Abstract
Pollinator nutrition and honey production potential depend on nectar quantity, nectar availability across flowering phases, and sugar concentration. For chestnut (Castanea spp.), cultivar- and flowering phase-specific nectar data remain limited. This study analyzed nectar traits of four Castanea cultivars to evaluate their potential importance for pollinators and apiculture. A two-year field study (2023–2024) was conducted on four major South Korean cultivars (‘Daebo’, ‘Okkwang’, ‘Riheiguri’, ‘Tsukuba’) to quantify catkin floral traits, nectar volume, free sugar concentration (sucrose, glucose, fructose), and estimated nectar yields across four flowering phases. Standardized catkin-scale sampling and multivariate modeling revealed that flowering phase, rather than catkin size, determined nectar rewards in all cultivars. Nectar volume and sugar concentration per catkin peaked at mid anthesis (phase 3), while sugar concentration and hexose proportion increased in late anthesis (phase 4). ‘Daebo’ led in phase 3 nectar yields, ‘Okkwang’ was intermediate, and ‘Tsukuba’ and ‘Riheiguri’ provided more hexose-rich, concentrated nectar during phase 4. Notably, cultivar × flowering phase interactions determined both the amount and sugar profile of nectar resources. These findings indicate that phase 3 measurements are optimal for yield comparisons, while phase 4 profiles guide honey chemistry and handling. Mixed-cultivar plantings combining ‘Daebo’ (high honey yield) with late-phase hexose sources (‘Riheiguri’, ‘Tsukuba’) can help stabilize pollinator resources.
1. Introduction
Pollinators, especially Apis spp., are critically important to both global food security and the maintenance of biodiversity [1,2]. However, multiple anthropogenic and environmental stressors, including habitat loss, climate change, pesticide exposure, and poor floral nutrition, are driving widespread pollinator decline [3,4,5]. Among these, the quantity and quality of floral nectar have emerged as central factors influencing bee health, colony resilience, and foraging efficiency [6,7]. Nectar serves not only as a primary carbohydrate source but also modulates bee gut microbiota, immune function, and reproductive fitness [8,9,10,11].
The nutritional value of floral nectar is largely determined by its sugar composition, most notably the concentration and ratio of sucrose, glucose, and fructose [12]. Nectar rich in monosaccharides is generally favored by bees due to its digestibility [13,14,15]. Moreover, the sugar profile of nectar directly influences the physical and sensory properties of honey, affecting crystallization tendency, flavor, and pharmacological efficacy [16,17,18,19]. For example, high-glucose honeys crystallize rapidly, while more balanced sugar ratios can enhance both shelf life and taste [12].
Chestnut trees (Castanea spp.) are extensively cultivated for nut production throughout the temperate zones of Asia and Europe and are gaining recognition as important melliferous resources. Chestnut honey, especially from Castanea sativa and C. crenata, is distinctive for its dark color, high polyphenol content, and bioactive properties, including antioxidant, antibacterial, and anti-inflammatory activities [20,21,22,23]. Notably, the male flowers of C. sativa possess high concentrations of kynurenic acid, a neuroprotective metabolite rare among nectar sources [24]. Korean chestnut honey has demonstrated both antibacterial activity against Helicobacter pylori and tyrosinase-inhibiting properties with potential cosmetic applications [21,25,26,27].
Despite its ecological and pharmacological significance, detailed quantitative data on chestnut nectar production remain scarce. Prior studies have primarily focused on morphological and agronomic metrics such as fruit size, harvest date, or leaf phenology rather than nectar characteristics [28,29,30,31]. Comparative analyses of nectar output across cultivars and flowering stages, especially under standardized, replicated multi-year field conditions, are almost absent. Although some recent studies report differences in nectar volume, sugar profile, and volatile organic compounds among major chestnut cultivars [28], most prior research lacks systematic, multi-phase comparisons performed with standardized protocols. This gap in the literature limits understanding of how cultivar and flowering phase influence floral nectar traits, which this study aims to address. This research gap is deepened by the unique floral structure of chestnut trees, which produce long, densely flowered male catkins that bloom sequentially and make individual nectar measurements highly challenging. Such complexities result in the need for standardized classification and extraction protocols to allow for meaningful comparison among cultivars.
We selected four widely grown chestnut cultivars in South Korea, ‘Daebo’, ‘Okkwang’, ‘Riheiguri’, and ‘Tsukuba’, because they are commonly used for commercial nut production [27,32]. ‘Daebo’ was developed by the National Institute of Forest Science (NIFoS) by crossing the local line ‘Sangmyeon-1’ as the seed parent with ‘Riheiguri’ as the pollen parent in 1976. Selection was finalized in 1998. Trees show strong vigor, long catkins, and abundant flowering. ‘Okkwang’, also bred at NIFoS, originated from selection within a native Castanea crenata population in Gyeonggi Province, South Korea, and was released in 1965. It shows robust growth with rapid canopy expansion and high fruit set [32]. ‘Riheiguri’ is a Japanese introduction identified among seedlings from natural hybridization between Japanese and Chinese chestnuts and named in 1950. It combines vigorous growth with cold hardiness and tolerance to pests and diseases [32]. ‘Tsukuba’, one of Japan’s most widely planted cultivars, was developed at the Japanese Horticultural Research Station from ‘Ganne’ (seed) × ‘Hayadama’ (pollen) and released in 1959. Trees are vigorous with fast canopy development and profuse branching, although they are relatively susceptible to cold and biotic stresses [32,33]. These breeding histories provide an informative basis for evaluating genotype effects on floral and nectar attributes.
Through a 2-year replicated field survey (2023–2024), we systematically quantified floral traits, nectar yield, sugar composition, and estimated honey yield across four flowering phases for these cultivars. The objectives of this work were (1) to elucidate cultivar-specific differences in nectar quantity and quality; (2) to identify optimal cultivar–flowering phase combinations for maximizing estimated honey yield and pollinator support; and (3) to generate foundational data to guide Castanea cultivar selection for sustainable agroforestry, pollinator conservation, and apicultural practice. In summary, this study aims to compare nectar traits among four chestnut cultivars and to assess their implications for pollinators and estimated honey yield.
2. Materials and Methods
2.1. Study Site and Plant Materials
This study was conducted in a managed chestnut orchard in Hwaseong, Gyeonggi province, South Korea (37°15′45″ N, 126°55′29″ E). To minimize exogenous variability, no supplemental irrigation or fertilization was applied during the study period; routine pruning and pest management followed local practices. For each cultivar (‘Daebo,’ ‘Okkwang,’ ‘Riheiguri,’ ‘Tsukuba’), three plants of comparable age, vigor, and canopy architecture were randomly selected within the same block (total n = 12 plants). Mean plant height was ~8 m (diameter at breast height ~30 cm). The male flowering period (catkin emergence to senescence) spanned ~20 days each year. For each cultivar and flowering phase, nectar was collected at peak anthesis from 10 catkins per plant in each of three plants (total 30 catkins per phase per cultivar). All collected catkins for each phase and cultivar were pooled to form one composite sample for chemical analysis.
2.2. Catkin Sampling and Nectar Collection
To characterize flowering phenology and quantify nectar secretion in chestnut cultivars, floral observations and catkin sampling were conducted during the early to mid-June flowering season in 2023 and 2024. Flowering phase was determined at the catkin scale based on the proportion of staminate flowers open within a single catkin, rather than on catkin elongation. Four phenological phases were defined at the catkin scale by the proportion of open staminate flowers within a single catkin: phase 1, <25% open; phase 2, 25–50% open; phase 3, >50–100% open; and phase 4, post-anthesis browning/senescence after all staminate flowers had opened (Figure 1). Phase assignments were made by visual inspection of representative catkins on each plant immediately before nectar sampling, enabling standardized comparisons of floral and nectar traits across cultivars and phases.

Figure 1.
Male catkin flowering phases in chestnut (Castanea spp.), scored per catkin by the proportion of open staminate flowers. (A) Phase 1, <25% open; (B) phase 2, 25–50% open; (C) phase 3, >50–100% open; (D) phase 4, post-anthesis browning/senescence after all staminate flowers had opened.
For morphological analysis, 30 fully elongated male catkins were randomly collected from four cardinal directions per plant. Catkin length and diameter were measured using a digital caliper (±0.03 mm) (NA500-150S, Bluebird, Zhejiang, China) to assess intra-cultivar variation and provide context for nectar output.
