Abstract
Plant pathogenic fungi pose a persistent global threat to food security, causing severe yield losses in staple crops and increasing dependence on chemical fungicides. However, the ecological and toxicological drawbacks of synthetic fungicides have intensified the search for safer, plant-derived alternatives. This review synthesizes current advances on the antifungal mechanisms of plant essential oils (EOs) and their prospects for biofungicide development. The literature reveals that the antifungal activity of EOs arises from their diverse phytochemical composition, principally terpenes, phenolics, and aldehydes that target multiple fungal cellular sites. These compounds disrupt membrane integrity through ergosterol depletion, inhibit chitin and β-glucan synthesis, interfere with mitochondrial energy metabolism, and induce oxidative stress, leading to lipid peroxidation and cell death. Morphological and transcriptomic evidence confirms that EOs alter hyphal growth, spore germination, and key gene expression pathways associated with fungal virulence. Furthermore, emerging nanotechnological and encapsulation strategies enhance EO stability, bioavailability, and field persistence, addressing major barriers to their large-scale agricultural application. The integration of EO-based biofungicides within sustainable and precision agriculture frameworks offers a promising route to reduce chemical inputs, mitigate resistance development, and promote ecological balance. This review underscores the need for interdisciplinary research linking phytochemistry, nanotechnology, and agronomy to translate EO-based antifungal mechanisms into next-generation, environmentally compatible crop protection systems.
1. Introduction
Plant pathogenic fungi continue to represent one of the most persistent threats to global agriculture, causing substantial yield losses in crops of high economic and nutritional relevance, including wheat, rice, maize, and potatoes [1]. To mitigate these impacts, farmers have historically depended on the use of chemical fungicides and other synthetic control agents as part of intensive disease management programs [2]. Although these compounds have proven highly effective in reducing the prevalence of fungal infections, their prolonged and excessive use has generated new challenges that extend far beyond plant protection.
The intensive application of synthetic fungicides has become a paradigm of growing concern in modern agriculture. Their widespread use has not only altered the ecological balance of agroecosystems but has also introduced significant human health and socio-economic risks. Residual chemical compounds persist in food commodities, posing latent dangers to consumers, while their non-target effects compromise beneficial soil microorganisms, aquatic ecosystems, and pollinators essential to ecological stability [3]. In addition, the increasing financial burden associated with purchasing these inputs, together with the emergence of resistant fungal strains, creates a vicious cycle that weakens the economic sustainability of agricultural systems [4,5].
Within this scenario, the limitations of conventional fungicides have become increasingly evident. Continuous exposure to chemical molecules has driven the evolution of resistant fungal populations, diminishing the effectiveness of widely used fungicides such as triazoles, strobilurins, and dithiocarbamates [6]. Moreover, their environmental persistence, bioaccumulation, and toxicity to non-target organisms have raised serious regulatory and ethical concerns. As agriculture undergoes a global transition toward sustainability, these limitations underscore the necessity of developing innovative, eco-friendly alternatives that maintain efficacy while reducing environmental impact [7].
In response to these challenges, plant-derived essential oils (EOs) have emerged as promising candidates for the development of next-generation biofungicides. These natural, volatile compounds are synthesized by aromatic plants as part of their secondary metabolism and are characterized by a complex mixture of terpenes, phenolics, aldehydes, and alcohols [8].
Their broad-spectrum biological activity, biodegradability, and low environmental persistence make them attractive for integration into sustainable crop protection systems. Numerous studies have demonstrated that EOs exert potent antifungal effects by disrupting the fungal cell membrane, inhibiting enzyme activity, and inducing oxidative stress that culminates in cell death [9]. For instance, thyme (Thymus vulgaris) essential oil, rich in thymol and carvacrol, has exhibited strong inhibitory activity against Botrytis cinerea and Fusarium oxysporum, two of the most economically damaging plant pathogens [10,11].
Recent research has expanded our understanding of the mechanistic complexity of these natural products, revealing that their antifungal action involves not only direct toxicity but also modulation of gene expression and interference with fungal development pathways [12]. Furthermore, advances in nanotechnology and formulation science are enhancing EO stability, controlled release, and bioavailability, paving the way for their practical application under field conditions.
Given this context, the present review aims to critically analyze and synthesize recent research concerning the antifungal mechanisms of plant essential oils and their potential role in developing innovative, sustainable biofungicides. By integrating biochemical, morphological, and molecular evidence, this work seeks to provide a comprehensive framework that supports the rational design of EO-based products capable of replacing or complementing synthetic fungicides in modern agriculture. Ultimately, this review underscores that the strategic combination of plant-derived antifungal compounds with advanced formulation and delivery systems represents a crucial step toward achieving environmentally responsible and resilient crop protection.
2. Essential Oils
Essential oils (EOs) are concentrated, volatile compounds extracted from various parts of aromatic plants, including leaves, flowers, stems, and roots [13]. They have complex chemical composition with a diverse array of phytochemicals such as terpenes, phenolics, and aldehydes, contributing to their distinctive aromas and flavors [14].
EOs are not only valued for their aromatic properties but also for their rich chemical compositions, which include a great variety of phytochemicals such as terpenes, phenolics, and aldehydes [15]. This diverse array of compounds not only defines their unique scents and flavors but also underpins their biological activities, including antifungal properties [16].
The antifungal activity of EOs has garnered considerable attention in recent years, as researchers explore their potential as biofungicides [17]. Studies have demonstrated that they effectively inhibit the growth of various plant pathogenic fungi, including species from genera such as Botrytis [18].
Eos, besides their antifungal properties, offer the advantage of a lower environmental impact compared to conventional chemical fungicides [19]. Their natural origins and biodegradability position them as promising alternatives in integrated pest management (IPM) strategies [20].
Great potential is expected for EOs when they are used in combination with other biological control agents, which could enhance their effectiveness and broaden their application spectrum [21]. Such synergistic approaches not only improve disease management but also contribute to the overall sustainability of agricultural practices, aligning with the increasing demand for environmentally friendly solutions [22].
3. Antifungal Properties of Plant Essential Oils Against Fungal Pathogens
The antifungal properties of plant essential oils have emerged as a significant area of research, particularly in their capacity to combat both human and plant pathogenic fungi [23]. Essential oils derived from a diverse range of plants, such as tea tree, oregano, and Eucalyptus, have demonstrated substantial antifungal activity against various fungal species [24].
The essential oil of Melaleuca alternifolia (tea tree) is well documented for its efficacy against dermatophytes and other opportunistic pathogens in humans, including Candida albicans [25]. The bioactive compounds within these oils, such as terpinen-4-ol and α-terpineol, play critical roles in disrupting fungal cell membranes and inhibiting key metabolic pathways, thereby leading to fungal cell death [26].
In the context of agriculture, EOs are gaining recognition for their effectiveness against economically significant plant pathogens [27]. Oils such as thyme and clove have shown remarkable antifungal activity against fungi like Botrytis cinerea and Fusarium oxysporum which are responsible for substantial crop losses [28].
EOs derived from oregano, thyme, and clove have demonstrated antifungal effects on various Fusarium species, including Fusarium oxysporum and Fusarium solani [29]. These oils contain bioactive compounds, particularly phenolic compounds and terpenes can lead to reduced fungal biomass and spore germination, highlighting their potential as effective biofungicides in agricultural settings [30].
EOs derived from plants such as oregano and thyme have demonstrated significant inhibitory effects on the growth and spore germination of Alternaria linariae, the causal agent of early blight in tomato plants [31]. Thyme essential oil (TEO) enhances the activity of defense related enzymes peroxidase, polyphenol oxidase and β-1,3-glucanase and promote phenolic compound accumulation indicates a dual mode of action both direct antifungal activity and the induction of systemic resistance in tomato plants. TEO was considered a promising product with multiple effects and high potential for biological control of early blight in tomato plants [32].
The antifungal activity of oregano essential oil against Rhizoctonia solani has been well documented, establishing it as an effective natural biofungicide. With an effective concentration (EC50) of 22.89 μg/mL, oregano essential oil not only inhibits mycelial growth but also induces significant alterations in mycelial morphology. Ultrastructural analyses reveal that the oil impacts the fungal cell membrane, leading to increased permeability and integrity loss [33].
Some essential oils (EOs) had antifungal activity against Colletotrichum lindemuthianum the causal agent of the common bean anthracnose [34]. Induced defense enzymes like Chitinase (CHI), Polyphenol oxidase (PPO), Guaiacol peroxidase (POX) and Phenylalanine ammonia-lyase (PAL) were activated on bean plants after essential oil were applied [35]. Additionally, EOs damaged the conidia’s ultrastructure by causing vacuolization, cytoplasmic leakage, and plasma membrane invagination of C. lindemuthianum isolates [34].
The in vitro antifungal activity of nine monoterpenes against Sclerotinia sclerotiorum has revealed that carvacrol is a particularly potent antifungal agent, demonstrating strong toxicity. When S. sclerotiorum was treated with carvacrol, the mycelial surface exhibited severe morphological alterations, characterized by a shriveled, uneven texture and the leakage of cytoplasmic contents, indicative of cellular damages [36].
Evaluation of the efficacy of essential oils (EOs) from grapefruit, rosemary, pine, sage, and thyme against Cercospora beticola indicated that thyme EO exhibited the highest antifungal activity, with a minimum inhibitory concentration (MIC) of 0.313 mL/L for most isolates tested. Moreover, thyme EO effectively inhibited the growth of multi-resistant C. beticola strains against chemical fungicides, highlighting its potential as a biocontrol agent [37].
4. Antifungal Mechanisms of Biofungicides Based on Essential Oils
4.1. Fungal Cell Membrane Disruption and Structural Damage
Essential oils (EOs) exhibit diverse chemical compositions that confer a wide range of biological activities, including potent antifungal properties [38]. Among their principal mechanisms of action, disruption of the fungal cell membrane is the most extensively documented. Terpenoids, phenolics, and aldehydes such as thymol, carvacrol, and citral interact with the lipid bilayer of fungal membranes, altering their fluidity and permeability [39]. This alteration promotes leakage of intracellular constituents, ionic imbalance, and ultimately the collapse of membrane integrity [26].
The hydrophobic compounds present in EOs embed within the phospholipid bilayer, displacing essential sterols such as ergosterol and key phospholipids, thereby weakening the overall structural framework of the fungal cell envelope [40]. Recent findings demonstrate that thymol and carvacrol specifically target ergosterol, a pivotal sterol for fungal membrane stability, which accounts for their selective antifungal activity [41].
