Abstract
The global demand for sustainable food systems requires innovative strategies that reconcile productivity with environmental stewardship, particularly in biodiversity-rich regions such as the Amazon. This study evaluated the cultivation of Acmella oleracea (jambu) using effluent from Colossoma macropomum (tambaqui) aquaculture as a partial substitute for chemical fertilizer. Five treatments were tested under greenhouse conditions: 100% fertilizer, 75% fertilizer, 50% fertilizer, 25% chemical, and 0% fertilizer. Significant treatment effects were observed for leaf number, plant height, stem diameter, and shoot biomass, while root biomass showed no differences. Treatments with 100%, 75%, and 50% fertilizer exhibited statistically similar performance across several growth parameters, indicating that up to 50% of the chemical fertilizer can be replaced by aquaculture effluent without significant yield reduction. Treatments with 50% fertilizer and 0% fertilizer showed reduced growth and higher tissue accumulation of nitrate and ammonium, reflecting nutritional imbalances. In parallel, tambaqui showed 100% survival and satisfactory growth, confirming the stability of the integrated system. These results highlight that, although exclusive use of effluent is insufficient to match chemical fertilizer, partial substitution represents a viable strategy to reduce input costs and recycle nutrients, reinforcing the bioeconomic potential of aqua-culture–agriculture integration in the Amazon.
1. Introduction
The growing global demand for multifunctional crops that meet nutritional, medicinal, and economic needs has become increasingly prominent within the broader contexts of climate resilience, food sovereignty, and the expanding bioeconomy [1,2,3,4]. Acmella oleracea (L.) R.K. Jansen, commonly known as jambu or paracress, is an herbaceous species with high agronomic potential and strong cultural significance, especially within the Amazon region. In Brazil, and particularly in the Amazon, jambu holds a distinctive place in regional cuisine, featuring not only in traditional dishes but also in the development of value-added products such as jambu-infused cachaça and gin [5].
The characteristic organoleptic properties of jambu—most notably its pungency and the distinctive tingling sensation it produces—are primarily attributed to spilanthol, a major alkamide known for its diverse bioactive properties [6,7].
Beyond its gastronomic importance, A. oleracea has attracted significant attention from the pharmaceutical, nutraceutical, and cosmetic industries. Spilanthol has been identified as a potent bioactive compound exhibiting analgesic, anti-inflammatory, antimicrobial, and antifungal properties [8,9,10,11]. These attributes have led to its incorporation into diverse commercial formulations, including oral care products, dermo cosmetics, and natural preservatives [8,12,13]. Moreover, jambu is recognized for its high antioxidant content and mineral profile, reinforcing its classification as a functional food with emerging global market interest [5,9].
Despite its growing relevance, jambu remains largely cultivated by smallholders and traditional farmers in the Amazon, often under low-input or subsistence-level systems. The limited adoption of modern agronomic practices constrains productivity and quality consistency, particularly in regions vulnerable to climatic variability, soil degradation, and market exclusion. Concurrently, sustainable intensification of agriculture, particularly through integrated resource management, is imperative in tropical regions where environmental pressure and socio-economic disparities converge [2].
In this context, aquaculture has rapidly expanded in the Amazon basin, with Colossoma macropomum (Tambaqui) emerging as the most widely cultivated native fish species due to its ecological plasticity and adaptability to intensive systems. However, the increase in aquaculture productivity is often accompanied by environmental concerns related to nutrient-rich effluent discharge, which contributes to eutrophication in receiving water bodies. Harnessing the nutrient load in aquaculture effluents presents a promising pathway to close nutrient loops, reduce environmental impacts, and improve water-use efficiency [14,15].
Effluent-based fertigation—the application of aquaculture wastewater as a nutrient source via irrigation—is increasingly recognized as a viable alternative to synthetic fertilizer, particularly in small-scale, diversified agricultural systems. Unlike aquaponics, which depends on closed-loop and often technologically complex systems, fertigation offers a flexible, low-cost solution suitable for traditional cropping systems. This approach not only recycles nutrients and conserves freshwater but also aligns with the principles of agroecology and circular economy by integrating food production, waste recovery, and environmental stewardship [4,16,17].
Despite the theoretical and practical advantages, the scientific understanding of crop performance under fertigation with fish effluents remains limited, particularly for underutilized or regionally important species such as jambu. Moreover, there is a paucity of empirical data addressing the synergistic effects of combining effluent-based fertigation with conventional regimes on crop physiology, productivity, and quality traits. These knowledge gaps are especially critical for the development of sustainable, context-specific agricultural models in biodiverse and socioeconomically complex regions such as the Amazon [18].
In this context, this study aims to evaluate the chemical fertilizer substitution by tambaqui aquaculture effluent in A. oleracea cultivation. We hypothesize that the partial replacement of chemical fertilizers with aquaculture effluent will not only maintain or enhance biomass accumulation but also improve nutrient use efficiency and environmental sustainability.