The collection of nectar was conducted at 14:00, under conditions characterized by dry weather and abundant sunlight (no precipitation within the previous 24 h), which is known to coincide with the peak in daily nectar availability. The catkins were covered with pollination bags, a method employed to prevent the loss of nectar due to visits from honeybees and other insect pollinators.
Individual male catkins were centrifuged at 4000 rpm for 4 min at room temperature (RT) using a 50 mL conical tube equipped with a pre-inserted nylon mesh filter in a benchtop centrifuge (ROTOFIX 32 A, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). The extracted nectar was immediately quantified using a 100 μL Hamilton micro-syringe (Hamilton Co., Reno, NV, USA). To stabilize and preserve the nectar samples, each was diluted tenfold with an 80% ethanol solution in a sterile 15 mL tube.
After dilution, the nectar solutions were purified by filtration through a centrifugal nylon filter with a 0.45 μm pore size (Millipore Sigma, Billerica, MA, USA) using a 2 mL collection tube. The samples were centrifuged at 4000 rpm for 2 min to remove pollen, debris, and other particulate matter. The purified nectar samples were stored at –20 °C until analysis of sugar concentration and composition.
All procedures followed Na et al. [34,35] with minor modifications to accommodate the elongated, sequentially blooming male catkins of Castanea.
2.3. Analysis of Sugar Contents
Free sugars (sucrose, glucose, fructose) were quantified using HPLC (Dionex Ultimate 3000; Dionex, Sunnyvale, CA, USA) with RI detection (Showa Denko, New York, NY, USA) with an Aminex HPX-87P column (300 × 7.8 mm, Bio-Rad Laboratories, Hercules, CA, USA). Deionized water served as the mobile phase at a flow rate of 0.5 mL/min; oven temperature was set at 80 °C. External calibration used ≥99.5% standards (Sigma Aldrich, St. Louis, MO, USA) over 0.05–2.0 mg mL−1 (R2 ≥ 0.999).
2.4. Estimation of Honey Yield
Potential honey yield per tree was calculated based on nectar sugar content and catkin abundance using the formula:
where 1.15 is the conversion factor representing the sugar-to-honey transformation ratio of 85:100, as applied in previous studies [36,37].
Honey yield (g/plant) = Nectar sugar content (mg/catkin) × Number of catkins (ea/plant) × 0.001 × 1.15,
2.5. Statistical Analysis
All statistical analyses were performed using JMP Pro 18.1.1 (SAS Institute Inc., Cary, NC, USA), with statistical significance set at p < 0.05. To identify predictors of nectar volume and free sugar content, generalized regression models were fitted with a Gamma distribution and log link using elastic net regularization. Continuous predictor variables were standardized before modeling, and penalty parameters (α, λ) were selected via 5-fold cross-validation following the 1-SE rule. Model performance was summarized using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and Root Mean Square Error (RMSE).
For analyses of catkin length, nectar volume, free sugar concentration, catkins per tree, and estimated honey yield, generalized linear mixed models (GLMMs; Gamma–log link) were applied. Year and tree (nested within cultivar) were treated as random effects, while cultivar, flowering phase, and their interaction were treated as fixed effects. Post hoc comparisons were conducted with Tukey’s HSD on least-squares means (reported as LSM ± SE).
To assess sugar composition (sucrose, glucose, fructose), multivariate analysis of variance (MANOVA) was performed on standardized data, including fixed effects of cultivar, flowering phase, year, and their two- and three-way interactions. Wilks’ λ and Pillai’s trace were reported for multivariate significance. When significant, univariate ANOVAs with Tukey’s adjustment were subsequently examined. Principal component analysis (PCA) was applied to standardized sugars, retaining principal components based on explained variance (PC1–PC3) and visualized via biplots. Canonical discriminant analysis (CDA) was then used to classify samples by cultivar, with 95% confidence ellipses shown in canonical space, and leave-one-out cross-validation evaluating classification accuracy.
Assumptions of normality, homogeneity of variance, and distributional adequacy were verified prior to all modeling. Shapiro–Wilk tests assessed normality, and Levene’s test assessed homogeneity.
3. Results
3.1. Variation in Floral and Nectar Traits by Cultivar and Flowering Phase
Catkin length and nectar volume were consistently highest at phase 3, with phase-specific peak values observed for each cultivar (Figure 2). For example, in 2023, ‘Daebo’ exhibited a maximum catkin length of 199.80 ± 20.78 mm at phase 3, while ‘Tsukuba’ reached 216.80 ± 50.47 mm at phase 2, and ‘Riheiguri’ peaked at 180.00 ± 30.62 mm at phase 4. In 2024, maximum catkin lengths were generally observed at phase 3 for ‘Daebo’ (250.67 ± 22.64 mm), ‘Riheiguri’ (198.40 ± 21.27 mm), and ‘Tsukuba’ (218.97 ± 27.69 mm), with ‘Okkwang’ showing similar lengths at phases 2 and 3.
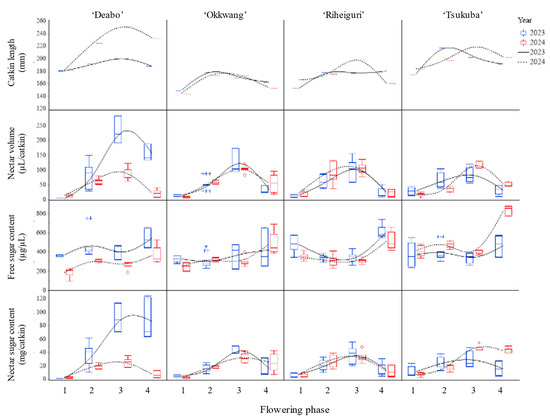
Figure 2.
Variation in catkin length (mm), nectar volume (µL/catkin), free sugar content (µg/µL), and nectar sugar content (mg/catkin) of four chestnut cultivars (‘Daebo’, ‘Okkwang’, ‘Riheiguri’, and ‘Tsukuba’) across four flowering phases in 2023 and 2024. Boxes denote median and IQR; whiskers = 1.5 × IQR; markers show means ± SD. Curves are loess smoothers by year (solid = 2023, dashed = 2024). Error bars indicate standard deviation.
Nectar volume (µL/catkin) peaked at phase 3 in both years (Figure 2). In 2023, ‘Daebo’ showed the highest nectar volume (232.7 ± 40.63), followed by ‘Okkwang’ (123.7 ± 37.25), ‘Riheiguri’ (101.4 ± 35.42), and ‘Tsukuba’ (81.50 ± 24.60). In 2024, phase 3 maxima shifted, with ‘Tsukuba’ (116.1 ± 9.55), ‘Riheiguri’ (107.3 ± 18.80), ‘Okkwang’ (103.6 ± 11.23), and ‘Daebo’ (94.00 ± 19.89).
Free sugar content (µg/µL) increased from early to late phases, with phase 4 showing the highest values for each cultivar (Figure 2). In 2023, peak free sugar content at phase 4 was 544.4 ± 89.21 in ‘Daebo’, 610.4 ± 58.85 in ‘Riheiguri’, 468.3 ± 93.09 in ‘Tsukuba’, and 420.7 ± 179 in ‘Okkwang’. In 2024, ‘Tsukuba’ reached the highest phase 4 value (833.2 ± 45.92), with other cultivars ranging from 365.8 to 513.1.
Nectar sugar content (mg/catkin) displayed phase-dependent maxima at phase 3 in both years (Figure 2). For 2023, ‘Daebo’ reached 91.39 ± 18.45, while ‘Okkwang’, ‘Riheiguri’, and ‘Tsukuba’ showed lower maxima. The 2024 values were highest in ‘Tsukuba’ (46.94 ± 3.53), with remaining cultivars between 25.36 and 33.51.
Significant effects of cultivar, phase, and their interaction for catkin length and free sugar content (p < 0.001) (Table 1). For nectar volume and nectar sugar per catkin, cultivar effects were not significant, but the overall phase dependence remained consistent across years.

Table 1.
Two-way ANOVA (analyzed by year) and GLMM (pooled years; random = year) testing effects of cultivar, flowering phase, and cultivar × flowering phase on floral/nectar traits. GLMM family/link: Gamma/log. Year was non-significant as a random effect.
