Effects of Differential Tobacco Straw Incorporation on Functional Gene Profiles and Functional Groups of Soil Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Test Site
2.2. Experimental Design
2.3. Test Method
2.3.1. Experimental Soil
2.3.2. Determination of Soil Physical and Chemical Properties
2.3.3. Soil DNA Extraction and Metagenomic Sequencing
2.3.4. Soil Microbial Function Annotation
2.3.5. Data Analysis
3. Results and Analysis
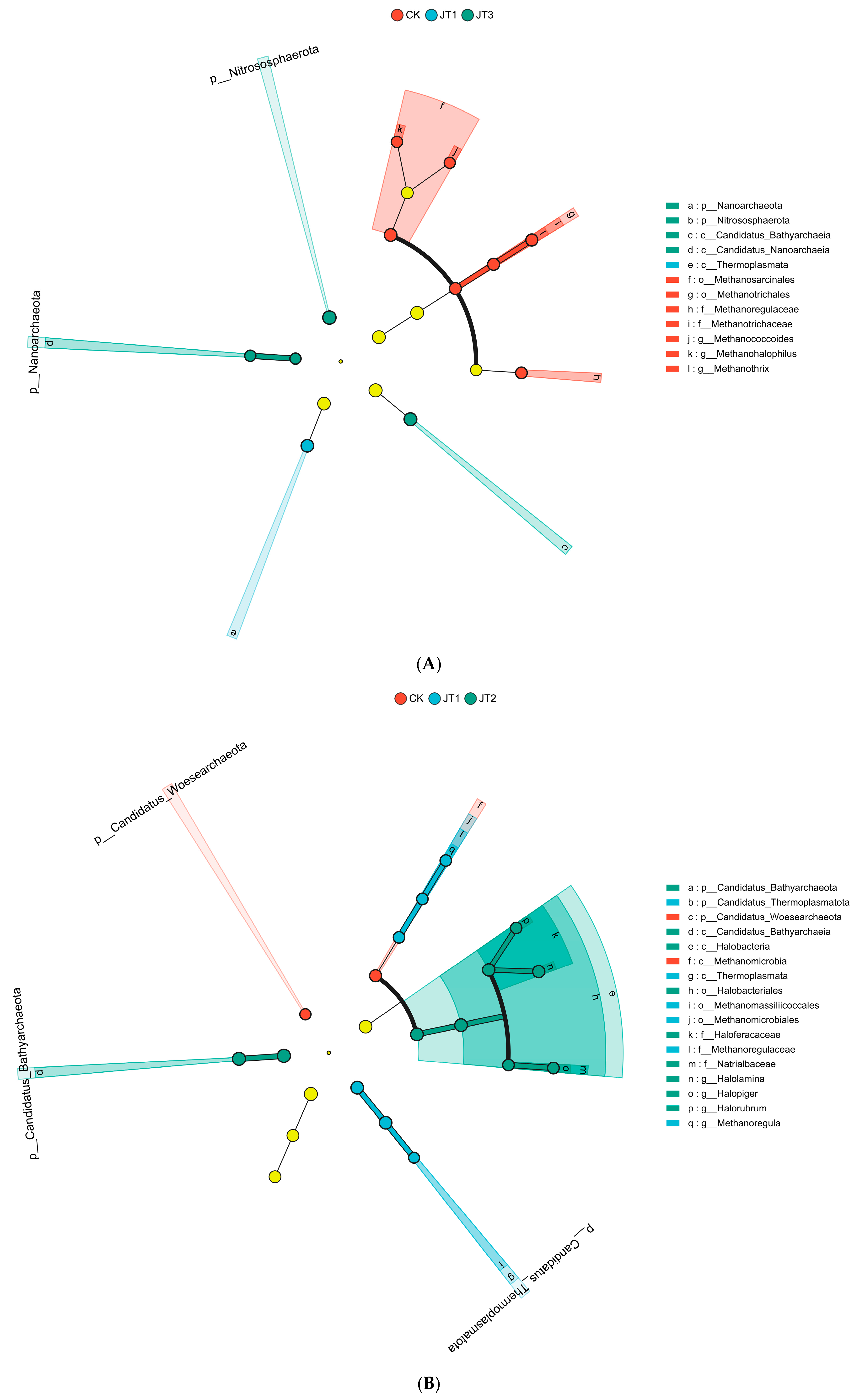
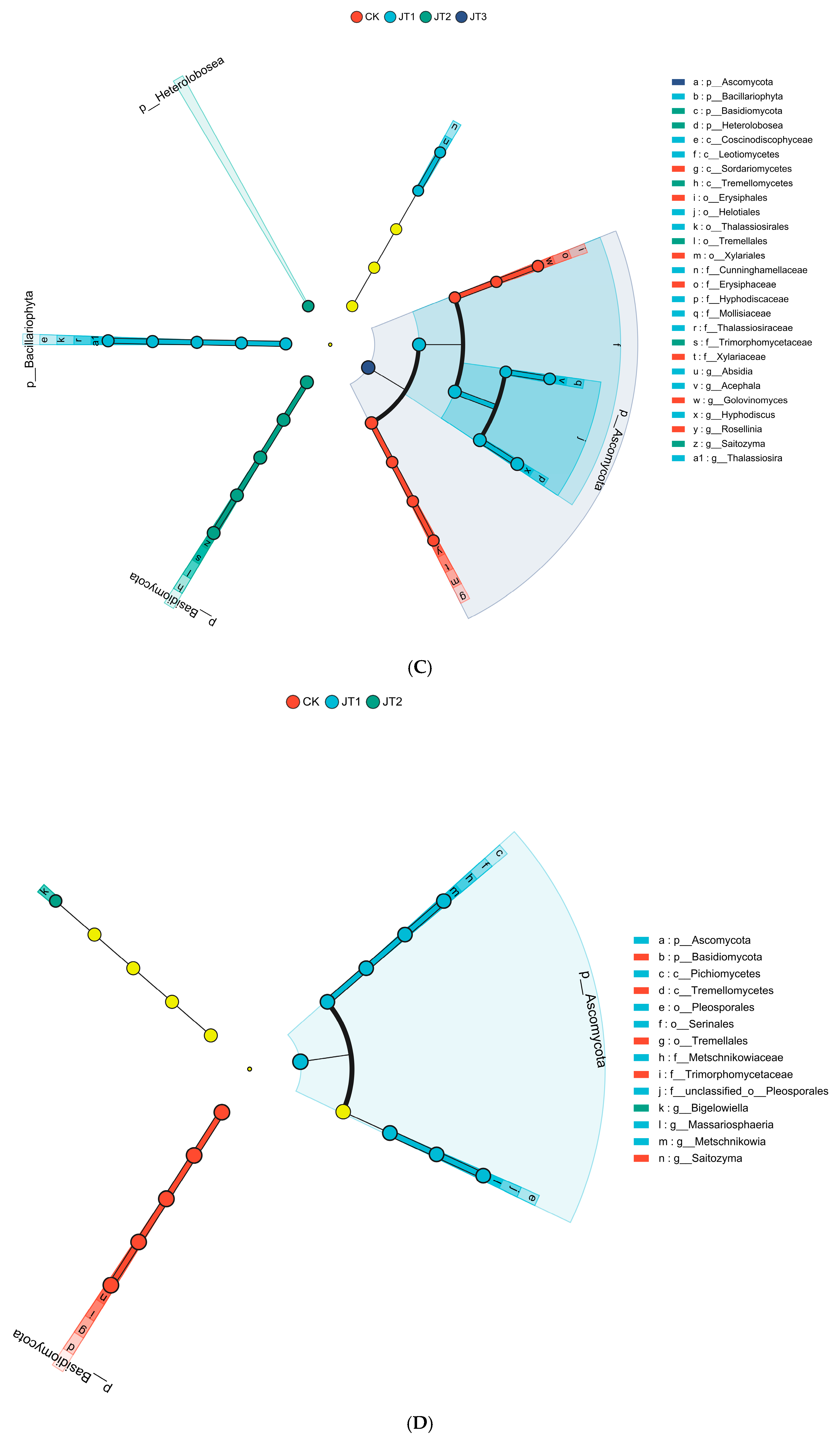
3.1. Effects of Different Tobacco Straw Returning Modes on Soil Microbial Functional Genes and Functional Group Diversity
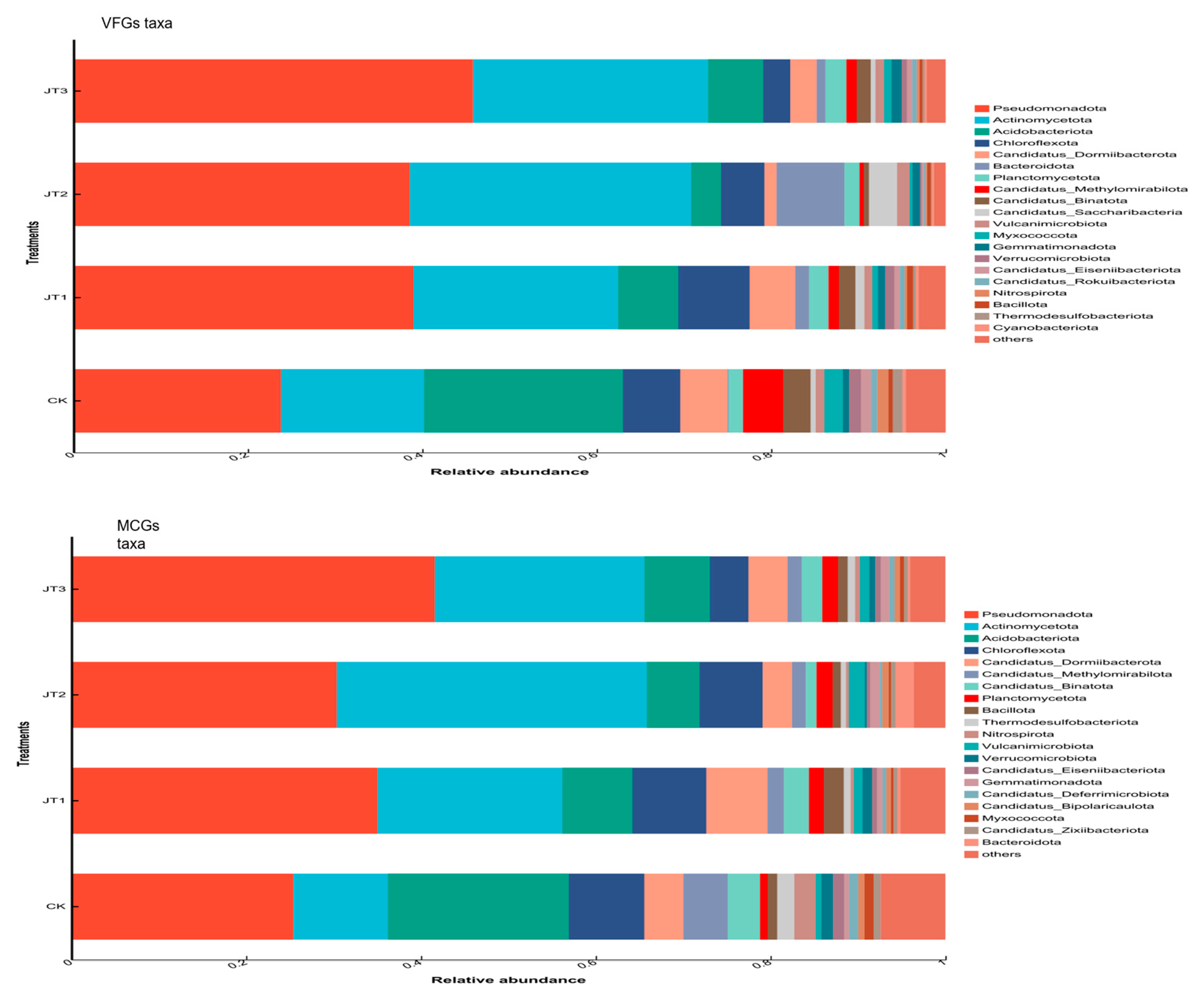
3.2. Effects of Different Tobacco Straw Returning Modes on the Abundance of Soil Microbial Functional Groups
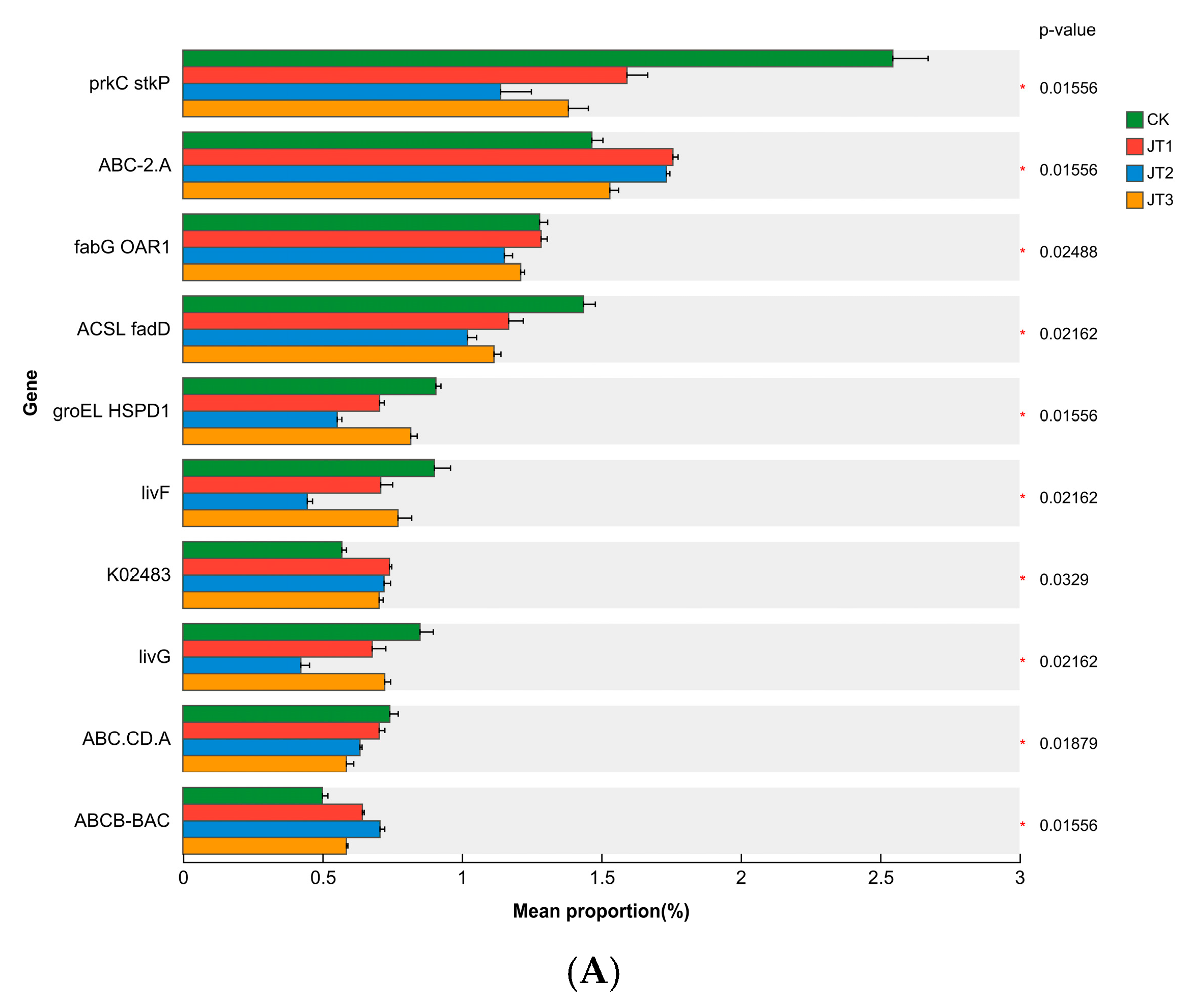
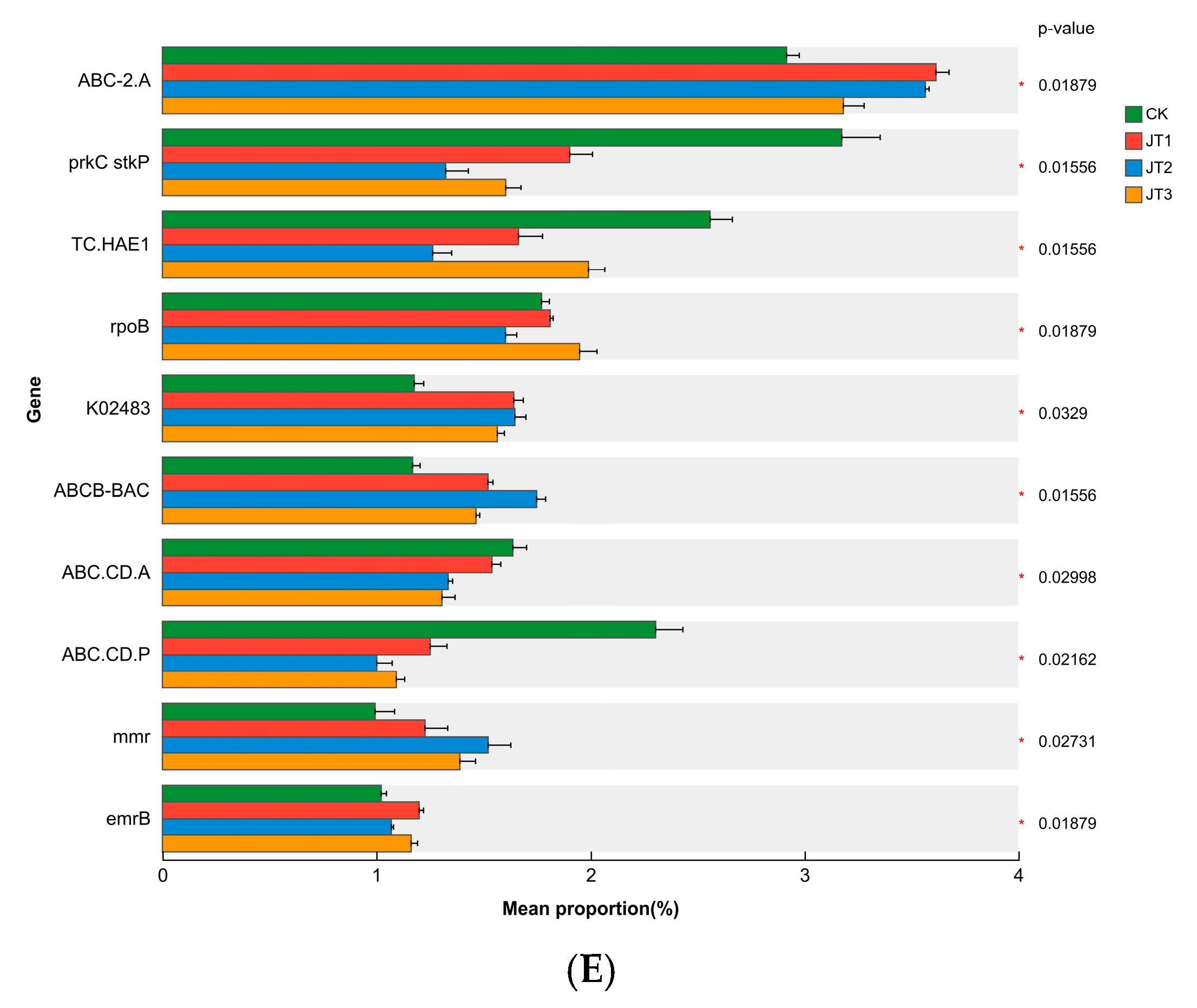
3.3. Effects of Different Tobacco Straw Returning Modes on the Abundance of Soil Microbial Functional Genes
3.4. The Potential Impact of Different Tobacco Straw Returning Modes on the Ecological Environment
3.5. Effects of Environmental Factors on Soil Microbial Functional Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, X.H. The development status, characteristics and future prospects of China’s tobacco industry. China Circ. Econ. 2021, 29, 8–10. [Google Scholar]
- Xiong, D.; Cai, H.; Luo, G. Analysis of the physical and chemical characteristics of Fujian tobacco-growing soils. Chin. J. Eco-Agric. 2007, 15, 21–24. [Google Scholar]
- Xiong, Y.M.; Huang, G.Q.; Wang, S.B.; Liu, L.W. Effect of crop rotation in paddy field on soil physical and chemical characteristics and crop yield. Rev. China Agric. Sci. Technol. 2004, 4, 42–45. [Google Scholar]
- Zhao, M.; Liu, Y.; Zhang, X. A review of research advances on carbon sinks in farmland ecosystems. Acta Ecol. Sin. 2022, 23, 9405–9416. [Google Scholar] [CrossRef]