2. Materials and Methods
The experiment was conducted in December/2024 in a rectilinear, naturally ventilated greenhouse with a convective roof structure, located at the Amazonian Aquatic Biosystems Laboratory (BIOAQUAM), Federal Rural University of Amazonia (UFRA), in Belém, Pará, Brazil (1°27′30″ S, 48°28′12″ W). The greenhouse measures 8.0 m in width and 12.0 m in length and is covered with low-density polyethylene (LDPE) film and lateral shading to mitigate thermal stress and excessive solar radiation. The region has a humid equatorial climate (Köppen classification: Af), characterized by consistently high temperatures and high relative humidity throughout the year.
2.1. Plant Material and Seedling Preparation
Seeds of A. oleracea (L.) R.K. Jansen (jambu) were sourced from the Ver-o-Peso Market, a traditional repository of regional biodiversity and ethnobotanical resources. Following manual threshing from floral capitula, seeds were air-dried under ambient conditions and sown in polystyrene trays (128 cells per tray) filled with pre-washed coconut fiber as substrate. Trays were kept under controlled conditions within the greenhouse, and seedlings were irrigated with dechlorinated water twice daily. After 25 days, when seedlings reached an average height of 7 cm and developed two true leaf pairs, they were transplanted into 0.004 m3 polyethylene pots.
2.2. Soil Preparation and Fertilization
The growth substrate consisted of a medium-textured dystrophic Yellow Latosol (Oxisol), collected from a nearby experimental field. Prior to use, the soil was sieved (5 mm mesh) and chemically characterized (Table 1). Liming was performed using calcium carbonate to raise base saturation to 60%, in accordance with the soil fertility guidelines for the Amazon region (EMBRAPA Amazônia Ocidental, Belém, Brazil 2020). The liming material was thoroughly mixed into the soil and incubated for 30 days at field capacity. Post-incubation pH (in H2O) was raised from 3.8 to 5.63, indicating adequate reactivity and neutralization of exchangeable acidity.

Table 1.
Chemical characterization of the soil in the 0–20 cm layer prior to experimental setup.
2.3. Aquaculture
The RAS (Recirculating Aquaculture System) unit (Figure 1) consisted of four main components: (i) an 8000 L fish culture tank serving as the main rearing environment for tambaqui (C. macropomum); (ii) a 1000 L sedimentation tank for solid waste removal; (iii) two 1000 L tanks designated for sedimentation and biofiltration processes; and (iv) production benches with planting pots positioned above the fish tank, enabling integrated crop cultivation. To ensure appropriate dissolved oxygen levels, a 1.0-horsepower (0.75 kW) radial air blower with an air flow rate of approximately 2920 L min−1 (175 m3 h−1) connected to a stone diffuser was employed to aerate the tambaqui rearing tanks. To account for water losses due to evaporation, dechlorinated tap water was regularly replenished, maintaining system volume and consistency across replicates.
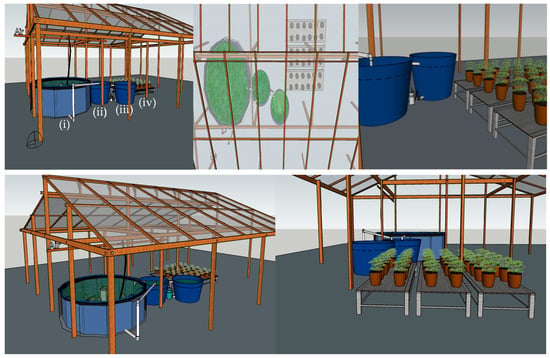
Figure 1.
Overview of the experimental recirculating aquaculture system (RAS) integrating a tambaqui cultivation and posterior fertigation of A. oleracea. (i) 8000 L fish tank (tambaqui); (ii) 1000 L sedimentation tank; (iii) two 1000 L sedimentation/biofiltration tanks; (iv) crop benches above the fish tank.
2.4. Experimental Design
The experiment was structured as a completely randomized design (CRD), consisting of five distinct treatments with six replicates each, totaling 30 experimental units. The objective was to evaluate the effects of chemical fertilizer substitution with fish wastewater in the cultivation of A. oleracea.
The control treatment was formulated with 100% chemical fertilizer and applied only once at the beginning of the experiment according to regional agronomic recommendations (Embrapa Amazônia Ocidental, 2020). The fertilization was based on soil analysis and consisted of a mixture of urea (CO(NH2)2), containing 46% nitrogen (N); potassium chloride (KCl), with 60% potassium oxide (K2O); and triple superphosphate [Ca(H2PO4)2·H2O], containing 41–46% phosphorus pentoxide (P2O5) and 14–15% calcium (Ca). The amount and proportion of each fertilizer were calculated according to the nutrient requirements identified in the soil analysis, and the total dose was applied directly to the soil in a single application before planting to ensure uniform nutrient availability throughout the experiment. The control treatment was irrigated with dechlorinated tap water. To assess the fertilizer-sparing potential, treatments were established with decreasing amounts of chemical fertilizer: 75%, 50%, and 25% of the full fertilization rate, followed by a treatment without any chemical fertilizer application.