3.2. Catkin and Nectar Traits: Mixed Model and Regression Analysis
Flowering phase shaped catkin length and nectar characteristics across cultivars and years (Table A1, Table 2 and Table 3). Catkin length was highest in ‘Daebo’ and ‘Tsukuba’ at phase 3. Nectar volume also peaked at phase 3 in all cultivars, while free sugar concentration reached its highest values at phase 4 for ‘Tsukuba’ and ‘Riheiguri’. Nectar sugar content per catkin was greatest at phase 3 in all cultivars, but the ranking among cultivars changed depending on phase and year. Catkin length showed no consistent association with nectar volume or free sugar content across phases. Both nectar volume and free sugar content displayed increased variability at higher mean levels.

Table 2.
Generalized regression analysis using elastic net regularization (gamma distribution with identity link) to determine significant predictors of nectar volume and free sugar content. Flowering phase and cultivar × flowering phase interaction were the most influential factors.

Table 3.
Estimated parameters from elastic net regression models with gamma distribution (identity link) showing coefficient (φ) estimates, standard errors (SE), Wald Chi-square (χ2) statistics with p-values, and 95% confidence intervals for nectar volume and free sugar content.
Specifically, Catkin length was generally greater in ‘Daebo’ and ‘Tsukuba’, and the maximum in ‘Daebo’ at phase 3 reached 5.40 ± 0.49. Nectar volume was highest at phase 3 for all cultivars, exemplified by ‘Daebo’ at 5.14 ± 0.56, ‘Okkwang’ at 4.74 ± 0.48, and ‘Riheiguri’ at 4.63 ± 0.47. Phase 1 consistently showed the lowest volumes. Free sugar content increased toward late anthesis and was highest at phase 4, exceeding 6.3 in ‘Tsukuba’ and ‘Riheiguri’.
Nectar sugar content per catkin reached a maximum at phase 3 across cultivars, with ‘Daebo’ at 4.09 ± 0.41, ‘Okkwang’ at 3.62 ± 0.39, ‘Tsukuba’ at 3.56 ± 0.36, and ‘Riheiguri’ at 3.52 ± 0.34 (Table A1). The ordering of cultivars varied by phase and year, and phase-dependent differences in magnitude were observed rather than a fixed hierarchy. Catkin length showed no consistent association with either nectar volume or free sugar content across phases.
Across models, flowering phase was the dominant predictor of nectar traits. Strong effects were recorded for nectar volume (χ2 = 40.61, p < 0.0001) and for free sugar concentration (χ2 = 302.5, p < 0.0001). A significant cultivar by phase interaction was recorded for nectar volume (χ2 = 17.63, p < 0.001), and phase-dependent differences in magnitude among cultivars were observed rather than a uniform ordering across phases (Table 2). Catkin length did not predict either response (all p > 0.05).
Dispersion estimates were greater than one for both traits, with nectar volume (φ = 4.308 ± 0.831) and free sugar content (φ = 5.581 ± 1.598) (Table 3). Both traits showed larger variability at higher mean levels. This pattern reflected biological heterogeneity among catkins and trees across years. After this heterogeneity was modeled in the mixed effects specification, the effect of flowering phase remained significant.
3.3. Sugar Composition in Floral Nectar
Sucrose and glucose in floral nectar generally increased toward phase 4. Fructose showed late-phase increases that were similar or steeper depending on the combination of cultivar and year (Figure 3). In 2023, ‘Daebo’ showed the highest sucrose in phase 4 (71.27 ± 18.84 µg/µL). In the same year, ‘Riheiguri’ recorded the highest glucose and fructose in phase 4 (136.46 ± 32.78 and 456.97 ± 94.72 µg/µL, respectively). In 2024, ‘Tsukuba’ recorded four maxima across all three sugars (sucrose 77.36 ± 5.98; glucose 206.33 ± 12.10; fructose 549.55 ± 34.16 µg/µL).
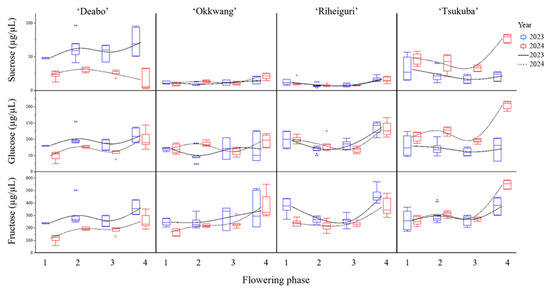
Figure 3.
Variation in nectar sugar composition (sucrose, glucose, fructose concentrations in μg/μL) across four flowering phases for each cultivar in 2023 and 2024.
The composition of sucrose, glucose, and fructose varied among cultivars and across flowering phases within each year. Each cultivar showed a characteristic progression of sugars through the flowering sequence, and the magnitude and direction of late-phase enrichment differed between years. The combination of cultivar, phase, and year defined discrete nectar sugar profiles that were separable (Table 4).

Table 4.
Multivariate analysis of variance results for nectar sugar composition (sucrose, glucose, fructose) by cultivar, flowering phase, year, and their interactions.
Late-phase enrichment of free sugars concentration was observed (Table 5). In 2023, ‘Daebo’ showed a concurrent rise in all three sugars at phase 4 with sucrose at +2.28, glucose at +0.81, and fructose at +0.87 in standardized units. In 2024, ‘Daebo’ showed a modest elevation in glucose at phase 4 with +0.404, while sucrose was slightly below average with −0.398 and fructose remained below average with −0.362. These patterns show that ‘Daebo’ expressed late-phase enrichment in 2023, while the same phase in 2024 showed a selective increase confined to glucose.

Table 5.
Standardized values of sucrose, glucose, and fructose concentrations (μg/μL) for each combination of cultivar, year, and flowering phase used in MANOVA analysis.
‘Okkwang’ was generally at or below average across many combinations. In 2024, glucose rose above average at phases 1 and 2 with +0.694 and +0.544 and dropped below average at phase 3 with −0.773, then returned to a modestly positive value at phase 4 with +0.296. Fructose in ‘Okkwang’ reached a positive value at phase 4 with +0.938, while sucrose values remained below average across phases in both years. This pattern shows weak late-phase enrichment dominated by glucose in early phases of 2024 and by fructose at phase 4.
‘Riheiguri’ showed higher monosaccharides in specific phases. In 2023, fructose was above average at phases 1 and 2 with +0.870 and +0.920, decreased at phase 3 with −0.934, and returned to a positive value at phase 4 with +0.264. In 2024, ‘Riheiguri’ again showed a late-phase increase in fructose at phase 4 of +1.254 and glucose at phase 4 of +1.022, while sucrose values were below average across phases. These observations show cultivar-specific late-phase accumulation of fructose and glucose in ‘Riheiguri’, with year-dependent magnitude.
‘Tsukuba’ had the strongest late-phase enrichment in 2024. Phase 4 z-scores were +2.572 for sucrose, +3.773 for glucose, and +2.970 for fructose. In 2023, ‘Tsukuba’ showed early phase elevations for glucose and fructose with phase 1 values of approximately +1.002 and +1.054, while later phases were near zero or negative for some sugars. These results show that ‘Tsukuba’ concentrated all sugars strongly at late anthesis in 2024, whereas the 2023 profile was more mixed with early phase elevations.
3.4. Principal Component and Discriminant Analyses of Sugar Composition
PCA of standardized sucrose, glucose, and fructose identified a dominant concentration axis (Figure 4). PC1 had positive loadings for glucose at 0.932, fructose at 0.868, and sucrose at 0.718 and explained 71.3% of the variance. PC2 explained 22.2% of the variance with a positive loading for sucrose at 0.689 and negative loadings for fructose at −0.412 and glucose at −0.147. PC1 and PC2 together accounted for 93.5% of the total variance. PC3 accounted for 6.5% and showed opposite signs for glucose at −0.331 and fructose at 0.277 with a weak sucrose loading at 0.095.