- Gou, H.; Liu, J.C. Study on comprehensive utilization of discard tobacco. Jiangsu Agric. Sci. 2014, 42, 220–223. [Google Scholar]
- Cao, X.H.; Tang, X.B.; Tian, W.H.; Peng, Y.B.; Jiang, S.; Song, C.H. Recycling of wasted tobacco stems in Chongqing tobacco-growing area. J. Anhui Agric. Sci. 2017, 45, 86–88. [Google Scholar]
- Xiao, H.Q.; Tu, N.M.; Guan, G.S.; Wang, Z.M. Effects offlue-cured tobacco straw returning on late season rice production under tobacco-rice cropping pattern. J. Hunan Agric. Univ. 2008, 34, 154–158. [Google Scholar]
- He, L.S.; Liu, C.C. Study on the effect of tobacco straw manuring. Hunan Agric. Sci. 2002, 6, 34–35. [Google Scholar]
- Han, F.; Wang, R. Status quo and development strategy of green development of tobacco stalks organic fertilizer industrialization. Acta Tabacaria Sin. 2016, 22, 126–132. [Google Scholar]
- Chen, Y.; Chen, W.; Lin, Y.C.; Cheng, J.Z.; Pan, W.J. Effects of biochar on the micro-ecology of tobacco-planting soil and physiology of flue-cured tobacco. Chin. J. Appl. Ecol. 2015, 26, 3781–3787. [Google Scholar]
- Jiang, C.Q.; Shen, J.; Wang, H.Y.; Li, D.C.; Li, T.; Wang, W.J.; Zu, C.L. Effect of tobacco straw incorporation on rice yield and nutrient absorption and its substitute for potassium fertilizer. Chin. J. Appl. Ecol. 2016, 27, 3969–3976. [Google Scholar]
- Conrad, R. Microbial ecology of methanogens and methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Kuypers, M.M.; Hannah, K.M.; Boran, K. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Lombard, V.; Hemalatha, G.R.; Bernard, H.; Pedro, M.C.; Bernard, H. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Y.; Bing, L.; Huang, C.; Zhang, S. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef]
