Cytokinin- and Auxin-Based Plant Growth Regulators Enhance Cell Expansion, Yield Performance, and Fruit Quality in ‘Maxi Gala’ Apple Fruits in Southern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Experimental Protocol
- (a)
- BA (MaxCel®, Valent BioSciences, Libertyville, IL, USA);
- (b)
- NAA, 95% concentration;
- (c)
- L-Tryptophan (C11H12N2O2).
2.3. Histological Parameters of Fruit Flesh Cells
2.4. Yield Performance and Fruit Quality Parameters
2.5. Experimental Design and Data Analysis
3. Results
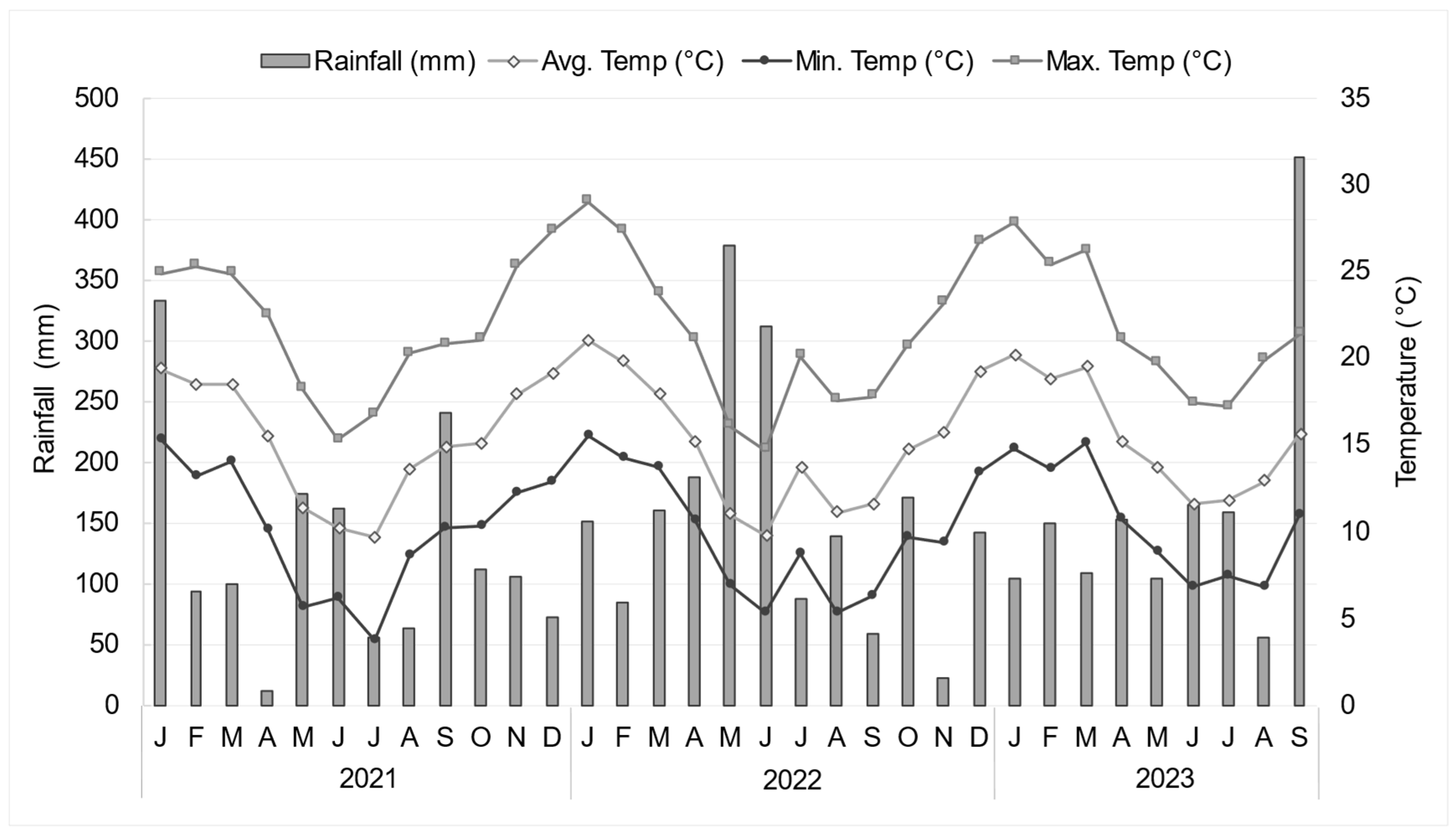
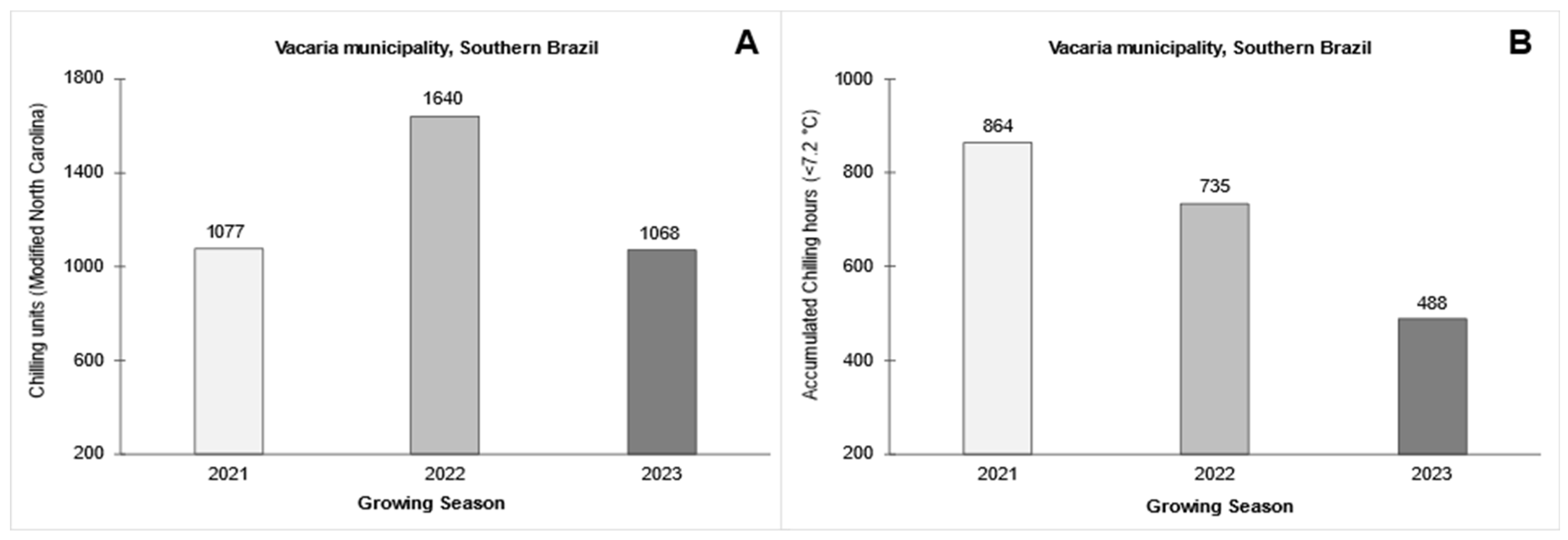
3.1. Climatic Conditions During the Growing Seasons
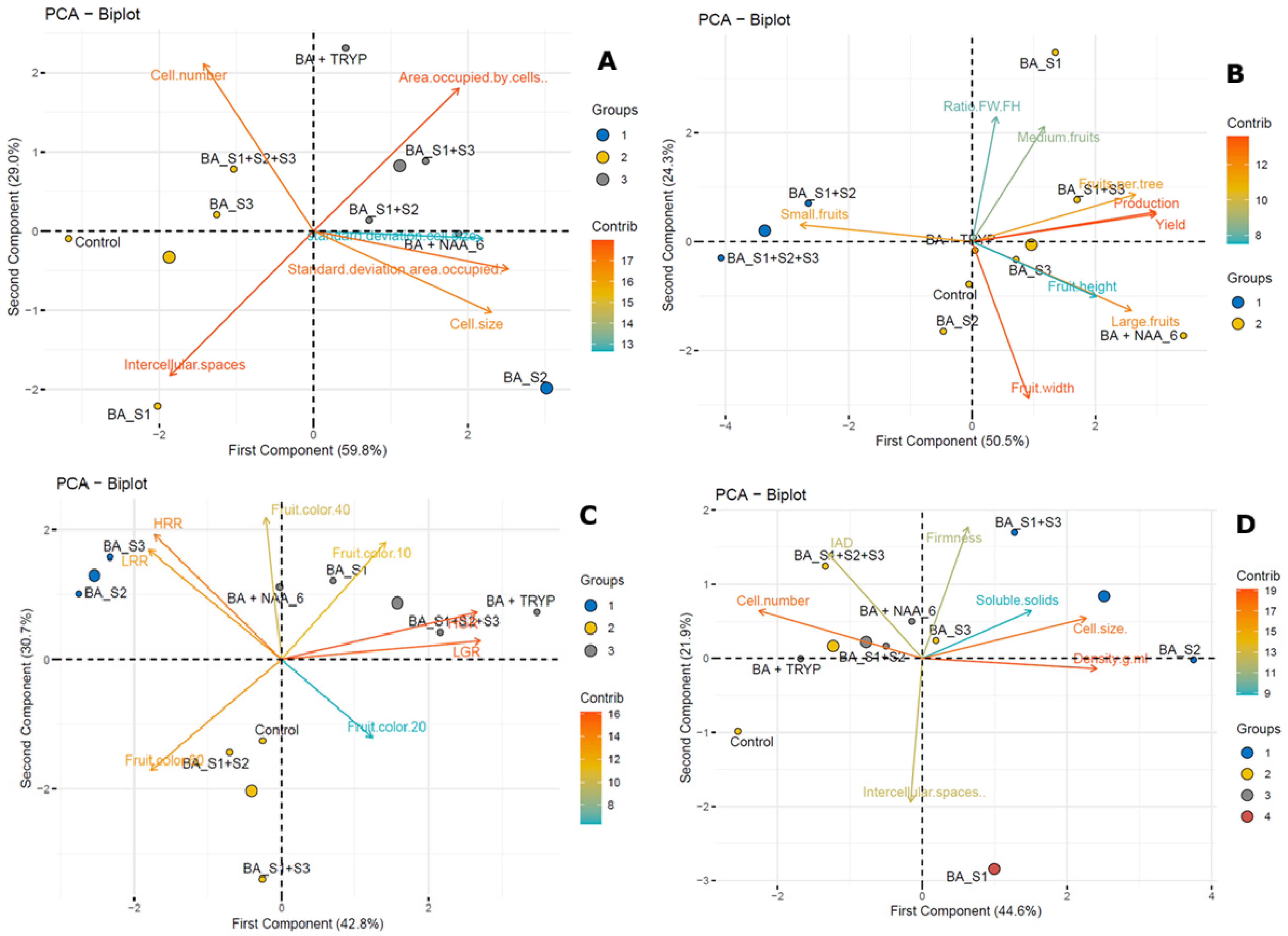
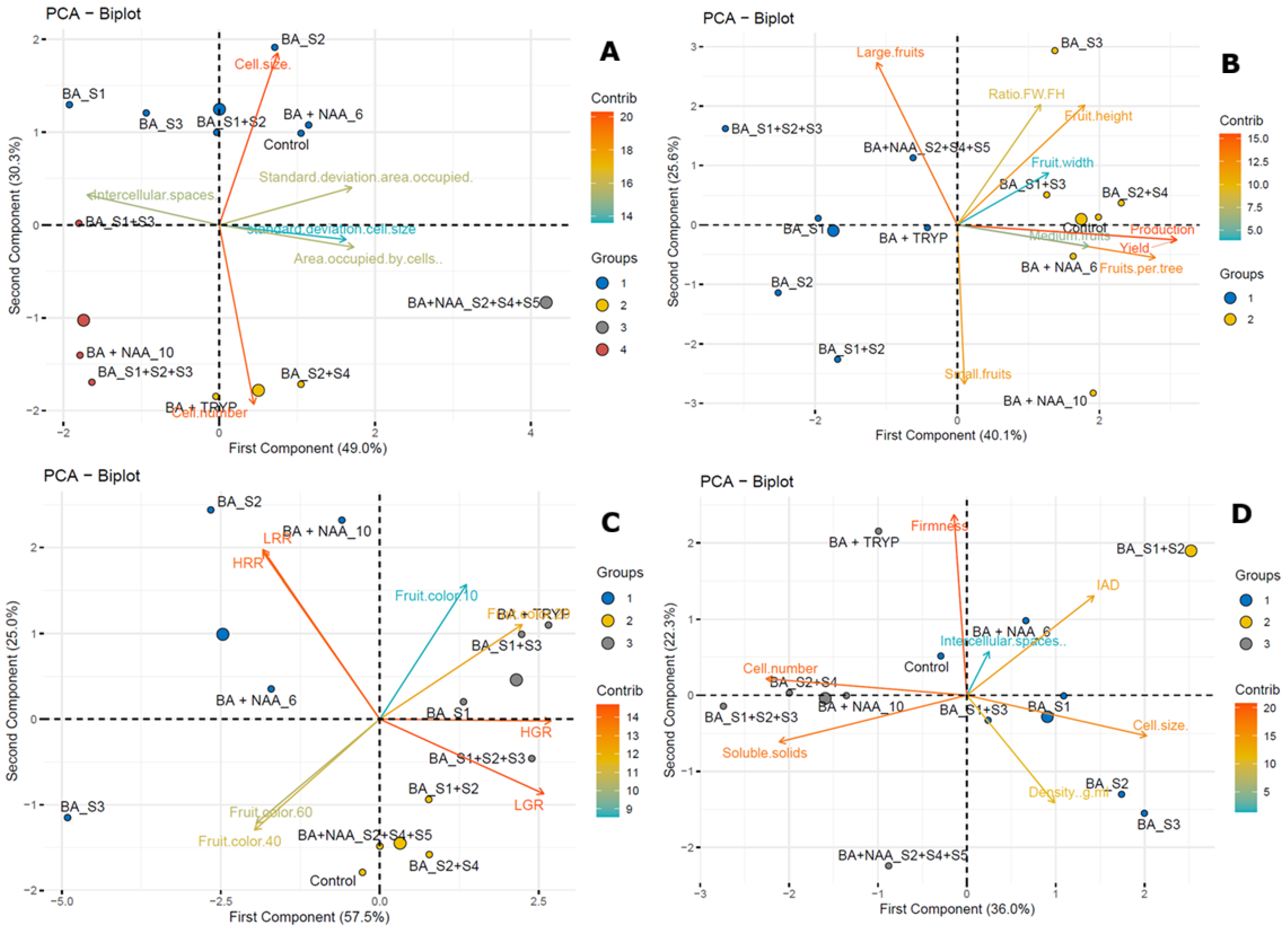
3.2. Histological Parameters, Yield Performance, and Fruit Quality Parameters
3.3. Pulp Firmness and Firmness Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahir, M.M.; Fan, L.; Liu, Z.; Raza, H.; Aziz, U.; Shehzaib, A.; Li, S.; He, Y.; Lu, Y.; Ren, X.; et al. Physiological and molecular mechanisms of cytokinin involvement in nitrate-mediated adventitious root formation in apples. J. Integr. Agric. 