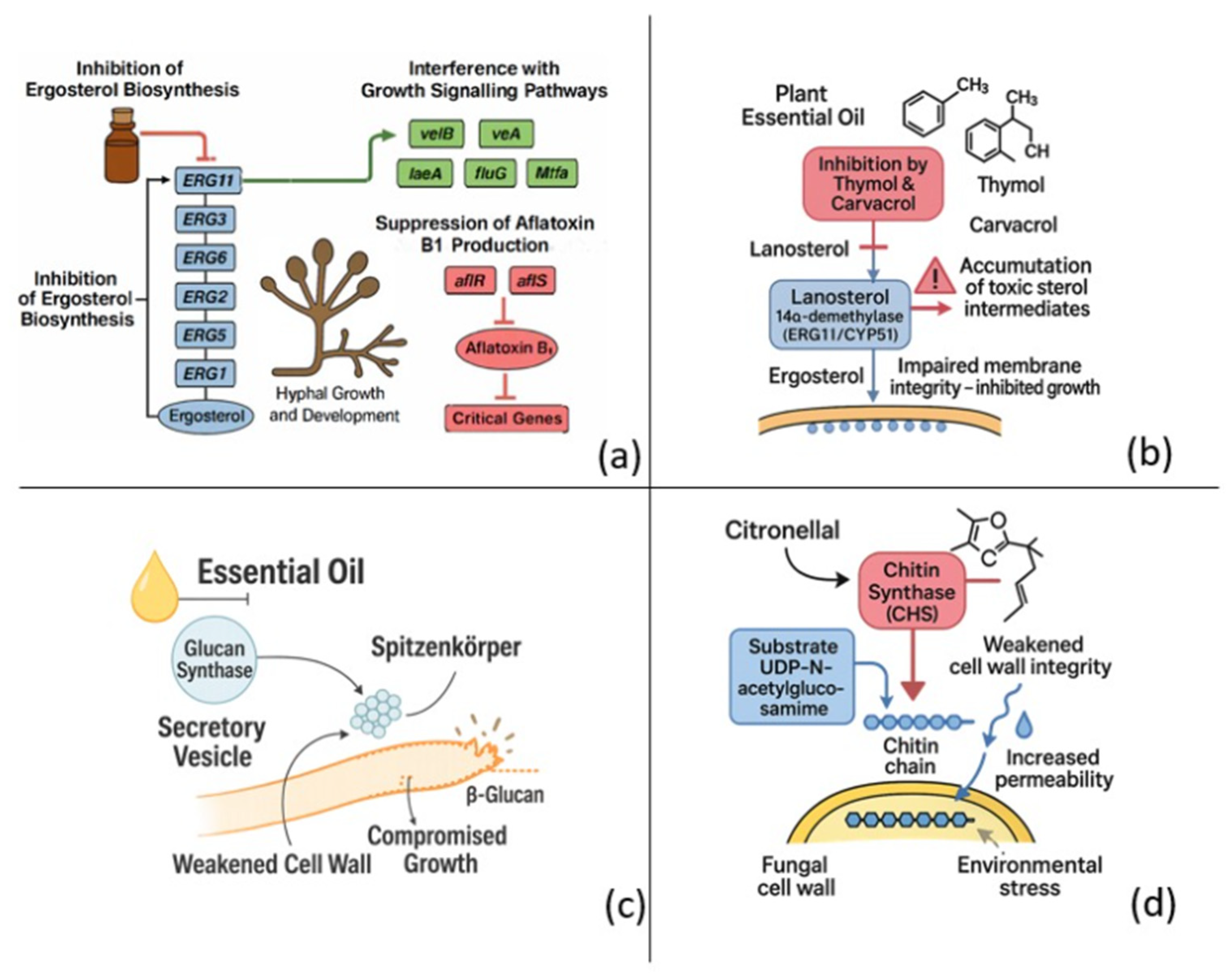
Figure 1 schematically illustrates the antifungal mechanisms of plant-derived essential oils, highlighting their interactions with fungal cell walls, membranes, and intracellular organelles. The diagram emphasizes how EOs trigger cellular leakage, oxidative imbalance, and enzymatic inhibition that collectively lead to fungal cell death.
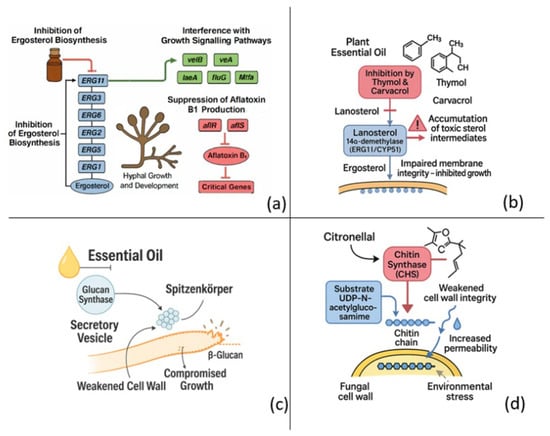
Figure 1.
Antifungal targets of plant-derived essential oils (EOs). (a) Multi-target blockade of ergosterol biosynthesis (ERG11, ERG3, ERG6, ERG2, ERG5, ERG1) coupled to interference with the velB/vea/laeA/fluG/Mtfa signaling module and down-regulation of the aflR/aflS activators, collectively inhibiting hyphal growth and aflatoxin B1 production. (b) Phenolic monoterpenes thymol and carvacrol inhibit lanosterol 14α-demethylase (ERG11/CYP51), causing lanosterol accumulation, toxic sterol build-up and loss of membrane integrity. (c) EO exposure prevents delivery of glucan synthase–containing secretory vesicles addressed to the fungal membrane, depleting β-glucan at the hyphal tip and weakening the cell wall. (d) The aldehyde citronellal binds chitin synthase (CHS), blocks incorporation of UDP-N-acetyl-glucosamine into nascent chitin chains and increases wall permeability, ultimately promoting osmotic stress and lysis. Together, these mechanisms highlight the antifungal potential of EOs to compromise fungal viability by simultaneously disrupting membrane sterol homeostasis, cell wall polysaccharide synthesis.
Furthermore, EOs often act synergistically with conventional fungicides, potentiating antifungal efficacy while mitigating resistance development [42]. This synergy enables lower dosages and reduces environmental residues, aligning EO-based strategies with the principles of sustainable agriculture [43].
To illustrate the breadth of antifungal action, Table 1 summarizes representative studies reporting the inhibitory effects of EOs against key plant pathogens. The table highlights the diversity of EO sources, targeted fungal species, and observed disease suppression outcomes under both laboratory and field conditions. These interactions collectively underscore the potential of EOs as eco-friendly alternatives to synthetic fungicides.

Table 1.
Antifungal activity of plant-derived essential oils against major phytopathogenic fungi and associated crop diseases.
4.2. Enzymatic and Molecular Inhibition of Key Fungal Pathways
The antifungal efficiency of essential oils (EOs) is closely related to their capacity to inhibit key enzymatic systems involved in fungal metabolism, energy production, and cell-wall biogenesis. Table 2 summarizes the main enzymatic targets reported in the literature, detailing their substrates, regulatory pathways, and the resulting cellular effects that contribute to growth suppression and loss of viability.

Table 2.
Primary enzymatic targets for essential oil antifungal activity: substrates, cellular effects, and modes of action.
Beyond physical disruption, EOs exert biochemical interference by inhibiting critical fungal enzymes involved in energy production, sterol biosynthesis, and cell wall construction. Ajwain essential oil, for example, suppresses ergosterol biosynthesis and downregulates regulatory genes in Aspergillus flavus, including velB, veA, laeA, fluG, and Mtfa, as well as developmental genes brlA and abaA [54]. It also interferes with aflR and aflS, key activators of aflatoxin biosynthesis.
Other EO components inhibit essential enzymes such as lanosterol demethylase [55], and glucan synthase [56], Chitin synthase [57]chitin synthase [58], thereby impeding the synthesis of chitin and β-glucans major structural polymers of fungal cell walls. Inhibition of these enzymes compromises rigidity, increases permeability, and renders fungi more vulnerable to environmental stress.
Mitochondrial enzymes are also major EO targets. Cytochrome c oxidase (Complex IV) inhibition disrupts electron transfer and ATP synthesis, leading to energy deprivation and the accumulation of reactive oxygen species (ROS). Likewise, NADH dehydrogenase inhibition halts oxidative phosphorylation, resulting in metabolic imbalance and oxidative stress [58].
Additionally, EOs inhibit antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD), enhancing intracellular ROS accumulation and oxidative injury [59]. Compounds like terpinen-4-ol and eugenol also block phospholipases, compromising lipid metabolism and cellular signaling [60].
Several terpenes directly target the mevalonate pathway, the metabolic route responsible for ergosterol synthesis, by inhibiting HMG-CoA reductase (HMGR) [61], mevalonate-5-pyrophosphate decarboxylase (MVD) [62], and farnesyl pyrophosphate synthase (FPPS) [63]. Moreover, lanosterol synthase (LSS) [64], C-14 sterol demethylase (CYP51) [65], and C-24 methyltransferase (ERG6) [66] are key enzymatic targets through which EO constituents especially thymol and carvacrol impair ergosterol biosynthesis and membrane assembly.
As summarized in Table 3, the antifungal efficiency of EOs is closely linked to the structural diversity of their secondary metabolites. The table outlines the principal biosynthetic pathways namely the mevalonate (MEV) and methylerythritol phosphate (MEP) routes, alongside representative metabolites and their proposed biochemical targets. Understanding these pathways clarifies how EOs interfere with vital fungal metabolic systems.

Table 3.
Major biosynthetic pathways and metabolites responsible for antifungal activity in plant essential oils.