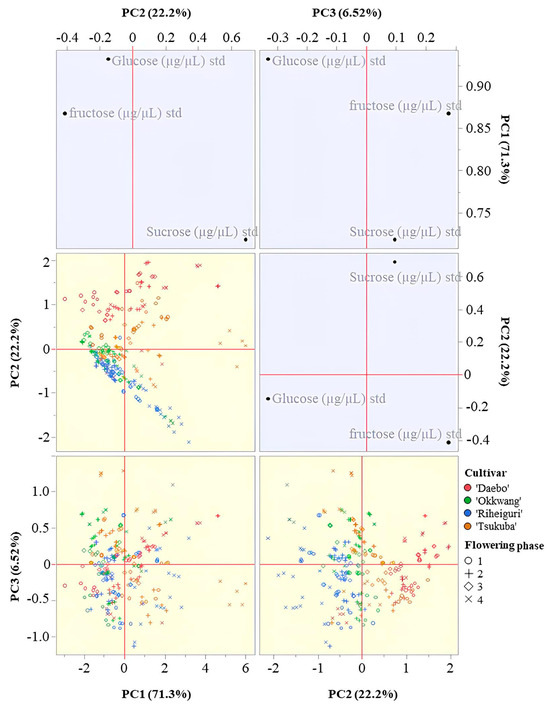
Figure 4.
PCA biplot of nectar sugar composition in the four chestnut cultivars based on standardized values of sucrose, glucose, and fructose concentrations (μg/μL).
In the PC1 versus PC2 plane, cultivar positions followed these loading patterns. ‘Tsukuba’ at phase 4 was positioned at high PC1 and negative PC2, which corresponds to nectar with high total sugar and a hexose-rich balance. ‘Daebo’ extended toward high PC1 and positive PC2, which corresponds to high total sugar with a larger sucrose share. ‘Okkwang’ clustered near the origin or slightly negative on both axes, and ‘Riheiguri’ shifted toward negative values on both axes.
CDA based on the same standardized sugars separated cultivar score clouds in the CF1 versus CF2 plot (Figure 5). The 95% confidence ellipses were distinct for ‘Daebo’, ‘Okkwang’, and ‘Riheiguri’, and a partial overlap was present between ‘Okkwang’ and ‘Tsukuba’. The confusion matrix recorded correct classification rates of 100.0% for ‘Riheiguri’ (27 out of 27), 87.3% for ‘Daebo’ (55 out of 63), 67.2% for ‘Okkwang’ (45 out of 67), and 36.4% for ‘Tsukuba’ (28 out of 44). The misclassification of the subjects was most prevalent in the ‘Tsukuba’ category and was most common in the ‘Okkwang’ category, with 15 out of 44 subjects falling into this category. It was determined that no ‘Riheiguri’ samples were misclassified (Table 6).
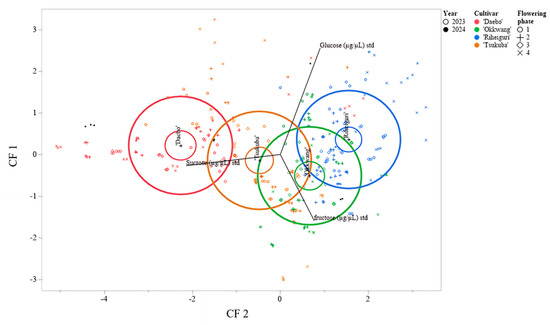
Figure 5.
Canonical discriminant analysis plot for cultivar separation based on standardized sugar composition. Ellipses represent 95% confidence intervals for cultivar groups.

Table 6.
Classification accuracy of canonical discriminant analysis for predicting cultivar identity based on standardized sugar composition.
3.5. Estimated Honey Yield
Across all cultivars, yields increased from phase 1 to phase 3 and declined in phase 4. Phase 1 consistently showed the lowest values (p < 0.001) (Figure 6).
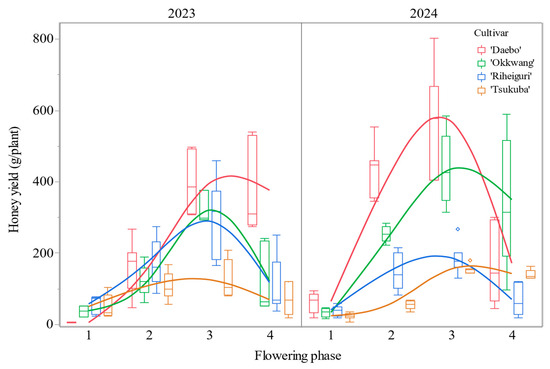
Figure 6.
Estimated honey yield (g/plant) by cultivar and flowering phase in 2023 and 2024 (all p < 0.001). Values are presented as mean ± standard deviation.
By cultivar, ‘Daebo’ increased sharply through phases 2–3 to the highest peak among all groups in both years (2023: 397.4 ± 80.21 g/plant; 2024: 572.9 ± 148.8 g/plant), followed by a reduction in phase 4 (2023: 373.6 ± 121.5; 2024: 167.3 ± 106.1 g/plant). ‘Okkwang’ exhibited a monotonic rise to phase 3 (2023: 324.2 ± 40.76; 2024: 437.5 ± 94.14 g/plant) and a subsequent decrease in phase 4 (2023: 118.3 ± 89.95; 2024: 346.1 ± 184.2 g/plant). ‘Riheiguri’ showed intermediate phase 3 maxima (2023: 289.6 ± 109.8; 2024: 186.8 ± 42.47 g/plant) and lower phase 4 values. In contrast, ‘Tsukuba’ yielded the smallest amounts across phases and years, with phase 3 values of 124.8 ± 51.71 (2023) and 155.8 ± 11.73 g/plant (2024). In all cultivars, standard deviations were larger around the phase 3 peak, indicating greater among-tree variability at maximum yield. Furthermore, within phase 3, ‘Daebo’ yielded the most, ‘Okkwang’ and ‘Riheiguri’ were intermediate, and ‘Tsukuba’ remained the lowest (Figure 6).
The model-based estimates of honey yield per plant demonstrated an increase from phase 1 to phase 3 across all cultivars, followed by a decrease in phase 4. Greater within-cultivar variability was observed near the phase 3 peak. The variance component of year as a random intercept was found to be minimal (0.0038) and non-significant (SE = 0.0082; 95% CI = −0.012 to 0.020; p > 0.05) (Table A1).
4. Discussion
4.1. Floral and Nectar Trait Dynamics Across Cultivars and Flowering Phases
Across two years, flowering phase rather than catkin size influenced nectar traits across cultivars. Nectar volume and nectar sugar per catkin reached maxima at phase 3, whereas free sugar concentration peaked at phase 4. Although cultivars differed in the magnitude and timing of this phase-dependent pattern, the overall trajectory was conserved across years (Figure 2; Table 1, Table 2 and Table 3).
‘Daebo’ and ‘Tsukuba’ frequently developed longer catkins, a trait commonly associated with floral display and forager access in previous literature [38,39,40]. However, in this study, catkin length was not associated with nectar volume or free sugar concentration across phases, which is consistent with findings from other woody taxa, where gross inflorescence size does not directly regulate nectar provisioning [41,42,43]. Thus, these results underscore the importance of phenology and secretory physiology over display traits as the dominant controls of nectar supply.
The observed offset between nectar quantity and concentration aligns with established physiological mechanisms. Nectar secretion typically increases toward anthesis and then declines, while evaporation and post-secretory resorption during late anthesis concentrate the solutes. Consequently, nectar sugar per catkin was greatest at phase 3 in both years (Figure 2). The subsequent increase in free sugar concentration at phase 4 did not compensate for the concurrent decrease in volume, which supports the robustness of this phase-dependent pattern [44].
Consistent with this offset, related studies indicate that cultivar- and taxon-level differences in nectar chemistry can influence pollinator activity. Higher nectar sugar content in some cultivars enhances the attraction of Apis spp., resulting in variation in pollination efficiency and estimated honey yield among varieties [43]. Furthermore, the composition of nectar sugars governs Apis spp. foraging preferences and ultimately shapes pollination networks within agricultural landscapes [43]. Marked differences in nectar volume and sugar content have been documented among Chinese chestnut cultivars, influencing both nectar quality and the chemical attributes of honey across ecological regions [44]. Comparative analyses of honeydew honeys from chestnut and beech trees demonstrate that plant taxa determine nectar sugar composition and mineral profiles, leading to unique honey characteristics and influencing selective bee visitation [45].