- Liu, Y. Meta-analysis of the impact of straw return on soil organic carbon sequestration in paddy fields. Sci. Total Environ. 2021, 799, 149398. [Google Scholar]
- Qin, X. A global synthesis of the trade-offs between greenhouse gas emissions and crop yield under straw retention. Glob. Change Biol. 2023, 29, 559–576. [Google Scholar]
- Zhu, D. Straw returning interacts with fertilizer to influence the soil antibiotic resistome and microbial community: A meta-analysis. J. Hazard. Mater. 2023, 454, 131469. [Google Scholar]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Li, C. Evaluation of soil fertility status of tobacco planting in Sanming City, Fujian Province. Soil Fertil. Sci. China 2020, 1, 147–154. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistly Analys, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Dane, J.H.; Topp, C.G. Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, MI, USA, 1982; pp. 159–165. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. S2), W316–W322. [Google Scholar] [CrossRef]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef]
- Richardson, D.; Heather, F.; Nick, W.; Andrew, T.; Elizabeth, B. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle: Could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jiang, C.Q.; Shen, J.; Li, T.; Wang, W.J.; Cui, Q.R.; Jing, L.L.; Zu, C.L. Decomposition rates and nutrient release patterns of tobacco straw. Soils 2017, 49, 543–549. [Google Scholar]
- Zhou, X.D.; Han, T.H.; Shen, Y.X. Response characteristics of soil microecology in long-term continuous cropping tobacco field under 4 rotation patterns. J. Agric. Sci. Technol. 2024, 26, 174–187. [Google Scholar]
- Zhao, C.; Li, Y.; Liu, Y.; Wang, X.; Zhao, W.; Huang, Y.; Li, H.; Ji, W. Effects of Rotating Cropping and Continuous Cropping on Soil Nutrients, Enzyme Activities and Microbial Community Structure of Rhizosphere Soil in Tobacco. Biotechnol. Bull. 2025, 41, 312–322. [Google Scholar]
