The Effect of Rice–Frog Co-Cropping Systems on Heavy Metal Availability and Accumulation in Rice in Reclaimed Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Profile of the Research Site
2.2. Experimental Design
2.3. Sample Collection
2.3.1. Collection of Soil Samples
2.3.2. Collection of Rice Sample
2.4. Measurement of Soil Samples’ Physical and Chemical Properties
2.5. Analysis of Rice Samples
2.6. Data Processing
3. Results
3.1. Differences in the Physical and Chemical Properties of Paddy Soil
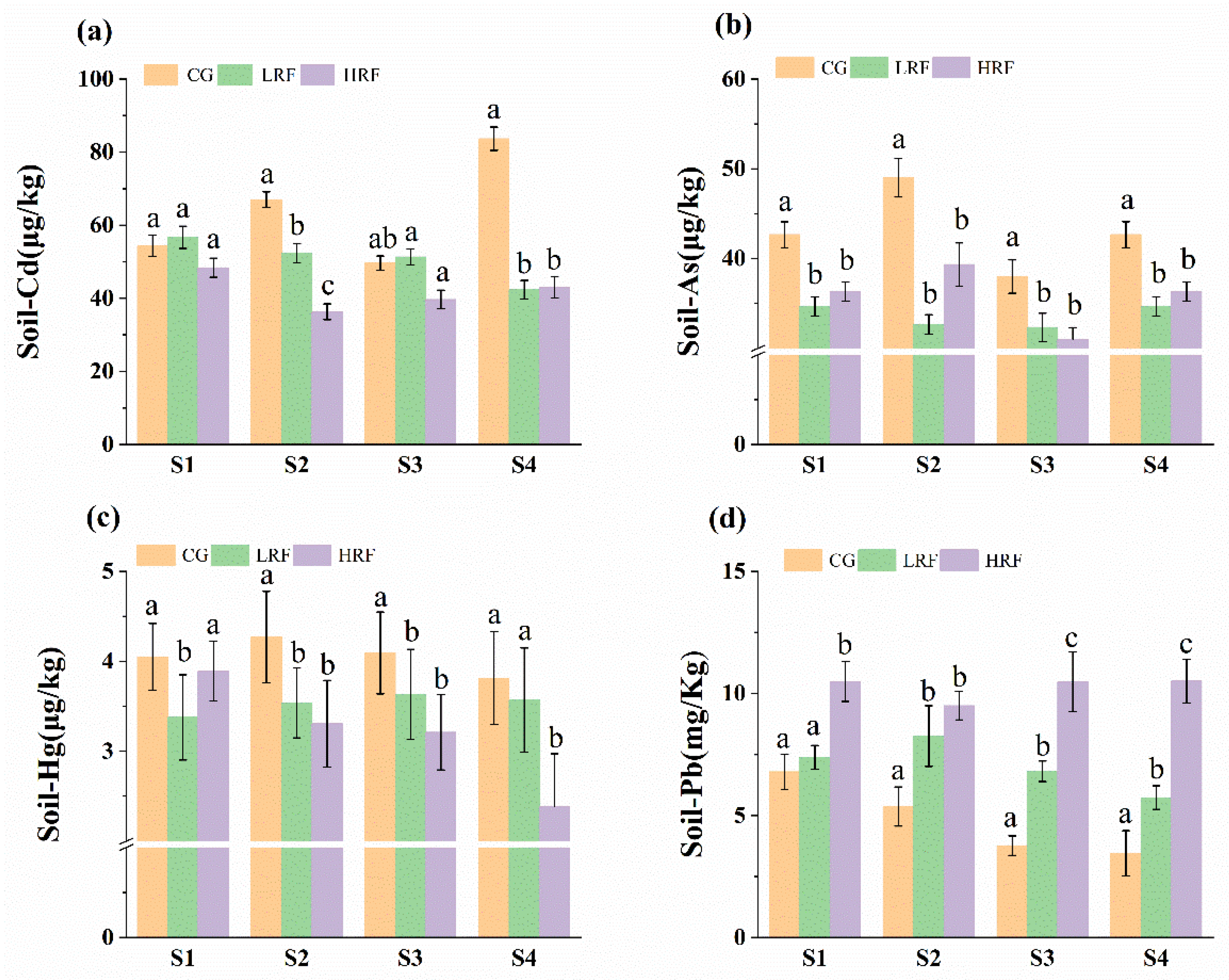
3.2. Differences in the Available Forms of Heavy Metals in Paddy Soil
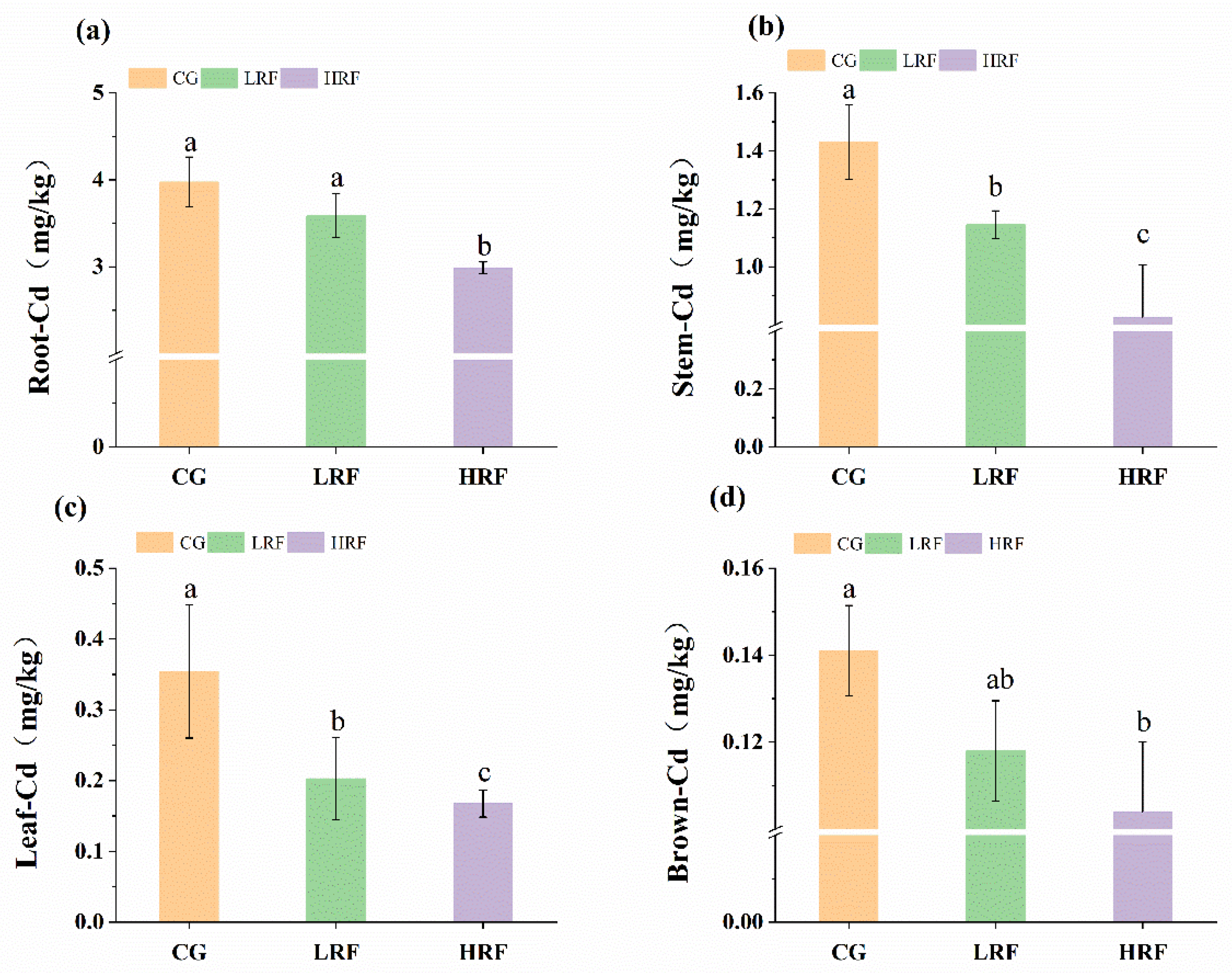
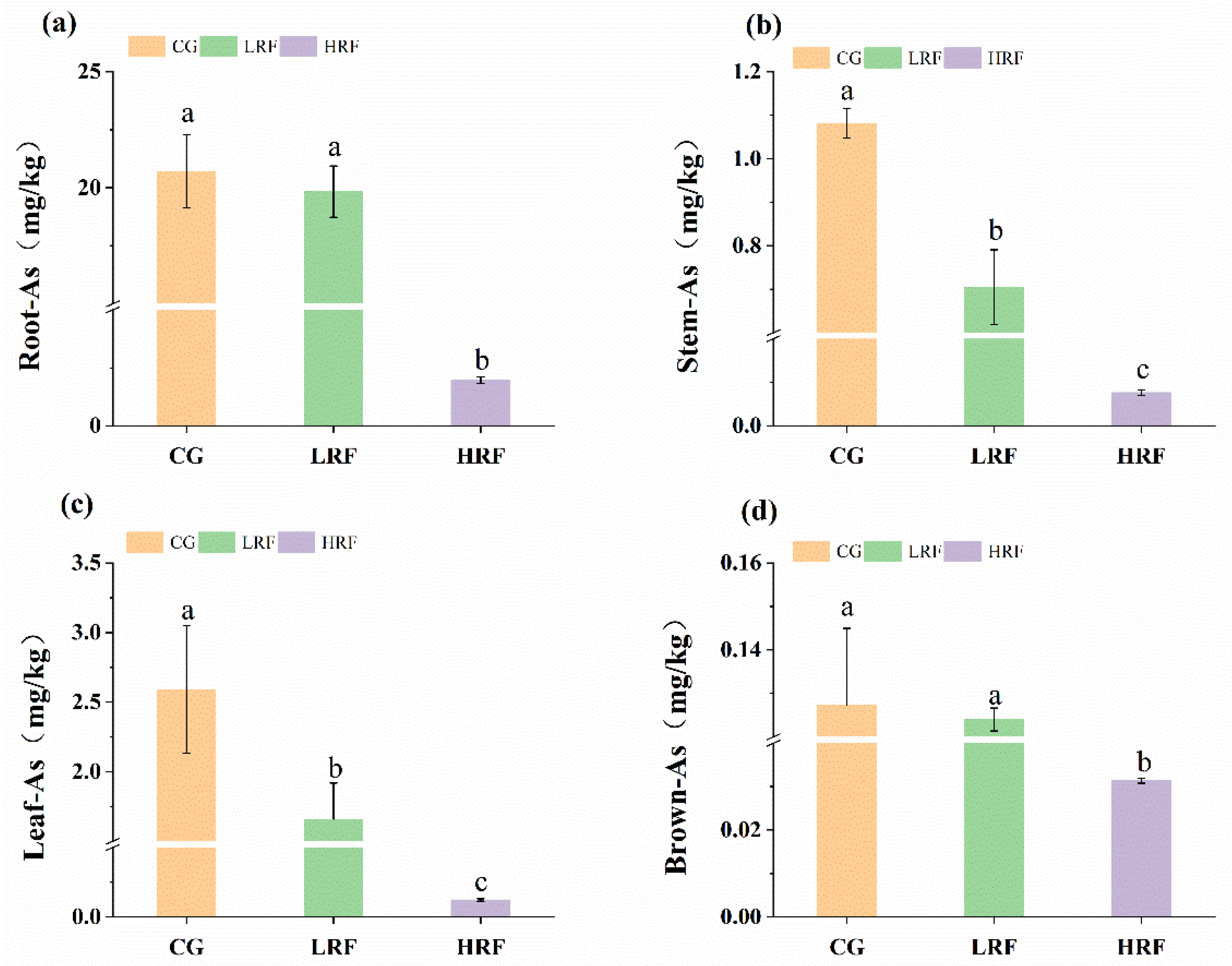
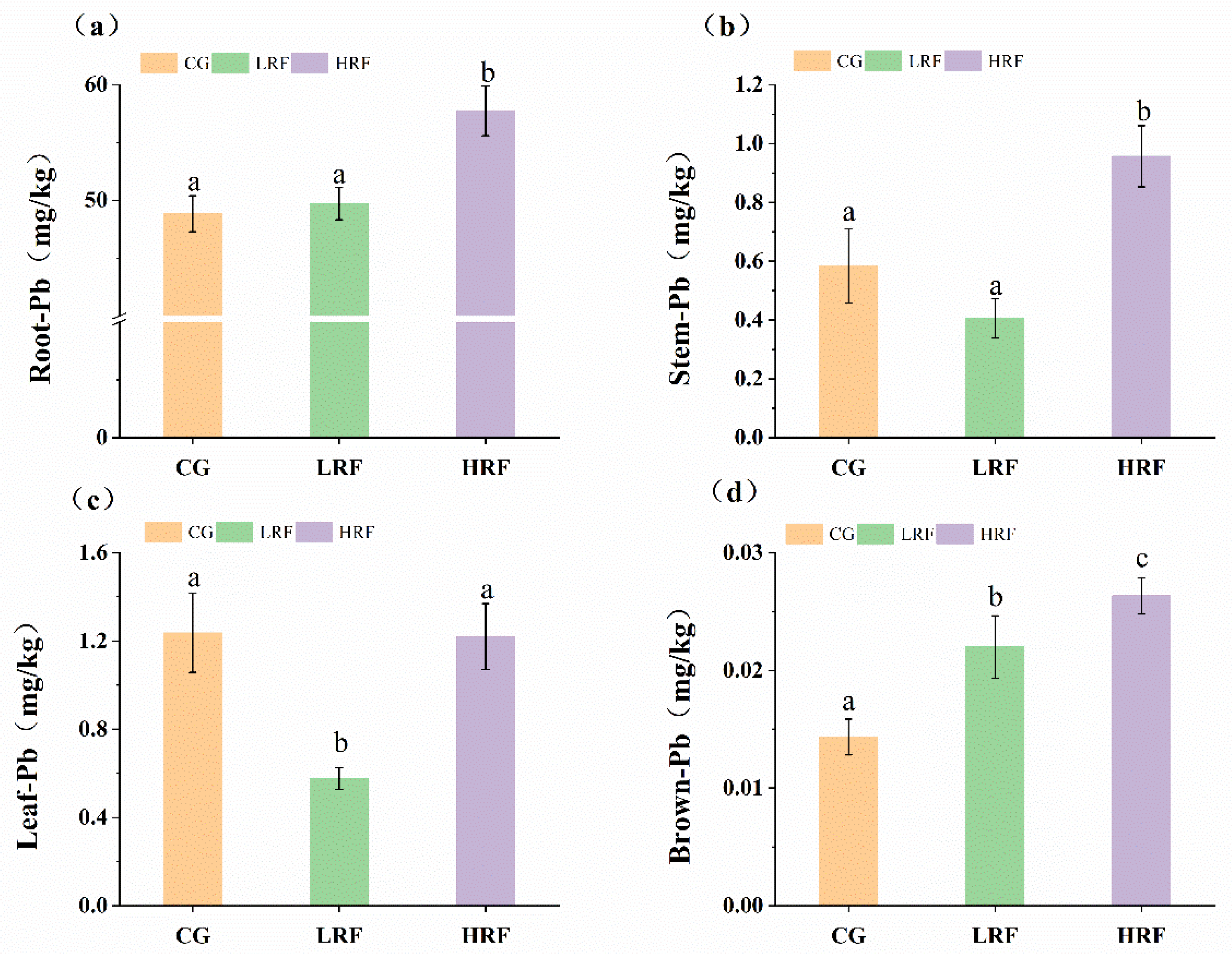
3.3. Differences in Heavy Metal Content in Different Parts of Rice Plants
3.4. Heavy Metal Accumulation Characteristics of Rice
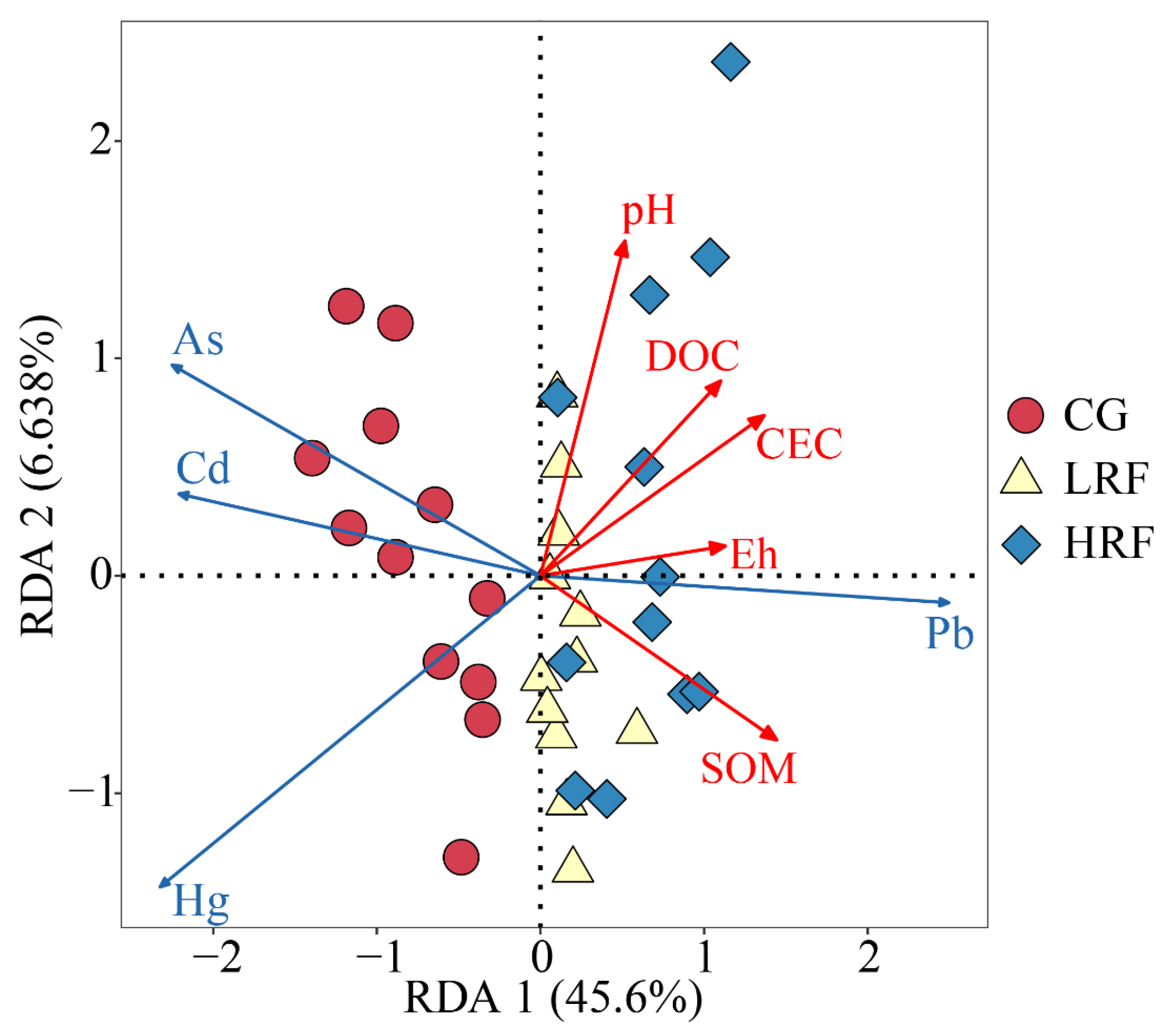
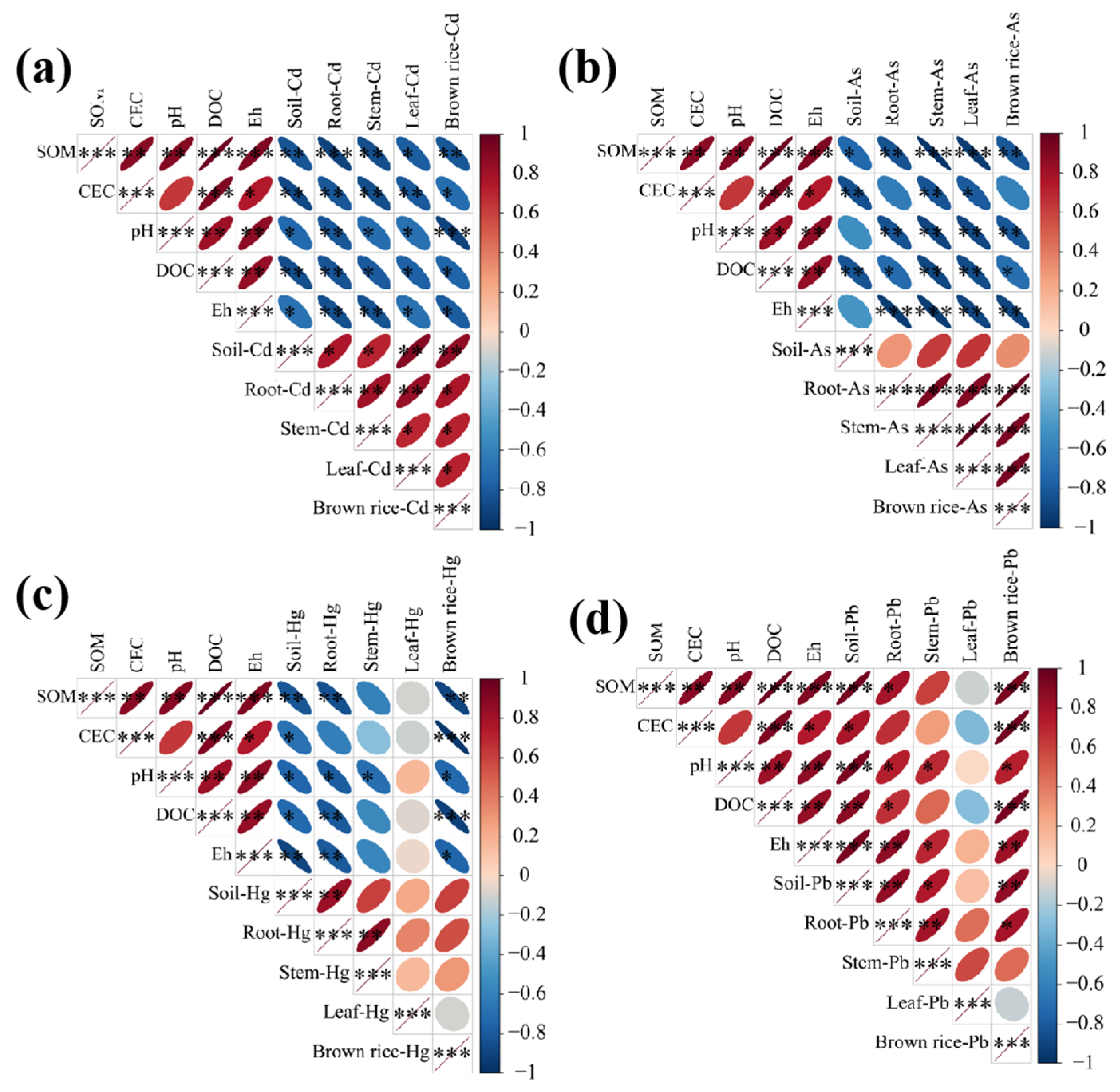
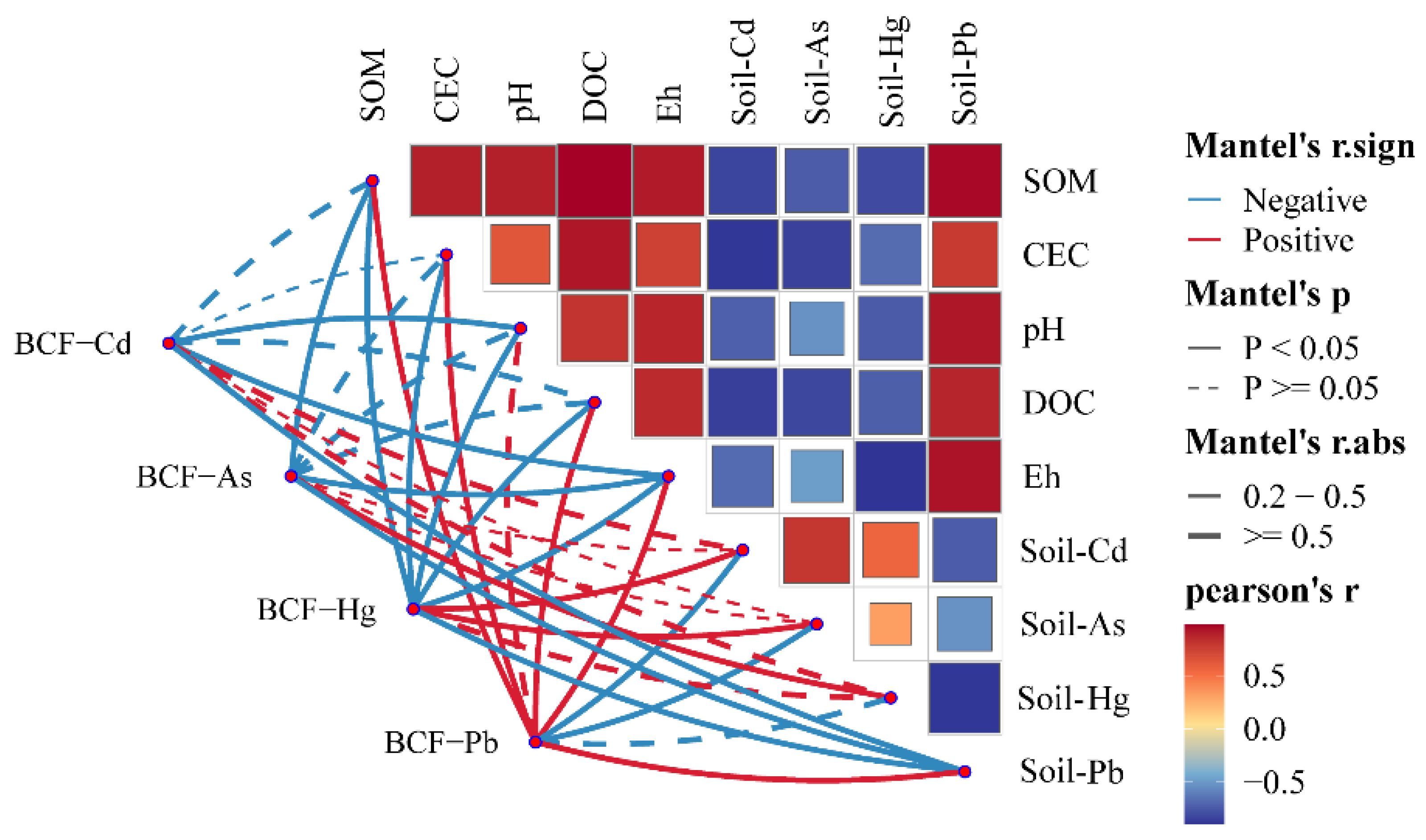
3.5. Correlation Analysis Between Soil Physicochemical Properties and the Bioavailable Forms of Heavy Metals in Rice Fields
4. Discussion
4.1. The Effects of the Rice–Frog Co-Cultivation Model on the Physical and Chemical Properties of Paddy Soil
4.2. Influence of Soil Physicochemical Properties on Bioavailable Forms of Heavy Metals in Rice Fields
4.3. Effects of Rice–Frog Co-Cultivation Model on Heavy Metal Content in Rice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil-Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Lin, L.; Zhang, Y.; Huang, H.; Deng, W.; He, Y.; Tao, J.; Hu, Y.; Nan, L.; et al. Distribution characteristics, sources and risk assessment of heavy metals in the surface sediments from the largest tributary of the Lancang River in the Tibet Plateau, China. Env. Geochem. Health 2024, 46, 414. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Wang, L.; Song, C.; Chen, L.; Zhang, J.; Liang, Y. Using Caenorhabditis elegans to assess the ecological health risks of heavy metals in soil and sediments around Dabaoshan Mine, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 16332–16345. [Google Scholar] [CrossRef]
- Ren, S.; Song, C.; Ye, S.; Cheng, C.; Gao, P. The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci. Total Environ. 2022, 806 Pt 2, 150322. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.; Han, Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, A.S.; Chaogejilatu; Iizumi, T. A climate impact attribution of historical rice yields in Sri Lanka using three crop models. Sci. Rep. 2025, 15, 15360. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.; Zhang, Y.; Chen, S.; Ying, Q.; Lv, Z.; Che, X.; Wang, D. Heat stress may cause a significant reduction of rice yield in China under future climate scenarios. Sci. Total Environ. 2022, 818, 151746. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, W.; Lu, T.; Hu, W.; Lin, J.; Fu, W.; Liang, Y.; Zeng, Y.; Fu, G.; Xiong, J.; et al. Increased Photosynthetic Capacity and Energy Status Contribute to Higher Grain Yield in Early Rice. Int. J. Mol. Sci. 2025, 26, 1508. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Deng, Y.; Zhang, X.; Wu, D.; Wang, X.; Huang, S. Distribution, source identification, and health risk assessment of heavy metals in the soil-rice system of a farmland protection area in Hubei Province, Central China. Environ. Sci. Pollut. Res. Int. 2021, 28, 68897–68908. [Google Scholar] [CrossRef]
- Ma, X.; Sharifan, H.; Dou, F.; Sun, W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem. Eng. J. 2020, 384, 123802. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhu, Y.; Shao, K.; Zhang, Q.; Ye, G.; Shen, J. Metals source apportionment in farmland soil and the prediction of metal transfer in the soil-rice-human chain. J. Environ. Manag. 2020, 260, 110092. [Google Scholar] [CrossRef] [PubMed]
- Shahab, A.; Hui, Z.; Rad, S.; Xiao, H.; Siddique, J.; Huang, L.L.; Ullah, H.; Rashid, A.; Taha, M.R.; Zada, N. A comprehensive review on pollution status and associated health risk assessment of human exposure to selected heavy metals in road dust across different cities of the world. Env. Geochem. Health 2023, 45, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287 Pt. 4, 132369. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy metal stabilization remediation in polluted soils with stabilizing materials: A review. Env. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef]
- Guo, W.; Yao, X.; Chen, Z.; Liu, T.; Wang, W.; Zhang, S.; Xian, J.; Wang, Y. Recent advance on application of biochar in remediation of heavy metal contaminated soil: Emphasis on reaction factor, immobilization mechanism and functional modification. J. Environ. Manag. 2024, 371, 123212. [Google Scholar] [CrossRef]
- Kou, B.; Yuan, Y.; Zhu, X.; Ke, Y.; Wang, H.; Yu, T.; Tan, W. Effect of soil organic matter-mediated electron transfer on heavy metal remediation: Current status and perspectives. Sci. Total Environ. 2024, 917, 170451. [Google Scholar] [CrossRef]
