Rebound of Antibiotic Resistance Genes in Composting: Mechanisms, Challenges, and Control Strategies
Abstract
1. Introduction
2. ARG Dynamics During Composting
2.1. The Composting Process
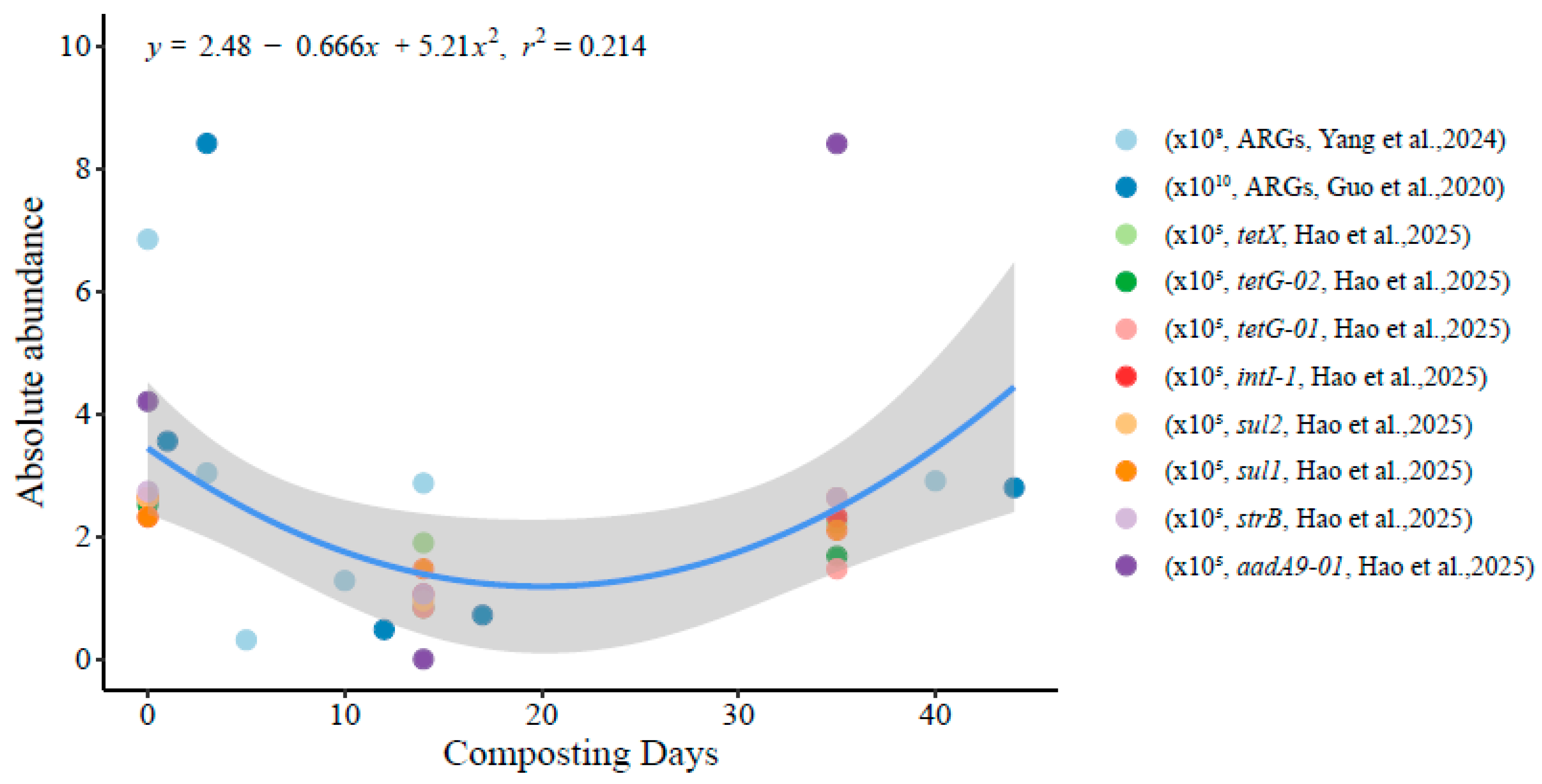
2.2. Dynamic Changes in ARGs During Livestock Manure Composting
3. Drivers of ARG Rebound in the Curing Stage of Composting
3.1. Inheritance and Dissemination of ARGs: Roles of HGT and VGT
3.2. Phylogenetic Shifts in Bacterial Hosts Influencing ARG Dynamics
3.3. Selective Pressure of Heavy Metals on ARG Persistence
3.4. Extracellular Polymeric Substance (EPS)-Mediated Protection and Dissemination of ARGs
4. Control Strategies to Mitigate ARG Rebound in Composting
4.1. Conventional Approaches for ARG Suppression
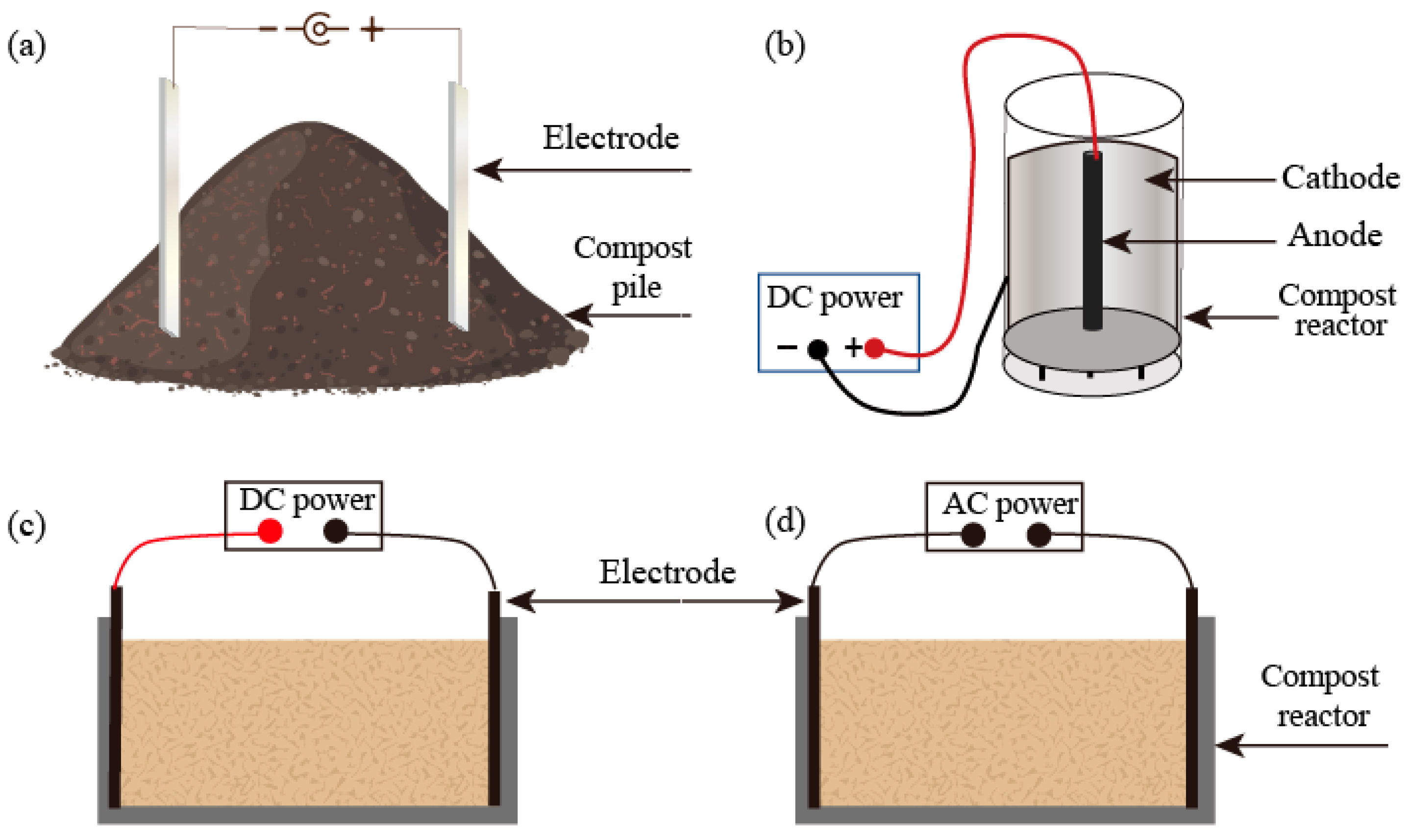
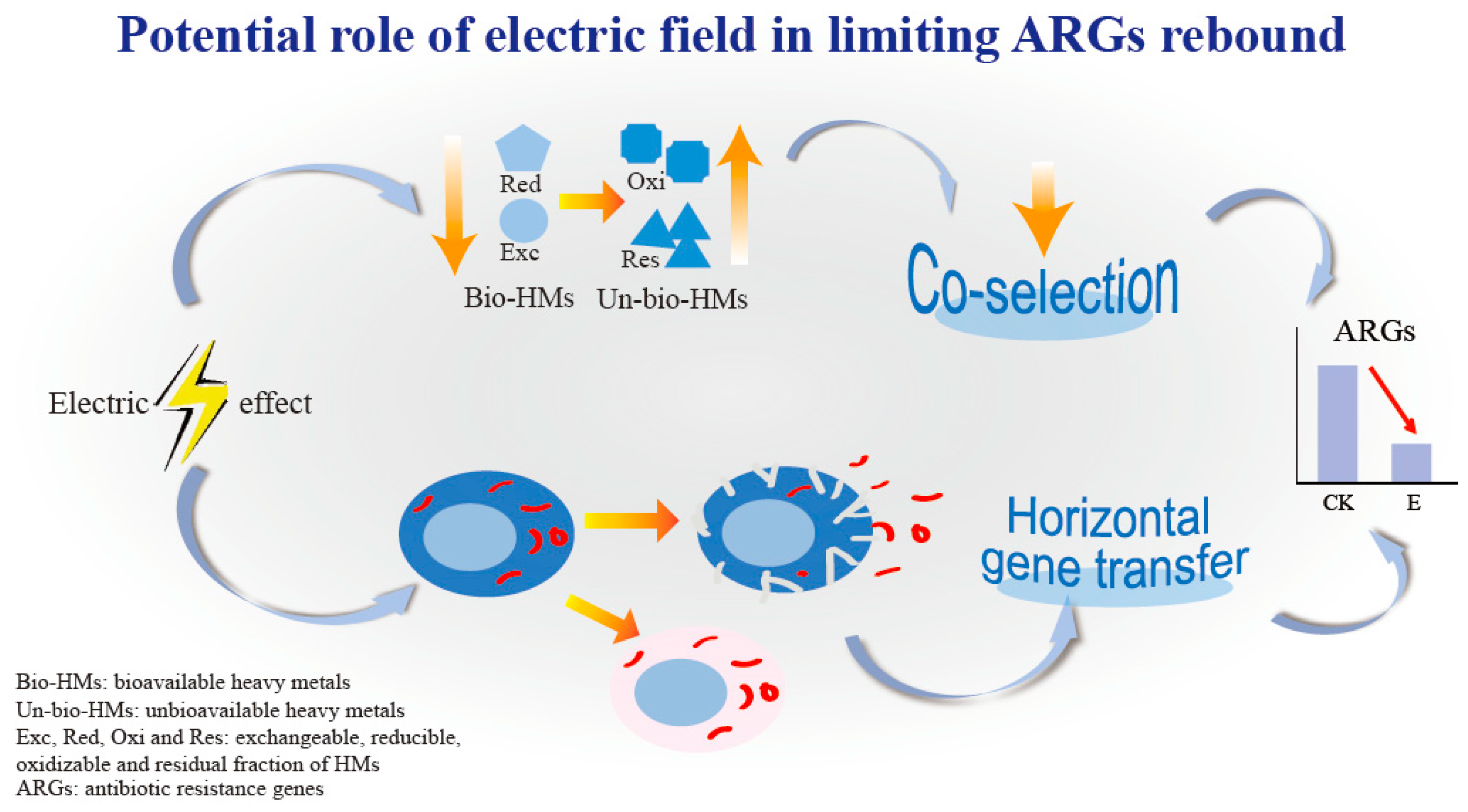
4.2. Electric Field-Assisted Composting—A Potential Strategy Targeting Heavy Metals and EPS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef] [PubMed]
- Rountree, P.M.; Barbour, R.G.H.; Thomson, E.F. Incidence of penicillin-resistant and streptomycin-resistant staphylococci in a hospital. Lancet 1951, 260, 435–436. [Google Scholar] [CrossRef]
- Serck-Hanssen, F. Penicillin-resistant staphylococci in a hospital’s environment and in acute puerperal mastitis. Acta Chir. Scand. 1952, 104, 236–243. [Google Scholar]
- Bobate, S.; Mahalle, S.; Dafale, N.A.; Bajaj, A. Emergence of environmental antibiotic resistance: Mechanism, monitoring and management. Environ. Adv. 2023, 13, 100409. [Google Scholar] [CrossRef]
- Cedeño-Muñoz, J.S.; Aransiola, S.A.; Reddy, K.V.; Ranjit, P.; Victor-Ekwebelem, M.O.; Oyedele, O.J.; Pérez-Almeida, I.B.; Maddela, N.R.; Rodríguez-Díaz, J.M. Antibiotic resistant bacteria and antibiotic resistance genes as contaminants of emerging concern: Occurrences, impacts, mitigations and future guidelines. Sci. Total Environ. 2024, 952, 175906. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’neill, J.I.M. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 20, 1–16. [Google Scholar]
- Sun, Z.; Hong, W.; Xue, C.; Dong, N. A comprehensive review of antibiotic resistance gene contamination in agriculture: Challenges and AI-driven solutions. Sci. Total Environ. 2024, 953, 175971. [Google Scholar] [CrossRef] [PubMed]
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; et al. State of the World’s Antibiotics 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; One Health Trust: Washington, DC, USA, 2021. [Google Scholar]
- Chee-Sanford, J.C.; Aminov, R.I.; Krapac, I.J.; Garrigues-Jeanjean, N.; Mackie, R.I. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 2001, 67, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235, 178–185. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Hu, H.-W.; Gou, M.; Wang, J.-T.; Chen, D.; He, J.-Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Kong, L.-C.; Wang, B.; Wang, Y.-M.; Hu, R.-G.; Atiewin, A.; Gao, D.; Gao, Y.-H.; Ma, H.-X. Characterization of bacterial community changes and antibiotic resistance genes in lamb manure of different incidence. Sci. Rep. 2019, 9, 10101. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.