2024, 23, 4046–4057. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Argenta, L.C.; do Amarante, C.V.T.; de Freitas, S.T.; Brancher, T.L.; Nesi, C.N.; Mattheis, J.P. Fruit quality of ‘Gala’ and ‘Fuji’ apples cultivated under different environmental conditions. Sci. Hortic. 2022, 303, 111195. [Google Scholar] [CrossRef]
- Karim, S.K.A.; Allan, A.C.; Schaffer, R.J.; David, K.M. Cell Division Controls Final Fruit Size in Three Apple (Malus × domestica) Cultivars. Horticulturae 2022, 8, 657. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Su, Q.; Li, X.; Wang, L.; Wang, B.; Feng, Y.; Yang, H.; Zhao, Z. Variation in Cell Wall Metabolism and Flesh Firmness of Four Apple Cultivars during Fruit Development. Foods 2022, 11, 3518. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J.; Ross, J.J.; Hallett, I.C.; Gunaseelan, K.; Dayatilake, G.A.; et al. A genomics approach to understanding the role of auxin in apple (Malus × domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Rojas-Candelas, L.E.; Chanona-Pérez, J.J.; Méndez, J.V.; Perea-Flores, M.J.; Cervantes-Sodi, H.F.; Hernández-Hernández, H.M. Physicochemical, structural and nanomechanical study elucidating the differences in firmness among four apple cultivars. Postharvest Biol. Technol. 2021, 171, 111342. [Google Scholar] [CrossRef]
- Tan, M.; Li, G.; Chen, X.; Xing, L.; Ma, J.; Zhang, D.; Ge, H.; Han, M.; Sha, G.; An, N. Role of Cytokinin, Strigolactone, and Auxin Export on Outgrowth of Axillary Buds in Apple. Front. Plant Sci. 2019, 15, 616. [Google Scholar] [CrossRef]
- Bu, H.; Yu, W.; Yuan, H.; Yue, P.; Wei, Y.; Wang, A. Endogenous Auxin Content Contributes to Larger Size of Apple Fruit. Front. Plant Sci. 2020, 11, 592540. [Google Scholar] [CrossRef]
- Mian, G.; Consolini, M.; Cellini, A.; Strano, A.; Magoni, T.; Mastroleo, M.; Donati, I.; Spinelli, F. A Multi-Year Study of Forchlorfenuron’s Effects on Physical Fruit Quality Parameters in A. chinensis var. chinensis. Agronomy 2025, 15, 215. [Google Scholar] [CrossRef]