- Gao, H.; Dai, W.; Fang, K.; Yi, X.; Chen, N.; Penttinen, P.; Sha, Z.; Cao, L. Rice-duck co-culture integrated different fertilizers reduce P losses and Pb accumulation in subtropical China. Chemosphere 2020, 245, 125571. [Google Scholar] [CrossRef]
- Ngoc, N.P.; Dang, L.V.; Qui, N.V.; Hung, N.N. Chemical processes and sustainability of rice-shrimp farming on saline acid sulfate soils in mekong delta. Heliyon 2023, 9, e13532. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, R.; Zhou, L.; Zhang, L.; Sun, W.; Li, B.; Zhu, J. Integrated rice-fish farming dynamically altered the metal resistances and microbial-mediated iron, arsenic, and mercury biotransformation in paddy soil. Environ. Pollut. 2025, 373, 126107. [Google Scholar] [CrossRef]
- Yan, T.; Xie, Y.Y.; Zhou, B.; Kuang, X.; Li, Q.Z.; Zhao, F.Q.; Li, Q.D.; He, B. Rice-fish farming improved antioxidant defences, glucose metabolism, and muscle nutrient of Carassius auratus in Sichuan province. Metabolites 2024, 14, 710. [Google Scholar] [CrossRef]
- Yan, J.; Yu, J.; Huang, W.; Pan, X.; Li, Y.; Li, S.; Tao, Y.; Zhang, K.; Zhang, X. Initial studies on the effect of the rice-duck-crayfish ecological co-culture system on physical, chemical, and microbiological properties of soils: A field case study in Chaohu Lake Basin, Southeast China. Int. J. Environ. Res. Public Health 2023, 20, 2006. [Google Scholar] [CrossRef]
- Yang, G.; Juncang, T.; Zhi, W. Composition and functional diversity of soil and water microbial communities in the rice-crab symbiosis system. PLoS ONE 2025, 20, e0316815. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Zhang, J.; Wu, T.; Liu, S.; Hei, Z.; Qiu, S. Effects of the integration of mixed-cropping and rice-duck co-culture on rice yield and soil nutrients in southern China. J. Sci. Food Agric. 2020, 100, 277–286. [Google Scholar] [CrossRef]
- Ahmed, N.; Hornbuckle, J.; Turchini, G.M. Blue-green water utilization in rice-fish cultivation towards sustainable food production. Ambio 2022, 51, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jia, R.; Zhou, L.; Zhang, L.; Wei, S.; Li, B.; Zhu, J. Alterations in microbial-mediated methane, nitrogen, sulfur, and phosphorus cycling within paddy soil induced by integrated rice-fish farming. J. Environ. Manag. 2025, 388, 126056. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Styles, D.; Cao, Y.; Ye, X. The sustainability of rice-crayfish coculture systems: A mini review of evidence from Jianghan plain in China. J. Sci. Food Agric. 2021, 101, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, A.; Zhang, L.; Zheng, R. Effects of Rice-Frog Co-Cropping on the Soil Microbial Community Structure in Reclaimed Paddy Fields. Biology 2024, 13, 396. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, M.; Zhang, P.; Shu, H.; Li, Y.; Guo, Z.; Li, Y. Amelioration of Cd-induced bioaccumulation, oxidative stress and intestinal microbiota by Bacillus cereus in Carassius auratus gibelio. Chemosphere 2020, 245, 125613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Ma, Y.; Yu, A.; Zheng, R. Frog Density and Growth Stage of Rice Impact Paddy Field and Gut Microbial Communities in Rice-Frog Co-Cropping Models. Microorganisms 2025, 13, 1700. [Google Scholar] [CrossRef]
- Lin, K.; Wu, J. Effect of introducing frogs and fish on soil phosphorus availability dynamics and their relationship with rice yield in paddy fields. Sci. Rep. 2020, 10, 21. [Google Scholar] [CrossRef]
- Fang, K.; Dai, W.; Chen, H.; Wang, J.; Gao, H.; Sha, Z.; Cao, L. The effect of integrated rice-frog ecosystem on rice morphological traits and methane emission from paddy fields. Sci. Total Environ. 2021, 783, 147123. [Google Scholar] [CrossRef]
- Qiao, M.; Zhang, L.; Chang, J.; Li, H.; Li, J.; Wang, W.; Yuan, G.; Su, J. Rapid and sensitive detection of pathogenic Elizabethkingia miricola in black spotted frog by RPA-LFD and fluorescent probe-based RPA. Fish Shellfish Immunol. Rep. 2022, 3, 100059. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Lu, Y.; Wang, J.; Yu, D.; Zhou, Z.; Wei, J.; Liu, L.; Liu, J.; Liu, F.; et al. Co-infections of Klebsiella pneumoniae and Elizabethkingia miricola in black-spotted frogs (Pelophylax nigromaculatus). Microb. Pathog. 2023, 180, 106150. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, Y.; He, T.; Tang, H. Effects of integrated rice-frog farming on soil bacterial community composition. Chil. J. Agric. Res. 2023, 83, 525–538. [Google Scholar] [CrossRef]
- Bose, H.; Saha, A.; Sahu, R.P.; Dey, A.S.; Sar, P. Characterization of the rare microbiome of rice paddy soil from arsenic contaminated hotspot of West Bengal and their interrelation with arsenic and other geochemical parameters. World J. Microbiol. Biotechnol. 2022, 38, 171. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E.; Lauenroth, W.K. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Liu, L.; Cao, H.; Geng, Y.; Zhang, Q.; Bu, X.; Gao, D. Response of soil microecology to different cropping practice under Bupleurum chinense cultivation. BMC Microbiol. 2022, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Maul, A.; Masfaraud, J.F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Qu, B.; Liu, Y.; Sun, X.; Li, S.; Wang, X.; Xiong, K.; Yun, B.; Zhang, H. Effect of various mulches on soil physico-chemical properties and tree growth (Sophora japonica) in urban tree pits. PLoS ONE 2019, 14, e0210777. [Google Scholar] [CrossRef]