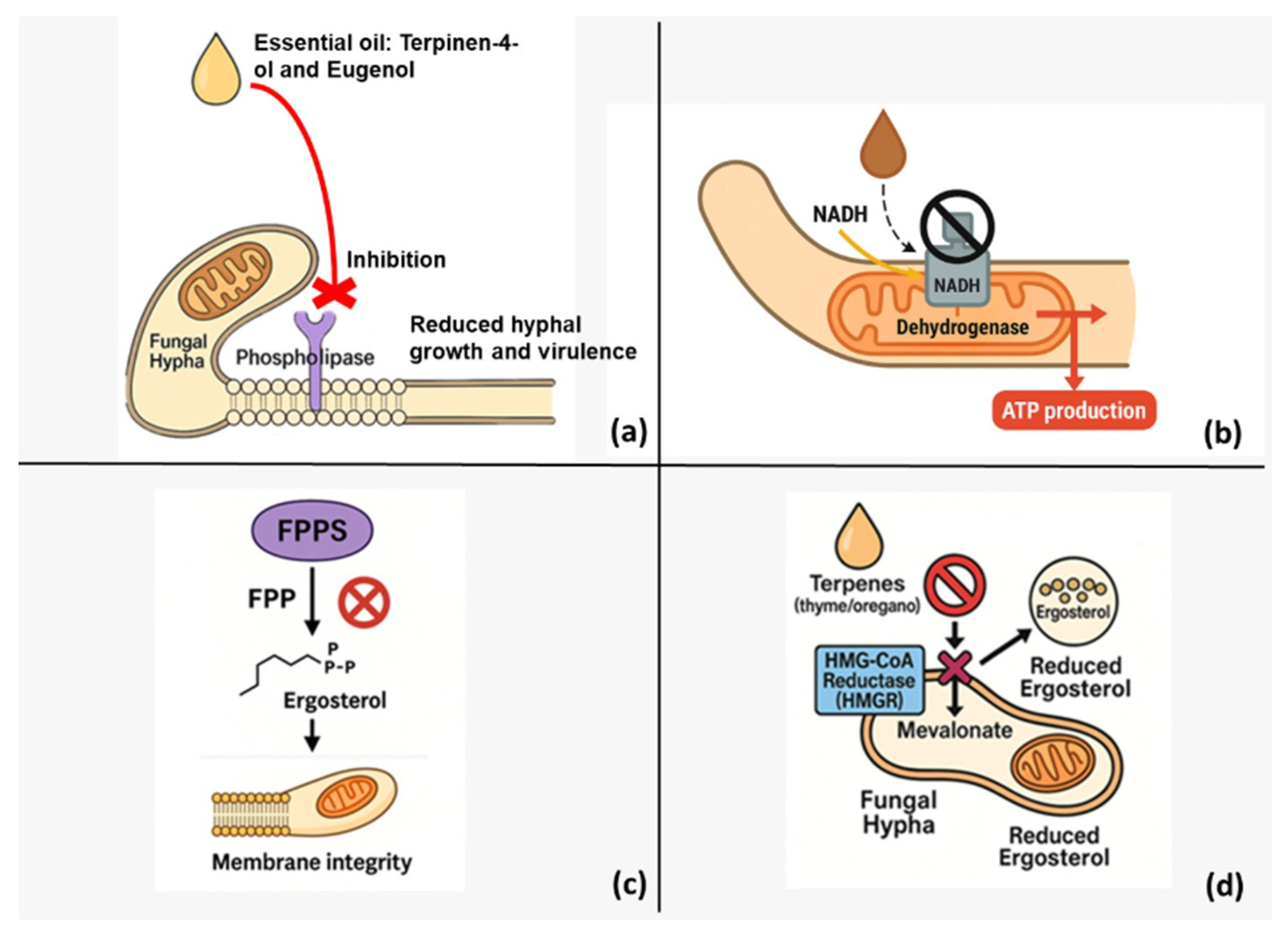
Figure 2 complements these data by illustrating the parallel enzymatic targets and the convergence of multiple inhibition routes that disrupt ergosterol biosynthesis and mitochondrial energy metabolism.
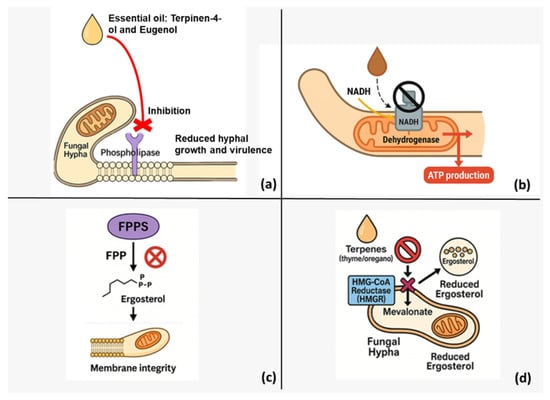
Figure 2.
Multifaceted antifungal mechanisms of plant-derived essential oils (EOs). (a) Monoterpenoids terpinen-4-ol and eugenol bind to membrane-embedded fungal phospholipases, blocking phospholipid turnover and thereby weakening the cell wall, arresting hyphal extension and attenuating virulence. (b) EO constituents inhibit mitochondrial NADH dehydrogenase (Complex I), interrupting electron flow to ubiquinone, collapsing the proton-motive force and lowering ATP output. (c) Targeting farnesyl pyrophosphate synthase (FPPS) reduces the cellular pool of farnesyl pyrophosphate, limiting downstream sterol (ergosterol) synthesis and compromising plasma-membrane integrity. (d) Terpene-rich EOs from thyme and oregano inhibit HMG-CoA reductase (HMGR)—the rate-limiting enzyme of the mevalonate pathway—decreasing mevalonate flux and further depleting ergosterol. Collectively, these parallel disruptions of lipid metabolism and respiratory energy production undermine fungal growth and viability.
4.3. Morphological and Cytoskeletal Alterations in Fungi
In addition to altering fungal morphology, essential oils have demonstrated a potent capacity to inhibit spore and conidial germination across diverse phytopathogens. This effect is highly dependent on EO composition, concentration, and fungal species. Table 4 compiles recent evidence on the inhibitory potential of different essential oils against agriculturally important fungi, highlighting both complete and partial suppression under controlled experimental conditions.

Table 4.
Effect of essential oils in spore germination of fungal plant pathogens.
Morphogenesis, the differentiation of hyphae, spores, and reproductive structures is crucial for fungal pathogenicity [74]. Essential oils disturb these morphological processes by targeting cytoskeletal components and interfering with microtubule and actin organization [75].
Clove oil, rich in eugenol, alters microtubule assembly and stability, disrupting vesicle transport and cell division, ultimately reducing fungal proliferation [76]. Actin filaments concentrated at hyphal tip are also disrupted by thymol, which affects polymerization dynamics and hinders vesicle transport and hyphal elongation [77]. These structural disturbances impair nutrient absorption, colonization capacity, and virulence.
During reproductive phases, EOs inhibit the formation and germination of conidia, the asexual spores responsible for fungal dissemination [78]. Compounds such as thymol, carvacrol, and eugenol decrease spore size, viability, and germination rates, reducing infection potential [79]. Tea tree and cinnamon oils alter hyphal and mycelial morphology, suppressing aerial mycelium development and metabolic activity [80].
Similarly, neem (Azadirachta indica) and tea tree EOs significantly inhibit urediniospore germination in Hemileia vastatrix, demonstrating their potential as natural fungicides for agricultural systems [81].
4.4. Oxidative Stress and Lipid Peroxidation as Determinants of Antifungal Activity
One of the central antifungal mechanisms of EOs is the induction of oxidative stress through excessive generation of reactive oxygen species (ROS) [82]. When EO constituents penetrate fungal membranes, phenolic and terpene compounds disrupt mitochondrial cytochrome c oxidase activity, leading to electron leakage and one-electron reduction of oxygen to superoxide radicals (O2·−) [83].
These radicals are subsequently converted to hydrogen peroxide (H2O2) by SOD and further transformed into hydroxyl radicals (•OH) via Fenton reactions [84]. The accumulation of ROS overwhelms the fungal antioxidant defenses, resulting in oxidative damage to lipids, proteins, and nucleic acids [85].
Thymol and carvacrol are particularly effective in triggering ROS-mediated apoptosis-like pathways in Candida albicans and Aspergillus niger [86]. Similarly, cinnamaldehyde and eugenol raise intracellular ROS levels, causing lipid peroxidation, mitochondrial dysfunction, and membrane collapse [87].
Lipid peroxidation represents a critical downstream event following EO exposure, involving the oxidative degradation of polyunsaturated fatty acids within the fungal phospholipid bilayer. This reaction yields reactive lipid peroxides that propagate further oxidative damage [88], disrupt membrane permeability, and lead to cellular lysis [89].
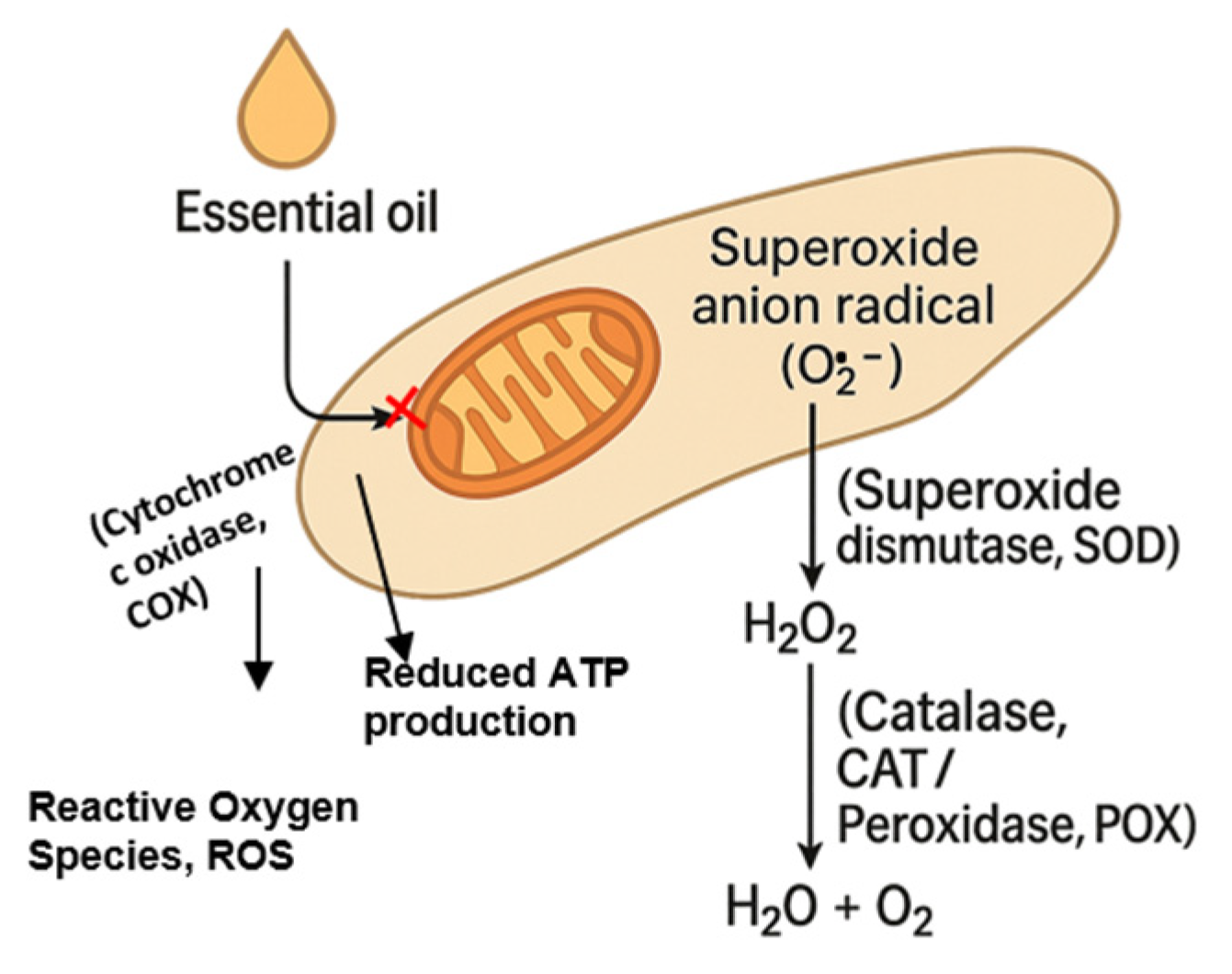
Figure 3 illustrates the oxidative-stress cascade triggered by EO exposure, highlighting its role in reducing ATP production and compromising fungal viability.