Plants were irrigated daily with effluent from tambaqui cultivation or tap water (control), in two equal applications. The irrigation volume was adjusted to maintain soil moisture at approximately 60% of field capacity, following the methodology described by Klar (1966) [19]. The initially estimated daily volume of 384 mL was determined from the soil’s water-holding capacity and pot dimensions, ensuring the supply of water necessary to meet the plants’ physiological demand and maintain adequate soil moisture throughout the experimental period.
All treatments were applied under uniform environmental conditions within the same climate-controlled greenhouse. Nutrient solutions were delivered through a floating hydroponic system using standardized application rates and schedules to ensure consistency across treatments. A single-factor design with multiple treatment levels was used to evaluate the effect of chemical and biological nutrient sources on plant biomass productivity and nutrient use efficiency.
2.5. Analysis of the Water
Water samples were collected daily throughout the experimental period to assess general physicochemical characteristics, while nitrogenous compounds were analyzed on a weekly basis. Key parameters such as water temperature, dissolved oxygen (DO), electrical conductivity (EC), and pH were monitored daily, whereas ammonia, nitrite, and nitrate concentrations were determined weekly.
The DO measurements were obtained using a calibrated oximeter (YSI ProODO, OH, USA; accuracy ±0.01 mg L−1), which was adjusted to atmospheric saturation. The pH was measured with a pH meter (AKSO®, RS, Brazil; accuracy ±0.01), calibrated using standard buffer solutions at pH 4.0 and 7.0. Electrical conductivity was assessed using a conductivity meter (TDS&EC, SP, Brazil; accuracy ±2% FS), previously calibrated with a 1413 μS/cm standard solution (AKSO, RS, Brazil).
Nitrogenous compounds were monitored weekly throughout the experimental period by collecting water samples from the inlet of the production benches with pots, located immediately after the biofilter compartment in the recirculating aquaculture system. This point ensured sampling of water enriched with nutrients following microbial nitrification. Each weekly sample (250 mL) was collected in sterile polyethylene containers and transported to the Laboratory of Aquatic Ecology and Tropical Aquaculture (LECAT/UFRA). Upon arrival, samples were filtered through 0.7 μm GF/F glass fiber membranes to remove suspended particles and then stored at –20 °C until analysis, following the standard protocols of the American Public Health Association [20].
Chemical analyses focused on total ammonia, nitrite, and nitrate concentrations. Total ammonia was determined using the colorimetric method of Bolleter et al. (1961) [21] with a precision of ±0.03 mg L−1, while nitrite was quantified via the Griess reaction [20], with absorbance read at 540 nm (RSD 4%). Nitrate levels were measured using dual-wavelength spectrophotometry (220 and 270 nm), following APHA guidelines and the protocol adapted by Sterzelecki et al. (2021) [18,22] for aquaponic systems (RSD 1.14%). All parameters were assessed in duplicate using a Kasuaki IL-593 spectrophotometer.
2.6. Fish Growth
Tambaqui (C. macropomum) were cultivated in a recirculating aquaculture system (RAS) designed to provide a stable and controlled environment for effluent collection. A total of 51 fish, with a combined biomass of 25.295 kg, were maintained under continuous water circulation to ensure optimal water quality and oxygenation during the grow-out phase.
At the beginning of the experiment, 51 Colossoma macropomum individuals with an average initial weight of 216.89 ± 53.72 g were stocked in the system. The animals were fed three times daily with a total of 758.88 g day−1, corresponding to 3% of the total biomass, using a commercial growth-phase diet (Nutripiscis SI Crescimento 32% PB, 6 mm). The feed was evenly distributed across the three meals to ensure uniform nutrient input and minimize feed waste. The system operated under continuous aeration and recirculation to maintain stable water parameters and reduce environmental stress.
To assess growth and survival, individuals of C. macropomum were counted, measured, and weighed at the beginning of the experiment (initial length and weight: 56.00 mm and 1.75 g, respectively) and again after 30 days, at the end of the experimental period. A precision analytical balance (BEL, ±0.1 mg; São Paulo, Brazil) was used to determine body mass, and a digital caliper (ZTTO, São Paulo, Brazil) was used to measure total length. Weight gain was calculated by subtracting the initial weight from the final weight.
2.7. Plant Growth
The growth performance of A. oleracea (jambu) was evaluated through systematic measurements of key morphological and biomass-related variables throughout the cultivation cycle. Phytometric assessments were conducted at three distinct time points: (i) on the day of transplanting (20 days after sowing), when seedlings had developed their second pair of definitive leaves; (ii) 15 days after transplanting; and (iii) at the end of the experimental period (50 days of total cultivation, consisting of 20 days of germination and 30 days of fertigation). Plant height (PH) was measured from the stem base to the apical meristem using a millimeter-scale ruler, while stem diameter (SD) was measured at the collar region using a digital caliper (±0.01 mm precision). The number of leaves (NL) was manually counted per plant at each phytometric interval to monitor foliar development dynamics.