- Yao, B.M.; Wang, S.Q.; Xie, S.T.; Li, G.; Sun, G.X. Optimal soil Eh, pH for simultaneous decrease of bioavailable Cd, As in co-contaminated paddy soil under water management strategies. Sci. Total Environ. 2022, 806 Pt. 3, 151342. [Google Scholar] [CrossRef]
- Junusbekov, M.M.; Akbasova, A.D.; Seidakbarova, A.D.; Koishiyeva, G.Z.; Sainova, G.A. Ecological assessment of soil contamination by heavy metals affected in the past by the lead-zinc mining and processing complex in Kentau, Kazakhstan. Environ. Monit. Assess. 2023, 195, 586. [Google Scholar] [CrossRef]
- Li, L.; Mao, K.; Ippolito, J.A.; Xing, W.; Chen, X.; Zhu, W.; Cheng, Y. Calcium amendments affect heavy metal bioavailability in acidic and calcareous soils. Int. J. Environ. Sci. Technol. 2022, 19, 10067–10076. [Google Scholar] [CrossRef]
- GB/T 17141-1997; Soil Quality—Determination of Lead and Cadmium—Graphite Furnace Atomic Absorption Spectrometry. Issuer: State Administration for Market Regulation (Formerly State Bureau of Quality and Technical Supervision): Beijing, China, 1997.
- DB35/T 1459-2014; Determination of Available Arsenic and Available Mercury in Acidic Soil—Atomic Fluorescence Spectrometry. Issuer: Fujian Provincial Administration for Market Regulation: Fuzhou, China, 2014.
- Xu, L.; Cao, S.; Wang, J.; Lu, A. Which factors determine metal accumulation in agricultural soils in the severely human-coupled ecosystem? Int. J. Environ. Res. Public Health 2016, 13, 510. [Google Scholar] [CrossRef]
- HJ 680-2013; Soil and Sediment—Determination of Mercury, Arsenic, Selenium, Bismuth and Antimony—Microwave Digestion/Atomic Fluorescence Spectrometry. Issuer: Ministry of Ecology and Environment of the People’s Republic of China (Formerly Ministry of Environmental Protection): Beijing, China, 2013.
- Sun, G.; Zhang, L.; Cheng, P.; Yao, B.M. Silicon fertilizers mitigate rice cadmium and arsenic uptake in a 4-year field trial. J. Soils Sediments 2021, 21, 163–171. [Google Scholar] [CrossRef]
- GB 5009.268-2016; National Food Safety Standard—Determination of Multi-Elements in Foods. Issuer: National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GBW(E)100348; Reference Material for Component Analysis of Rice Flour. Developer (Corresponding Issuing Entity): CNAS Testing Technology Co., Ltd.: Beijing, China, 2020.
