Integrative Feeding Strategies with Essential Oils and Probiotics to Improve Raw Meat Quality and Carcass Traits in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
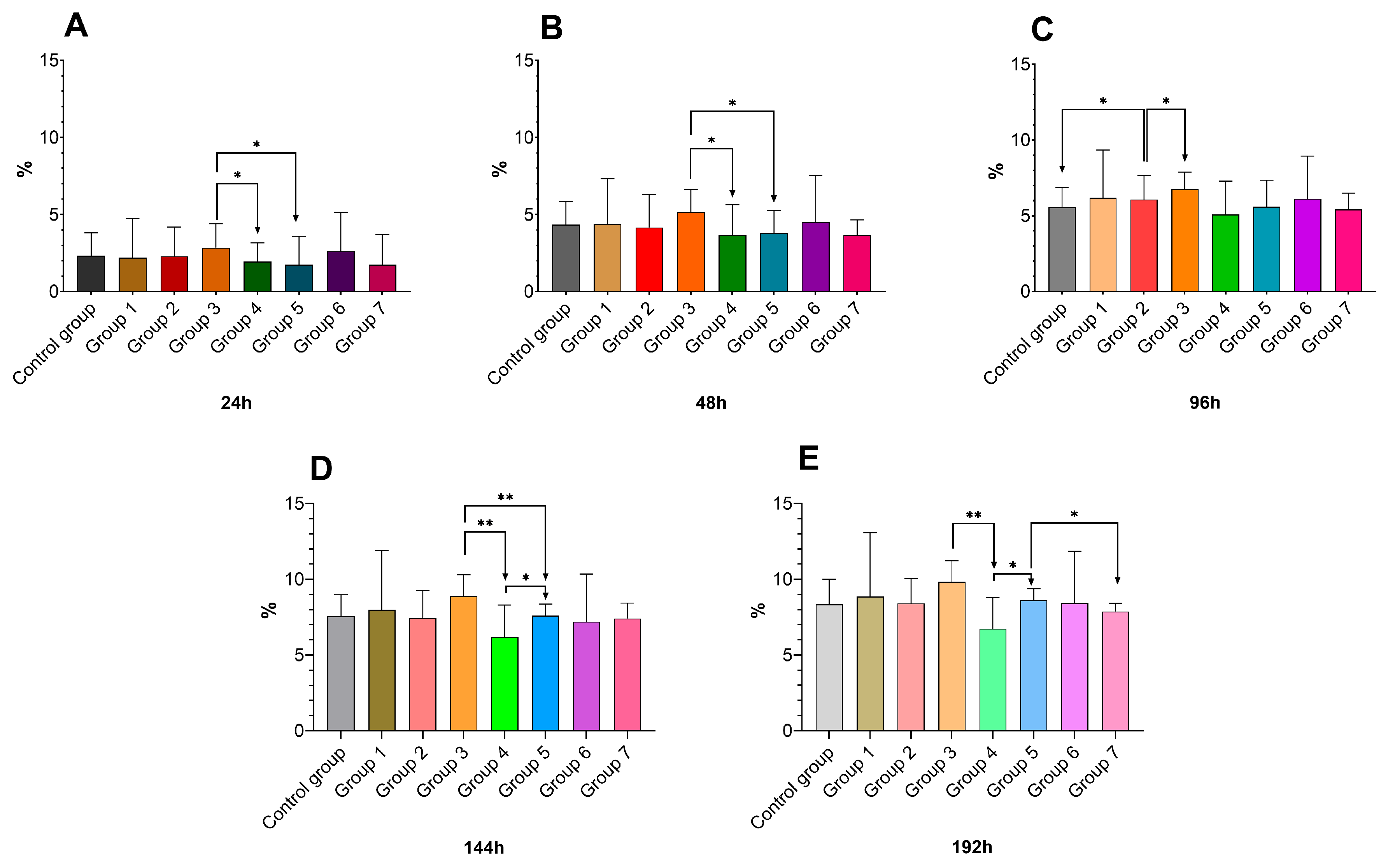
2.2. Assessment of Drip Loss
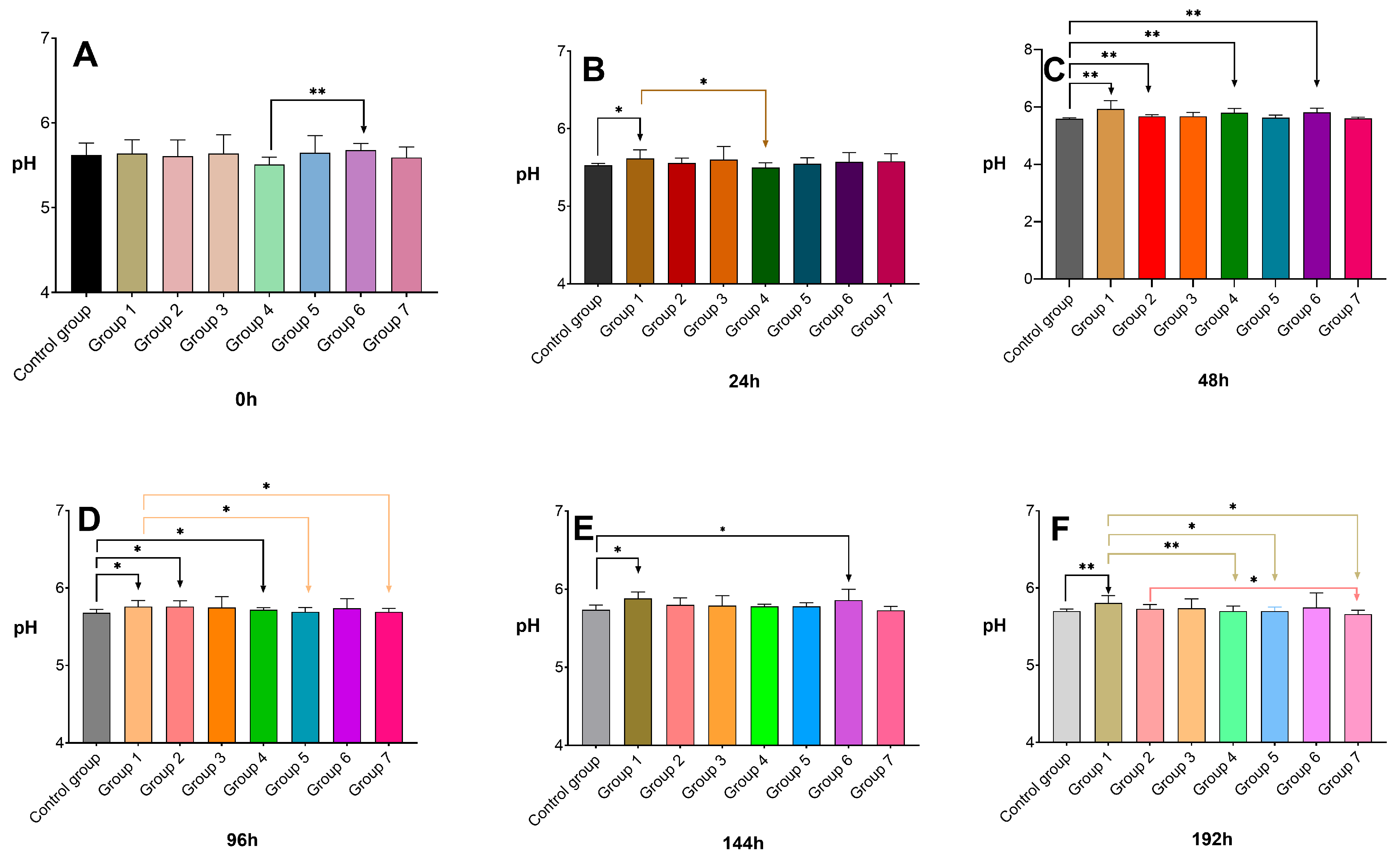
2.3. Determination of Breast Meat pH
2.4. Establishing the Carcass Weight
2.5. Essential Oil Analysis with Gas Chromatography-Mass Spectrometry (GC-MS)
2.6. Statistical Analysis
3. Results
3.1. Nutritional and Bio-Productive Indices Recorded in Chickens from the Experimental Variants
3.2. Carcass Portion, Meat Cuts and Organs Percentages
3.3. Drip Loss Impact on Breast Meat Quality
3.4. pH Analysis in Broiler Breast Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| TTEO | Tea tree essential oil |
| TEO | Thyme essential oil |
References
- Lam, D. The Next 2 Billion: Can the World Support 10 Billion People? Popul. Dev. Rev. 2025, 51, 63–102. [Google Scholar] [CrossRef]
- Alabdallah, Z.A.; Nikishov, A.A.; Jhonn Lenon, C.J.; Ortiz Manzano, M.L. Influence of using probiotic on the productivity and morphometry of the organs in the visceral cavity of broiler chicks. Casp. J. Environ. Sci. 2023, 21, 431–437. [Google Scholar]
- Ramlucken, U.; Lalloo, R.; Roets, Y.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 2020, 241, 104215. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe 2024, 5. [Google Scholar] [CrossRef]
- WOAH. Annual Report on Antimicrobial Agents Intended for Use in Animals: 9th Report; WOAH: Paris, France, 2025; p. 49. [Google Scholar]
- Deeb, S.E.; Ashour, E.A.; Abd El-Hack, M.E.; El-Maaty, M.A.; Youssef, I.M.; Adil, S.; Elolimy, A.A.; Swelum, A.A. Impacts of dietary different levels of thyme leave powder as a natural growth promoter on growth performance, carcass characteristics, and blood indices of broilers. Poult. Sci. 2024, 103, 104396. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, M.; Arslan, M.; Javaid, A.; Farooq, U.; Asad, T.; Nisa, Q.; Azhar, M.; Bughio, E.; Liaqat, S. Effect of Blend of Essential Oils on Growth Performance, Carcass Characteristics, Meat Quality, Intestinal Morphology, Serum Biochemistry, and Immune Response of Broiler Chickens. Braz. J. Poult. Sci. 2024, 26, eRBCA-2023-1853. [Google Scholar]
- Hassan, A.H.A.; Youssef, I.M.I.; Abdel-Atty, N.S.; Abdel-Daim, A.S.A. Effect of thyme, ginger, and their nano-particles on growth performance, carcass characteristics, meat quality and intestinal bacteriology of broiler chickens. BMC Vet. Res. 2024, 20, 269. [Google Scholar] [CrossRef]
- Darmawan, A.; Öztürk, E.; Güngör, E.; Özlü, Ş.; Jayanegara, A. Effects of essential oils on egg production and feed efficiency as influenced by laying hen breed: A meta-analysis. Vet. World 2024, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Pellegrini, F.; Fracchiolla, G.; Mrenoshki, D.; Zarea, A.A.K.; Bianco, A.; Del Sambro, L.; Capozzi, L.; Schiavone, A.; Saleh, M.S.; et al. Pilot Study on the Action of Thymus vulgaris Essential Oil in Treating the Most Common Bacterial Contaminants and Salmonella enterica subsp. enterica Serovar Derby in Poultry Litter. Antibiotics 2023, 12, 436. [Google Scholar] [CrossRef]