-J.; Su, J.-Q.; Stedfeld, R. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Johnson, T.A.; Su, J.-Q.; Qiao, M.; Guo, G.-X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Hou, L.; Yang, Y.; van Boeckel, T.P.; Zheng, Y.; Li, Y. Global biogeography and projection of soil antibiotic resistance genes. Sci. Adv. 2022, 8, eabq8015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.-H.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y.-G. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2159–2196. [Google Scholar] [CrossRef]
- Vaddella, V.; Pandey, P.; Cao, W.; Biswas, S.; Chiu, C.; Zheng, Y.; Wu, T.; Ghanem, N.; Buyuksonmez, F. Assessment of Pathogen Inactivation under Sub-composting Temperature in Lab-scale Compost Piles. J. Food Res. 2018, 7, 64. [Google Scholar] [CrossRef][Green Version]
- Kadir, A.A.; Azhari, N.W.; Jamaludin, S.N. An Overview of Organic Waste in Composting. MATEC Web Conf. 2016, 47, 5025. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Lin, H.; Zhou, S. Enhanced removal of antibiotic resistance genes during chicken manure composting after combined inoculation of Bacillus subtilis with biochar. J. Environ. Sci. 2024, 135, 274–284. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhang, L.; Zhang, X.; Cao, Y.; Xiao, R.; Bai, Z.; Ma, L. Meta-analysis addressing the potential of antibiotic resistance gene elimination through aerobic composting. Waste Manag. 2024, 182, 197–206. [Google Scholar] [CrossRef]
- Liu, B.; Yu, K.; Ahmed, I.; Gin, K.; Xi, B.; Wei, Z.; He, Y.; Zhang, B. Key factors driving the fate of antibiotic resistance genes and controlling strategies during aerobic composting of animal manure: A review. Sci. Total Environ. 2021, 791, 148372. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Chang, J.; Kong, Y.; Jiang, T.; Wang, J.; Yuan, J. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting. Bioresour. Technol. 2021, 323, 124620. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, Q.; Tong, J.; Buhe, C.; Wang, R.; Chen, M.; Wei, Y. Sludge bio-drying: Effective to reduce both antibiotic resistance genes and mobile genetic elements. Water Res. 2016, 106, 62–70. [Google Scholar] [CrossRef]
- Fukuda, A.; Suzuki, M.; Makita, K.; Usui, M. Low-frequency transmission and persistence of antimicrobial-resistant bacteria and genes from livestock to agricultural soil and crops through compost application. PLoS ONE 2024, 19, e0301972. [Google Scholar] [CrossRef]
- Buta, M.; Korzeniewska, E.; Harnisz, M.; Hubeny, J.; Zieliński, W.; Rolbiecki, D.; Bajkacz, S.; Felis, E.; Kokoszka, K. Microbial and chemical pollutants on the manure-crops pathway in the perspective of “One Health” holistic approach. Sci. Total Environ. 2021, 785, 147411. [Google Scholar] [CrossRef] [PubMed]
- Nemet, F.; Perić, K.; Lončarić, Z. Microbiological activities in the composting process: A review. Columella 2021, 8, 41–53. [Google Scholar] [CrossRef]
- Wikurendra, E.; Nurika, G.; Herdiani, N.; Lukiyono, Y.T. Evaluation of the Commercial Bio-Activator and a Traditional Bio-activator on Compost Using Takakura Method. J. Ecol. Eng. 2022, 23, 278–285. [Google Scholar] [CrossRef]