These phase-dependent patterns have important implications for pollinator use. Apis spp. typically collect nectars with sugar concentrations ranging from thirty to fifty percent by weight and can process somewhat higher concentrations depending on their drinking mechanics [46,47]. In phase 3, the combination of high nectar volume and substantial nectar sugar content per catkin resulted in the largest energetic rewards per visit, making this stage the most attractive for foragers. In contrast, phase 4 offered higher concentration but reduced volume, leading to a lower absolute return per visit. The tree-to-tree heterogeneity observed in this study is consistent with previous reports of temporal and intraspecific variation in nectar production and likely organizes fine-scale differences in visitation within orchards [28,41,48]. Trees expressing greater nectar volume during optimal phases attract more foragers and contribute disproportionately to pollen transfer and estimated honey yield at the stand level. Reports from Castanea and other woody taxa describe cultivar- or taxon-level contrasts in nectar chemistry and associated honey properties [43,44,45], supporting the cultivar-specific patterns and ecological implications observed in this study.
4.2. Sugar Composition and Its Functional Implications for Pollinator Nutrition
Across cultivars and years, nectar sugars followed a phase-dependent pattern that shifted toward a hexose-rich balance at late anthesis, while the extent of this shift and the persistence of residual sucrose differed by cultivar and by year (Figure 3; Table 4) [49,50]. Free sugar composition varied along two biological features (Figure 4 and Figure 5; Table 5 and Table 6). One feature was the overall abundance of free sugars. The other feature was the balance between sucrose and the hexoses glucose and fructose. As flowers progress from mid anthesis to late anthesis, extracellular invertases split sucrose into glucose and fructose, and evaporation reduces water content [51,52]. Together these processes shift nectar toward a hexose-rich mixture at late anthesis, and the magnitude of the shift differs among cultivars [53,54].
Such diversity in nectar sugar composition is widely observed among woody species and reflects both genetic and environmental drivers. For example, black locust has documented variability in sugar types and concentrations by tree genotype and floral stage with direct consequences for bee preferences and nectar use [49]. Comparative studies of chestnut fruit chemistry have shown that local environment and plant genotype interact to produce distinct chemical profiles [50]. At the physiological level, invertase activity and evaporative concentration regulate age-related changes in floral sugar ratios across angiosperms [51,52,53].
Nectar composition affects not only energy value but also physical properties and forager behavior. Honeybees are sensitive to viscosity and temperature and often prefer warmer and less viscous nectar even at comparable concentrations [54,55,56]. The functional value of nectar also depends on the fit between floral traits such as corolla tube length and sugar ratios and the mouthpart design and physiological capacities of visiting insects [54]. In addition, nectar nutrition shapes bee gut microbiota and immune function [51,57].
From an ecological and practical perspective, these changing sugar profiles have clear implications for pollinators. Analyses in species such as linden and acacia have reported that seasonal fluctuations in nectar composition can redirect Apis spp. foraging across sites and years, which underscores the importance of maintaining a continuous and diverse nectar supply for pollinator health [49,58]. Furthermore, a precise understanding of these nectar sugar dynamics is important because modest shifts in sucrose to hexose balance or in total concentration can alter resource quality for Apis spp. and influence colony performance [53].
Phase 4 provided hexose-rich and energetically dense nectar, and the balance of sugar contents in nectar differed among cultivars and between years (Table 4, Table 5 and Table 6; Figure 3, Figure 4 and Figure 5). These differences are compatible with the preferences of Apis spp. and help explain cultivar-specific visitation patterns and their consequences for estimated honey yield at the stand level [43,44,45]. Planting mixtures of cultivars could therefore enhance the reliability and quality of nectar resources through the blooming season, when local conditions support the recorded phase-dependent pattern [12,50,58].
Taken together, variation in nectar sugar profiles driven by genotype, phenology, and environment organizes temporal and spatial patterns of pollinator visitation. Similar relationships have been documented in other trees, supporting the view that nectar chemistry is a key determinant of pollinator attraction and ecosystem service delivery [49,51].
4.3. Cultivar Differences in Estimated Honey Yield and Management Implications
Integrating nectar volume per catkin, free sugar concentration, nectar sugar per catkin, and catkin production revealed cultivar- and phase-dependent differences in estimated honey yield (Figure 6; Table A1). Across all cultivars, nectar volume per catkin and nectar sugar per catkin increased from early bloom, reached a maximum level at flowering phase 3, and declined at phase 4. Similar phase-sensitive trends and genotype by environment influences on nectar provisioning have been reported in melliferous resources [34,59,60,61,62].
In these data, ‘Daebo’ repeatedly formed the highest estimated honey yield at phase 3 across years, and ‘Okkwang’ followed a similar mid anthesis maximum. ‘Tsukuba’ did not achieve high estimated honey yield at any phase. However, in phase 4, it expressed a high free sugar concentration with a compositionally rich sugar profile as described in Section 2.3, which supports a role in honey quality rather than yield alone [34,63,64]. These cultivar-specific outcomes are consistent with biological and ecological processes that shape nectar supply and with reports that link genotype and phenology to resource profiles in Castanea and related systems [59,61,65].
Variation in nectar traits influences foraging by Apis spp. and informs the timing of hive placement and honey extraction [12,13,15,34]. Phase 3 aligns the peak of nectar sugar per catkin with foraging by Apis spp., which raises the energetic return per visit and supports pollen transfer, fruit set, and stand-level estimated honey yield [62,65]. Phase 4 provides more concentrated but smaller volumes, so absolute return per visit is lower even when monosaccharides are abundant. In orchard settings, cultivar mixtures can buffer temporal gaps because ‘Daebo’ and ‘Okkwang’ deliver high nectar sugar mass at mid anthesis, while ‘Tsukuba’ and ‘Riheiguri’ extend hexose-rich resources into late bloom. This approach is consistent with recommendations that diverse nectar supply stabilizes pollinator foraging and improves productivity under variable seasonal conditions [66,67].
Across the flowering season, the four Castanea cultivars contribute complementary strengths to the temporal pattern of nectar resources. ‘Daebo’ consistently provides the greatest nectar sugar per catkin and the highest estimated honey yield at mid anthesis under comparable conditions. ‘Okkwang’ maintains an intermediate yield with a relatively uniform and hexose-leaning composition. ‘Riheiguri’ shows late-phase fructose enrichment and a distinct sugar signature separable in the multivariate analyses, which offers opportunities for targeted honey differentiation [66,67]. Although ‘Tsukuba’ shows the lowest estimated honey yield, its late-bloom nectar is highly concentrated and compositionally distinct, which can contribute to its honey flavor and nutritional quality [62,64,67].
These cultivar-specific nectar profiles support a diversified planting strategy as a rational way to manage honey resources. ‘Daebo’-dominated stands secure high throughput at mid anthesis, and interplanting with ‘Tsukuba’ and ‘Riheiguri’ extends high-density hexose and nectar sugar availability into late bloom. When the single objective is to maximize yield, a greater share of ‘Daebo’ is justified with a trade off in seasonal and compositional stability relative to mixtures [60,61,65]. In summary, phase 3 is the key stage for maximizing nectar sugar per catkin and estimated honey yield, whereas phase 4 provides greater chemical distinctiveness. Selecting cultivars with higher nectar volume per catkin, higher free sugar concentration within the preferred range of Apis spp., and higher nectar sugar per catkin will improve estimated honey yield. Additionally, because commercial chestnut orchards are managed primarily for nut production, hive placement during phase 3 enhances pollen transfer and nut set, and late bloom provides a differentiated honey crop. Coordinated management of apiculture and chestnut production therefore delivers complementary returns under typical orchard conditions [34,64,67].
4.4. Limitations and Future Perspectives
This study offers new insights into cultivar- and phase-specific variations in chestnut nectar traits, but several important limitations must be acknowledged. First, the chemical analysis was restricted to free sugars, excluding other nectar constituents such as amino acids, polyphenols, proteins, and volatiles that may play major roles in pollinator attraction and honey characteristics. Second, all samples were derived from a single orchard and two years, limiting the representation of broader environmental, geographic, and interannual variability. Third, direct measurements of pollinator visitation, bee foraging behavior, and honey processing outcomes were not conducted in parallel with nectar profiling.
To address these limitations, future research should expand chemical analyses to include a wider spectrum of nectar constituents, implement multi-location and multi-year sampling designs, and incorporate explicit linkages between nectar composition, pollinator foraging, colony health, and honey properties using interdisciplinary and field-based approaches [11,12,68]. Landscape-scale studies integrating metabolomic, genomic, and ecological data will provide a deeper understanding of nectar resource stability and pollination services. These approaches are essential for developing sustainable and resilient agroforestry and apicultural systems in the face of ongoing environmental change [12,68,69,70].