- Puvača, N.; Čabarkapa, I.; Petrović, A.; Bursić, V.; Prodanović, R.; Soleša, D.; Lević, J. Tea tree (Melaleuca alternifolia) and its essential oil: Antimicrobial, antioxidant and acaricidal effects in poultry production. World’s Poult. Sci. J. 2019, 75, 235–246. [Google Scholar] [CrossRef]
- Raza, Q.S.; Saleemi, M.K.; Gul, S.; Irshad, H.; Fayyaz, A.; Zaheer, I.; Tahir, M.W.; Fatima, Z.; Chohan, T.Z.; Imran, M. Role of essential oils/volatile oils in poultry production—A review on present, past and future contemplations. Agrobiol. Rec. 2022, 7, 40–56. [Google Scholar]
- Zaazaa, A.; Mudalal, S.; Alzuheir, I.; Samara, M.; Jalboush, N.; Fayyad, A.; Petracci, M. The Impact of Thyme and Oregano Essential Oils Dietary Supplementation on Broiler Health, Growth Performance, and Prevalence of Growth-Related Breast Muscle Abnormalities. Animals 2022, 12, 3065. [Google Scholar] [CrossRef]
- Bo, R.; Zhan, Y.; Wei, S.; Xu, S.; Huang, Y.; Liu, M.; Li, J. Tea tree oil nanoliposomes: Optimization, characterization, and antibacterial activity against Escherichia coli in vitro and in vivo. Poult. Sci. 2023, 102, 102238. [Google Scholar] [CrossRef]
- Puvača, N.; Tufarelli, V.; Giannenas, I. Essential Oils in Broiler Chicken Production, Immunity and Meat Quality: Review of Thymus vulgaris, Origanum vulgare, and Rosmarinus officinalis. Agriculture 2022, 12, 874. [Google Scholar] [CrossRef]
- Saim, M.; Nagi, A.B.; Noor, I.; Zaheer, H.; Zaheer, W.; Tahir, R.; Rafay, A.; Fayaz, A.; Fatima, J.; Naeem, L. Effect of Essential Oils as Natural Alternatives and Antioxidants for the Growth of Poultry Broilers. In Complementary and Alternative Medicine: Essential Oils; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 170–182. [Google Scholar]
- Purnamasari, L. Productivity of young broilers fed Phyllanthus niruri Linn and Melaleuca cajuput leaf meal as a phytogenic feed additive. Thai J. Vet. Med. 2024, 53, 131–137. [Google Scholar] [CrossRef]
- Fawaz, M.; Ismail, Z.; Hassan, H.; Abdel-Wareth, A. Effect of thyme essential oil on productive performance of broiler chickens a-review. SVU-Int. J. Environ. Res. 2021, 3, 8–18. [Google Scholar] [CrossRef]
- Rocha, R.F.; da Silva Fidelis, P.H.; Massuquetto, A.; Sobrane Filho, S.T.; de Araújo, M.S.; Silva, C.M.; da Costa Lopes, C.; de Medeiros, E.S.; Moreira, R.H.R. Essential oils as a strategy to improve gut histomorphometry and performance of broilers: Systematic review and meta-analysis. J. Agric. Sci. 2024, 162, 404–416. [Google Scholar] [CrossRef]
- Noruzi, S.; Torki, M.; Mohammadi, H. Effects of supplementing diet with Thyme (Thymuas vulgaris L.) essential oil and/or selenium yeast on production performance and blood variables of broiler chickens. Vet. Med. Sci. 2022, 8, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Kareem, K.Y.; Matuszewski, A.; Bień, D.; Ciborowska, P.; Lutostański, K.; Michalczuk, M. Enhancing broiler chicken health and performance: The impact of phytobiotics on growth, gut microbiota, antioxidants, and immunity. Phytochem. Rev. 2024, 24, 2131–2145. [Google Scholar] [CrossRef]
- Gumus, R.; Gelen, S.U. Effects of dietary thyme and rosemary essential oils on performance parameters with lipid oxidation, water activity, pH, colour and microbial quality of breast and drumstick meats in broiler chickens. Arch. Anim. Breed. 2023, 66, 17–29. [Google Scholar] [CrossRef]