- Yang, X.; Sun, P.; Liu, B.; Ahmed, I.; Xie, Z.; Zhang, B. Effect of Extending High-Temperature Duration on ARG Rebound in a Co-Composting Process for Organic Wastes. Sustainability 2024, 16, 5284. [Google Scholar] [CrossRef]
- Qian, X.; Sun, W.; Gu, J.; Wang, X.-J.; Zhang, Y.-J.; Duan, M.-L.; Li, H.-C.; Zhang, R.-R. Reducing antibiotic resistance genes, integrons, and pathogens in dairy manure by continuous thermophilic composting. Bioresour. Technol. 2016, 220, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.F.; Zhu, C.; Geng, B.; Ahmad, H.R.; Song, T.; Li, H. The fate of antibiotic resistance genes in cow manure composting: Shaped by temperature-controlled composting stages. Bioresour. Technol. 2021, 320, 124403. [Google Scholar] [CrossRef]
- Hao, X.X.; Sang, W.P.; Li, F.T.; Shen, L.Y.; Zhu, L.; Rong, L.; Jiang, D.M.; Bai, L. Regulation of antibiotic resistance gene rebound by degrees of microecological niche occupation by microbiota carried in additives during the later phases of swine manure composting. Ecotoxicol. Environ. Saf. 2025, 294, 118112. [Google Scholar] [CrossRef]
- Wen, X.; Chen, M.J.; Ma, B.H.; Xu, J.J.; Zhu, T.; Zou, Y.D.; Liao, X.D.; Wang, Y.; Worrich, A.; Wu, Y.B. Removal of antibiotic resistance genes during swine manure composting is strongly impaired by high levels of doxycycline residues. Waste Manag. 2024, 177, 76–85. [Google Scholar] [CrossRef]
- Tong, Z.Y.; Liu, F.W.; Tian, Y.; Zhang, J.Z.; Liu, H.; Duan, J.Z.; Bi, W.L.; Qin, J.M.; Xu, S.Z. Effect of biochar on antibiotics and antibiotic resistance genes variations during co-composting of pig manure and corn straw. Front. Bioeng. Biotechnol. 2022, 10, 960476. [Google Scholar] [CrossRef]
- Guo, H.; Gu, J.; Wang, X.; Nasir, M.; Yu, J.; Lei, L.; Wang, Q. Elucidating the effect of microbial inoculum and ferric chloride as additives on the removal of antibiotic resistance genes from chicken manure during aerobic composting. Bioresour. Technol. 2020, 309, 122802. [Google Scholar] [CrossRef]
- Liao, H.; Bai, Y.; Liu, C.; Wen, C.; Yang, Q.; Chen, Z.; Banerjee, S.; Zhou, S.; Friman, V.-P. Airborne and indigenous microbiomes co-drive the rebound of antibiotic resistome during compost storage. Environ. Microbiol. 2021, 23, 7483–7496. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shao, Z.; Li, X.; Zheng, X.; Xu, J. Fate of antibiotic resistance genes in farmland soil applied with three different fertilizers during the growth cycle of pakchoi and after harvesting. J. Environ. Manag. 2021, 289, 112576. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Lanzén, A.; Mijangos, I.; Garbisu, C. The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl. Soil Ecol. 2019, 135, 73–84. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, X.; Zhao, J.; Li, H.; Xu, J.; Li, Z.; Wang, M.; Peng, Y.; Tian, T.; Yuan, G.; et al. Regional antimicrobial resistance gene flow among the One Health sectors in China. Microbiome 2025, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.O. Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim. Biotechnol. 2006, 17, 125–135. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Knight, R.; Fierer, N.; Dantas, G. Bacterial phylogeny structures soil resistomes across habitats. Nature 2014, 509, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Farhan, M.H.R.; Yuan, L.; Sui, Y.; Chu, J.; Yang, X.; Li, Y.; Huang, L.; Cheng, G. Transfer dynamics of antimicrobial resistance among gram-negative bacteria. Sci. Total Environ. 2024, 954, 176347. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Yang, X.-P.; Li, Q.; Wu, L.-H.; Shen, Q.-R.; Zhao, F.-J. Changes in antibiotic concentrations and antibiotic resistome during commercial composting of animal manures. Environ. Pollut. 2016, 219, 182–190. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Ren, Q.; Lu, J.; Cao, S.; Liu, C.; Li, Y. Phages in sludge from the A/O wastewater treatment process play an important role in the transmission of ARGs. Sci. Total Environ. 2024, 926, 172111. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Topp, E. Abundance of Antibiotic Resistance Genes in Bacteriophage following Soil Fertilization with Dairy Manure or Municipal Biosolids, and Evidence for Potential Transduction. Appl. Environ. Microbiol. 2015, 81, 7905–7913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, C.; Yin, S.; Chang, X.; Chen, K.; Xing, Y.; Yang, Y. Transmission and retention of antibiotic resistance genes (ARGs) in chicken and sheep manure composting. Bioresour. Technol. 2023, 382, 129190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-M.; Liu, X.; Wang, S.-L.; Fang, L.-X.; Sun, J.; Liu, Y.-H.; Liao, X.-P. Distribution patterns of antibiotic resistance genes and their bacterial hosts in pig farm wastewater treatment systems and soil fertilized with pig manure. Sci. Total Environ. 2021, 758, 143654. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Che, Z.; Xue, L. Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting. Biology 2022, 11, 1181. [Google Scholar] [CrossRef]
- Meng, Q.; Yang, W.; Men, M.; Bello, A.; Xu, X.; Xu, B.; Deng, L.; Jiang, X.; Sheng, S.; Wu, X.; et al. Microbial Community Succession and Response to Environmental Variables During Cow Manure and Corn Straw Composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Wang, K.; Shen, D.; Guo, Z.; Zhong, Q.; Huang, K. Contamination Characteristics of Antibiotic Resistance Genes in Multi-Vector Environment in Typical Regional Fattening House. Toxics 2024, 12, 916. [Google Scholar] [CrossRef]
- Korry, B.J.; Belenky, P. Trophic level and proteobacteria abundance drive antibiotic resistance levels in fish from coastal New England. Anim. Microbiome 2023, 5, 16. [Google Scholar] [CrossRef]
- Shen, D.; Li, C.; Guo, Z. Dynamics of antibiotic resistance in poultry farms via multivector analysis. Poult. Sci. 2025, 104, 104673. [Google Scholar] [CrossRef]
- Zuo, X.; Suo, P.; Li, Y.; Xu, Q. Diversity and distribution of antibiotic resistance genes associated with road sediments transported in urban stormwater runoff. Environ. Pollut. 2022, 292, 118470. [Google Scholar] [CrossRef]