At final harvest, both the aerial and root portions were carefully separated and rinsed with distilled water to remove any residual substrate. Fresh mass (FM) of shoots and roots was recorded using a high-precision analytical balance (BEL Engineering, Monza, Italy; accuracy ±0.1 mg). To determine dry biomass, plant material was placed in pre-labeled Kraft paper envelopes and subjected to oven-drying in a forced-air convection chamber at 65 °C for 72 h or until a constant mass was achieved. Post-drying, the dry mass (DM) of each plant component was measured using a digital precision balance (accuracy ±0.01 g), allowing robust quantification of biomass accumulation under each treatment condition. This protocol ensured accurate and reproducible evaluation of jambu’s morphological responses to the experimental factors tested.
2.8. Statistical Analysis
The statistical analysis began with the evaluation of normality and homoscedasticity, which are essential assumptions for parametric testing. Normality was assessed using the Shapiro–Wilk test, and homoscedasticity (homogeneity of variances) was evaluated using Levene’s test. Upon confirmation of these assumptions, a one-way Analysis of Variance (ANOVA) was employed to assess the effects of different water treatment groups. When significant differences were detected (p < 0.05), Tukey’s post hoc test was applied to identify pairwise differences among treatment means. All statistical analyses and graphical representations were performed using GraphPad Prism version 10, ensuring robust and reliable interpretation of the experimental data.
3. Results
3.1. Water Quality
The monitoring of water quality parameters indicated conditions within suitable ranges for aquatic organisms throughout the sampling period (Table 2). Dissolved oxygen averaged 5.3 ± 0.6 mg/L, ranging from 4.19 to 6.70. The mean water temperature remained stable at 28.7 ± 0.7 °C (27.3–29.4). Electrical conductivity was 259.0 ± 30.0 µS/cm, with total dissolved solids averaging 288.0 ± 118.0 mg/L (189–758).

Table 2.
Water quality parameters at the sampling points used for fertigation. Values are mean ± SD (min–max).
Monitoring of water quality parameters throughout the experiment revealed distinct patterns among the compounds analyzed. Total ammonia showed a slight initial increase, rising from 0.094 mg L−1 (Day 0) to 0.116 mg L−1 (Day 6), reaching a peak of 0.337 mg L−1 on Day 21, and then decreasing to 0.185 mg L−1 by Day 29 (Figure 2A). Nitrate exhibited a continuous upward trend, increasing from 1.136 mg L−1 (Day 0) to 1.665 mg L−1 (Day 29), with the steepest rise observed between Day 6 and Day 21 (Figure 2B). Nitrite fluctuated more markedly, increasing from 0.082 mg L−1 (Day 0) to a maximum of 0.430 mg L−1 on Day 21, before declining to 0.248 mg L−1 on Day 29 (Figure 2C). Regarding alkalinity and hardness, values oscillated moderately: alkalinity ranged between 2.1 and 3.9 (×20), while hardness increased progressively from 5.3 to 8.7 (×40) during the experimental period (Figure 2D).
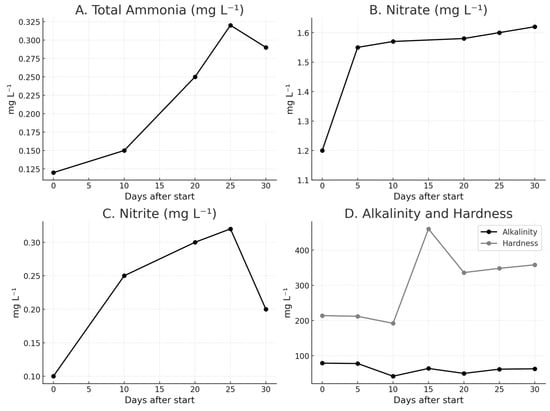
Figure 2.
Changes in (A) total ammonia, (B) nitrate, (C) nitrite, and (D) alkalinity and hardness (ppm) over 0, 6, 21, and 29 days after the start of the experiment.
3.2. Plant Growth
Significant treatment effects were observed for stem diameter (SD) only (Table 3). Plants under 100% fertilizer exhibited the highest SD values, while 75% and 50% fertilizer treatments were intermediate but significantly different only for SD. Other growth parameters—leaf number, aerial shoot length, and biomass—showed slight reductions in the 75% and 50% fertilizer treatments compared to 100% fertilizer, but these differences were not statistically significant. Growth was clearly reduced under lower inputs (25% and 0% fertilizer).

Table 3.
Growth performance of jambu (A. oleracea) under different fertigation treatments. Numbers followed by different letters indicate statistically significant differences between the densities p ≤ 0.05.
3.3. Tambaqui Growth
Juvenile tambaqui (C. macropomum) were cultivated for 30 days in a recirculating aquaculture system (RAS) designed to maintain optimal water quality and enable effluent reuse. The system included a sedimentation tank, an 8000 L fish culture unit, a hydroponic grow bed, and an equalization reservoir, all operated under continuous aeration and water recirculation. At the beginning of the experiment, 51 Colossoma macropomum individuals with an average initial length of 23.43 ± 3.20 cm and a mean weight of 228.57 ± 110.37 g were stocked, totaling a biomass of 11.66 kg. The fish were fed 726 g day−1 of commercial feed (32% crude protein, 6 mm pellet) divided into three equal portions. After 30 days, survival was 100%, and the final mean length and weight reached 27.6 ± 3.2 cm and 400 ± 149 g (n = 51), respectively, resulting in a final biomass of 20.4 kg and a net gain of 8.7 kg.