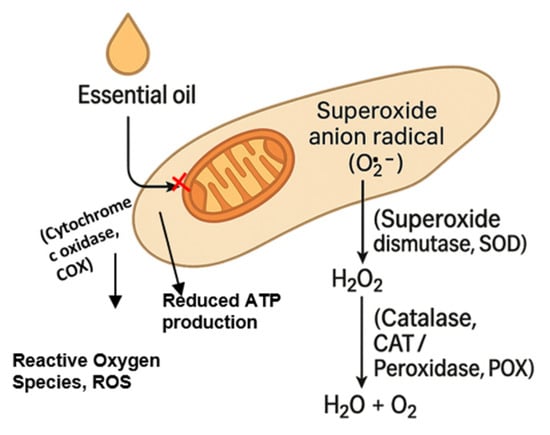
Figure 3.
Essential oil-induced oxidative stress cascade in a fungal hypha. Contact with a plant essential oil (EO) inhibits mitochondrial cytochrome c oxidase (Complex IV), promoting electron leakage and the one-electron reduction of molecular oxygen to the superoxide anion radical (O2·−). Superoxide dismutase (SOD) rapidly dismutates O2·− to hydrogen peroxide (H2O2), which is normally detoxified to water and molecular oxygen by catalase (CAT) and peroxidase (POX). Excessive ROS generation, together with the limited capacity of antioxidant enzymes, leads to the intracellular accumulation of O2·− and H2O2, initiating oxidative damage to lipids, proteins and nucleic acids and thereby compromising fungal viability.
4.5. Technological, Formulation, and Regulatory Challenges in the Development of EO-Based Biofungicides for Field Application
While the biochemical and physiological mechanisms of essential oils (EOs) as antifungal agents are increasingly well characterized, their translation from laboratory assays to consistent field performance remains constrained by several technological and regulatory barriers [90]. Unlike synthetic fungicides, EO-based products face intrinsic physicochemical limitations such as high volatility, poor water solubility, and susceptibility to oxidation that significantly reduce their persistence under agricultural conditions [91,92]. These limitations hinder dose standardization and long-term disease suppression, especially in open-field environments exposed to fluctuating temperature, humidity, and UV radiation [93].
From a formulation standpoint, ensuring the controlled release and stability of EO constituents is one of the most critical technological challenges. Conventional emulsions and wettable powders often fail to protect the active compounds from rapid degradation or evaporation [94]. Consequently, research has increasingly focused on encapsulation and nanotechnology-based systems, such as liposomes, nanoemulsions, polymeric nanoparticles, and cyclodextrin complexes that enhance bioavailability, reduce volatility, and extend field efficacy [95]. Despite promising results in controlled environments, scalability and cost-efficiency remain limiting factors for large-scale production [96].
Furthermore, the regulatory framework for botanical biofungicides presents another barrier. Registration procedures for EO-based products are frequently adapted from chemical pesticide frameworks, which do not always account for the complex, multicomponent nature of EOs. This misalignment delays approval processes and constrains innovation in developing novel formulations [97]. Harmonized international guidelines that balance efficacy, safety, and sustainability are urgently needed to facilitate market integration.
As summarized in Table 5, these challenges encompass volatility, environmental degradation, formulation stability, production costs, regulatory constraints, and limited field validation. Addressing them requires interdisciplinary collaboration that integrates formulation science, nanotechnology, agronomy, and environmental toxicology. Such an integrated approach will bridge the current gap between laboratory innovation and agricultural implementation, ultimately consolidating EO-based biofungicides as viable components of sustainable crop protection systems.

Table 5.
Key limitations hindering development and field application of essential oil-based biofungicides.
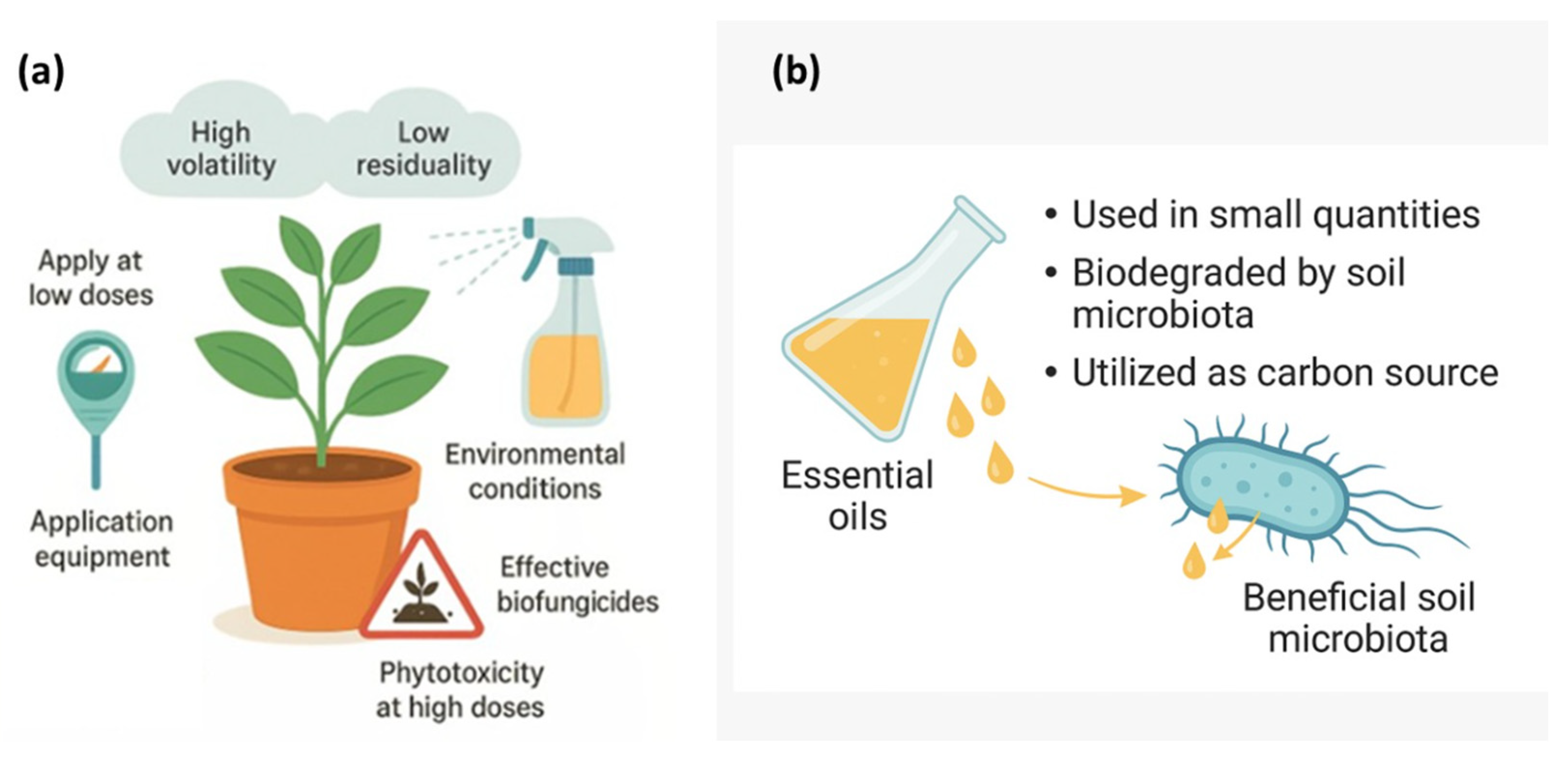
The practical implementation of essential oils (EOs) as biofungicides requires careful consideration of their physicochemical behavior and ecological interactions. When applied at appropriate doses, EOs can effectively suppress phytopathogenic fungi without causing phytotoxic effects or compromising plant vitality. Their high volatility and low residuality make them environmentally benign when used with optimized application equipment and under suitable environmental conditions, ensuring safety and efficacy in the field. Conversely, excessive concentrations or repeated applications may induce phytotoxicity, underscoring the importance of dosage calibration and controlled formulations.
Furthermore, evidence indicates that EOs pose minimal risk to beneficial soil microbiota, as they are typically applied in low quantities and are rapidly degraded by soil microorganisms, including Bacillus, Trichoderma, and other beneficial fungi. These organisms can metabolize EO constituents as carbon sources, contributing to their detoxification and integration into natural biogeochemical cycles. Consequently, EOs demonstrate strong potential as eco-compatible biofungicides, balancing antifungal efficacy with environmental safety (Figure 4).
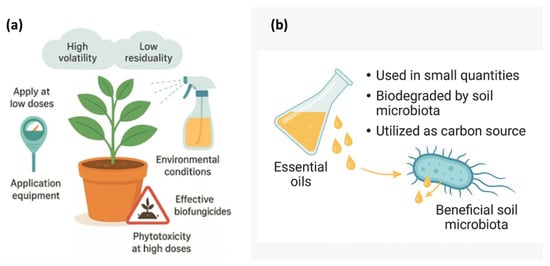
Figure 4.