- Ranwa, S.; Palod, J.; Sharma, R.K.; Kumar, S.; Rastogi, S.; Kumar, R. Influence of thyme and turmeric essential oils supplementation on growth performance, nutrient utilization and economics of Japanese quails. Indian J. Anim. Sci. 2023, 93, 505–509. [Google Scholar] [CrossRef]
- Fawaz, M.; Hassan, H.; Abdel-Wareth, A. The effect of dietary aflatoxin B1, thyme oil, and their combination on sustainability of meat production of broiler chickens. SVU-Int. J. Agric. Sci. 2022, 4, 131–135. [Google Scholar] [CrossRef]
- Behboodi, H.R.; Hosseini, D.; Salarieh, A.; Gholampour, M.; Panahi, M.; Alemi, M.; Baradaran, A.; Nazarpak, H.H. Impact of drinking water supplementation of a blend of peppermint, coneflower (Echinacea purpurea), thyme, propolis, and prebiotic on performance, serum constituents, and immunocompetence of broiler chickens. Trop. Anim. Health Prod. 2022, 54, 289. [Google Scholar] [CrossRef] [PubMed]
- Belali, M.; Seidavi, A.; Bouyeh, M.; Gorlov, I.F.; Slozhenkina, M.I.; Mosolov, A.A.; Ramírez, L.S.; Tirado-González, D.N.; Cuevas-Barragán, C.E.; Cipriano-Salazar, M. Substantiable bioconversion of the aromatic plant extracts biomass as feed additives in broiler performance: Effects and prefeasibility comparison of thyme (Thymus vulgaris). Biomass Convers. Biorefinery 2024, 14, 6097–6109. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils on Antibiotic-Resistant Bacterial Biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Yuan, C.; Ma, X.; Jiang, M.; Yang, T.; Lin, M.; Zhao, G.; Zhan, K. Effects of Tea Tree Oil on Production Performance, Serum Parameter Indices, and Immunity in Postpartum Dairy Cows. Animals 2023, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qiu, G.; Yu, X.; Zhao, J.; Liu, J.; Wang, H.; Dong, L. Terpinen-4-ol Improves the Intestinal Barrier Function of the Colon in Immune-Stressed Weaning Piglets. Animals 2025, 15, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, L.; Ling, H.; Zhou, L. Protective Effect of Melaleuca alternifolia Essential Oil on Broiler Growth Performance, Intestinal Morphology, Gut Microflora, and Gut Redox Status: An Alternative Growth Promoter to Antibiotics. Pak. J. Zool. 2025; Online First Articles. [Google Scholar]
- Deminicis, R.G.d.S.; Meneghetti, C.; Garcia, A.A.P.; Deminicis, B.B.; Maciel, B.M. Gut microbiome and morphometry of quails fed diets containing essential oils. Ciênc. Rural 2024, 54, e20230372. [Google Scholar] [CrossRef]
- Qu, H.; Cheng, Y.; Chen, Y.; Zhao, Y.; Li, J.; Wen, C.; Zhou, Y. Dietary tea tree (Melaleuca alternifolia) oil supplementation improves growth performance, cecal microflora, immunity, and antioxidant capacity of partridge shank chickens. J. Poult. Sci. 2019, 56, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Chand, N.; Naz, S.; Khan, R.U. Dietary tea tree (Melaleuca alternifolia) essential oil as alternative to antibiotics alleviates experimentally induced Eimeria tenella challenge in Japanese quails. J. Anim. Physiol. Anim. Nutr. 2023, 107, 643–649. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Du, H.; Feng, J.; Zhang, W.; Li, H.; Xu, F.; Lin, J.; Fu, H.; Zhao, X.; et al. Effects of adding tea tree oil on growth performance, immune function, and intestinal function of broilers. Poult. Sci. 2023, 102, 102936. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiang, L.; Hu, M.; Luo, X.; Cheng, T.; Wang, Y. Tea tree oil inhibits hydrogen sulfide-induced oxidative damage in chicken lungs through CYP450s/ROS pathway. Poult. Sci. 2024, 103, 103860. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Shirazi, M.R.J.; Moradi, H.R.; Kazemi, K.; Akbarabadi, Z.K.; Jazi, V. Effects of drinking water supplemented with apple vinegar, essential oils, or colistin sulfate on growth performance, blood lipids, antioxidant status, intestinal morphology, and gut microflora of broiler chickens. Poult. Sci. 2025, 104, 104801. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- Yang, T.; Feng, F.; Zhan, K.; Ma, X.; Jiang, M.; Datsomor, O.; Zhu, X.; Huo, Y.; Zhao, G. Effect of the Tea Tree Oil on Growth Performance, Meat Quality, Serum Biochemical Indices, and Antioxidant Capacity in Finishing Pigs. Front. Vet. Sci. 2022, 9, 916625. [Google Scholar] [CrossRef]
- Puvača, N.; Lika, E.; Cocoli, S.; Shtylla Kika, T.; Bursić, V.; Vuković, G.; Tomaš Simin, M.; Petrović, A.; Cara, M. Use of Tea Tree Essential Oil (Melaleuca alternifolia) in Laying Hen’s Nutrition on Performance and Egg Fatty Acid Profile as a Promising Sustainable Organic Agricultural Tool. Sustainability 2020, 12, 3420. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Huang, Y.; Wang, W.; Long, X.; Jiang, L.; Cheng, T.; Du, J.; Luo, X. The mechanism of tea tree oil regulating the damage of hydrogen sulfide to spleen and intestine of chicken. Poult. Sci. 2025, 104, 104605. [Google Scholar] [CrossRef]
- Wade, M.; Manwar, S.; Kuralkar, S.; Waghmare, S.; Ingle, V.; Hajare, S. Effect of thyme essential oil on performance of broiler chicken. J. Entomol. Zool. Stud. 2018, 6, 25–28. [Google Scholar]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, F.-F.; Hu, J.-Y.; Mohammed, A.; Cheng, H.-W. Bacillus subtilis-Based Probiotic Improves Skeletal Health and Immunity in Broiler Chickens Exposed to Heat Stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Hernández, R.; Gonzalez-Galaviz, J.R.; Rodriguez-Anaya, L.Z.; Gil-Núñez, J.C.; Rodríguez-Jaramillo, M.d.C. Dietary Use of Methionine Sources and Bacillus amyloliquefaciens CECT 5940 Influences Growth Performance, Hepatopancreatic Histology, Digestion, Immunity, and Digestive Microbiota of Litopenaeus vannamei Fed Reduced Fishmeal Diets. Animals 2023, 13, 43. [Google Scholar] [CrossRef]
- de Oliveira, M.J.K.; Sakomura, N.K.; de Paula Dorigam, J.C.; Doranalli, K.; Soares, L.; Viana, G.d.S. Bacillus amyloliquefaciens CECT 5940 alone or in combination with antibiotic growth promoters improves performance in broilers under enteric pathogen challenge. Poult. Sci. 2019, 98, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, X.; Liu, H. Effects of Dietary Probiotic (Bacillus subtilis) Supplementation on Carcass Traits, Meat Quality, Amino Acid, and Fatty Acid Profile of Broiler Chickens. Front. Vet. Sci. 2021, 8, 767802. [Google Scholar] [CrossRef]