- Su, Y.; Yu, M.; Xi, H. Soil microbial community shifts with long-term of different straw retum in wheat-orn rotation system. Sci. Rep. 2020, 10, 6360. [Google Scholar]
- Qiao, Y.Q.; Cao, C.F.; Zhao, Z. Effects of straw-returning and N-fertilizer application on yield, quality and occurrence of Fusarium head blight of wheat. J. Triticeae Crops 2013, 33, 727–731. [Google Scholar]
- Taobing, Y.; Lang, C.; Qing, Z.; Jida, Y.; Huadong, Z. Characterization of antibiotic resistance genes and virulence factors in organic managed tea plantation soils in southwestern China by metagenomics. Front. Microbiol. 2025, 16, 1580450. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wei, J.; Li, Z.; Zhou, X. Effects of Long-term Straw Returning on Fungal Community, Enzyme Activity and Wheat Yield in Fluvo-aquic Soil. Environ. Sci. 2022, 10, 4755–4764. [Google Scholar]
- Li, S.; Zhang, W.; Li, X.; Cui, J. Effect of straw returning on fertility and stem rot of black soil with different land fertility. J. Agric. Sci. Technol. 2021, 23, 80–90. [Google Scholar]
- Chen, Y. The effect of different straw returning measures on soil fungal community structure and the occurrence of wheat sheath blight. Plant Soil 2018, 433, 207–219. [Google Scholar]
- Yu, F.Y.; Chen, Y.; Huang, X.W. Does straw returning affect the root rot disease of crops in soil? A systematic review and meta-analysis. J. Environ. Manag. 2023, 336, 117673. [Google Scholar] [CrossRef]
- Zeng, Y.; Fu, Y.; Ye, X.; Meng, L.; Yan, S.; Li, G.; Yang, X.; Ding, L.; Li, S. Influence of Rotten Wheat Straw on Yield and Quality of Flue-Cured Tobacco. Chin. Tob. Sci. 2014, 5, 40–44. [Google Scholar]
- He, Y.D. Study on Fermentation, Soil Colonization and Control of Tobacco Soil-Borne Diseases of Two Biocontrol Agents; Fujian Agriculture and Forestry University: Fuzhou, China, 2019. [Google Scholar]