- Ao, G.; Wang, Z.; Shi, Y.; Ling, H.; Sun, S.; Ping, W. Effects of exogenously added humic acid on the fate of aminoglycoside antibiotics and humification process during aerobic compost. Chem. Eng. J. 2024, 498, 155704. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Yang, Y.; Ma, R.; Li, L.; Li, G.; Yuan, J. Composting temperature directly affects the removal of antibiotic resistance genes and mobile genetic elements in livestock manure. Environ. Pollut. 2022, 303, 119174. [Google Scholar] [CrossRef]
- Qiu, T.; Huo, L.; Guo, Y.; Gao, M.; Wang, G.; Hu, D.; Li, C.; Wang, Z.; Liu, G.; Wang, X. Metagenomic assembly reveals hosts and mobility of common antibiotic resistome in animal manure and commercial compost. Environ. Microbiome 2022, 17, 42. [Google Scholar] [CrossRef]
- Wang, J.; Ben, W.; Yang, M.; Zhang, Y.; Qiang, Z. Dissemination of veterinary antibiotics and corresponding resistance genes from a concentrated swine feedlot along the waste treatment paths. Environ. Int. 2016, 92–93, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ren, P.; Wang, L.; Li, S.; Luo, C.; Zhao, Y.; Zhao, H.; Sun, J.; Ji, P. Material flow analysis of heavy metals in large-scale cattle farms and ecological risk assessment of cattle manure application to fields. J. Environ. Manag. 2024, 364, 121452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of heavy metals in animal feeds and manures from farms of different scales in northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Li, N.; Yang, Z.; Li, B.; Zhang, X.; Li, H. Prioritized regional management for antibiotics and heavy metals in animal manure across China. J. Hazard. Mater. 2024, 461, 132706. [Google Scholar] [CrossRef]
- Hasman, H.; Aarestrup, F.M. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: Occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob. Agents Chemother. 2002, 46, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Hasman, H.; Jensen, L.B.; Moreno, M.; Herrero, I.A.; Domínguez, L.; Finn, M.; Franklin, A. Antimicrobial resistance among enterococci from pigs in three European countries. Appl. Environ. Microbiol. 2002, 68, 4127–4129. [Google Scholar] [CrossRef]
- Fang, L.; Li, X.; Li, L.; Li, S.; Liao, X.; Sun, J.; Liu, Y. Co-spread of metal and antibiotic resistance within ST3-IncHI2 plasmids from E. coli isolates of food-producing animals. Sci. Rep. 2016, 6, 25312. [Google Scholar] [CrossRef]
- Aendekerk, S.; Ghysels, B.; Cornelis, P.; Baysse, C. Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology 2002, 148, 2371–2381. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Tang, Z.; Zhang, W.; Yu, G.; Shen, Q.; Zhao, F.-J. Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manag. 2017, 64, 333–339. [Google Scholar] [CrossRef]
- Yin, Y.; Gu, J.; Wang, X.; Song, W.; Zhang, K.; Sun, W.; Zhang, X.; Zhang, Y.; Li, H. Effects of Copper Addition on Copper Resistance, Antibiotic Resistance Genes, and intl1 during Swine Manure Composting. Front. Microbiol. 2017, 8, 344. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wen, Q.; Ji, Y. Variation of heavy metal speciation, antibiotic degradation, and potential horizontal gene transfer during pig manure composting under different chlortetracycline concentration. Environ. Sci. Pollut. Res. Int. 2020, 28, 1224–1234. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Poté, J.; Prabakar, K. Extracellular DNA (eDNA): Neglected and Potential Sources of Antibiotic Resistant Genes (ARGs) in the Aquatic Environments. Pathogens 2020, 9, 874. [Google Scholar] [CrossRef]