- Chen, B.; Guo, L.; Tang, J.; Li, Y.; Li, C. Comprehensive impacts of different integrated rice-animal co-culture systems on rice yield, nitrogen fertilizer partial factor productivity and nitrogen losses: A global meta-analysis. Sci. Total Environ. 2024, 915, 169994. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Huang, L.; Zhang, Y.; Fang, T.; He, J. Dynamics of soil fertility and microbial community response to stocking density in rice-turtle co-culture. Aquac. Rep. 2021, 20, 100765. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Wegner, C.E.; Luo, Y.; Xiao, K.Q.; Cui, Z.; Zhang, F.; Liesack, W.; Peng, J. Deciphering microbial mechanisms underlying soil organic carbon storage in a wheat-maize rotation system. Sci. Total Environ. 2021, 788, 147798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ma, H.; Zhao, Q.L.; Yang, J.; Xin, C.Y.; Chen, B. Bacterial communities in paddy soil and ditch sediment under rice-crab co-culture system. AMB Express 2021, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Barnes, M.E.; Moreland, K.; Graham, R.C.; Berhe, A.A.; Hart, S.C. Depth dependence of climatic controls on soil microbial community activity and composition. ISME Commun. 2021, 1, 78. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Delgado-Baquerizo, M.; Fanin, N.; Chen, X.; Zhou, Y.; Du, G.; Hu, F.; Jiang, L.; Hu, S.; Liu, M. Nutrient-induced acidification modulates soil biodiversity-function relationships. Nat. Commun. 2024, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Chu, Q.; Zhao, Z.; Yue, Y.; Lu, L.; Yuan, J.; Cao, L. Variations in nutrient and trace element composition of rice in an organic rice-frog coculture system. Sci. Rep. 2017, 7, 15706. [Google Scholar] [CrossRef]
- Fang, K.; Yi, X.; Dai, W.; Gao, H.; Cao, L. Effects of integrated rice-frog farming on paddy field greenhouse gas emissions. Int. J. Environ. Res. Public Health 2019, 16, 1930. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Ding, K.; Shi, X.; Kalkhajeh, Y.K.; Wei, Z.; Zhang, Z.; Ma, C. Co-culture of rice and aquatic animals enhances soil organic carbon: A meta-analysis. Sci. Total Environ. 2024, 955, 176819. [Google Scholar] [CrossRef]