- Grant, A.Q.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Ugwuodo, C.J.; Nwagu, T.N. Stabilizing enzymes by immobilization on bacterial spores: A review of literature. Int. J. Biol. Macromol. 2021, 166, 238–250. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Pinheiro, S.R.F.; Dumont, M.A.; Pires, A.V.; Boari, C.A.; Miranda, J.A.; de Oliveira, R.G.; Ferreira, C.B. Carcass yield and meat quality of meat quails fed with diets with different levels of protein and essential amino acids supplementation/Rendimento de carcaca e qualidade da carne de codornas de corte alimentadas com racoes de diferentes niveis de proteina e suplementadas com aminoacidos essenciais. Cienc. Rural 2015, 45, 292–298. [Google Scholar]
- Aviagen. Ross Broiler Management Handbook; Aviagen: Huntsville, AL, USA, 2025; pp. 1–137. [Google Scholar]
- Aviagen. Ross 308/308 FF Broiler: Performance Objectives; Aviagen: Huntsville, AL, USA, 2022; pp. 1–14. [Google Scholar]
- Jitariuc Sava, Ş.E. Contributions to the Knowledge of Meat Production Obtained from Kabir Hens Breed. Ph.D. Thesis, Iasi University of Life Sciences (IULS), Iasi, Romania, 2020. [Google Scholar]
- Vázquez, A.; Tabanca, N.; Kendra, P.E. HPTLC Analysis and Chemical Composition of Selected Melaleuca Essential Oils. Molecules 2023, 28, 3925. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.; Linstrom, P.; Zenkevich, I. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Cao, Q.-q.; Shaukat, A.; Zhang, C.; Huang, S.-c. Insights into the evaluation, influential factors and improvement strategies for poultry meat quality: A review. npj Sci. Food 2024, 8, 62. [Google Scholar] [CrossRef]
- Choi, J.; Kong, B.; Bowker, B.C.; Zhuang, H.; Kim, W.K. Nutritional Strategies to Improve Meat Quality and Composition in the Challenging Conditions of Broiler Production: A Review. Animals 2023, 13, 1386. [Google Scholar] [CrossRef]
- Jaspal, M.H.; Ijaz, M.; Haq, H.A.u.; Yar, M.K.; Asghar, B.; Manzoor, A.; Badar, I.H.; Ullah, S.; Islam, M.S.; Hussain, J. Effect of oregano essential oil or lactic acid treatments combined with air and modified atmosphere packaging on the quality and storage properties of chicken breast meat. LWT 2021, 146, 111459. [Google Scholar] [CrossRef]
- Gümüş, R.; Kara, A.; Özkanlar, S.; İmik, H.; Celep, N.A. Effects of dietary thyme and rosemary essential oils on biochemical parameters, anti-oxidant metabolism, small intestinal morphology and myofiber structure of superficial pectoral and biceps femoris muscles in broilers. In Proceedings of the Veterinary Research Forum; Ferdowsi University of Mashhad: Mashhad, Iran, 2023; p. 249. [Google Scholar]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Saracila, M.; Turcu, R.P.; Dumitru, M. Effect of Basil, Thyme and Sage Essential Oils as Phytogenic Feed Additives on Production Performances, Meat Quality and Intestinal Microbiota in Broiler Chickens. Agriculture 2023, 13, 874. [Google Scholar] [CrossRef]
- Wade, M.; Manwar, S.; Kuralkar, S.; Waghmare, S.; Ingle, V.; Hajare, S. Effect of dietary thyme essential oil on carcass parameter of broiler chickens. Chem. Sci. Rev. Lett. 2018, 7, 669–672. [Google Scholar]
- Onel, S.E.; Aksu, T. The effect of thyme (Thymbra spicata L. var. spicata) essential oil on the antioxidant potential and meat quality of Japanese quail fed in various stocking densities. Atatürk Üniv. Vet. Bilim. Derg. 2019, 14, 129–136. [Google Scholar]
- Amirahmadi, E.; Safamehr, A.; Nobakht, A.; Mehmannavaz, Y. The effects of thyme essential oil and enzyme supplementation to corn or wheat based diets on performance, carcass traits and blood metabolites in broilers. J. Hell. Vet. Med. Soc. 2022, 73, 3799–3808. [Google Scholar] [CrossRef]