5. Conclusions
This study demonstrates clear differences in nectar traits among four chestnut cultivars, affecting both pollinator behavior and honey yield. The results indicate that a diversified planting approach, combining cultivars with varied nectar sugar characteristics, can help maintain stable nectar resources for Apis spp. and improve both honey yield and quality across the flowering season. In systems managed for nut production, synchronizing apiculture activities at mid anthesis may also support effective pollen transfer. Further studies with multi-year and multi-location designs will be needed to better understand the impact of environmental factors on nectar composition and its implications for orchard management.
Author Contributions
J.-M.P., designed study, methodology, planned and performed the experiments, data analysis, graphic design, formal analysis, statistical analysis, draft writing, collecting references; H.-J.K., planned and performed the experiments, data curation, writing—review and editing; S.-J.W., planned and performed the experiments, data curation, writing—review and editing; S.-J.N., project administration, designed study, methodology, writing—review and editing, collecting references. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Forest Science, Project No. FG0403-2023-03-2025.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Tukey’s HSD pairwise comparisons on GLMM classified cultivar × flowering stage combinations into homogeneous groups for catkin length (mm), number of catkins per each plant, nectar volume (µL) per catkin, free sugar content (µg/µL), nectar sugar content (mg) per catkin, and estimated honey yield (g) per plant across cultivars and flowering phases.
Table A1.
Tukey’s HSD pairwise comparisons on GLMM classified cultivar × flowering stage combinations into homogeneous groups for catkin length (mm), number of catkins per each plant, nectar volume (µL) per catkin, free sugar content (µg/µL), nectar sugar content (mg) per catkin, and estimated honey yield (g) per plant across cultivars and flowering phases.
| Trait | Cultivar | Flowering Phase | Grouping | LSD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catkin length | ‘Daebo’ | 3 | A | 5.403 | |||||||||
| ‘Tsukuba’ | 2 | A | B | 5.368 | |||||||||
| ‘Tsukuba’ | 3 | B | C | 5.345 | |||||||||
| ‘Daebo’ | 4 | B | C | D | 5.335 | ||||||||
| ‘Daebo’ | 2 | C | D | 5.315 | |||||||||
| ‘Tsukuba’ | 4 | D | 5.278 | ||||||||||
| ‘Riheiguri’ | 3 | E | 5.218 | ||||||||||
| ‘Daebo’ | 1 | E | F | 5.191 | |||||||||
| ‘Tsukuba’ | 1 | E | F | 5.187 | |||||||||
| ‘Riheiguri’ | 2 | E | F | 5.186 | |||||||||
| ‘Okkwang’ | 2 | E | F | 5.184 | |||||||||
| ‘Riheiguri’ | 4 | F | 5.155 | ||||||||||
| ‘Okkwang’ | 3 | F | 5.145 | ||||||||||
| ‘Okkwang’ | 4 | G | 5.076 | ||||||||||
| ‘Riheiguri’ | 1 | G | 5.064 | ||||||||||
| ‘Okkwang’ | 1 | H | 4.998 | ||||||||||
| No. of catkins | ‘Daebo’ | 1 | A | 9.181 | |||||||||
| ‘Daebo’ | 4 | A | B | 9.181 | |||||||||
| ‘Daebo’ | 3 | A | B | 9.084 | |||||||||
| ‘Okkwang’ | 3 | A | B | 9.075 | |||||||||
| ‘Okkwang’ | 1 | A | B | 9.065 | |||||||||
| ‘Okkwang’ | 4 | A | B | 9.056 | |||||||||
| ‘Okkwang’ | 2 | A | B | 9.046 | |||||||||
| ‘Daebo’ | 2 | A | B | 8.915 | |||||||||
| ‘Riheiguri’ | 2 | B | 8.889 | ||||||||||
| ‘Riheiguri’ | 3 | B | 8.827 | ||||||||||
| ‘Riheiguri’ | 4 | B | 8.811 | ||||||||||
| ‘Riheiguri’ | 1 | B | 8.301 | ||||||||||
| ‘Tsukuba’ | 2 | C | 8.256 | ||||||||||
| ‘Tsukuba’ | 4 | C | 8.236 | ||||||||||
| ‘Tsukuba’ | 3 | C | 8.236 | ||||||||||
| ‘Tsukuba’ | 1 | C | 8.236 | ||||||||||
| Nectar volume | ‘Daebo’ | 3 | A | 5.136 | |||||||||
| ‘Okkwang’ | 3 | A | B | 4.740 | |||||||||
| ‘Riheiguri’ | 3 | B | C | 4.628 | |||||||||
| ‘Daebo’ | 4 | B | C | 4.547 | |||||||||
| ‘Tsukuba’ | 3 | B | C | 4.544 | |||||||||
| ‘Daebo’ | 2 | B | C | D | 4.327 | ||||||||
| ‘Riheiguri’ | 2 | C | D | 4.232 | |||||||||
| ‘Tsukuba’ | 2 | D | E | 4.042 | |||||||||
| ‘Okkwang’ | 2 | D | E | 4.000 | |||||||||
| ‘Okkwang’ | 4 | E | F | 3.696 | |||||||||
| ‘Tsukuba’ | 4 | E | F | 3.620 | |||||||||
| ‘Tsukuba’ | 1 | F | G | 3.187 | |||||||||
| ‘Riheiguri’ | 4 | F | G | 3.149 | |||||||||
| ‘Riheiguri’ | 1 | G | H | 2.816 | |||||||||
| ‘Okkwang’ | 1 | H | 2.542 | ||||||||||
| ‘Daebo’ | 1 | H | 2.430 | ||||||||||
| Free sugar content | ‘Tsukuba’ | 4 | A | 6.378 | |||||||||
| ‘Riheiguri’ | 4 | A | 6.338 | ||||||||||
| ‘Daebo’ | 4 | A | B | 6.132 | |||||||||
| ‘Okkwang’ | 4 | A | B | C | 6.085 | ||||||||
| ‘Riheiguri’ | 1 | B | C | D | 6.044 | ||||||||
| ‘Tsukuba’ | 2 | B | C | D | E | 6.006 | |||||||
| ‘Daebo’ | 2 | B | C | D | E | 6.000 | |||||||
| ‘Tsukuba’ | 1 | B | C | D | E | F | 5.948 | ||||||
| ‘Tsukuba’ | 3 | B | C | D | E | F | 5.894 | ||||||
| ‘Okkwang’ | 3 | C | D | E | F | 5.832 | |||||||
| ‘Daebo’ | 3 | C | D | E | F | 5.818 | |||||||
| ‘Riheiguri’ | 3 | D | E | F | 5.802 | ||||||||
| ‘Riheiguri’ | 2 | E | F | 5.789 | |||||||||
| ‘Okkwang’ | 2 | F | G | 5.718 | |||||||||
| ‘Okkwang’ | 1 | F | G | 5.678 | |||||||||
| ‘Daebo’ | 1 | G | 5.485 | ||||||||||
| Nectar sugar content | ‘Daebo’ | 3 | A | 4.093 | |||||||||
| ‘Daebo’ | 4 | A | B | 3.884 | |||||||||
| ‘Okkwang’ | 3 | A | B | C | 3.620 | ||||||||
| ‘Tsukuba’ | 3 | A | B | C | 3.564 | ||||||||
| ‘Riheiguri’ | 3 | B | C | D | 3.520 | ||||||||
| ‘Daebo’ | 2 | B | C | D | 3.391 | ||||||||
| ‘Tsukuba’ | 4 | C | D | E | 3.203 | ||||||||
| ‘Riheiguri’ | 2 | C | D | E | 3.101 | ||||||||
| ‘Tsukuba’ | 2 | C | D | E | F | 3.080 | |||||||
| ‘Okkwang’ | 4 | D | E | F | 2.944 | ||||||||
| ‘Okkwang’ | 2 | E | F | 2.804 | |||||||||
| ‘Riheiguri’ | 4 | F | G | 2.570 | |||||||||
| ‘Tsukuba’ | 1 | G | H | 2.238 | |||||||||
| ‘Riheiguri’ | 1 | H | I | 1.934 | |||||||||
| ‘Okkwang’ | 1 | I | J | 1.348 | |||||||||
| ‘Daebo’ | 1 | J | 0.920 | ||||||||||
| Estimated honey yield | ‘Daebo’ | 3 | A | 6.160 | |||||||||
| ‘Okkwang’ | 3 | A | B | 5.923 | |||||||||
| ‘Daebo’ | 4 | A | B | C | 5.664 | ||||||||
| ‘Riheiguri’ | 3 | B | C | 5.580 | |||||||||
| ‘Daebo’ | 2 | B | C | 5.513 | |||||||||
| ‘Okkwang’ | 4 | B | C | D | 5.289 | ||||||||
| ‘Riheiguri’ | 2 | C | D | 5.153 | |||||||||
| ‘Okkwang’ | 2 | C | D | E | 5.100 | ||||||||
| ‘Tsukuba’ | 3 | D | E | 4.920 | |||||||||
| ‘Riheiguri’ | 4 | E | 4.587 | ||||||||||
| ‘Tsukuba’ | 2 | E | 4.578 | ||||||||||
| ‘Tsukuba’ | 4 | E | F | 4.517 | |||||||||
| ‘Riheiguri’ | 1 | F | G | 3.915 | |||||||||
| ‘Daebo’ | 1 | F | G | 3.788 | |||||||||
| ‘Tsukuba’ | 1 | G | 3.695 | ||||||||||
| ‘Okkwang’ | 1 | G | 3.599 | ||||||||||
Groupings with different letters (A–J) indicate significant differences at p < 0.05. Minimum significant difference (LSD) values are shown in the rightmost column.