- Sjogersten, S.; Wookey, P.A. Spatio-temporal variability and environmental controls of methane fluxes at the forest-tundra ecotone in the Fennoscandian mountains. Glob. Change Biol. 2002, 8, 885–894. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, T.; Liu, Z.; Yin, Z.; Wang, Z. Effects of experimental warming on the decomposition properties of winter wheat root residue and straw. Ecol. Environ. Sci. 2019, 3, 472–480. [Google Scholar]
- Jiang, Y.; Qian, H.Y.; Huang, S.; Zhang, X.; Wang, L. Acclimation of methane emissions from rice paddy fields to straw addition. Sci. Adv. 2019, 5, eaau9038. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, T.; Liu, Z.; Yin, Z.; Wang, Z. Higher rice yields and lower methane emissions can be reconciled for rice cultivation: A review. J. Nanjing Agric. Univ. 2022, 5, 839–847. [Google Scholar]
- Li, F.Y.; Cao, X.D.; Zhao, L.; Fan, Y.; Wang, J. Short-term effects of raw rice straw and its derived biochar on greenhouse gas emission in five typical soils in China. Soil Sci. Plant Nutr. 2013, 59, 800–811. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.K.; Liao, S.P.; Ren, T.; Li, X. Differences in responses of ammonia volatilization and greenhouse gas emissions to straw return and paddy upland rotations. Environ. Sci. Pollut. Res. 2022, 29, 25296–25307. [Google Scholar] [CrossRef]
- Hu, N.J.; Wang, B.J.; Gu, Z.H.; Tao, B.; Zhang, Z.W. Effects of different straw returning modes on greenhouse gas emissions and crop yields in a rice—Wheat rotation system. Agric. Ecosyst. Environ. 2016, 223, 115–122. [Google Scholar] [CrossRef]
- Yang, S.; He, T.; Yang, L.; Zhao, Q.; Zhang, T. Effects of straw and biochar mulching on the soil nutrients and greenhouse gas emissions. J. Hunan Agric. Univ. 2022, 1, 75–81. [Google Scholar]