- Ramos, F.T.; Dores, E.F.C.; Weber, O.L.D.S.; Beber, D.C.; Campelo, J.H., Jr.; Maia, J.C.S. Soil organic matter doubles the cation exchange capacity of tropical soil under no-till farming in Brazil. J. Sci. Food Agric. 2018, 98, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Li, C.; Cao, C. Long-term rice-crayfish farming aggravates soil gleying and induced changes of soil iron morphology. Soil Use Manag. 2020, 38, 757–770. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Wang, Q.; Yu, X.; Hang, Y. Integrated rice-duck farming mitigates the global warming potential in rice season. Sci. Total Environ. 2017, 575, 58–66. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Q.; Sun, Z.; Sun, H. Effects of natural organic matter on cadmium mobility in paddy soil: A review. J. Environ. Sci. 2021, 104, 204–215. [Google Scholar] [CrossRef]
- Xiong, R.; Li, Y.; Gao, X.; Xue, Y.; Huang, J.; Li, N.; Chen, C.; Chen, M. Distribution and migration of heavy metals in the sediment-plant system: Case study of a large-scale constructed wetland for sewage treatment. J. Environ. Manag. 2024, 349, 119428. [Google Scholar] [CrossRef]
- Zou, Q.; Wei, H.; Chen, Z.; Ye, P.; Zhang, J.; Sun, M.; Huang, L.; Li, J. Soil particle size fractions affect arsenic (As) release and speciation: Insights into dissolved organic matter and functional genes. J. Hazard. Mater. 2023, 443 Pt. B, 130100. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M.; Antoniadis, V. The release of Cd, Cu, Fe, Mn, Ni, Pb, and Zn from clay loam and sandy loam soils under the influence of various organic amendments and low-molecular-weight organic acids. J. Hazard. Mater. 2023, 459, 132111. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, X.; Peng, Y.; Zheng, Z.; Gao, X.; Ma, Y.; Chen, S.; Cui, S.; Fan, B.; Chen, Q. Effects of phosphorus-containing material application on soil cadmium bioavailability: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 42372–42383. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Dong, Z.; Wijayawardena, M.A.A.; Liu, Y.; Li, Y.; Naidu, R. The source of lead determines the relationship between soil properties and lead bioaccessibility. Environ. Pollut. 2019, 246, 53–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Tu, C.; Huang, X.; Zhang, J.; Fang, H.; Huo, H.; Lin, C. Relationships between soil properties and the accumulation of heavy metals in different Brassica campestris L. growth stages in a Karst mountainous area. Ecotoxicol. Environ. Saf. 2020, 206, 111150. [Google Scholar] [CrossRef]