- Jahed, K.R.; Hirst, P.M. Fruit growth and development in apple: A molecular, genomics and epigenetics perspective. Front. Plant Sci. 2023, 14, 1122397. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Malladi, A. Identification of potential regulators of cell production and early fruit growth in apple (Malus × domestica Borkh.). Sci. Hortic. 2022, 297, 1109–1139. [Google Scholar] [CrossRef]
- Köeppen, W. (Ed.) Climatologia: Con un Estudio de los Climas de la Tierra; Fundo de Cultura Econômica: Mexico City, México, 1948. [Google Scholar]
- Agroconnect. Climatic Data for Lages, Santa Catarina, Brazil. 2024. Available online: https://ciram.epagri.sc.gov.br/agroconnect/ (accessed on 5 November 2025).
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1094001/brazilian-soil-classification-system (accessed on 2 November 2024).
- Meier, U. BBCH-Monograph. Growth Stages of Plants; Entwick-lungsstadienvonpflanzen. Estadios de Las Plantas; Julius Kühn-Institut: Quedlinburg, Germany, 1994. [Google Scholar]
- Pereira, M.F.G.; Luz, A.R.; Sander, G.F.; Ferreira, A.S.; Woitexen, J.F.W.; Kretzschmar, A.A.; Rufato, D.P.; Rios, P.D.; Bogo, A.; Rufato, L. Cytokinins improve quality of ‘Maxi Gala’ apples produced in southern Brazil. Cienc. Rural 2025, 55, e20240112. [Google Scholar] [CrossRef]
- Olabinjo, O.; Ogunlowo, A.S.; Ajayi, O.O.; Olalusi, A.P. Analysis of Physical and Chemical Composition of Sweet Orange (Citrus sinensis) Peels. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2201–2206. [Google Scholar] [CrossRef]
- Mendiburu, F. R Package, Version 1.3-7; Agricolae: Statistical Procedures for Agricultural Research; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=agricolae (accessed on 5 November 2025).
- R Core Team. R: A Language and Environment for Statistical Computing R Version 4.5.2; Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 5 November 2025).