At the end of the 30-day period, survival was 100%, and final mean length and weight were 27.60 ± 3.20 cm and 399.80 ± 149.31 g (n = 51), respectively. The final biomass reached 37.177 kg, reflecting a net gain of 11.882 kg. A total of 36.3 kg of feed was supplied throughout the experiment. These results demonstrate robust growth performance and system stability, reinforcing the suitability of RAS for C. macropomum cultivation in integrated aquaculture–agriculture models.
4. Discussion
The integration of C. macropomum aquaculture with A. oleracea cultivation via effluent fertigation in a recirculating aquaponic system demonstrated high compatibility between fish and plant production, confirming the technical and ecological viability of integrated bioeconomic strategies in tropical environments. Throughout the 30-day experimental period, water quality parameters remained within ideal thresholds for both organisms, highlighting the stability and buffering capacity of the system. Dissolved oxygen levels remained above 4 mg/L across all compartments, with significantly higher values in the fish culture tank (5.28 ± 0.58 mg/L), likely due to continuous aeration and active fish metabolism. Similar patterns have been observed in other tropical aquaponic systems, where water circulation and biological uptake stabilize oxygen availability [23,24,25].
Thermal conditions (~28.6 °C) and pH values (average 7.18) remained relatively stable, reinforcing the effectiveness of the recirculating system design in maintaining environmental consistency. Electrical conductivity (EC) ranged from 259 to 388 µS/cm—levels well below the salinity stress threshold (commonly above 500 µS/cm) for herbaceous crops such as lettuce, coriander, and mint [26,27,28]. These values indicate a favorable ionic balance for plant growth, particularly in systems combining fish effluent with supplemental fertilizers. Comparable studies with lettuce and basil in aquaponics confirm that EC levels below 400 µS/cm favor vegetative development, provided nutrient ratios are adequate [24,29,30].
The concentrations of nitrogenous compounds measured in this study were below the critical thresholds reported for Colossoma macropomum. Total ammonia ranged from 0.094 to 0.337 mg L−1, values far lower than the 96 h LC50 of 1.63 mg L−1 for the hybrid tambacu (C. macropomum × Piaractus mesopotamicus) [31]. Nitrite fluctuated between 0.082 and 0.430 mg L−1, remaining well below the LC50-96 h of 1.82 mg L−1 determined for tambaqui [32]. Nitrate concentrations increased gradually (1.136–1.665 mg L−1), but still represented a negligible risk compared to chronic toxicity thresholds (>60 mg L−1) reported for freshwater fish [33].
Supporting parameters, such as alkalinity (62–78 mg L−1 CaCO3) and hardness (212–348 mg L−1 CaCO3), ensured stable ionic conditions and buffering capacity, which are known to attenuate the toxicity of ammonia and nitrite. Together, these results indicate that the system operated under safe conditions, with a substantial margin before reaching harmful thresholds for tambaqui. Therefore, the relatively low concentrations observed here demonstrate the potential to safely increase nitrogenous inputs through higher proportions of pisciculture effluents. This strategy could enhance nutrient recycling and improve the fertilization potential of the effluent, while maintaining water quality within physiologically safe limits for C. macropomum.
Growth performance of A. oleracea was significantly affected by nutrient availability and fertigation composition. Treatments with 100%, 75%, and 50% fertilizer showed statistically similar results for several growth parameters, including shoot height and leaf number. This indicates that aquaculture effluent can effectively replace up to 50% of synthetic fertilizer without causing significant reductions in plant productivity. From an economic perspective, these parameters—leaf number, aerial shoot height, and aboveground biomass—are directly associated with the visual quality, weight, and marketability of jambu, thereby exerting a strong influence on its commercial value. This aligns with previous reports in coriander and lettuce, where effluent inputs of 25–40% sustained acceptable growth performance while reducing reliance on chemical fertilizers. [26,28,34].
However, plants fertigated with 0% fertilizer showed the most severe reductions in vegetative development. Compared to 100% fertilizer, aerial part length was reduced by more than half, and dry shoot biomass dropped from 1.33 g to just 0.24 g. These results are consistent with observations in coriander seedlings subjected to high proportions of Euterpe oleracea offal, where phytotoxicity and nutrient imbalances hindered growth [26]. Nutritional analysis of fish effluents revealed deficiencies in critical elements such as potassium, calcium, and magnesium—nutrients essential for nitrogen assimilation and cell expansion [22,35]. While root biomass did not differ significantly among treatments, the pronounced variation in shoot growth confirms the prioritization of aerial development by jambu under nutrient-rich conditions, a physiological trait common among leaf crops with culinary and medicinal uses [5,36].
The relatively large variation observed in DRW among treatments can be explained by the high sensitivity of root biomass accumulation to nutrient availability and environmental heterogeneity within the cultivation system. According to Nendel et al. (2009) [37], small-scale differences in nitrogen supply and moisture distribution can produce non-linear and spatially variable growth responses in the root fraction of vegetable crops. In A. oleracea, the fibrous and shallow root system is particularly responsive to these microenvironmental variations, leading to substantial differences in dry root mass even under similar management conditions. This intrinsic variability is common in short-cycle leafy vegetables grown under fertigation, where nutrient and water gradients develop rapidly within the substrate.