Safe application and environmental compatibility of essential oils as biofungicides. (a) Appropriate use of essential oils on plants under field conditions considering volatility, residuality, dosage, equipment, and environmental factors. (b) Interaction between essential oils and beneficial soil microbiota showing their biodegradation and utilization as a carbon source.
4.6. Technological Frontiers in EO-Based Biofungicide Development: From Extraction to Field Application
It is true that promising laboratory results with EOs sometimes fail to translate to field-effective bioproducts [107]. The critical bridge between fundamental knowledge and practical application lies in addressing three interconnected technological domains: advanced extraction methodologies [108], stabilization through innovative formulation [109], and precision delivery systems [110].
The chemical complexity of EOs, while central to their multi-target action, presents a primary challenge for standardization [111]. Modern extraction has evolved beyond conventional steam distillation. Supercritical fluid extraction (SFE), particularly using CO2, has emerged as a superior technology [112]. SFE allows for precise modulation of temperature and pressure to selectively extract target bioactive compounds with higher yield and minimal thermal degradation, ensuring a more consistent and potent product [113].
Furthermore, the integration of metabolomic fingerprinting using GC-MS and LC-MS is becoming indispensable for quality control [114]. This is crucial for regulatory approval and for growers who require predictable performance [115]. For high-value compounds whose plant sources are rare or slow-growing, plant cell culture in bioreactors offers a revolutionary, climate-independent production platform, ensuring supply chain stability and freeing production from agricultural constraints [116].
The inherent volatility, photosensitivity, and hydrophobicity of EOs have long been an important limitation in field conditions [117]. The most significant progress in the last five years has come from nano- and micro-technology. EO droplets within a polymeric matrix is no longer a novel concept, but the sophistication of these systems has dramatically increased [118]. Current research focuses on stimuli-responsive nanocarriers. For instance, chitosan-based nanocapsules can be engineered to release their payload in response to the specific enzymatic activity (e.g., cutinases, cellulases) of the target fungus at the infection site [119]. This targeted delivery minimizes non-target losses and prolongs antifungal activity. Similarly, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) provide superior physical stability and protection against UV degradation compared to traditional emulsions [120].
To combat photodegradation, the simple addition of natural, food-grade antioxidants like rosmarinic acid and tocopherols directly into the oil phase before encapsulation has proven highly effective [121]. These compounds act by absorbing damaging radiation and quenching free radicals, thereby protecting the bioactive terpenes and phenols [122]. This approach significantly narrows the efficacy gap between laboratory and field conditions.
Electrostatic sprayers that impart a negative charge to droplets ensure that they wrap around and adhere to both sides of plant leaves, drastically improving coverage and reducing drift [123]. Coupled with ultra-low-volume (ULV) nozzles, which produce a uniform fine mist, these systems ensure optimal deposition with minimal product volume [124].
Furthermore, application timing must be intelligent. Integrating EO sprays into decision support systems (DSSs) that utilize disease forecasting models [125] allows for prophylactic application just before critical infection periods [126]. This strategic timing maximizes the impact of the EO’s protective action, disrupting the pathogen’s life cycle at its most vulnerable stage.
5. Conclusions
Plant essential oils (EOs) are a relevant ecologically alternative for sustainable crop protection. Their multifaceted antifungal mechanisms including membrane disruption, enzyme inhibition, and induction of oxidative stress provide a robust, nature-derived arsenal with a broad spectrum to control devastating phytopathogenic fungi. However, the central thesis emerging from this review is that the primary barrier to their widespread adoption is no longer a question of efficacy, but one of translational science. The volatility, hydrophobicity, and chemical complexity of essential oil also give rise to critical challenges in photostability, field persistence, and phytotoxicity. Consequently, the historical focus on bio-prospecting and in vitro screening has reached a point of diminishing returns. The path forward demands a paradigm shift from discovery to innovation in formulation and delivery. The future of EO-based biofungicides hinges on the strategic convergence of advanced material science for nanoencapsulation, bioprocess engineering for the scalable production of standardized metabolites in bioreactors, and precision agriculture for targeted application. Next, the thematics of research must be fundamentally interdisciplinary. It is only through the synergistic integration of these fields that EOs can transcend their current status as promising laboratory candidates and emerge as reliable, cornerstone tools in next-generation Integrated Pest Management (IPM) systems. This integrated approach is the indispensable key to unlocking their full potential, creating a route for their transition from a niche alternative to a mainstream solution for building more resilient and sustainable global agriculture.
Author Contributions
Conceptualization, M.L.-M.; writing—original draft preparation, M.L.-M.; D.B.; C.A.; K.H.; D.G.-F.; O.L.-H.; L.R.S.; P.S.P. and A.B.; review and editing, M.L.-M.; and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the support of the Dirección de Investigación y Desarrollo of the Universidad Técnica de Ambato (UTA), as well as the contributions of the researchers and individuals involved in this project. Special thanks to the authorities of the Faculty of Agricultural Sciences and DIDE-UTA. This work was funded by Michel Leiva-Mora under the research project EVALUACIÓN DE LA ACTIVIDAD ANTIFÚNGICA DE ACEITES ESENCIALES MICROENCAPSULADOS MEDIANTE MÉTODOS DE DILUCIÓN EN AGAR Y RESARZURINA. (Resolución Nro. UTA-CONIN-2025-0046-R).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the Dirección de Investigación y Desarrollo (DIDE) of the Universidad Técnica de Ambato for its administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EOs | Essential Oils |
| ROS | Reactive Oxygen Species |
| IPM | Integrated Pest Management |
| TEO | Thyme Essential Oil |
| EC50 | Effective Concentration 50 (concentration at which 50% of maximal effect is observed) |
| CHI | Chitinase |
| PPO | Polyphenol Oxidase |
| POX | Guaiacol Peroxidase |
| PAL | Phenylalanine Ammonia-Lyase |
| CcO | Cytochrome c Oxidase |
| NADH | Nicotinamide Adenine Dinucleotide (reduced form) |
| CAT | Catalase |
| SOD | Superoxide Dismutase |
| QS | Quorum Sensing |
| HMGR | 3-hydroxy-3-methylglutaryl-CoA Reductase |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-Coenzyme A |
| PMK | Phosphomevalonate Kinase |
| MVD | Mevalonate-5-pyrophosphate Decarboxylase |
| IPP | Isopentenyl Pyrophosphate |
| FPPS | Farnesyl Pyrophosphate Synthase |
| FPP | Farnesyl Pyrophosphate |
| LSS | Lanosterol Synthase |
| CYP51 | C-14 Sterol Demethylase (Cytochrome P450 51) |
| MIC | Minimum Inhibitory Concentration |
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance mechanisms of plant pathogenic fungi to fungicide, environmental impacts of fungicides, and sustainable solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Mešić, A.; Jurić, M.; Donsì, F.; Maslov Bandić, L.; Jurić, S. Advancing climate resilience: Technological innovations in plant-based, alternative and sustainable food production systems. Discov. Sustain. 2024, 5, 423. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides Based on Plant Extracts: On the Road to Organic Farming. Int. J. Mol. Sci. 2024, 25, 6879. [Google Scholar] [CrossRef]
- Siddiqui, T.; Sharma, V.; Khan, M.U.; Gupta, K. Terpenoids in Essential Oils: Chemistry, classification, and potential impact on human health and industry. Phytomedicine Plus. 2024, 4, 100549. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.; Beresford, R.; Berlin, A.; Bock, C.; Fox, A.; Gold, K.M.; Newton, A.C.; Vicent, A.; Xu, X. Impact of novel methods and research approaches in plant pathology: Are individual advances sufficient to meet the wider challenges of disease management? Plant Pathol. 2024, 73, 1629–1655. [Google Scholar] [CrossRef]
- Sakthiyavathi, S.S.K.; Malaisamy, K.; Kamalakannan, M.; Murugesan, V.; Palanisamy, S.R.; Theerthagiri, A.; Kasivelu, G. Eco-nanotechnology: Phyto essential oil-based pest control for stored products. Environ. Sci. Nano 2025, 12, 4150–4180. [Google Scholar] [CrossRef]
- Zahraoui, E.M. Essential Oils: Antifungal activity and study methods. Moroc. J. Agric. Sci. 2025, 6, 99–108. [Google Scholar] [CrossRef]
- Ismail, A.; Elshewy, E.; Ali, I.; Abd, N.; Khafagi, E. Encapsulation of Clove Oil Nanoemlusion in Chitosan-Based Nano-Composite. Phyton 2024, 93, 2787. [Google Scholar] [CrossRef]