- Ji, X.; Pan, X. Intra-/extra-cellular antibiotic resistance responses to sewage sludge composting and salinization of long-term compost applied soils. Sci. Total Environ. 2022, 838, 156263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Q. Single gene retrieval from thermally degraded DNA. J. Biosci. 2005, 30, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gao, S.; Lou, L.; Zhou, Z. Removal of Intracellular and Extracellular Antibiotic Resistance Genes from Swine Wastewater by Sequential Electrocoagulation and Electro-Fenton Processes. Environ. Eng. Sci. 2021, 38, 74–80. [Google Scholar] [CrossRef]
- Dong, P.; Wang, H.; Fang, T.; Wang, Y.; Ye, Q. Assessment of extracellular antibiotic resistance genes (eARGs) in typical environmental samples and the transforming ability of eARG. Environ. Int. 2019, 125, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Haffiez, N.; Zakaria, B.S.; Azizi, S.M.M.; Dhar, B.R. Fate of intracellular, extracellular polymeric substances-associated, and cell-free antibiotic resistance genes in anaerobic digestion of thermally hydrolyzed sludge. Sci. Total Environ. 2023, 855, 158847. [Google Scholar] [CrossRef]
- He, T.; Jin, L.; Xie, J.; Yue, S.; Fu, P.; Li, X. Intracellular and Extracellular Antibiotic Resistance Genes in Airborne PM 2.5 for Respiratory Exposure in Urban Areas. Environ. Sci. Technol. Lett. 2021, 8, 128–134. [Google Scholar] [CrossRef]
- Li, D.; Gao, J.; Dai, H.; Duan, W.; Wang, Z.; Zhou, Z. Fates of intracellular and extracellular antibiotic resistance genes during a pilot-scale aerobic granular sludge cultivation process. Chem. Eng. J. 2021, 421, 127737. [Google Scholar] [CrossRef]
- Zarei-Baygi, A.; Smith, A.L. Intracellular versus extracellular antibiotic resistance genes in the environment: Prevalence, horizontal transfer, and mitigation strategies. Bioresour. Technol. 2021, 319, 124181. [Google Scholar] [CrossRef]
- Guo, N.; Wang, M.; Shen, Y.; Li, B.; Zhao, D.; Zou, S.; Yang, Y. Detection of extracellular antibiotic resistance genes in river water: Application of ultrafiltration-magnetic beads method. Environ. Res. 2024, 263, 120259. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, L.; Li, Z.-H.; Zhang, X.; Leung, K.M.Y.; Sheng, G.-P. Extracellular polymeric substances (EPS) associated extracellular antibiotic resistance genes in activated sludge along the AAO process: Distribution and microbial secretors. Sci. Total Environ. 2022, 816, 151575. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Y.; Cao, B.; Huang, Q.; Cai, P. An invisible workforce in soil: The neglected role of soil biofilms in conjugative transfer of antibiotic resistance genes. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2720–2748. [Google Scholar] [CrossRef]