- Zhang, X.; Li, S.; Yu, F.; Cheng, D.; Gao, J.; Gu, X.; Liu, L. Research progresses on the effects of crop straw returning on greenhouse gas emission in paddy field. Hybrid Rice 2021, 5, 1–7. [Google Scholar]
- Ma, E.D.; Zhang, G.B.; Ma, J.; Xu, H.; Cai, Z. Effects of rice straw returning methods on N2O emission during wheat-growing season. Nutr. Cycl. Agroecosystems 2010, 88, 463–469. [Google Scholar] [CrossRef]
- Hu, F.L.; Chai, Q.; Gan, Y.T. Characteristics of soil carbon emission and water utilization in wheat/maize inter cropping with minimal/zero tillage and straw retention. Sci. Agric. Sin. 2016, 49, 120–131. [Google Scholar]
- Zhu, K.J.; Xie, W.X.; Liu, W.L. Progress in research on plant effect on N2O emission flux from terrestrial ecosystem. Earth Environ. 2014, 42, 456–463. [Google Scholar]
- Song, H.; Wang, C.Y.; Chen, Q.; Cao, W.; Wang, J. Effects of Long-term Amendment of Residue on Denitrification Characteristics and N2O Emissions in Greenhouse Soil. Chin. J. Agrometeorol. 2014, 6, 628–634. [Google Scholar]
- Guo, J.; Jin, W.; Liu, Z.; Cheng, Z.; Zhao, W.; Meng, Y. Effects of straw and biochar on NH3 volatilization and N2O emission from alkaline soils planted with cotton. J. Agro-Environ. Sci. 2024, 43, 442–451. [Google Scholar]
- Wang, Q.; Chen, X.; Yu, M.; Shen, A. Research progress on effects of straw returning on nitrogen cycling microbes and functional genes in paddy soil. Acta Agric. Zhejiangensis 2019, 2, 333–342. [Google Scholar]




| Experimental Design | Treatment | |||
|---|---|---|---|---|
| CK | JT1 | JT2 | JT3 | |
| Plot area (m2) | 40 | 40 | 40 | 40 |
| Number of plots | 3 | 3 | 3 | 3 |
| Straw return amount | Full rice straw + No tobacco straw | Full rice straw + Half tobacco straw | Full rice straw + Full tobacco straw | Full rice straw + doubled tobacco straw |
