World; Springer: Dordrecht, The Netherlands, 2014; Volume 2. [Google Scholar] [CrossRef]
- Bartholy, J.; Pongracz, R.; Torma, C.; Pieczka, I.; Kardos, P.; Hunyady, A. Analysis of regional climate change modelling experiments for the Carpathian basin. Int. J. Glob. Warm. 2009, 1, 238–252. [Google Scholar] [CrossRef]
- Mezősi, G.; Bata, T.; Meyer, B.C.; Blanka, V.; Ladányi., Z. Climate change impacts on environmental hazards on the Great Hungarian Plain, Carpathian Basin. Int. J. Disaster Risk Sci. 2014, 5, 136–146. [Google Scholar] [CrossRef]
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Bernáth, J. (Ed.) Poppy: The Genus Papaver; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Kara, N. The effects of autumn and spring sowing on yield, oil and morphine contents in the Turkish poppy (Papaver somniferum L.) cultivars. Turk. J. Field Crops 2017, 22, 39–46. [Google Scholar] [CrossRef]
- Chung, B. The effect of irrigation on the growth and yield components of poppies (Papaver somniferum L.). J. Agric. Sci. 1987, 108, 389–394. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. The influence of sowing and planting seedlings at different dates in autumn on the yield and quality of the opium poppy (Papaver somniferum L.). J. Appl. Res. Med. Aromat. Plants 2021, 21, 100290. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; Volume 7, pp. 1–9. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 1–16. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Maleki, A.; Naderi, A.; Naseri, R.; Fathi, A.; Bahamin, S.; Maleki, R. Physiological performance of soybean cultivars inder drought stress. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 38–44. [Google Scholar]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies–A review. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Kandoudi, W.; Németh-Zámboriné, É. Stimulating secondary compound accumulation by elicitation: Is it a realistic tool in medicinal plants in vivo? Phytochem. Rev. 2022, 21, 2007–2025. [Google Scholar] [CrossRef]
- Esfahani, S.T.; Karimzadeh, G.; Naghavi, M.R.; Vrieling, K. Altered gene expression and root thebaine production in polyploidized and methyl jasmonate-elicited Papaver bracteatum Lindl. Plant Physiol. Biochem. 2021, 158, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Májer, P.; Sotkó, G.; Zámboriné Németh, É. Termésnövelő anyagok szerepe az aszály okozta stresszhatások kivédésében ipari mák kultúrában. Hortic./Kertgazdaság 2022, 54, 51–65. [Google Scholar]
- Májer, P.; Németh, É.Z. Alkaloid accumulation and distribution within the capsules of two opium poppy (Papaver somniferum L.) varieties. Plants 2024, 13, 1640. [Google Scholar] [CrossRef]
- Reynolds, S.G. The gravimetric method of soil moisture determination. Part I. A study of equipment, and methodological problems. J. Hydrol. 1970, 11, 258–273. [Google Scholar] [CrossRef]
- Ádám, A.; Bestwic, C.S.; Barna, B.; Mansfield, J.W. Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. phaseolicola. Planta 1995, 197, 240–249. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Guthenberg, C. Glutathione transferase (Human placenta). Methods Enzymol. 1981, 77, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Molnár, M.; Ács-Szabó, L.; Papp, L.A.; Cziáky, Z.; Miklós, I. Insight into the Yeast Diversity of Hungarian Honeys. Diversity 2025, 17, 325. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sheteawi, S.A. Improving growth and yield of salt-stressed soybean by exogenous application of jasmonic acid and ascobin. Int. J. Agric. Biol. 2007, 9, 473–478. [Google Scholar]