Although a complete nutrient analysis was not conducted, the nutritional status of Acmella oleracea plants could be inferred from their visual and physiological characteristics. Visual diagnosis is a well-established approach for identifying nutrient deficiencies in jambu, as alterations in leaf color, size, or morphology are reliable indicators of nutritional imbalance [38]. In the present study, plants exhibited vigorous growth, with no visible symptoms such as chlorosis, necrosis, or reduced leaf area, suggesting that nutrient availability was adequate for plant development. Similar findings were reported by Carmo et al. (2024) [39], who observed consistent morphological responses in jambu cultivated under different nutrient solutions. Moreover, studies such as Sampaio et al. (2021) [40] highlight that adequate electrical conductivity and nutrient balance in hydroponic or fertigation systems are crucial to maintain plant health, even when minor nutrients are not individually quantified. Therefore, despite the absence of a full nutrient profile, the lack of deficiency symptoms supports the assumption that the nutrient supply was sufficient to sustain optimal growth under the experimental conditions.
The tambaqui displayed robust adaptability to the integrated system, achieving 100% survival and a total biomass gain of 11.88 kg across the 51 individuals stocked. These results are comparable to growth patterns reported by Santos et al. (2021) [25] and Petillo et al. (2025) [15] under similar RAS conditions. Moreover, the stability of water parameters across compartments reinforces the regulatory role of plant uptake in nutrient cycling. Previous studies have shown that plant integration into RAS designs helps mitigate nitrogenous waste accumulation, reducing the need for mechanical or chemical filtration [24,30]. Such configurations are particularly suitable for the Amazonian context, where environmental constraints and fertilizer costs demand resource-efficient, closed-loop systems [16,17].
In summary, these findings demonstrate that integrated aquaponic systems utilizing fish effluents can support sustainable and productive cultivation of A. oleracea. Partial substitution of fertilizers up to 50% with aquaculture wastewater presents a promising strategy for reducing input costs and improving circularity in agricultural production. However, exclusive use of effluent requires supplementation or pre-treatment to ensure adequate macronutrient and micronutrient supply. This bio-integrated model holds considerable potential for smallholder and riparian farmers in the Amazon, where access to external inputs is limited and locally available residues can be harnessed to support agroecological intensification.
5. Conclusions
The findings of this study demonstrate that irrigation with effluent from tambaqui (Colossoma macropomum) farming effectively enhanced the growth of jambu (Acmella oleracea), enabling a reduction of up to 50% in chemical fertilizer inputs without impairing plant performance. These results provide strong evidence that aquaculture effluent can serve as a viable partial substitute for conventional fertilizers, thereby reducing production costs and minimizing environmental impacts. Consequently, fertigation with pisciculture effluent represents a practical and sustainable strategy to foster the integration of fish farming and vegetable production, offering particular relevance for the development of circular and resource-efficient agricultural systems in the Amazon region.
Author Contributions
Conceptualization, G.D.A.P. and F.C.S.; methodology, A.M.B.S.d.J., L.M.L., S.L.d.M.G. and G.D.A.P.; software, N.F.A.C.d.M.; validation, J.R.G., S.L.d.M.G. and N.F.A.C.d.M.; formal analysis, S.L.d.M.G.; investigation, A.M.B.S.d.J., S.L.d.M.G. and J.R.G.; resources, R.K.L. and G.D.A.P.; data curation, A.M.B.S.d.J. and L.M.L., S.L.d.M.G.; writing—original draft preparation, A.M.B.S.d.J. and L.M.L., S.L.d.M.G.; writing—review and editing, S.L.d.M.G., G.D.A.P., F.C.S. and R.K.L.; visualization, S.L.d.M.G. and G.D.A.P.; supervision, G.D.A.P.; project administration, G.D.A.P.; funding acquisition, F.C.S. and G.D.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Brazilian National Research Council (CNPq), through projects CNPq-441536/2023-9. It was also supported by the Brazilian Coordination for Higher Education Personnel Training (CAPES) through PROCAD AMAZÔNIA 2018 (project no. 88887.200588/2018-00), Finance code 001, Fundação de Amparo e Pesquisa de Minas Gerais (FAEMIG APQ-00132-23), and Edital PDPG—Pós doutorado Estratégico Nº 16/2022/CAPES—postdoctoral fellowship to Sávio Lucas de Matos Guerreiro (process no. 88887.939510/2024-00).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Use (CEUA) of the Federal Rural University of Amazon (UFRA), under protocol number CEUA/UFRA Nº 1457260820.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fanzo, J.; Haddad, L.; McLaren, R.; Marshall, Q.; Davis, C.; Herforth, A.; Jones, A.; Beal, T.; Tschirley, D.; Bellows, A.; et al. The Food Systems Dashboard is a new tool to inform better food policy. Nat. Food 2020, 1, 243–246. [Google Scholar] [CrossRef]