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services-IPBES. Summary for policymakers of the thematic assessment on pollinators, pollination and food production. Biota Neotrop. 2016, 16, e20160101. [Google Scholar]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Wright, G.A. Plant—Pollinator interactions and threats to pollination. Funct. Ecol. 2017, 31, 22–25. [Google Scholar] [CrossRef]
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76. [Google Scholar] [CrossRef]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Lee, F.J.; Rusch, D.B.; Stewart, F.J.; Mattila, H.R.; Newton, I.L.G. Saccharide breakdown and fermentation by the honey bee gut microbiome. Environ. Microbiol. 2015, 17, 796–815. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Moran, N.A. The honeybee microbiota and its impact on health and disease. Nat. Rev. Microbiol. 2024, 22, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W. Sweet solutions: Nectar chemistry and quality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210163. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.C.; Walters, K.F.A. Potential use of floral nectar sugar characteristics in plant selection for pollinator habitats. J. Apic. Res. 2023, 62, 266–273. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kessler, M.; Hanley, D.; Karger, D.N.; Müller, M.P.; Knauer, A.C.; Keller, F.; Schwerdtfeger, M.; Humphreys, A.M. Pollinator adaptation and the evolution of floral nectar sugar composition. J. Evol. Biol. 2017, 30, 112–127. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Mallinger, R.E.; Portlas, Z.M.; Hulke, B.S.; Fugate, K.K.; Paradis, T.; Hampton, M.E.; Carter, C.J. Using nectar-related traits to enhance crop-pollinator interactions. Front. Plant Sci. 2018, 9, 812. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2022, 102, 139–146. [Google Scholar] [CrossRef]
- Ntakoulas, D.D.; Pasias, I.N.; Raptopoulou, K.G.; Proestos, C. Authenticity of Greek honey based on phenolic compounds and physicochemical characteristics. Food Chem. 2025, 476, 143465. [Google Scholar] [CrossRef]
- Rodríguez, I.; Tananaki, C.; Galán-Soldevilla, H.; Pérez-Cacho, P.R.; Serrano, S. Sensory profile of Greek islands thyme honey. Appl. Sci. 2021, 11, 9548. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Papastephanou, C.; Badeka, A.V. New insights into the typification of Hellenic monofloral honeys using selected physico-chemical and bio-chemical indicators coupled with z score analysis and chemometric models. Eur. Food Res. Technol. 2021, 247, 169–182. [Google Scholar] [CrossRef]
- Temizer, I.K.; Güder, A.; Temel, F.A.; Cüce, H. Antioxidant activities and heavy metal contents of Castanea sativa Honey. Glob. Nest J. 2019, 20, 541–550. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Bae, S.-H.; Kim, H.-K.; Lee, M.-L.; Choi, Y.-S.; Jin, B.-R.; Lee, H.J.; Jeong, H.Y.; Lee, Y.G.; Moon, J.-H. New quinolinone alkaloids from chestnut (Castanea crenata Sieb) honey. J. Agric. Food Chem. 2015, 63, 3587–3592. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- González-Paramás, A.M.; García-Villanova, R.J.; Gómez Bárez, J.A.; Sánchez Sánchez, J.; Ardanuy Albajar, R. Botanical Origin of Monovarietal Dark Honeys (from Heather, Holm Oak, Pyrenean Oak and Sweet Chestnut) Based on Their Chromatic Characters and Amino Acid Profiles. Eur. Food Res. Technol. 2007, 226, 87–92. [Google Scholar] [CrossRef]
- Turski, M.P.; Chwil, S.; Turska, M.; Chwil, M.; Kocki, T.; Rajtar, G.; Parada-Turska, J. An exceptionally high content of kynurenic acid in chestnut honey and flowers of chestnut tree. J. Food Compos. Anal. 2016, 48, 67–72. [Google Scholar] [CrossRef]
- Cviljević, S.; Bilić Rajs, B.; Primorac, L.; Strelec, I.; Gal, K.; Cvijetić Stokanović, M.; Penava, A.; Mindum, A.; Flanjak, I. Antibacterial activity OF chestnut honey (Castanea sativa Mill.) against Helicobacter pylori and correlation to its antioxidant capacity. Hrana Zdr. Boles. Znan. Stručni Cas. Nutr. Dijetetiku 2020, 9, 52–56. [Google Scholar]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio Petronio, G.; Straquadanio, C.; Crascì, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2019, 33, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, A.; Kwon, H.Y.; Lee, U.; Kim, M.S. Analysis of flowering and nectar characteristics of major four chestnut cultivars (Castanea spp.). J. Apic. 2017, 32, 237–246. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, S.; Song, J.H.; Kim, M.J.; Yunusbaev, U.; Lee, M.-L.; Kim, M.S.; Kwon, H.W. Comparison of biochemical constituents and contents in floral nectar of Castanea spp. Molecules 2020, 25, 4225. [Google Scholar] [CrossRef]
- Silva, D.M.; Zambon, C.R.; Techio, V.H.; Pio, R. Floral characterization and pollen germination protocol for Castanea crenata Siebold & Zucc. S. Afr. J. Bot. 2020, 130, 389–395. [Google Scholar] [CrossRef]
- Gurjar, T.D.; Pandey, A.K.; Rodge, R.R.; Biology, F. Pollination, and genetic resources in nut crops. In Temperate Nut Crops; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 51–108. [Google Scholar]
- Şahin, H.; Kolaylı, S.; Kara, Y.; Can, Z.; Güler, H.İ.; Özkök, A.; Serdar, Ü. A study on recognizing the value of chestnut (Castanea sativa) blossom waste. J. Agric. Sci. 2023, 30, 79–89. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.C.; Lee, W. Chestnut Cultivars in Korea; Korea Forest Research Institute: Seoul, Republic of Korea, 2006. [Google Scholar]
- Yoo, H.; Kim, C.; Lee, U.; Park, Y.; Jung, H.; Kim, Y. ‘EePi No. 1’: A high-yielding cultivar of chestnut (Castanea crenata Siebold and Zucc.) with high pellicle removability. Korean J. Breed. Sci. 2025, 57, 199–204. [Google Scholar] [CrossRef]
- Na, S.-J.; Kim, Y.-K.; Park, J.-M. Nectar characteristics and honey production potential of five rapeseed cultivars and two wild-flower species in in South Korea. Plants 2024, 13, 419. [Google Scholar] [CrossRef]
- Na, S.-J.; Park, J.-M.; Kwon, H.-Y.; Kim, Y.-K. Assessment of floral nectar and amino acid yield in eight landscape trees for enhanced pollinator food resources in urban forests. Plants 2025, 14, 1924. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing plants for bee-keeping at any cost?—Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2003, 238, 155–168. [Google Scholar] [CrossRef]
- Adgaba, N.; Al-Ghamdi, A.; Tadesse, Y.; Getachew, A.; Awad, A.M.; Ansari, M.J.; Owayss, A.A.; Mohammed, S.E.A.; Alqarni, A.S. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 180–191. [Google Scholar] [CrossRef]
- da Cunha, N.L.; Aizen, M.A. Pollen production per flower increases with floral display size across animal-pollinated flowering plants. Am. J. Bot. 2023, 110, e16180. [Google Scholar] [CrossRef]
- Aguirre, L.A.; Junker, R.R. Floral and pollinator functional diversity mediate network structure along an elevational gradient. Alp. Bot. 2024, 134, 193–206. [Google Scholar] [CrossRef]