- Ebert, A.; Petri, J.L.; Bender, R.J.; Braga, H.J. First experiences with chill units models is southern Brazil. Acta Hortic. 1986, 184, 89–96. [Google Scholar] [CrossRef]
- Jing, S.; Malladi, A. Higher growth of the apple (Malus × domestica Borkh.) fruit cortex is supported by resource intensive metabolism during early development. BMC Plant Biol. 2020, 20, 75. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, S.; Wani, A.W.; Mhaske, T.; Hanmant, S.S.; Saran, R.; Gupta, A. Exploring plant growth regulators (PGRs): Classification, structural features, and functional significance. Int. J. Res. Agron. 2024, 7 (Suppl. S7), 316–330. [Google Scholar] [CrossRef]
- Gao, H.N.; Jiang, H.; Cui, J.Y.; You, C.X.; Li, Y.Y. Review: The effects of hormones and environmental factors on anthocyanin biosynthesis in apple. Plant Sci. 2021, 312, 111024. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Petrasek, J. Auxins and cytokinins–the dynamic duo of growth-regulating phytohormones heading for new shores. New Phytologist. 2019, 221, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, A. Recent Advances in Phytohormone Regulation of Apple-Fruit Ripening. Plants 2021, 10, 2061. [Google Scholar] [CrossRef]
- Malladi, A. Molecular Physiology of Fruit Growth in Apple. In Horticultural Reviews; Warrington, I., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–42. [Google Scholar] [CrossRef]
- Trejo, E.J.O.; Brizzolara, S.; Cardillo, V.; Ruperti, B.; Bonghi, C.; Tonutti, P. The impact of PGRs applied in the field on the postharvest behavior of fruit crops. Sci. Hortic. 2023, 318, 112103. [Google Scholar] [CrossRef]
- Yang, L.; Cong, P.; He, J.; Bu, H.; Qin, S.; Lyu, D. Differential pulp cell wall structures lead to diverse fruit textures in apple (Malus domestica). Protoplasma 2022, 259, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni-Putman, S.M.; Brumos, J.; Zhao, C.; Alonso, J.M.; Stepanova, A.N. Auxin Interactions with Other Hormones in Plant Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039990. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Yaseen, T.; Ahmad, H. Role of cytokinin and auxin interaction in fruit growth and ripening: Molecular perspectives. Hortic. Int. J. 2021, 5, 100–109. [Google Scholar]
- Attia, S.M. Enhancing fruit set, yield and quality of LeConte pear trees by preharvest foliar spray of some plant growth regulators. SVU Int. J. Agric. Sci. 2022, 4, 1–7. [Google Scholar] [CrossRef]
- Narváez, A.; Olivares, C.; Gómez, M.; Contreras, D.; Espinoza, J.; Sepúlveda, R. Improving Productivity and Reducing Drop Percentages of Fruits in Pear by Foliar Application of PGRs. Bioresources 2023, 19, 5880–5894. [Google Scholar]
- Ornelas-Paz, J.J.; Quintana-Gallegos, B.M.; Escalante-Minakata, P.; Reyes-Hernández, J.; Pérez-Martínez, J.D.; Rios-Velasco, C.; Ruiz-Cruz, S. Relationship between the firmness of Golden Delicious apples and the physicochemical characteristics of the fruits and their pectin during development and ripening. J. Food Sci. Technol. 2018, 55, 33–41. [Google Scholar] [CrossRef]
| Treatments | Spray Timing at Different Phenological Stages | ||||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | |
| 10 Days After Bud Break BBCH * 01 | Full Bloom BBCH 65 | Petal Fall BBCH 67 | Fruits 8–10 mm BBCH 71 | Fruits 25–27 mm BBCH 74 | |