- Ren, Y.; Yang, F.; Dai, W.; Yuan, C.; Qin, Y.; Li, J.; Zhang, M. The effect and potential mechanism of fulvic acid on flavonoids in lemon leaves. Horticulturae 2024, 10, 144. [Google Scholar] [CrossRef]
- Nofal, E.M.S.; Menesy Fardous, A.; Abd El-hady, W.M.; Shehab El-Deen Eman, G. Effect of bio-stimulants (humic acid, salicylic acid and chitosan) on rose periwinkle (Catharanthus roseus L.). Appl. Ecol. Environ. Res. 2021, 19, 971–980. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Alsanam, M.A.; Al-Ma’athedi, A.F.; Zahwan, T.A. Response of tuberose (Polianthes tuberosa L.) cormels to humic acid and phosphorus fertilization to increase the production, quality and lycorine alkaloid contents of the produced corms. Int. J. Agric. Stat. Sci. 2022, 18, 619. [Google Scholar]
- Ghasemi Pirbalouti, A.; Rahmani Samani, M.; Hashemi, M.; Zeinali, H. Salicylic acid affects growth, essential oil and chemical compositions of thyme (Thymus daenensis Celak.) under reduced irrigation. Plant Growth Regul. 2014, 72, 289–301. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Rakkammal, K.; Largia, M.J.V.; Thamilarasan, S.K.; Balaji, S.; Zoclanclounon, Y.A.B.; Silpha, J.; Ramesh, M. Jasmonates in plant growth and development and elicitation of secondary metabolites: An updated overview. Front. Plant Sci. 2022, 13, 942789. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- Jászberényi, C.; Lukacs, L.; Inotai, K.; Németh, É. Soluble sugar content in poppy (Papaver somniferum L.) and its relationship to winter hardiness. Z. Für Arznei Gewürzpflanzen 2012, 17, 169–174. [Google Scholar]
- Chen, Y.L.; Zou, Z.R.; Yang, S.L. Effect of exogenous methyl jasmonate on osmotic adjustment capacity and proline metabolism of Jatropha curcas seedlings under salt stress. Acta Bot. Boreali-Occident. Sin. 2023, 43, 794–804. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Wang, X.; Feng, J.; Zhu, S. Potassium fulvic acid alleviates salt stress of citrus by regulating rhizosphere microbial community, osmotic substances and enzyme activities. Front. Plant Sci. 2023, 14, 1161469. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Li, J.; Yang, F.; Dai, W.; Xiang, C.; Zhang, M. The positive effects of humic/fulvic acid fertilizers on the quality of lemon fruits. Agronomy 2022, 12, 1919. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Wang, Y.; Chen, Y.; Hou, H.; Dai, Q. Methyl jasmonate alleviates the deleterious effects of salinity stress by augmenting antioxidant enzyme activity and ion homeostasis in rice (Oryza sativa L.). Agronomy 2022, 12, 2343. [Google Scholar] [CrossRef]
- Keramat, B.; Kalantari, K.M.; Arvin, M.J. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr. J. Microbiol. Res. 2009, 3, 240–244. [Google Scholar]
- Harshavardhan, V.T.; Wu, T.M.; Hong, C.Y. Glutathione Reductase and Abiotic Stress Tolerance in Plants. In Glutathione in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Mostofa, M.G., Diaz-Vivancos, P., Burritt, D.J., Fujita, M., Tran, L.-S.P., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 265–286. [Google Scholar] [CrossRef]


| Time (Day) | Phenophase | Temperature Day/Night, (°C) | Light Cycle Day/Night (Hour) | SWC Saturation (%) | Relative Humidity (%) | Light Intensity (lux) | |
|---|---|---|---|---|---|---|---|
| Control | Drought | ||||||
| 16 (day 1–16) | germination | 13/8 | 10/14 | 75 | 60 ± 2 | 16,000 | |
| 59 (day 17–75) | rosette-formation | 20/12 | 12/12 | 75 | 60 ± 2 | 16,000 | |
| 27 (day 76–102) | stem growth | 22/12 | 14/10 | 75 | 50 | 50 ± 2 | 16,000 |
| 7 (day 103–109) | flowering | 26/15 | 14/10 | 75 | 50 | 50 ± 2 | 16,000 |
| 14 (day 110–124) | capsule growth | 26/15 | 14/10 | 75 | 50 | 50 ± 2 | 16,000 |