- Deng, W.; Wang, F.; Liu, W. Identification of factors controlling heavy metals/metalloid distribution in agricultural soils using multi-source data. Ecotoxicol. Environ. Saf. 2023, 253, 114689. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Łukowiak, R.; Kunzová, E. Effect of long-term application of pig slurry and NPK fertilizers on trace metal content in the soil. Environ. Sci. Pollut. Res. Int. 2024, 31, 60004–60022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Li, Y.; Liang, C.; Huang, H.; Wang, S. Available heavy metals concentrations in agricultural soils: Relationship with soil properties and total heavy metals concentrations in different industries. J. Hazard. Mater. 2024, 471, 134410. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, Q.; Zhang, L.; Huang, Z.; Zhao, Z.; Zhao, H.; Du, J.; Zhou, J. Toxic metals in rice-fish co-culture systems and human health. Ecotoxicol. Environ. Saf. 2022, 241, 113797. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, K.; Guo, Z.; Li, H. Effects of fiddler crab bioturbation on the geochemical migration and bioavailability of heavy metals in coastal wetlands. J. Hazard. Mater. 2022, 437, 129380. [Google Scholar] [CrossRef]
- Liu, M.; Shen, D.; Zhang, W.; Jiang, Y.; Cao, C. Health risk assessment and bioaccumulation of heavy metal(loid)s in rice from the rice-crayfish coculture system. Environ. Pollut. 2025, 381, 126451. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Bioconcentration Factor (BCF) | |||
|---|---|---|---|---|
| BCF-Cd | BCF-As | BCF-Hg | BCF-Pb | |
| CG | 0.7464 ± 0.0574 | 0.0197 ± 0.0025 a | 0.0030 ± 7.8671 × 10−5 a | 0.00037 ± 5.803 × 10−5 a |
| LRF | 0.7123 ± 0.0696 | 0.0204 ± 0.0004 a | 0.0003 ± 0.0005 b | 0.00049 ± 5.5526 × 10−5 b |
| HRF | 0.6101 ± 0.0868 | 0.0081 ± 0.0036 b | 0.0000 b | 0.00052 ± 1.3703 × 10−5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Wang, Z.; Zhu, Z.; Li, H.; Ma, Y.; Zheng, R. The Effect of Rice–Frog Co-Cropping Systems on Heavy Metal Availability and Accumulation in Rice in Reclaimed Fields. Agriculture 2025, 15, 2374. https://doi.org/10.3390/agriculture15222374
Xia X, Wang Z, Zhu Z, Li H, Ma Y, Zheng R. The Effect of Rice–Frog Co-Cropping Systems on Heavy Metal Availability and Accumulation in Rice in Reclaimed Fields. Agriculture. 2025; 15(22):2374. https://doi.org/10.3390/agriculture15222374
Chicago/Turabian StyleXia, Xinni, Zhigang Wang, Zhangyan Zhu, Han Li, Yunshuang Ma, and Rongquan Zheng. 2025. "The Effect of Rice–Frog Co-Cropping Systems on Heavy Metal Availability and Accumulation in Rice in Reclaimed Fields" Agriculture 15, no. 22: 2374. https://doi.org/10.3390/agriculture15222374
APA StyleXia, X., Wang, Z., Zhu, Z., Li, H., Ma, Y., & Zheng, R. (2025). The Effect of Rice–Frog Co-Cropping Systems on Heavy Metal Availability and Accumulation in Rice in Reclaimed Fields. Agriculture, 15(22), 2374. https://doi.org/10.3390/agriculture15222374