- Fonseca-Guerra, I.R.; Posada, A.M.V.; Rozo, M.E.B.; Pineda, M.E.B. Essential oils of thyme (Thymus vulgaris) and oregano (Origanum vulgare) as an alternative for the control of pesticide-resistant Fusarium spp. in quinoa seeds. J. Sci. Food Agric. 2024, 105, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Deepa, N.; Chauhan, S.; Tandon, S.; Verma, R.S.; Singh, A. Antifungal action of 1, 8 cineole, a major component of Eucalyptus globulus essential oil against Alternaria tenuissima via overproduction of reactive oxygen species and downregulation of virulence and ergosterol biosynthetic genes. Ind. Crops Prod. 2024, 214, 118580. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Sulaiman, G.M.; Al-Saffar, A.Z.; Mohsin, M.H.; Khan, R.A.; Hadi, N.A.; Ismael, S.B.; Elshibani, F.; Ismail, A.; Abomughaid, M.M. Aromatic Volatile Compounds of Essential Oils: Distribution, Chemical Perspective, Biological Activity, and Clinical Applications. Food Sci. Nutr. 2025, 13, e70825. [Google Scholar] [CrossRef]
- Ben Miri, Y. Essential Oils: Chemical Composition and Diverse Biological Activities: A Comprehensive Review. Nat. Prod. Commun. 2025, 20, 1934578X241311790. [Google Scholar] [CrossRef]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Mekky, R.H.; Ibrahim, A.E.; Abouelela, M.E.; Kedra, T.A.; Al-Harrasi, A. Essential Oils: The Science of Extraction and Its Implications for Composition and Biological Activity—A Review. Food Anal. Methods 2025, 18, 1483–1513. [Google Scholar] [CrossRef]
- Salem, M.Z.; Abo-Elgat, W.A.; Farahat, M.G.; El-Settawy, A.A.; Selim, S. The Essential Oil and Recoverable Extract from Callistemon viminalis Leaves as Wood-and Textile Biofungicides with the GC-MS and HPLC Analyses. Chem. Afr. 2025, 8, 1–13. [Google Scholar] [CrossRef]
- Dehghanpour, S.; Abdollahi, F.; Mirzaalian Dastjerdi, A.; Yavari, A. Antifungal activity of plant essential oils against gray mold (Botrytis cinerea) under In Vitro conditions. Curr. Res. Environ. Appl. Mycol. (J. Fungal Biol.) 2025, 15, 77–97. [Google Scholar] [CrossRef]
- Siddiqui, A.; Ahmad, S.; Afaq, U. Innovations in Biopesticides: The Role of Plant-Based Biopesticides in Sustainable Agriculture. J. Biopestic. 2025, 18, 18–35. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant essential oils as biopesticides: Applications, mechanisms, innovations, and constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 6477–6497. [Google Scholar] [CrossRef]
- Wang, D.; Chu, F.; Wei, H.; Zhang, J.; Zhai, H. Recent Advances in the Synergistic Application of Essential Oils and Emerging Technologies for Fresh Food Preservation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70244. [Google Scholar] [CrossRef]
- Rama, J.F.; Coelho, A.C.; Leal, F.M. Testing the potential antifungal activity of Origanum vulgare against Aspergillus fumigatus, Aspergillus niger and Talaromyces marneffei isolated from pets. Vet. Stanica 2024, 55, 559–567. [Google Scholar] [CrossRef]
- Diass, K.; Oualdi, I.; Benabbas, R.; Zaki, H.; Ouabane, M.; Hammouti, B.; Touzani, R.; Bouachrine, M. Use of Essential Oils for the Treatment of Fusarium oxysporum f. sp. Albedinis: Chemical Profile, In Vitro Antifungal Activity, and In Silico Investigation by Molecular Docking Study. Curr. Chem. Biol. 2024, 18, 193–214. [Google Scholar] [CrossRef]
- Shala, A.; Singh, S.; Hameed, S.; Khurana, S.P. Essential oils as alternative promising anti-candidal agents: Progress and prospects. Curr. Pharm. Des. 2022, 28, 58–70. [Google Scholar] [CrossRef]
- Li, K.; Guo, J.; Liang, R.; Wang, X.; Chen, Q.; Wang, J.; Ge, X.; Chen, C.; Sun, J.; Zhao, C. A Review on Antifungal Activity of Plant Essential Oils. Phytother. Res. 2025, 39, 3736–3761. [Google Scholar] [CrossRef]
- Ansari, T.; Prasanna, N.L.; Nigam, R.; Shankar, U.; Mohapatra, D.; Sahoo, L.; Prasad, S.S. A Review on the Potential of Ginger Essential Oil-based Nanotechnology for Controlling Tropical Plant Diseases. J. Adv. Biol. Biotechnol. 2025, 28, 230–248. [Google Scholar] [CrossRef]
- Bunevičius, V.; Morkeliūnė, A.; Griauzdaitė, J.; Valiuškaitė, A.; Rasiukevičiūtė, N. The Use of Clove and Rosemary Plant Extracts Against Colletotrichum acutatum and Botrytis cinerea. Agronomy 2025, 15, 1728. [Google Scholar] [CrossRef]
- Christova, P.K.; Dobreva, A.M.; Dzhurmanski, A.G.; Dincheva, I.N.; Dimkova, S.D.; Zapryanova, N.G. The impact of plant essential oils on the growth of the pathogens Botrytis cinerea, Fusarium solani, and Phytophthora pseudocryptogea. Life. 2024, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N. Origanum dictamnus essential oil in vapour or aqueous solution application for pepper fruit preservation against botrytis cinerea. Agronomy 2024, 14, 257. [Google Scholar] [CrossRef]
- Faghih-Imani, M.H.; Taheri, P.; Tarighi, S. Antifungal and virulence-modulating effects of thyme essential oil against Fusarium spp., causing wheat diseases. Appl. Microbiol. Theory Technol. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Saltos-Rezabala, L.A.; Silveira, P.R.D.; Tavares, D.G.; Moreira, S.I.; Magalhães, T.A.; Botelho, D.M.D.S.; Alves, E. Thyme essential oil reduces disease severity and induces resistance against Alternaria linariae in tomato plants. Horticulturae 2022, 8, 919. [Google Scholar] [CrossRef]
- Wu, T.L.; Zhang, B.Q.; Luo, X.F.; Li, A.P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; Liu, Y.Q. Antifungal efficacy of sixty essential oils and mechanism of oregano essential oil against Rhizoctonia solani. Ind. Crops Prod. 2023, 191, 115975. [Google Scholar] [CrossRef]
- Hoyos, J.M.A.; Dorigan, A.F.; da Silveira, P.R.; Labory, C.R.G.; Júnior, P.M.R.; Fernandes, R.; Alves, E. Antifungal activity of essential oils in Colletotrichum lindemuthianum and alternative control of bean anthracnose. Eur. J. Plant Pathol. 2025, 172, 373–389. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Guan, Y.; Saren, G. Effects of plant essential oil treatment on the growth of pathogenic fungi and the activity of defense-related enzymes of fungi-inoculated blueberry. Horticulturae 2024, 10, 318. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; Wang, L.; Yang, G.; Zhou, L.; Zhang, Z.; Li, X. In vitro antifungal activity and mechanism of action of carvacrol against Sclerotinia sclerotiorum (Lib.) de Bary. Plant Prot. Sci. 2024, 60, 172–180. [Google Scholar] [CrossRef]
- Kiniec, A.; Spychalski, M.; Miziniak, W.; Palacz, M.; Kukawka, R. The Use of Thyme (Thymus vulgaris) Essential Oil for Controlling Cercospora Leaf Spot (Cercospora beticola) on Sugar Beets (Beta vulgaris). Agriculture 2024, 14, 2017. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove essential oil: Chemical profile, biological activities, encapsulation strategies, and food applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef]
- Alhantoobi, W.A.; Alkatheri, A.H.; Parusheva, T.; Lai, K.S.; Thomas, W.; Lim, S.H.E. Antimicrobial Activity of Essential Oils and Their Mechanism of Action Against Bacterial and Fungal Infections. Saudi J. Biomed. Res. 2025, 10, 168–186. [Google Scholar] [CrossRef]
- Połeć, K.; Olechowska, K.; Klejdysz, A.; Dymek, M.; Rachwalik, R.; Sikora, E.; Hąc-Wydro, K. The influence of ergosterol on the action of the hop oil and its major terpenes on model fungi membranes. Towards understanding the mechanism of action of phytocompounds for food and plant protection. Chem. Phys. Lipids 2021, 238, 105092. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Mishra, S.; Barua, S.; Tyagi, V.; Kumar, A.; Kumar, M.; Kamle, M. Use of essential oils and phytochemicals against the mycotoxins producing fungi for shelf-life enhancement and food preservation. Int. J. Food Sci. Technol. 2022, 57, 2171–2184. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Diogo Gonçalves, S.; Paiva-Cardoso, M.D.N.; Caramelo, A. Green Preservation Strategies: The Role of Essential Oils in Sustainable Food Preservatives. Sustainability 2025, 17, 7326. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal activities and mode of action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum essential oil vapors against Botrytis cinerea and their potential application to control postharvest strawberry gray mold. Foods 2021, 10, 2451. [Google Scholar] [CrossRef]
- Ghuffar, S.; Irshad, G.; Naz, F.; Khan, M.A.; Ahmed, M.Z.; Gleason, M.L. Efficacy of plant essential oils against Alternaria alternata causing bunch rot of grapes in Pakistan. J. Anim. Plant Sci. 2022, 32, 150–162. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Mohafrash, S.M.; Ziedan, E.S.H.; Abdelsalam, I.S.; Sahab, A.F. Development of eco-friendly nanoemulsions of some natural oils and evaluating of its efficiency against postharvest fruit rot fungi of cucumber. Ind. Crops Prod. 2021, 159, 113049. [Google Scholar] [CrossRef]
- Danielewicz, J.; Grzanka, M.; Sobiech, Ł.; Jajor, E.; Horoszkiewicz, J.; Korbas, M.; Blecharczyk, A.; Stuper-Szablewska, K.; Matysiak, K. Impact of various essential oils on the development of pathogens of the Fusarium genus and on health and germination parameters of winter wheat and maize. Molecules 2024, 29, 2376. [Google Scholar] [CrossRef] [PubMed]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal activity of plant-derived essential oils on pathogens of pulse crops. Plant Disease 2021, 105, 1692–1701. [Google Scholar] [CrossRef]
- Danielewicz, J.; Horoszkiewicz, J.; Jajor, E.; Korbas, M.; Zamojska, J.; Dworzańska, D.; Węgorek, P.; Grzanka, M.; Sobiech, Ł.; Idziak, R.; et al. The Use of Selected Essential Oils as an Alternative Method of Controlling Pathogenic Fungi, Weeds and Insects on Oilseed Rape (Brassica napus L.). Agriculture 2025, 15, 2214. [Google Scholar] [CrossRef]