- Ali, N.S.A.; Muda, K.; Mohd Amin, M.F.; Najib, M.Z.M.; Ezechi, E.H.; Darwish, M.S.J. Initialization, enhancement and mechanisms of aerobic granulation in wastewater treatment. Sep. Purif. Technol. 2021, 260, 118220. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Gu, P.; Yang, K.; Jia, Y.; Miao, H. The effect of extracellular polymeric substances on the distribution and transmission of antibiotic resistance genes treating antibiotic wastewater via microbial electrolysis cells. Chemosphere 2024, 364, 143284. [Google Scholar] [CrossRef]
- Hu, X.; Kang, F.; Yang, B.; Zhang, W.; Qin, C.; Gao, Y. Extracellular Polymeric Substances Acting as a Permeable Barrier Hinder the Lateral Transfer of Antibiotic Resistance Genes. Front. Microbiol. 2019, 10, 736. [Google Scholar] [CrossRef]
- Li, S.; Bai, Y.; Li, Z.; Wang, A.; Ren, N.-Q.; Ho, S.-H. Overlooked role of extracellular polymeric substances in antibiotic-resistance gene transfer within microalgae-bacteria system. J. Hazard. Mater. 2025, 488, 137206. [Google Scholar] [CrossRef]
- Ben, W.; Wang, J.; Pan, X.; Qiang, Z. Dissemination of antibiotic resistance genes and their potential removal by on-farm treatment processes in nine swine feedlots in Shandong Province, China. Chemosphere 2017, 167, 262–268. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zheng, L.; Cai, Q.J.; Xu, Y.B.; Xie, Z.F.; Liu, J.Y.; Ning, X.N. Simultaneous reduction of antibiotics and antibiotic resistance genes in pig manure using a composting process with a novel microbial agent. Ecotoxicol. Environ. Saf. 2021, 208, 111724. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Liao, H.; Lu, X.; Rensing, C.; Friman, V.P.; Geisen, S.; Chen, Z.; Yu, Z.; Wei, Z.; Zhou, S.; Zhu, Y. Hyperthermophilic Composting Accelerates the Removal of Antibiotic Resistance Genes and Mobile Genetic Elements in Sewage Sludge. Environ. Sci. Technol. 2018, 52, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, A.; Guo, T.; Zhu, Y.; Shao, Y. Biochar and Hyperthermophiles as Additives Accelerate the Removal of Antibiotic Resistance Genes and Mobile Genetic Elements during Composting. Materials 2021, 14, 5428. [Google Scholar] [CrossRef]
- Cheng, D.; Xiong, J.; Chen, J.; Chang, H.; Wong, J.W.C. Effect of biochar addition on antibiotic and heavy metal resistance genes during sewage sludge composting. J. Environ. Chem. Eng. 2025, 13, 115732. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, L.; Yan, W.; Tao, H.; Yao, C.; An, L.; Sun, Y.; Hu, T.; Sun, W.; Qian, X.; et al. Exogenous additives reshape the microbiome and promote the reduction of resistome in co-composting of pig manure and mushroom residue. J. Hazard. Mater. 2025, 481, 136544. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, X.; Zhao, W.; Wang, Y.; Cui, P.; Zeng, R.J.; Yu, L.; Zhou, S. Electric field induces electron flow to simultaneously enhance the maturity of aerobic composting and mitigate greenhouse gas emissions. Bioresour. Technol. 2019, 279, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Tang, J.; Wu, J.; Shen, C.; Shangguan, H.; Zeng, R.J.; Zhou, S. Alternating electric field enables hyperthermophilic composting of organic solid wastes. Sci. Total Environ. 2022, 828, 154439. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Zhang, X.; Misselbrook, T.; Bai, Z.; Ma, L. An electric field immobilizes heavy metals through promoting combination with humic substances during composting. Bioresour. Technol. 2021, 330, 124996. [Google Scholar] [CrossRef]