- Tittonell, P.; Klerkx, L.; Baudron, F.; Félix, G.F.; Ruggia, A.; van Apeldoorn, D.; Dogliotti, S.; Mapfumo, P.; Rossing, W.A.H. Ecological Intensification: Local Innovation to Address Global Challenges. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 19, pp. 1–34. [Google Scholar]
- Aktar, M.A.; Bhuia, M.S.; Molla, S.; Chowdhury, R.; Sarkar, C.; Al Shahariar, M.; Roy, P.; Reiner, Ž.; Sharifi-Rad, J.; Calina, D.; et al. Pharmacological and phytochemical review of Acmella oleracea: A comprehensive analysis of its therapeutic potential. Discov. Appl. Sci. 2024, 6, 412. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A. Ecology as the Paradigm for the Future of Aquaculture. In Ecological Aquaculture; Wiley: Hoboken, NJ, USA, 2002; pp. 337–372. [Google Scholar]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source—Review. Food Rev. Int. 2021, 37, 399–414. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Erickson, A.J.; Nair, M.G. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A Review. Adv. Pharmacol. Pharm. Sci. 2013, 2013, 423750. [Google Scholar]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) R.K. Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Franzen, F.d.L.; Boscariol Rasera, G.; Silva, K.F.C.e.; Castro, R.J.S.d.; Oliveira, M.S.R.d.; Bolini, H.M.A. Physicochemical characterization and antioxidant potential of plant extracts for use in foods. Braz. J. Food Technol. 2025, 28, e2024085. [Google Scholar] [CrossRef]
- Gerbino, A.; Schena, G.; Milano, S.; Milella, L.; Barbosa, A.; Armentano, M.; Procino, G.; Svelto, M.; Carmosino, M. Spilanthol from Acmella oleracea Lowers the Intracellular Levels of cAMP Impairing NKCC2 Phosphorylation and Water Channel AQP2 Membrane Expression in Mouse Kidney. PLoS ONE 2016, 11, e0156021. [Google Scholar] [CrossRef]
- Jayashan, S.S.; Darai, N.; Rungrotmongkol, T.; Dasuni Wasana, P.W.; Nwe, S.Y.; Thongphichai, W.; Suriyakala, G.; Towiwat, P.; Sukrong, S. Exploring the Therapeutic Potential of Spilanthol from Acmella paniculata (Wall ex DC.) R. K. Jansen in Attenuating Neurodegenerative Diseases: A Multi-Faceted Approach Integrating In Silico and In Vitro Methodologies. Appl. Sci. 2024, 14, 3755. [Google Scholar] [CrossRef]
- Shivananda, S.; Doddawad, V.G.; Bhuyan, L.; Shetty, A.; Pushpa, V.H. Assessment of the Antibacterial Activity of Spilanthes acmella Against Bacteria Associated with Dental Caries and Periodontal Disease: An In-vitro Microbiological Study. J. Pure Appl. Microbiol. 2024, 18, 476–482. [Google Scholar] [CrossRef]
- Spelman, K.; Depoix, D.; McCray, M.; Mouray, E.; Grellier, P. The traditional medicine Spilanthes acmella, and the alkylamides spilanthol and undeca-2E-ene-8,10-diynoic acid isobutylamide, demonstrate in vitro and in vivo antimalarial activity. Phytother. Res. 2011, 25, 1098–1101. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, H.; Zhang, Y.; Zhou, Q.; Wen, X.; Guo, W.; Zhang, Z. A systematic review on aquaculture wastewater: Pollutants, impacts, and treatment technology. Environ. Res. 2024, 262, 119793. [Google Scholar] [CrossRef]
- Petillo, E.C.; Ferreira, A.d.C.; Oliveira, C.P.F.d.; Brandão, L.V.; Marinho-Pereira, T.; Cavero, B.A.S. Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase. Aquac. J. 2025, 5, 1. [Google Scholar] [CrossRef]
- Masi, M.; Adinolfi, F.; Vecchio, Y.; Agnusdei, G.P.; Coluccia, B. Toward the Circular Economy in the Aquaculture Sector: Bibliometric, Network and Content Analyses. Sustainability 2024, 16, 5405. [Google Scholar] [CrossRef]
- Velasco-Muñoz, J.F.; Mendoza, J.M.F.; Aznar-Sánchez, J.A.; Gallego-Schmid, A. Circular economy implementation in the agricultural sector: Definition, strategies and indicators. Resour. Conserv. Recycl. 2021, 170, 105618. [Google Scholar] [CrossRef]
- Nascimento, E.T.d.S.; Pereira, R.F., Jr.; Reis, V.S.d.; Gomes, B.d.J.F.; Owatari, M.S.; Luz, R.K.; Melo, N.F.A.C.d.; Santos, M.d.L.S.; Palheta, G.D.A.; Sterzelecki, F.C. Production of Late Seedlings of Açai (Euterpe oleraceae) in an Aquaponic System with Tambaqui (Colossoma macropomum, Curvier, 1818). Agriculture 2023, 13, 1581. [Google Scholar] [CrossRef]