- Prakasam, V.; Savani, A.K.; Sukesh, P. Unveiling the potential antifungal role of essential oils in the management of Rhizoctonia solani causing sheath blight of rice. Eur. J. Plant Pathol. 2025, 171, 245–256. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, M.; Karami, A.; Cacciola, S.O. Defense mechanisms induced by celery seed essential oil against powdery mildew incited by Podosphaera fusca in cucumber. J. Fungi 2023, 10, 17. [Google Scholar] [CrossRef]
- Ahmed, H.F.; Seleiman, M.F.; Mohamed, I.A.; Taha, R.S.; Wasonga, D.O.; Battaglia, M.L. Activity of essential oils and plant extracts as biofungicides for suppression of soil-borne fungi associated with root rot and wilt of marigold (Calendula officinalis L.). Horticulturae 2023, 9, 222. [Google Scholar] [CrossRef]
- Henri, N.G.P.; Brahima, C.A.M.A.R.A.; Alban, M.B.K.A.; Didier, K.K.; Edouard, Y.K.J.; Martial, K.K.J.F.; Franck, Z.B.; Martial, K.N.G.; Daouda, K. Evaluation of the Sensitivity of Strains of phytophthora spp Cause of Brown Rot of Cocoa (Theobroma cacao L.) against to Limocide 60 ME (An Extract of Sweet Orange Essential Oil). Biotechnol. J. Int. 2025, 29, 69–83. [Google Scholar] [CrossRef]
- Wang, L.; Jin, J.; Liu, X.; Wang, Y.; Liu, Y.; Zhao, Y.; Xing, F. Effect of cinnamaldehyde on morphological alterations of Aspergillus ochraceus and expression of key genes involved in ochratoxin A biosynthesis. Toxins 2018, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Harmouzi, A.; Chlouchi, A.; Afroukh, R.; El Hamzaoui, N.; Kamari, F.; Chrachmy, M.; Ouallal, H.; Chebaibi, M.; Mssillou, I.; El Ammari, Y. Chemical Profiling of Two Eucalyptus Essential Oils: Insecticidal and in Silico Inhibitory Activities on Acetylcholinesterase and Chitin Synthase. In Plant Pathology, Fungal Diversity, and Biotechnological Advances in Agriculture; Springer Nature: Cham, Switzerland, 2025; pp. 9–20. [Google Scholar] [CrossRef]
- Kowalczyk, A. Essential oils against Candida auris—A promising approach for antifungal activity. Antibiotics 2024, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Wang, Y.; Yang, Q.; Wang, B.; Zhang, Y.; Tong, Z.; Zhang, J. Antifungal mechanisms of Phoebe bournei wood essential oil against Botryosphaeria dothidea and its application in apples. Postharvest Biol. Technol. 2025, 229, 113703. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wen, X.; Chen, H.; Du, H.; Liu, D. Chemical composition of Artemisia argyi essential oil and its antifungal activity against dermatophytes by inhibiting oxidative phosphorylation and causing oxidative damage. J. Ethnopharmacol. 2024, 331, 118344. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, X.; Chen, X.; Xian, Y. Health benefits of plant-derived essential oils in mitigating oxidative stress: A comprehensive review. Cogent Food Agric. 2025, 11, 2564258. [Google Scholar] [CrossRef]
- Raj, N.; Parveen; Khatoon, S.; Manzoor, N. Antifungal Efficacy of Terpenes and Mechanism of Action Against Human Pathogenic Fungi. In Advances in Antifungal Drug Development: Natural Products with Antifungal Potential; Springer Nature: Singapore, 2024; pp. 315–341. [Google Scholar] [CrossRef]
- Madrigal-Aguilar, D.A.; Gonzalez-Silva, A.; Rosales-Acosta, B.; Bautista-Crescencio, C.; Ortiz-Álvarez, J.; Escalante, C.H.; Sánchez-Navarrete, J.; Hernández-Rodríguez, C.; Chamorro-Cevallos, G.; Tamariz, J.; et al. Antifungal activity of fibrate-based compounds and substituted pyrroles that inhibit the enzyme 3-hydroxy-methyl-glutaryl-CoA reductase of Candida glabrata (CgHMGR), thus decreasing yeast viability and ergosterol synthesis. Microbiol. Spectr. 2022, 10, e01642-21. [Google Scholar] [CrossRef]
- Ganotra, J.; Supolia, D.; Sharma, A.; Raina, M.; Negi, N.P.; Gautam, V.; Kumar, D. Pathways of Important Metabolites and Enzymes Involved. In Metabolites of Medicinal Plants: Insightful Approaches; Bentham Science Publishers: Singapore, 2024; pp. 289–311. [Google Scholar] [CrossRef]
- Cardoza, R.E.; McCormick, S.P.; Lindo, L.; Mayo-Prieto, S.; González-Cazón, D.; Martínez-Reyes, N.; Carro-Huerga, G.; Rodríguez-González, Á.; Proctor, R.H.; Casquero, P.A.; et al. Effect of farnesol in Trichoderma physiology and in fungal–plant interaction. J. Fungi 2022, 8, 1266. [Google Scholar] [CrossRef]
- Fu, R.; Zhao, L.; Wang, J.; Chen, C.; Liu, Y.; Lu, D. Antifungal activity and inhibition of the molecular mechanism of lavender essential oil against Ustilaginoidea virens. LWT. 2024, 203, 116315. [Google Scholar] [CrossRef]
- Kumari, K.U.; Imam, M.W.; Luqman, S. Antifungal Efficacy of Plant Essential Oils Against Candida, Aspergillus and Cryptococcus Species. In Advances in Antifungal Drug Development: Natural Products with Antifungal Potential; Springer Nature: Singapore, 2024; pp. 159–191. [Google Scholar] [CrossRef]
- Kumari, D.; Kaur, H.; Palmo, T.; Nargotra, A.; Singh, K. Exploring natural product library as potential target against sterol C-24 methyltransferase protein of Leishmania donovani. Nat. Prod. Res. 2024, 381, 1–7. [Google Scholar] [CrossRef]
- Aoujil, F.; Loubna, D.R.A.; El Ghdaich, C.; Toufiq, S.; Yahyaoui, H.; Hafidi, M.; Aziz, A.Z.I.Z.; Habbadi, K. Antifungal efficacy of four plant-derived essential oils against Botrytis cinerea: Chemical profiles and biological activities. Phytopathol. Mediterr. 2025, 64, 109–127. [Google Scholar] [CrossRef]
- van Dyk, M.; Kellerman, H.; van Niekerk, J.M. The potential use of antifungal essential oil volatiles to manage citrus decay during degreening. Phytoparasitica 2025, 53, 22. [Google Scholar] [CrossRef]
- Mirmajlessi, M.; Najdabbasi, N.; Sigillo, L.; Haesaert, G. An implementation framework for evaluating the biocidal potential of essential oils in controlling Fusarium wilt in spinach: From in vitro to in planta. Front. Plant Sci. 2024, 15, 1444195. [Google Scholar] [CrossRef] [PubMed]
- Carmello, C.R.; Magri, M.M.R.; Cardoso, J.C. Cinnamon and clove aqueous extracts promote in vitro and postharvest control of Alternaria alternata in tomato fruit. Eur. J. Plant Pathol. 2025, 172, 261–274. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, X.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Effect of treatment of Fusarium head blight infected barley grains with hop essential oil nanoemulsion on the quality and safety of malted barley. Food Chem. 2023, 421, 136172. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, R.; Shang, Z.; Wang, K.; Zhang, C.; Wang, Z.; Lou, Y.; Liu, J.; Li, P. Peppermint essential oil for controlling Aspergillus flavus and analysis of its antifungal action mode. Curr. Microbiol. 2025, 82, 140. [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Merah, O.; Lahlali, R. In vitro and in vivo antifungal activities of nine commercial essential oils against brown rot in apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, 10–1128. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Shahina, Z.; Dahms, T.E. A comparative review of eugenol and citral anticandidal mechanisms: Partners in crimes against fungi. Molecules 2024, 29, 5536. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Qi, X.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Mechanisms of antifungal and mycotoxin inhibitory properties of Thymus vulgaris L. essential oil and their major chemical constituents in emulsion-based delivery system. Ind. Crops Prod. 2023, 197, 116575. [Google Scholar] [CrossRef]
- Schorr, R.R.; Ballesteros Garcia, M.J.; Petermann, D.; Moreira, R.R.; Sales Maia, B.H.; Marques, F.A.; May-De Mio, L.L. Eugenol, Isoeugenol, Thymol, Carvacrol, and Ester Derivatives as an Ecofriendly Option to Control Glomerella Leaf Spot and Bitter Rot on Apple. Plants 2024, 13, 3196. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.A.; Bicas, J.L. Antifungal activity of essential oils of tea tree, oregano, thyme, and cinnamon, and their components. Braz. J. Food Technol. 2024, 27, e2023071. [Google Scholar] [CrossRef]
- Santiago-Santiago, M.; Sánchez-Viveros, G.; Hernández-Adame, L.; Chiquito-Contreras, C.J.; Salinas-Castro, A.; Chiquito-Contreras, R.G.; Hernández-Montiel, L.G. Essential oils and antagonistic microorganisms as eco-friendly alternatives for coffee leaf rust control. Plants 2023, 12, 3519. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.; Tzortzakis, N. Application of rosemary and eucalyptus essential oils and their main component on the preservation of apple and pear fruits. Horticulturae 2021, 7, 479. [Google Scholar] [CrossRef]
- Kumar, P.S.; Nattudurai, G.; Islam, V.I.H.; Ignacimuthu, S. The effects of some essential oils on Alternaria alternata, a post-harvest phyto-pathogenic fungus in wheat by disrupting ergosterol biosynthesis. Phytoparasitica 2022, 50, 513–525. [Google Scholar] [CrossRef]
- Bakour, M.; Soulo, N.; Hammas, N.; Fatemi, H.E.; Aboulghazi, A.; Taroq, A.; Lyoussi, B. The antioxidant content and protective effect of argan oil and Syzygium aromaticum essential oil in hydrogen peroxide-induced biochemical and histological changes. Int. J. Mol. Sci. 2018, 19, 610. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: In vivo ROS detection and alterations in antioxidant enzymes. Biochem. Syst. Ecol. 2012, 44, 390–399. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Molina Hernandez, J.B.; Grande-Tovar, C.D.; Serio, A.; Valbonetti, L.; Chaves-López, C. Antifungal mechanism of Ruta graveolens essential oil: A Colombian traditional alternative against anthracnose caused by Colletotrichum gloeosporioides. Molecules 2024, 29, 3516. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E. Candida albicans reactive oxygen species (ROS)-dependent lethality and ROS-independent hyphal and biofilm inhibition by eugenol and citral. Microbiol. Spectr. 2022, 10, e03183-22. [Google Scholar] [CrossRef]