| Treatment | VFGs | MCGs | NCGs | |||
| Ace Index | Shannon Index | Ace Index | Shannon Index | Ace Index | Shannon Index | |
| CK | 2395 ± 382.3 a | 6.374 ± 0.189 a | 51.67 ± 3.667 a | 2.965 ± 0.178 a | 38.33 ± 0.33 a | 2.755 ± 0.128 a |
| JT1 | 2777 ± 387.7 b | 6.563 ± 0.232 b | 55.33 ± 0.333 a | 2.787 ± 0.329 b | 38.67 ± 2.00 a | 2.628 ± 0.135 b |
| JT2 | 2782 ± 352.7 b | 6.606 ± 0.202 b | 54.67 ± 0.667 a | 2.636 ± 0.180 c | 40.33 ± 1.67 a | 2.621 ± 0.088 bc |
| JT3 | 2747 ± 29.67 b | 6.576 ± 0.044 b | 54.00 ± 1.667 a | 2.785 ± 0.151 b | 40.00 ± 1.33 a | 2.668 ± 0.048 c |
| Treatment | CAZyGs | CARGs | ||||
| Ace Index | Shannon Index | Ace Index | Shannon Index | |||
| CK | 890.7 ± 167.0 a | 5.479 ± 0.139 a | 1033 ± 158.0 a | 5.472 ± 0.162 a | ||
| JT1 | 1058 ± 190.0 bd | 5.618 ± 0.123 b | 1191 ± 161.7 b | 5.634 ± 0.183 b | ||
| JT2 | 1081 ± 150.7 b | 5.602 ± 0.125 b | 1195 ± 142.7 b | 5.656 ± 0.161 b | ||
| JT3 | 1041 ± 23.0 cd | 5.604 ± 0.016 b | 1176 ± 19.0 b | 5.633 ± 0.022 b | ||
| Treatment | VFGs Taxa | MCGs Taxa | NCGs Taxa | |||
| Ace Index | Shannon Index | Ace Index | Shannon Index | Ace Index | Shannon Index | |
| CK | 6097 ± 184.30 a | 5.21 ± 0.805 a | 728.3 ± 239.7 a | 4.840 ± 0.487 a | 477.7 ± 118.3 a | 4.505 ± 0.346 a |
| JT1 | 9039 ± 334.08 b | 6.02 ± 0.294 b | 968.0 ± 173.7 bd | 5.326 ± 0.454 b | 596.0 ± 89.3 b | 4.851 ± 0.017 b |
| JT2 | 8952 ± 487.00 b | 5.51 ± 0.601 b | 902.0 ± 216.3 c | 5.294 ± 0.365 b | 567.0 ± 110.3 c | 4.489 ± 0.323 a |
| JT3 | 8855 ± 297.33 b | 5.81 ± 0.510 b | 944.7 ± 42.7 cd | 5.205 ± 0.032 c | 588.0 ± 29.0 bc | 4.828 ± 0.362 b |
| Treatment | CAZyGs Taxa | CARGs Taxa | ||||
| Ace Index | Shannon Index | Ace Index | Shannon Index | |||
| CK | 3689 ± 85.12 a | 5.073 ± 0.853 a | 3470 ± 169.33 a | 5.059 ± 0.775 a | ||
| JT1 | 5609 ± 38.00 bd | 5.926 ± 0.109 b | 5241 ± 73.67 b | 5.833 ± 0.296 b | ||