- Klar, A.E.; Villa Nova, N.A.; Marcos, Z.Z.; Cervellini, A. Determinação da umidade do solo pelo método das pesagens. An. Esc. Super. Agric. "Luiz Queiroz" 1966, 23, 15–30. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Bolleter, W.T.; Bushman, C.J.; Tidwell, P.W. Spectrophotometric determination of ammonia as indophenol. Anal. Chem. 1961, 33, 592–594. [Google Scholar] [CrossRef]
- Sterzelecki, F.C.; Santos, G.R.; de Gusmão, M.T.A.; de Carvalho, T.C.C.; dos Reis, A.R.; Guimarães, R.; Santos, M.d.L.S.; de Melo, N.F.A.C.; Luz, R.K.; Palheta, G.D.A. Effects of hydroponic supplementation on Amazon river prawn (Macrobrachium amazonicum Heller, 1862) and lettuce seedling (Lactuca sativa L.) development in aquaponic system. Aquaculture 2021, 543, 736916. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.; Losordo, T. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; SRAC Publication No. 454; SRAC: Penrith, Australia, 2006. [Google Scholar]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquacult. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Santos, F.A.C.; da Costa Julio, G.S.; Luz, R.K. Stocking density in Colossoma macropomum larviculture, a freshwater fish, in recirculating aquaculture system. Aquacult. Res. 2021, 52, 1185–1191. [Google Scholar] [CrossRef]
- Da Costa, J.A.S.; Sterzelecki, F.C.; Natividade, J.; Souza, R.J.F.; de Carvalho, T.C.C.; de Melo, N.F.A.C.; Luz, R.K.; Palheta, G.D.A. Residue from Açai Palm, Euterpe oleracea, as substrate for cilantro, Coriandrum sativum, seedling production in an aquaponic system with tambaqui, Colossoma macropomum. Agriculture 2022, 12, 1555. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Espinosa-Moya, A.; Alvarez-Gonzalez, A.; Albertos-Alpuche, P.; Guzman-Mendoza, R.; Martínez-Yáñez, R. Growth and development of herbaceous plants in aquaponic systems. Acta Univ. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G. Aquaponics Food Production Systems—Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Quaresma, F.d.S.; Santos, F.L.B.d.; Ribeiro, P.F.; Leite, L.A.; Sampaio, A.H. Acute toxicity of non-ionized ammonia on tambacu (Colossoma macropomum x Piaractus mesopotamicus). Rev. CiÊncia AgronÔmica 2020, 51, e20186277. [Google Scholar] [CrossRef]
- Ferreira da Costa, O.T.; dos Santos Ferreira, D.J.; Presti Mendonça, F.L.; Fernandes, M.N. Susceptibility of the Amazonian fish, Colossoma macropomum (Serrasalminae), to short-term exposure to nitrite. Aquaculture 2004, 232, 627–636. [Google Scholar] [CrossRef]
- Araújo, T.P.d.; Brighenti, L.S.; Santos, H.B.d.; Castro, A.H.F.; Thomé, R.G. Toxicidade de compostos nitrogenados em peixes influenciada por parâmetros físico-químicos da água: Uma revisão narrativa. Res. Soc. Dev. 2021, 10, e359101119779. [Google Scholar] [CrossRef]
- Kimera, F.; Sewilam, H.; Fouad, W.M.; Suloma, A. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential oils composition of Origanum majorana cultivation. Ann. Agric. Sci. 2021, 66, 1–7. [Google Scholar] [CrossRef]
- Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents—What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Mortensen, L. The Effect of Air Temperature on Growth of Eight Herb Species. Am. J. Plant Sci. 2014, 5, 1542–1546. [Google Scholar] [CrossRef][Green Version]
- Nendel, C.; Schmutz, U.; Venezia, A.; Piro, F.; Rahn, C.R. Converting simulated total dry matter to fresh marketable yield for field vegetables at a range of nitrogen supply levels. Plant Soil 2009, 325, 319–334. [Google Scholar] [CrossRef]
- Alves Peçanha, D.; Mendonça Freitas, M.; Evangelista Vieira, M.; Capato Lima, T.; de Souza Gonçalves, Y. Characterization of deficiency symptoms and mineral nutrient content in Acmella oleracea cultivated under macronutrient and boron omissions. J. Plant Nutr. 2019, 42, 879–890. [Google Scholar] [CrossRef]
- Carmo, A.P.M.d.; Freitas, M.S.M.; Machado, L.C.; Silva, L.d.S.; Petri, D.J.C.; Vimercati, J.C.; Matos, C.R.R.; Mathias, L.; Vieira, I.J.C.; de Carvalho, A.J.C. Electrical conductivity of nutrient solutions affects the growth, nutrient levels, and content and composition of essential oils of Acmella oleracea (L.) R. K. Jansen from southeastern Brazil. J. Agric. Food Res. 2024, 15, 100968. [Google Scholar] [CrossRef]
- Sampaio, I.M.G.; Silva Júnior, M.L.; Bittencourt, R.F.P.M.; Santos, G.A.M.d.; Nunes, F.K.M.; Costa, V.C.N. Productive and physiological responses of jambu (Acmella oleracea) under nutrient concentrations in nutrient solution. Hortic. Bras. 2021, 39, 65–71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).