| 12 (day 125–136) | maturation | 26/15 | 14/10 | 75 | 50 | 50 ± 2 | 16,000 |
| KONT | FULV | MEJA | |
|---|---|---|---|
| Seed yield (g/m2) | 73.25 ± 2.26 a | 85.00 ± 2.24 b | 109.3 ± 4.53 c |
| Capsule yield (g/m2) | 57.50 ± 2.07 a | 75.08 ± 2.27 b | 79.20 ± 2.77 b |
| Total yield (g/m2) | 130.75 ± 4.03 a | 160.08 ± 3.97 b | 188.50 ± 6.96 c |
| Seed ratio (% of total mass) | 56.02 ± 0.01 b | 53.10 ± 0.01 a | 57.98 ± 0.01 b |
| Capsule ratio (% of total mass) | 43.98 ± 0.01 a | 46.90 ± 0.01 b | 42.02 0.01 a |
| Morphine yield (g/m2) | 1.15 ± 0.05 a | 1.31 ± 0.04 a | 1.67 ± 0.09 b |
| Total alkaloid yield (g/m2) | 1.25 ± 0.05 a | 1.39 ± 0.04 a | 1.79 ± 0.10 b |
| Water Supply | Control Chamber | Drought Chamber | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | KONT | FULV | MEJA | Mean | KONT | FULV | MEJA | Mean |
| Plant height (cm) | 80.50 ± 2.73 b | 82.18 ± 1.27 b | 69.88 ± 2.69 a | 77.52 ± 2.20 B | 51.57 ± 0.94 a | 53.75 ± 1.23 a | 53.94 ± 0.92 a | 53.09 ± 1.02 A |
| Root length (cm) | 20.13 ± 1.15 a | 19.95 ± 0.66 a | 19.47 ± 0.82 a | 19.85 ± 0.90 A | 18.50 ± 1.11 a | 23.06 ± 2.30 a | 22.16 ± 1.42 a | 21.24 ± 1.60 A |
| Position of stem leaves | non stem-enclosing | stem-enclosing | ||||||
| Water Supply | Control Chamber | Drought Chamber | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | KONT | FULV | MEJA | Mean | KONT | FULV | MEJA | Mean |
| AC (mgAAE/mL) *** | 0.1585 ± 0.0010 a | 0.1777 ± 0.0013 b | 0.1725 ± 0.0027 b | 0.1696 ± 0.0022 A | 0.1776 ± 0.0011 a | 0.1849 ± 0.0022 b | 0.1981 ± 0.0015 c | 0.1884 ± 0.0022 B |
| TPC (mgGAE/mL) *** | 0.1420 ± 0.0090 a | 0.1900 ± 0.0038 c | 0.1728 ± 0.0069 b | 0.1683 ± 0.0060 A | 0.2009 ± 0.0011 b | 0.2300 ± 0.0033 c | 0.1836 ± 0.0021 a | 0.2048 ± 0.0049 B |
| proline (nmol/gFW) | 92.41 ± 6.40 a | 89.05 ± 13.85 a | 78.10 ± 3.52 a | 86.52 ± 4.78 A | 169.02 ± 28.96 a | 282.89 ± 87.17 a | 155.63 ± 18.03 a | 202.51 ± 30.89 B |
| GR (nkatal/gFW) *** | 16.93 ± 1.31 a | 12.87 ± 0.20 a | 12.48 ± 0.88 a | 14.09 ± 1.11 A | 17.11 ± 1.96 b | 17.13 ± 2.11 b | 9.02 ± 1.24 a | 14.42 ± 1.20 A |
| GST (nkatal/gFW) | 3.31 ± 0.49 a | 3.28 ± 0.28 a | 3.12 ± 0.31 a | 3.24 ± 0.18 B | 2.94 ± 0.34 a | 2.39 ± 0.39 a | 2.08 ± 0.22 a | 2.47 ± 0.22 A |
| APX (nkatal/gFW) * | 45.43 ± 2.73 a | 54.04 ± 3.51 a | 44.73 ± 0.77 a | 48.07 ± 1.93 A | 45.74 ± 1.67 ab | 51.48 ± 3.57 b | 36.80 ± 3.91 a | 44.67 ± 2.47 A |
| CAT (nkatal/gFW) | 1296 ± 585 a | 1158 ± 629 a | 1452 ± 454 a | 1302 ± 302 A | 1076 ± 483 a | 1479 ± 281 a | 2085 ± 443 a | 1547 ± 456 A |
| GPX (nkatal/gFW) | 209.81 ± 93.13 a | 93.47 ± 37.9 a | 183.14 ± 95.47 a | 162.14 ± 44.81 B | 20.91 ± 8.06 a | 30.17 ± 3.71 a | 25.47 ± 0.80 a | 25.51 ± 3.08 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Májer, P.; Németh, É.Z. Performance and Stress Tolerance of Poppy (Papaver somniferum L.) in Response to Biostimulant Treatments. Agriculture 2025, 15, 2386. https://doi.org/10.3390/agriculture15222386
Májer P, Németh ÉZ. Performance and Stress Tolerance of Poppy (Papaver somniferum L.) in Response to Biostimulant Treatments. Agriculture. 2025; 15(22):2386. https://doi.org/10.3390/agriculture15222386
Chicago/Turabian StyleMájer, Péter, and Éva Zámboriné Németh. 2025. "Performance and Stress Tolerance of Poppy (Papaver somniferum L.) in Response to Biostimulant Treatments" Agriculture 15, no. 22: 2386. https://doi.org/10.3390/agriculture15222386
APA StyleMájer, P., & Németh, É. Z. (2025). Performance and Stress Tolerance of Poppy (Papaver somniferum L.) in Response to Biostimulant Treatments. Agriculture, 15(22), 2386. https://doi.org/10.3390/agriculture15222386