| JT2 | 5647 ± 187.70 b | 5.182 ± 0.741 b | 5172 ± 281.11 b | 5.354 ± 0.610 c | ||
| JT3 | 5421 ± 225.70 cd | 5.814 ± 0.744 b | 5168 ± 502.00 b | 5.669 ± 0.479 b | ||
| Community | Correlation Coefficient | |||||
|---|---|---|---|---|---|---|
| pH | Soil Organic Carbon | Dissolved Organic Carbon | Alkali-Hydrolyzed Nitrogen | Available Phosphorus | Available Potassium | |
| VFGs | 0.107 | 0.561 * | 0.505 * | 0.584 * | 0.823 * | 0.509 * |
| MCGs | 0.128 | 0.531 * | 0.497 * | 0.589 * | 0.777 * | 0.499 * |
| NCGs | 0.109 | 0.516 * | 0.490 * | 0.559 * | 0.807 * | 0.467 * |
| CAZyGs | 0.108 | 0.601 * | 0.526 * | 0.625 * | 0.818 * | 0.555 * |
| CARGs | 0.112 | 0.548 * | 0.493 * | 0.569 * | 0.824 * | 0.496 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, L.; Yu, Y.; Lin, C.; Fang, Y.; Jia, X. Effects of Differential Tobacco Straw Incorporation on Functional Gene Profiles and Functional Groups of Soil Microorganisms. Agriculture 2025, 15, 2384. https://doi.org/10.3390/agriculture15222384
Zhang H, Chen L, Yu Y, Lin C, Fang Y, Jia X. Effects of Differential Tobacco Straw Incorporation on Functional Gene Profiles and Functional Groups of Soil Microorganisms. Agriculture. 2025; 15(22):2384. https://doi.org/10.3390/agriculture15222384
Chicago/Turabian StyleZhang, Hui, Longjun Chen, Yanshuang Yu, Chenqiang Lin, Yu Fang, and Xianbo Jia. 2025. "Effects of Differential Tobacco Straw Incorporation on Functional Gene Profiles and Functional Groups of Soil Microorganisms" Agriculture 15, no. 22: 2384. https://doi.org/10.3390/agriculture15222384
APA StyleZhang, H., Chen, L., Yu, Y., Lin, C., Fang, Y., & Jia, X. (2025). Effects of Differential Tobacco Straw Incorporation on Functional Gene Profiles and Functional Groups of Soil Microorganisms. Agriculture, 15(22), 2384. https://doi.org/10.3390/agriculture15222384