- Jemai, N.; Ben Mahmoud, K.; Jedidi, E.; Gargouri, S.; Hachana, A.; Jemmali, A.; Boulila, A. Chemical Composition and Antifungal Activity of Essential Oils from Lavandula dentata L.; Cymbopogon citratus DC. Stapf., and Salvia officinalis L. against Rhizoctonia solani in Potato Tubers. Potato Res. 2025, 68, 3179–3196. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Nayak, S.; Hegde, R.B.; Rao, A.S.; Poojary, R. Unlocking the potential of essential oils in aromatic plants: A guide to recovery, modern innovations, regulation and AI integration. Planta 2025, 262, 6. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Durofil, A.; Ríos-Núñez, S.; Tardugno, R.; Manfredini, S.; Abreu Naranjo, R.; Scalvenzi, L. Unlocking the Anti-Phytopathogenic Potential of Essential Oils: A Systematic Review of Plant-Based Botrytis cinerea Management for Regenerative Viticulture and Horticultural Products. ACS Agric. Sci. Technol. 2025, 5, 930–948. [Google Scholar] [CrossRef]
- Lippi, P.; Eichmeier, A.; Puccioni, S.; Mattii, G.B.; Cataldo, E. Essential Oils Usage on Vitis vinifera L.; from the Vineyard to Post-Harvest: Advantages, Limitations, and Future Perspectives. Phyton-Int. J. Exp. Bot. 2025, 94, 1047–1072. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Ivanitskikh, A.S.; Izmailov, A.Y.; Dorokhov, A.S.; Proshkin, Y.A.; Yanykin, D.V.; Sarimov, R.R.; Gudkov, S.V.; Chilingaryan, N.O. Impact of ultraviolet radiation on the pigment content and essential oil accumulation in sweet basil (Ocimum basilicum L.). Appl. Sci. 2022, 12, 7190. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in essential oils encapsulation: Development, characterization and release mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Kaspute, G.; Ivaskiene, T.; Mobasheri, A.; Viter, R.; Ramanavicius, A.; Prentice, U. Enhancing essential oils: Advanced extraction, sustainability, and nanotechnology for optimal use. Planta 2025, 262, 71. [Google Scholar] [CrossRef] [PubMed]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Abdul Wahab, M.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, M.I. Trends in nanotechnology and its potentialities to control plant pathogenic fungi: A review. Biology 2021, 10, 881. [Google Scholar] [CrossRef]
- Ivanova, S.; Ivanov, K.; Gvozdeva, Y.; Staynova, R.; Grekova-Kafalova, D.; Hristozova, M.; Koleva, N. Essential oils—A review of the regulatory framework in the European Union. Pharmacia 2025, 72, 1–12. [Google Scholar] [CrossRef]
- Sheikh, A.R.; Wu-Chen, R.A.; Matloob, A.; Mahmood, M.H.; Javed, M. Nanoencapsulation of volatile plant essential oils: A paradigm shift in food industry practices. Food Innov. Adv. 2024, 3, 305–319. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Moreira, J.V.; Akamine, I.T.; Cardoso, V.S.; Mansoldo, F.R. Agricultural pest management: The role of microorganisms in biopesticides and soil bioremediation. Plants 2024, 13, 2762. [Google Scholar] [CrossRef]
- Shen, X.; He, L.; Cui, Y.; Lin, Z.; Jafari, S.M.; Tan, C. Co-encapsulation of bioactive compounds in liposomal delivery systems for synergistic effects. Food Biosci. 2025, 68, 106306. [Google Scholar] [CrossRef]
- Omidian, H.; Cubeddu, L.X.; Gill, E.J. Harnessing nanotechnology to enhance essential oil applications. Molecules 2025, 30, 520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, Z.; Liu, Y.; Zhou, Z. Eugenol Nanoemulsion Loaded with Polymethoxyflavones Control Blue Mold in Apple and Citrus Fruits by Damaging the Cell Membrane of Penicillium Expansum. Available online: https://ssrn.com/abstract=5226717 (accessed on 28 October 2025). [CrossRef]
- Ullah, Z.; Iqbal, J.; Abbasi, B.A.; Ijaz, S.; Ahmad, S.; Khan, S.; Sampath, S.; Iqbal, R.; Murtaza, G.; Mehmood, Y.; et al. Nano-formulation for Agriculture Applicability. In Revolutionizing Agriculture: A Comprehensive Exploration of Agri-Nanotechnology; Springer Nature: Cham, Switzerland, 2024; pp. 325–367. [Google Scholar] [CrossRef]
- Herkins, M.; Zhao, L.; Zhu, H.; Jeon, H.; Castilho-Theodoro, J. Optimization and evaluation of electrostatic spraying systems and their effects on pesticide deposition and coverage inside dense canopy plants. Agronomy 2025, 15, 1401. [Google Scholar] [CrossRef]
- Dhotre, I. A comprehensive review on progression and innovations in microwave assisted extraction technology for essential oils. J. Chem. Technol. Biotechnol. 2025, 100, 894–907. [Google Scholar] [CrossRef]
- Singh, H. Bioengineering for Production of Biologically Active Compounds in Plants. In Biosynthesis of Natural Products in Plants: Bioengineering in Post-genomics Era; Springer Nature: Singapore, 2024; pp. 1–37. [Google Scholar] [CrossRef]
- Gonzaga, B.C.F.; Barrozo, M.M.; Coutinho, A.L.; Pereira e Sousa, L.J.M.; Vale, F.L.; Marreto, L.; Marchesini, P.; de Castro Rodrigues, D.; De Souza, E.D.F.; Sabatini, G.A.; et al. Essential oils and isolated compounds for tick control: Advances beyond the laboratory. Parasites Vectors. 2023, 16, 415–439. [Google Scholar] [CrossRef]
- Sultana, R.; Mohanto, S.; Bhunia, A.; Biswas, A.; Akhtar, M.S.; Mishra, V.; Faiyazuddin, M. Current progress and emerging role of essential oils in drug delivery therapeutics. Curr. Drug Deliv. 2025, 22, 332–357. [Google Scholar] [CrossRef]
- Sarmah, K.; Anbalagan, T.; Marimuthu, M.; Mariappan, P.; Angappan, S.; Vaithiyanathan, S. Innovative formulation strategies for botanical-and essential oil-based insecticides. J. Pest Sci. 2025, 98, 1–30. [Google Scholar] [CrossRef]
- Sivalingam, S.; Golla, G.; Arunachalam, L.; Singh, T.; Malaichamy, K. Encapsulation of essential oil to prepare environment friendly nanobio-fungicide against Fusarium oxysporum f. sp. lycopersici: An experimental and molecular dynamics approach. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132681. [Google Scholar] [CrossRef]
- Etri, K.; Pluhár, Z. Exploring chemical variability in the essential oils of the thymus genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef]
- Ghedira, W.; Souissi, M.; Boudokhane, C.; Dhaouadi, H. Eco-Friendly Extraction and Antioxidant Profiling of Rosmarinus officinalis L.: Advances in Supercritical Fluid Extraction, DFT, and ADMET Analyses. Results Eng. 2025, 28, 107422. [Google Scholar] [CrossRef]
- Eyube, M.; Ohwoevwo, A.; Okechukwu, F.; Babayanju, S.; Osadolor, E.; Boco, F.; Alimikhena, M.; Agbonghae, E. Sustainable Optimization of Extraction Techniques for Bioactive Compounds in Drug Discovery: Principles, Innovations, and Case Studies. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Khalafalla, M.M. Plant cell suspension culture for plant secondary metabolite production: Current status, constraints, and future solutions. Pol. J. Environ. Stud. 2025, 10, 1–14. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.V.; Singh, P.K.; Krishnan, N.; Devadasan, V.; Gopinath, S.C.; Raman, P. Characterization of phytoconstituents of vital herbal oils by GC–MS and LC–MS/MS and their bioactivities. J. Food Sci. Technol. 2024, 1–13. [Google Scholar] [CrossRef]
- Abd El-Kareem, M.S.; Rabbih, M.A.; Rashad, A.M.; EL-Hefny, M. Essential oils from fennel plants as valuable chemical products: Gas chromatography–mass spectrometry, FTIR, quantum mechanical investigation, and antifungal activity. Biomass Convers. Biorefinery 2025, 15, 9173–9191. [Google Scholar] [CrossRef]
- Abbas, A.; Lai, D.Y.; Peng, P.; She, D. Lignin-Based Functional Materials in Agricultural Application: A Review. J. Agric. Food Chem. 2025, 73, 5685–5710. [Google Scholar] [CrossRef]
- Nahas, E.O.; Furtado, G.F.; Lopes, M.S.; Silva, E.K. From Emulsions to Films: The Role of Polysaccharide Matrices in Essential Oil Retention Within Active Packaging Films. Foods 2025, 14, 1501. [Google Scholar] [CrossRef]
- Zhou, Q.; Xia, Z.; Zhang, Y.; Sun, Z.; Zeng, W.; Zhang, N.; Yuan, C.; Gong, C.; Zhou, Y.; Xue, W. Design of a delivery vehicle chitosan-based self-assembling: Controlled release, high hydrophobicity, and safe treatment of plant fungal diseases. J. Nanobiotechnol. 2024, 22, 121. [Google Scholar] [CrossRef]
- Jayaraj, G.; Balasubramaniam, M.; Raju, K. Nanoencapsulation of Agricultural Inputs. In Nanotechnology Applications in Modern Agriculture; Springer Nature: Cham, Switzerland, 2025; pp. 51–79. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; McClements, D.J.; Jin, Z.; Ji, H.; Qiu, C. Recent progress in the source, extraction, activity mechanism and encapsulation of bioactive essential oils. Crit. Rev. Food Sci. Nutr. 2024, 65, 6352–6370. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, D.; Warchoł, M.; Skrzypek, E. Rosmarinic Acid as Bioactive Compound: Molecular and Physiological Aspects of Biosynthesis with Future Perspectives. Cells. 2025, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Appah, S. Assessment of agrochemical spray droplet effect on substrates by electrostatic-conventional spraying. Agric. Sci. Technol. 2025, 17, 59–69. [Google Scholar] [CrossRef]
- Sun, H.; Liu, C.; Li, Y.; Shi, H.; Zhao, S.; Wu, M.; Hu, J. Design of a Contact-Type Electrostatic Spray Boom System Based on Rod-Plate Electrode Structure and Field Experiments on Droplet Deposition Distribution. Agronomy 2024, 14, 2715. [Google Scholar] [CrossRef]
- Ye, K.; Hu, G.; Tong, Z.; Xu, Y.; Zheng, J. Key intelligent pesticide prescription spraying technologies for the control of pests, diseases, and weeds: A review. Agriculture 2025, 15, 81. [Google Scholar] [CrossRef]
- Martini, F.; Jijakli, M.H.; Gontier, E.; Muchembled, J.; Fauconnier, M.L. Harnessing plant’s arsenal: Essential oils as promising tools for sustainable management of potato late blight disease caused by Phytophthora infestans—A comprehensive review. Molecules 2023, 28, 7302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).