- Glaettli, M.; Barrett, S.C.H. Pollinator responses to variation in floral display and flower size in dioecious Sagittaria latifolia (Alismataceae). New Phytol. 2008, 179, 1193–1201. [Google Scholar] [CrossRef]
- Frigero, M.L.P.; Boaro, C.S.F.; Galetto, L.; Tunes, P.; Guimarães, E. Extreme events induced by climate change alter nectar offer to pollinators in cross pollination-dependent crops. Sci. Rep. 2025, 15, 10852. [Google Scholar] [CrossRef]
- Romero-Bravo, A.; Castellanos, M.C. Nectar and floral morphology differ in evolutionary potential in novel pollination environments. New Phytol. 2024, 243, 753–764. [Google Scholar] [CrossRef]
- Liu, Y.; Dunker, S.; Durka, W.; Dominik, C.; Heuschele, J.M.; Honchar, H.; Hoffmann, P.; Musche, M.; Paxton, R.J.; Settele, J.; et al. Eco-evolutionary processes shaping floral nectar sugar composition. Sci. Rep. 2024, 14, 13856. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Pan, S.; Xu, C.; Xiong, Y.L. Chemical composition and quality traits of Chinese chestnuts (Castanea mollissima) produced in different ecological regions. Food Biosci. 2015, 11, 33–42. [Google Scholar] [CrossRef]
- Barbarić, A.; Saftić Martinović, L.; Milinčić, D.D.; Pešić, M.B.; Marijanović, Z.; Jakac, M.; Brčić Karačonji, I.; Brekalo, H.; Petrović, D.; Pavlešić, T.; et al. Characterization and differentiation of beech and chestnut honeydew honeys: A comparative study. Food Chem. 2025, 477, 143446. [Google Scholar] [CrossRef]
- Nganso, B.T.; Agboka, K.M.; Atagong, S.D.; Topé, S.F.; Massing, T.; Landmann, T.; Sevgan, S.; Mwiza, W.; Odera, F.; Piiru, E.D.; et al. A geospatial atlas of honey bee forage plants and their distribution patterns in Africa and beyond. Sci. Rep. 2025, 15, 34384. [Google Scholar] [CrossRef] [PubMed]
- Deksisa, K.; Gemechu, A.; Tolera, T. Does cluster beekeeping improve the efficiency of honey production in participant households in Southwestern Ethiopia? Heliyon 2024, 10, e38651. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Wen, S.-J.; Wang, H.; Ren, Z.-X. Floral nectar reabsorption and a sugar concentration gradient in two long-spurred Habenaria species (Orchidaceae). BMC Plant Biol. 2023, 23, 331. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, M.S.; Nam, J.I.; Song, J.H.; Kim, S.H. Analysis on floral nectar characteristics among the selected black locust (Robinia spp.) individuals. J. Apic. Res. 2023, 62, 923–931. [Google Scholar] [CrossRef]
- Turfan, N.; Kara, F.; Alay, M. Variations on the chemical compositions of chestnut fruits collected at different locations. BioResources 2024, 19, 8527–8541. [Google Scholar] [CrossRef]
- Roy, R.; Schmitt, A.J.; Thomas, J.B.; Carter, C.J. Review: Nectar biology: From molecules to ecosystems. Plant Sci. 2017, 262, 148–164. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Revell, M.; Noe, S.; Manley-Harris, M. Influence of genotype, floral stage, and water stress on floral nectar yield and composition of Mānuka (Leptospermum scoparium). Ann. Bot. 2018, 121, 501–512. [Google Scholar] [CrossRef]
- Bordin, D.M.; Latgé, S.G.C.; Pyke, G.; Kalman, J.; Doble, P.; Genta, F.A.; Blanes, L. A simple approach to analyze sugar nectar composition in flowers using capillary electrophoresis and enzymatic assays. J. Braz. Chem. Soc. 2020, 31, 2129–2134. [Google Scholar] [CrossRef]
- Torres, C.; Galetto, L. Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biol. 2002, 4, 360–366. [Google Scholar] [CrossRef]
- Nicolson, S.W.; De Veer, L.; Köhler, A.; Pirk, C.W.W. Honeybees prefer warmer nectar and less viscous nectar, regardless of sugar concentration. Proc. Biol. Sci. 2013, 280, 20131597. [Google Scholar] [CrossRef]
- Basari, N.; Ramli, S.N.; Abdul-Mutalid, N.A.; Shaipulah, N.F.M.; Hashim, N.A. Flowers morphology and nectar concentration determine the preferred food source of stingless bee, Heterotrigona itama. J. Asia Pac. Entomol. 2021, 24, 232–236. [Google Scholar] [CrossRef]
- Nicolson, S.W. Bee Food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204. [Google Scholar] [CrossRef]
- Quinlan, G.M.; Miller, D.A.W.; Grozinger, C.M. Examining spatial and temporal drivers of pollinator nutritional resources: Evidence from five decades of honey bee colony productivity data. Environ. Res. Lett. 2023, 18, 114018. [Google Scholar] [CrossRef]
- Sheridan, R.E. Environmental and Genetic Influences on Growth, Flowering, and Nectar Production in Mānuka (Leptospermum scoparium JR Forst. & G. Forst.): A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy in Plant Science. Ph.D. Thesis, Massey University, School of Agriculture and Environment, Palmerston North, New Zealand, 2019. [Google Scholar]
- Nickless, E.M.; Anderson, C.W.N.; Hamilton, G.; Stephens, J.M.; Wargent, J. Soil influences on plant growth, floral density and nectar yield in three cultivars of mānuka (Leptospermum scoparium). N. Z. J. Bot. 2017, 55, 100–117. [Google Scholar] [CrossRef]
- McPeek, S.J.; Erwin, C.L.; Brodie, E.D., III. Patterns of within- and among-plant variation in nectar production in the beetle-pollinated Amianthium muscaetoxicum. Am. J. Bot. 2025, 112, e70069. [Google Scholar] [CrossRef]
- Wei, J.; Rico-Guevara, A.; Nicolson, S.W.; Brau, F.; Damman, P.; Gorb, S.N.; Wu, Z.; Wu, J. Honey bees switch mechanisms to drink deep nectar efficiently. Proc. Natl. Acad. Sci. USA 2023, 120, e2305436120. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.R.; Kidd, N.A.C.; Pickard, R.S. A comparative evaluation of sampling methods for varroa destructor (acari: Varroidae) population estimation. Apidologie 2006, 37, 452–461. [Google Scholar] [CrossRef]
- Barberis, M.; Calabrese, D.; Galloni, M.; Nepi, M. Secondary metabolites in nectar-mediated plant-pollinator relationships. Plants 2023, 12, 550. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M.; Vesprini, J.L. Nectar biodiversity: A short review. Plant Syst. Evol. 2003, 238, 7–21. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. ISBN 978-1-4020-5936-0. [Google Scholar] [CrossRef]
- Camina, M.J.; Pellerano, G. Geographical and botanical classification of honeys and apicultural products by chemometric methods. A review. Curr. Anal. Chem. 2012, 8, 408–425. [Google Scholar] [CrossRef]
- Rudelli, C.; Bellei, E.; Andreani, G.; Isani, G. An innovative multi-omics approach reveals the interactions between honeybees and their environment. Animals 2025, 15, 2660. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.; Newburn, L.R.; Fitch, G. Food as medicine: A review of plant secondary metabolites from pollen, nectar, and resin with health benefits for bees. Insects 2025, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-L.; Huang, Z.Y.; Dai, W.-F.; Yang, Y.-P.; Duan, Y.-W. Mixed effects of honey bees on pollination function in the Tibetan alpine grasslands. Nat. Commun. 2024, 15, 8164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).