- Naderiboroojerdi, N.; Zeinali, A.; Hoseini, A. Comparison of different levels of thyme and rosemary ether extracts on growth performance and carcass characteristics of broiler chickens. J. Food Sci. Nutr. Res. 2022, 5, 682–689. [Google Scholar] [CrossRef]
- Irawan, A.; Hidayat, C.; Jayanegara, A.; Ratriyanto, A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: A meta-analysis. Anim. Biosci. 2020, 34, 1499. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Chang, X.; Oleforuh-Okoleh, V.U.; Onu, P.N.; Zhang, H.; Qiu, K.; Wu, S. Phytobiotics in poultry: Revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 2024, 15, 169. [Google Scholar] [CrossRef]
- Hassanin, O.; El-Sebai, A.; El-Motaal, S.A.; Khalifa, H.A. Experimental trials to assess the immune modulatory influence of thyme and ginseng oil on NDV-vaccinated broiler chickens. Open Vet. J. 2024, 14, 398–406. [Google Scholar] [CrossRef]
- Olfati, A.; Hosseini, S.M. The Effects of dietary supplementation of encapsulated thyme essential oil on growth, pro-inflammatory cytokines, and serum amino acid profiles of broiler chicks challenged with Salmonella Typhimurium. Ann. Anim. Sci. 2022, 22, 189–200. [Google Scholar] [CrossRef]
- Almremdhy, H.; Al-Khafaji, M.A. Drinking thyme extract on growth performance and immune response of broiler chickens. J. Artic. Indian Vet. J. 2020, 97, 32–35. [Google Scholar]
- Al-Abdullatif, A.A.; Al-Garadi, M.A.; Qaid, M.M.; Matar, A.M.; Alobre, M.M.; Al-Badwi, M.A.; Hussein, E.O.; Suliman, G.M. Effects of water and feed based RISCO-NUTRIFOUR probiotic supplementation on the technological and physicochemical quality of broiler breast meat. Front. Vet. Sci. 2025, 12, 1517078. [Google Scholar] [CrossRef]
- Moirangthem, S.; Patra, G.; Biswas, S.; Das, A.; Nath, S.; Verma, A.K.; Pal, S.; Chatterjee, N.; Bandyopadhyay, S.; Nanda, P.K.; et al. Effect of Nutmeg (Myristica fragrans) and Tea Tree (Melaleuca alternifolia) Essential Oils on the Oxidative and Microbial Stability of Chicken Fillets During Refrigerated Storage. Foods 2024, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Young, O.A.; West, J.; Hart, A.L.; van Otterdijk, F.F.H. A method for early determination of meat ultimate pH. Meat Sci. 2004, 66, 493–498. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the US: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, O.A.; Akinleye, S.B.; Simon, C.J.; Kayode, A.O.; Akande, M.O.; Ogunjobi, T.E.; Tijani, L.T.; Ayileye, K.T. Oxidative stability in meat (pectoralis major) of broiler orally supplemented with essential oils of allium sativum, Curcuma longa, Zingiber officinale, and Cinnamomum zeylanicum. Transl. Anim. Sci. 2024, 8, txae073. [Google Scholar] [CrossRef] [PubMed]
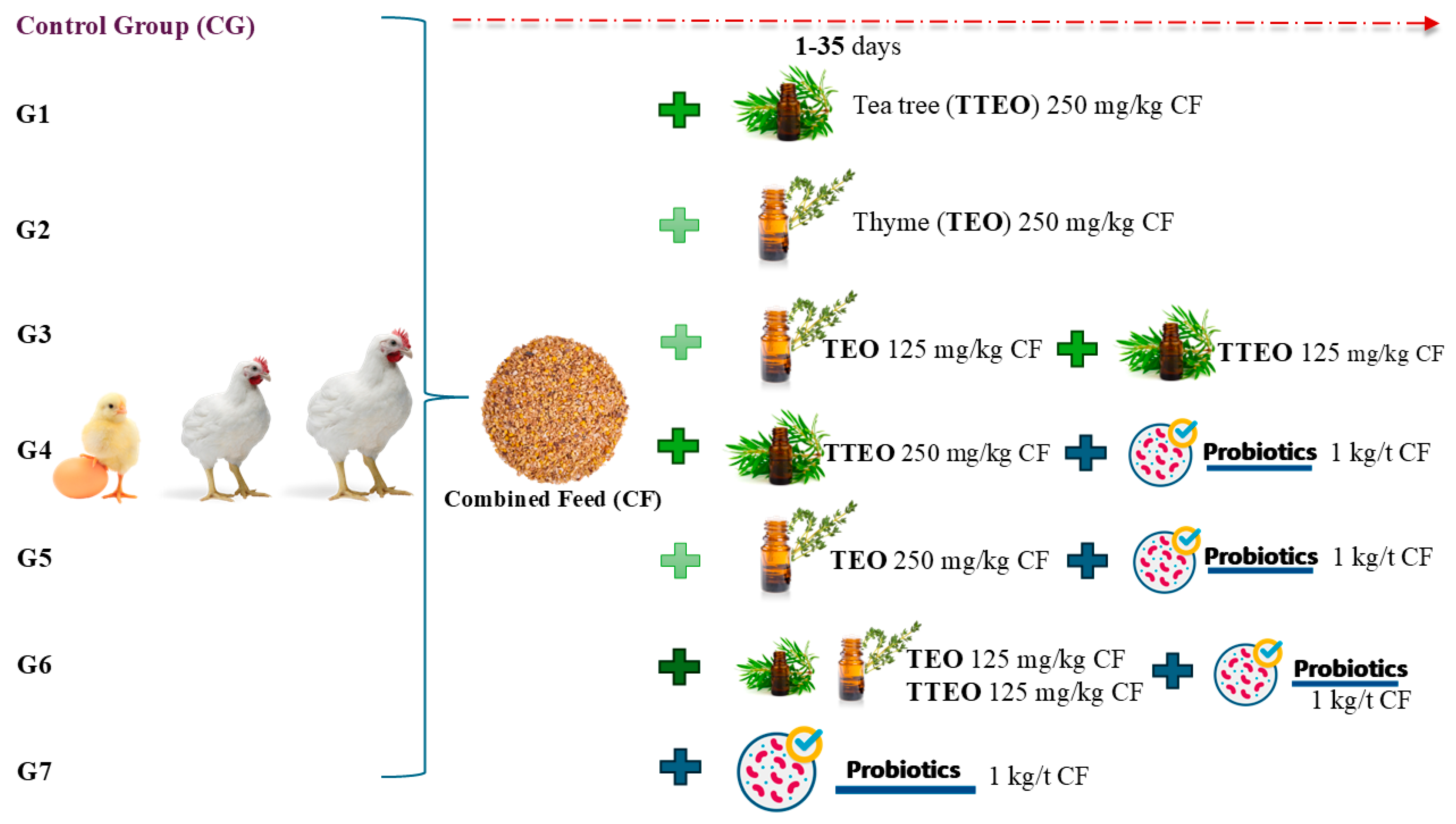


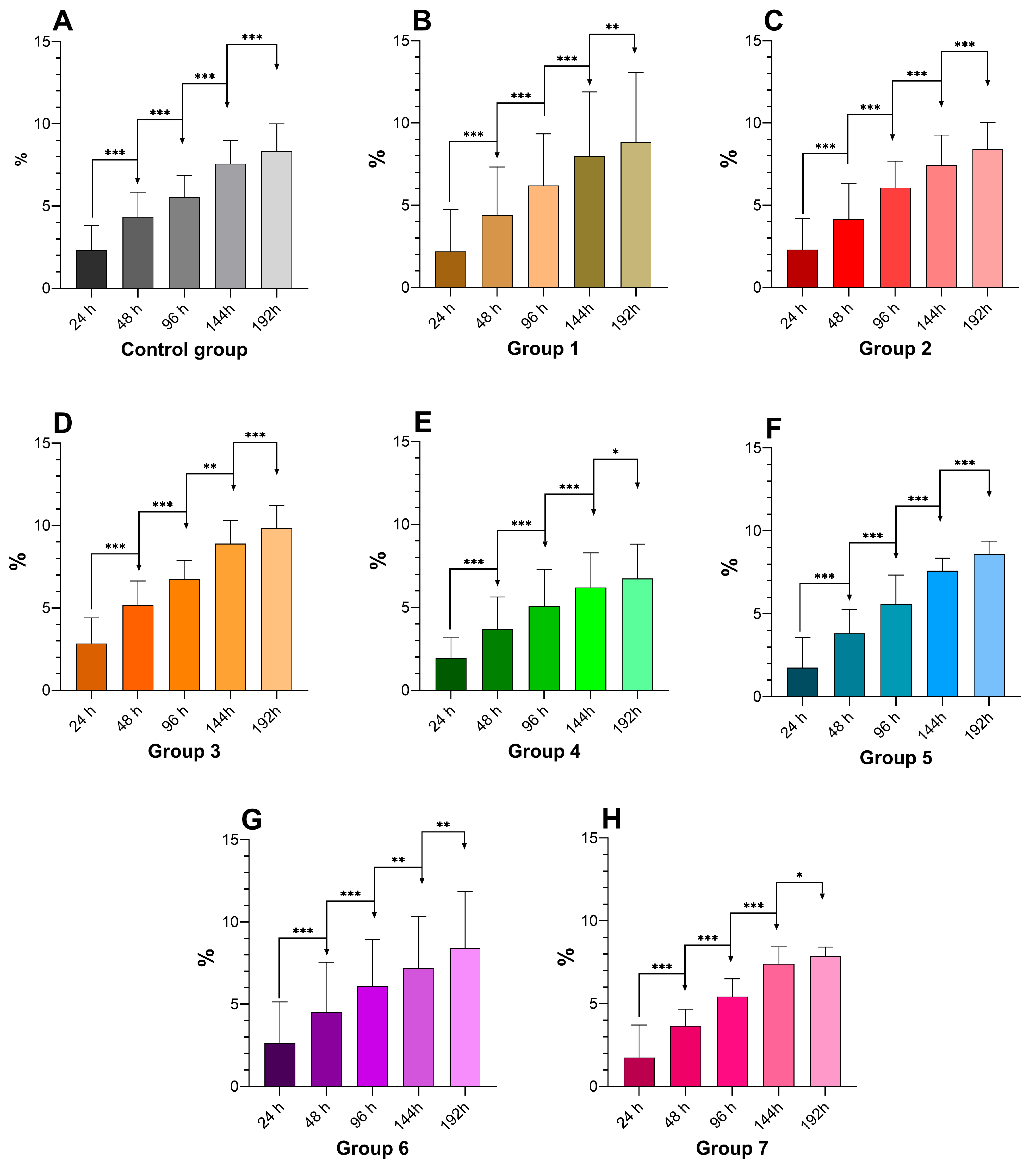

| Specification | Starter 1–10 Days | Grower 11–24 Days | Finisher 25–35 Days |
|---|---|---|---|
| % | |||
| Corn | 49.96 | 53.32 | 57.86 |
| Soybean meal | 41.65 | 37.88 | 32.70 |
| Sunflower oil | 4.22 | 5.10 | 5.80 |
| Calcium carbonate | 1.18 | 1.44 | 0.93 |
| Monocalcium phosphate | 1.52 | 0.80 | 1.30 |
| Salt | 0.26 | 0.19 | 0,20 |
| Sodium bicarbonate | 0.01 | 0.20 | 0,20 |
| L Lysine | 0.25 | 0.18 | 0.18 |