| Control | - | - | - | - | - |
| BA-S1 | BA-60 mg L−1 | - | - | - | - |
| BA-S2 | - | BA-10 mg L−1 | - | - | - |
| BA-S3 | - | - | BA-10 mg L−1 | - | - |
| BA-S1 + S3 | BA-60 mg L−1 | - | BA-10 mg L−1 | - | - |
| BA-S1 + S2 | BA-60 mg L−1 | BA-10 mg L−1 | - | - | - |
| BA-S1 + S2 + S3 | BA-60 mg L−1 | BA-10 mg L−1 | BA-10 mg L−1 | - | - |
| BA + TRYP | BA 60 mg L−1 | BA-10 mg L−1 + 2 g ha−1 tryptophan | BA-10 mg L−1 + 2 g ha−1 tryptophan | - | - |
| BA + NAA-6 | BA 60 mg L−1 | BA-10 mg L−1 + 6 mg L−1 NAA | BA-10 mg L−1 + 6 mg L−1 NAA | - | - |
| ** BA + NAA-10 | BA 60 mg L−1 | BA-10 mg L−1 + 10 mg L−1 NAA | BA-10 mg L−1 + 10 mg L−1 NAA | - | - |
| ** BA-S2 + S4 | - | BA-10 mg L−1 | - | BA-10 mg L−1 | - |
| ** BA + NAA-S2 + S4 + S5 | - | BA-10 mg L−1 + 6 mg L−1 NAA | - | BA-10 mg L−1 + 6 mg L−1 NAA | BA-10 mg L−1 |
| Treatment | Pre-Storage Firmness (N) | Post-Storage Firmness (N) | Loss of Fruit Firmness (N) |
|---|---|---|---|
| Control | 65.96 ns | 47.06 abcd | 18.90 ab |
| BA-S1 | 64.25 | 48.21 abcd | 16.04 ab |
| BA-S2 | 63.42 | 49.06 abc | 14.36 ab |
| BA-S3 | 65.06 | 49.41 abc | 12.65 ab |
| BA-S1 + S3 | 65.36 | 50.61 ab | 14.74 ab |
| BA-S1 + S2 | 68.16 | 52.84 a | 15.32 ab |
| BA-S1 + S2 + S3 | 63.61 | 52.83 a | 10.78 b |
| BA + TRYP | 69.95 | 44.24 cd | 25.71 a |
| BA + NAA-6 | 68.41 | 43.76 cd | 24.65 a |
| BA + NAA-10 | 65.01 | 44.75 bcd | 20.26 ab |
| BA-S2 + S4 | 67.34 | 42.43 d | 24.91 a |
| BA + NAA-S2 + S4 + S5 | 62.09 | 45.73 bcd | 16.36 ab |
| Mean | 65.47 | 47.58 | 17.89 |
| CV (%) | 7.09 | 5.02 | 30.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldissera, S.; Dias, A.F.; Ribeiro, J.d.C.; Andrade Júnior, R.B.d.; Pirolli, B.; Costa Júnior, E.d.S.; Francescatto, P.; Rios, P.D.; Rufato, D.P.; Bogo, A.; et al. Cytokinin- and Auxin-Based Plant Growth Regulators Enhance Cell Expansion, Yield Performance, and Fruit Quality in ‘Maxi Gala’ Apple Fruits in Southern Brazil. Agriculture 2025, 15, 2339. https://doi.org/10.3390/agriculture15222339
Baldissera S, Dias AF, Ribeiro JdC, Andrade Júnior RBd, Pirolli B, Costa Júnior EdS, Francescatto P, Rios PD, Rufato DP, Bogo A, et al. Cytokinin- and Auxin-Based Plant Growth Regulators Enhance Cell Expansion, Yield Performance, and Fruit Quality in ‘Maxi Gala’ Apple Fruits in Southern Brazil. Agriculture. 2025; 15(22):2339. https://doi.org/10.3390/agriculture15222339
Chicago/Turabian StyleBaldissera, Sabrina, Alex Felix Dias, Joel de Castro Ribeiro, Renaldo Borges de Andrade Júnior, Bruno Pirolli, Euvaldo de Sousa Costa Júnior, Poliana Francescatto, Polliana D’Angelo Rios, Daiana Petry Rufato, Amauri Bogo, and et al. 2025. "Cytokinin- and Auxin-Based Plant Growth Regulators Enhance Cell Expansion, Yield Performance, and Fruit Quality in ‘Maxi Gala’ Apple Fruits in Southern Brazil" Agriculture 15, no. 22: 2339. https://doi.org/10.3390/agriculture15222339
APA StyleBaldissera, S., Dias, A. F., Ribeiro, J. d. C., Andrade Júnior, R. B. d., Pirolli, B., Costa Júnior, E. d. S., Francescatto, P., Rios, P. D., Rufato, D. P., Bogo, A., & Rufato, L. (2025). Cytokinin- and Auxin-Based Plant Growth Regulators Enhance Cell Expansion, Yield Performance, and Fruit Quality in ‘Maxi Gala’ Apple Fruits in Southern Brazil. Agriculture, 15(22), 2339. https://doi.org/10.3390/agriculture15222339