- Chen, S.; Sun, X.; Zhang, H.; Chang, H.; Wang, Y.; Tan, Z.; Xi, B.; Xing, M.; Dong, B.; Zhu, H. Influence of Electric Fields on the Maturity and Microbial Communities During Sludge and Straw Composting. Waste Biomass Valor. 2024, 90, 44. [Google Scholar] [CrossRef]
- Shen, C.; Shangguan, H.; Fu, T.; Mi, H.; Lin, H.; Huang, L.; Tang, J. Electric field-assisted aerobic co-composting of chicken manure and kitchen waste: Ammonia mitigation and maturation enhancement. Bioresour. Technol. 2024, 391, 129931. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, W.; Zhuo, Q.; Cao, Z.; Chen, Q.; Xiao, L. Research Progress on Heavy Metal Passivators and Passivation Mechanisms of Organic Solid Waste Compost: A Review. Fermentation 2024, 10, 88. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, B.; Ren, R.; Shi, Y.; Xiong, J.; Zhang, W.; Wang, D. Correlation and mechanism of extracellular polymeric substances (EPS) on the effect of sewage sludge electro-dewatering. Sci. Total Environ. 2021, 801, 149753. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-P.; Yan, X.-F.; Yang, C.-F.; Zhu, N.-W. Enhancement of waste activated sludge dewaterability by electro-chemical pretreatment. J. Hazard. Mater. 2011, 187, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Zhang, M.; Meng, S.; Mao, Z.; Liang, D.; Fan, W.; Yang, L.; Dong, Z.; Liao, Y.; Wang, J.; et al. Integration of membrane bioreactor with a weak electric field: Mitigating membrane fouling and improving effluent quality targeting low energy consumption. Chem. Eng. J. 2024, 495, 153336. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, X.; Xie, J.; Guan, M.; Wang, E.; Lu, D.; Dong, T.; Zhang, X. Influence of alternating electric field on deep dewatering of municipal sludge and changes of extracellular polymeric substance during dewatering. Sci. Total Environ. 2022, 842, 156839. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Ren, Y.; Wang, Z.; Bai, Z.; Ma, L. Rebound of Antibiotic Resistance Genes in Composting: Mechanisms, Challenges, and Control Strategies. Agriculture 2025, 15, 2317. https://doi.org/10.3390/agriculture15222317
Zhang X, Wang X, Ren Y, Wang Z, Bai Z, Ma L. Rebound of Antibiotic Resistance Genes in Composting: Mechanisms, Challenges, and Control Strategies. Agriculture. 2025; 15(22):2317. https://doi.org/10.3390/agriculture15222317
Chicago/Turabian StyleZhang, Xinyuan, Xuan Wang, Yazhan Ren, Zihan Wang, Zhaohai Bai, and Lin Ma. 2025. "Rebound of Antibiotic Resistance Genes in Composting: Mechanisms, Challenges, and Control Strategies" Agriculture 15, no. 22: 2317. https://doi.org/10.3390/agriculture15222317
APA StyleZhang, X., Wang, X., Ren, Y., Wang, Z., Bai, Z., & Ma, L. (2025). Rebound of Antibiotic Resistance Genes in Composting: Mechanisms, Challenges, and Control Strategies. Agriculture, 15(22), 2317. https://doi.org/10.3390/agriculture15222317