| DL Methionine | 0.36 | 0.30 | 0.28 |
| L Threonine | 0.09 | 0.09 | 0.05 |
| Vitamin-mineral premix | 0.5 | 0.5 | 0.5 |
| 100 | 100 | 100 | |
| Nutritional characteristics | |||
| ME (kcal/kg feed) | 3004.07 | 3104.52 | 3200.80 |
| Crude protein (%) | 23.02 | 21.50 | 19.49 |
| Lysine (%) | 1.44 | 1.29 | 1.16 |
| Methionine + cystine (%) | 1.08 | 0.98 | 0.91 |
| Calcium (%) | 0.96 | 0.87 | 0.79 |
| Total phosphorus (%) | 0.67 | 0.52 | 0.60 |
| Crude cellulose (%) | 3.44 | 3.28 | 3.05 |
| TTEO | TEO | |||
|---|---|---|---|---|
| Compound | RIc | RIr | % | % |
| Tricyclene | 925 | 923 | n.i. | 0.13 |
| alpha-Thujene | 929 | 928 | 1.02 | 0.88 |
| alpha-Pinene | 934 | 936 | 2.64 | 1.20 |
| Camphene | 945 | 950 | n.i. | 2.14 |
| Sabinene | 973 | 970 | 0.19 | n.i. |
| beta-Pinene | 978 | 977 | 0.76 | 0.29 |
| 1-Octen-3-ol | 979 | 980 | n.i. | 0.68 |
| beta-Myrcene | 991 | 989 | 0.87 | 2.13 |
| alpha-Phellandrene | 1002 | 1004 | 0.58 | 0.22 |
| 3-Carene | 1007 | 1008 | n.i. | 0.16 |
| 4-Carene | 1010 | 1011 | 10.45 | 1.73 |
| p-Cymene | 1024 | 1025 | 2.77 | 21.11 |
| Limonene | 1030 | 1029 | n.i. | 0.87 |
| Eucalyptol | 1031 | 1032 | 5.50 | 1.93 |
| gamma-Terpinen | 1058 | 1060 | 19.88 | 9.33 |
| Terpinolen | 1090 | 1086 | 3.78 | 0.20 |
| Linalool | 1098 | 1099 | 0.03 | 5.92 |
| Camphor | 1140 | 1143 | n.i. | 1.66 |
| Terpinen-4-ol | 1164 | 1166 | 40.47 | 1.67 |
| alpha-Terpineol | 1188 | 1190 | 2.46 | 0.07 |
| Thymol methyl ether | 1233 | 1234 | n.i. | 1.65 |
| Carvone | 1243 | 1242 | n.i. | 0.15 |
| Bornyl acetate | 1283 | 1283 | n.i. | 0.07 |
| Thymol | 1288 | 1290 | n.i. | 28.77 |
| Carvacrol | 1298 | 1300.4 | n.i. | 6.13 |
| alpha-Cubebene | 1351 | 1352 | 0.04 | 0.08 |
| Isoledene | 1364 | 1360 | 0.06 | n.i. |
| Copaene | 1368 | 1370 | 0.18 | 0.13 |
| beta-Caryophyllene | 1403 | 1406 | 0.42 | 9.60 |
| alpha-Gurjunene | 1408 | 1410 | 0.36 | n.i. |
| (+)-Spathulenol | 1410 | 1412 | 0.06 | n.i. |
| Eudesma-3,7(11)-diene | 1412 | 1413 | 0.08 | n.i. |
| delta-Guaiene | 1417 | 1416 | 0.15 | n.i. |
| beta-Cubebene | 1419 | 1418 | 0.17 | n.i. |
| alpha-Caryophyllene | 1422 | 1420 | 0.08 | 0.09 |
| beta-Gurjunene | 1431 | 1433 | 0.30 | n.i. |
| Alloaromadendrene | 1460 | 1462 | 1.86 | n.i. |
| gamma-Gurjunene | 1472 | 1475 | 0.36 | n.i. |
| gamma-Muurolene | 1476 | 1477 | n.i. | 0.10 |
| Germacrene D | 1480 | 1510 | 0.79 | n.i. |
| alpha-Cubebene | 1490 | 1488 | 0.19 | n.i. |
| Viridiflorene | 1492 | 1493 | 1.19 | n.i. |
| alpha-Muurolene | 1498 | 1495 | 0.13 | 0.10 |
| gamma-Cadinene | 1512 | 1513 | n.i. | 0.08 |
| beta-Cadinene | 1518 | 1520 | 0.41 | n.i. |
| delta-Cadinene | 1525 | 1523 | 1.37 | 0.34 |
| alfa-Cadinene | 1533 | 1535 | 0.10 | n.i. |
| Caryophyllene oxide | 1578 | 1580 | n.i. | 0.21 |
| Monoterpene Hydrocarbons (MH) | 43.05 | 40.53 | ||
| Monoterpene Oxygenates (MO) | 48.52 | 48.10 | ||
| Sesquiterpene Hydrocarbons (SH) | 8.31 | 10.10 | ||
| Sesquiterpene Oxygenates (SO) | 0.19 | 0.21 | ||
| Other compounds (O) | n.i. | 0.68 |
| Growth Period | Treatment Group | SEM | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | G1 | G2 | G3 | G4 | G5 | G6 | G7 | ||||
| BW | 1 d | 43.9 | 44.0 | 43.8 | 43.9 | 43.9 | 43.9 | 44.0 | 44.0 | 0.26 | 0.99 |
| 10 d | 288.1 | 285.7 | 281.1 | 279.0 | 278.3 | 296.1 | 276.4 | 281.4 | 11.44 | 0.71 | |
| 24 d | 1201.5 | 1135.2 | 1160.8 | 1139.7 | 1136.8 | 1151.0 | 1117.7 | 1176.9 | 53.37 | 0.81 | |
| 35 d | 2166.3 | 2022.9 | 2102.5 | 2108.4 | 2098.7 | 1985.5 | 2052.8 | 2151.1 | 65.87 | 0.22 | |
| Growth Period | Treatment Group | SEM | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | G1 | G2 | G3 | G4 | G5 | G6 | G7 | ||||
| Total WG | 1–10 d | 244.2 | 21.7 | 237.3 | 235.1 | 234.4 | 252.2 | 232.4 | 237.4 | 11.30 | 0.70 |
| 11–24 d | 913.4 | 849.5 | 879.7 | 860.7 | 858.5 | 854.9 | 841.3 | 895.5 | 52.51 | 0.84 | |
| 25–35 d | 964.8 | 887.7 | 941.7 | 968.7 | 961.9 | 834.5 | 935.1 | 974.2 | 79.54 | 0.64 | |
| 1–35 d | 2122.4 | 1978.9 | 2058.7 | 2064.5 | 2054.8 | 1941.6 | 2008.8 | 2107.1 | 65.79 | 0.22 | |
| Growth Period | Treatment Group | SEM | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | G1 | G2 | G3 | G4 | G5 | G6 | G7 | ||||
| FI | 1–10 d | 249.1 | 259.2 | 247.8 | 233.5 | 237.8 | 247.7 | 247.9 | 256.1 | 7.13 | 0.08 |
| 11–24 d | 1282.3 | 1200.8 | 1231.3 | 1253.1 | 1222.6 | 1206.2 | 1204.3 | 1284.4 | 52.17 | 0.57 | |
| 25–35 d | 1637.1 | 1507.0 | 1554.6 | 1575.6 | 1614.9 | 1606.4 | 1563.4 | 1611.9 | 72.81 | 0.69 | |
| 1–35 d | 3168.5 | 2967.0 | 3033.7 | 3062.2 | 3075.3 | 3060.3 | 3015.6 | 3152.4 | 101.88 | 0.56 | |
| Growth Period | Treatment Group | SEM | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | G1 | G2 | G3 | G4 | G5 | G6 | G7 | ||||
| FCR | 1–10 d | 1.02 | 1.07 | 1.04 | 0.99 | 1.01 | 0.98 | 1.06 | 1.07 | 0.04 | 0.28 |
| 11–24 d | 1.40 | 1.41 | 1.40 | 1.45 | 1.42 | 1.41 | 1.43 | 1.43 | 0.05 | 0.94 | |
| 25–35 d | 1.69 | 1.69 | 1.65 | 1.62 | 1.67 | 1.69 | 1.67 | 1.65 | 0.25 | 0.69 | |
| 1–35 d | 1.49 | 1.49 | 1.47 | 1.48 | 1.49 | 1.58 | 1.50 | 1.49 | 0.06 | 0.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stef, L.; Corcionivoschi, N.; Julean, C.; Callaway, T.; Simiz, E.; Marcu, A.; Stef, D.S.; Pet, I.; Popescu, I.; Gradisteanu Pircalabioru, G.; et al. Integrative Feeding Strategies with Essential Oils and Probiotics to Improve Raw Meat Quality and Carcass Traits in Broiler Chickens. Agriculture 2025, 15, 2356. https://doi.org/10.3390/agriculture15222356
Stef L, Corcionivoschi N, Julean C, Callaway T, Simiz E, Marcu A, Stef DS, Pet I, Popescu I, Gradisteanu Pircalabioru G, et al. Integrative Feeding Strategies with Essential Oils and Probiotics to Improve Raw Meat Quality and Carcass Traits in Broiler Chickens. Agriculture. 2025; 15(22):2356. https://doi.org/10.3390/agriculture15222356
Chicago/Turabian StyleStef, Lavinia, Nicolae Corcionivoschi, Calin Julean, Todd Callaway, Eliza Simiz, Adela Marcu, Ducu Sandu Stef, Ioan Pet, Iuliana Popescu, Gratiela Gradisteanu Pircalabioru, and et al. 2025. "Integrative Feeding Strategies with Essential Oils and Probiotics to Improve Raw Meat Quality and Carcass Traits in Broiler Chickens" Agriculture 15, no. 22: 2356. https://doi.org/10.3390/agriculture15222356
APA StyleStef, L., Corcionivoschi, N., Julean, C., Callaway, T., Simiz, E., Marcu, A., Stef, D. S., Pet, I., Popescu, I., Gradisteanu Pircalabioru, G., Simiz, F. D., & Balta, I. (2025). Integrative Feeding Strategies with Essential Oils and Probiotics to Improve Raw Meat Quality and Carcass Traits in Broiler Chickens. Agriculture, 15(22), 2356. https://doi.org/10.3390/agriculture15222356












