Biotechnological and Oenological Potential of Advanced Genetic Lines of Grapevine Resistant to Powdery Mildew (Erysiphe necator)
Abstract
1. Introduction
2. Materials and Methods
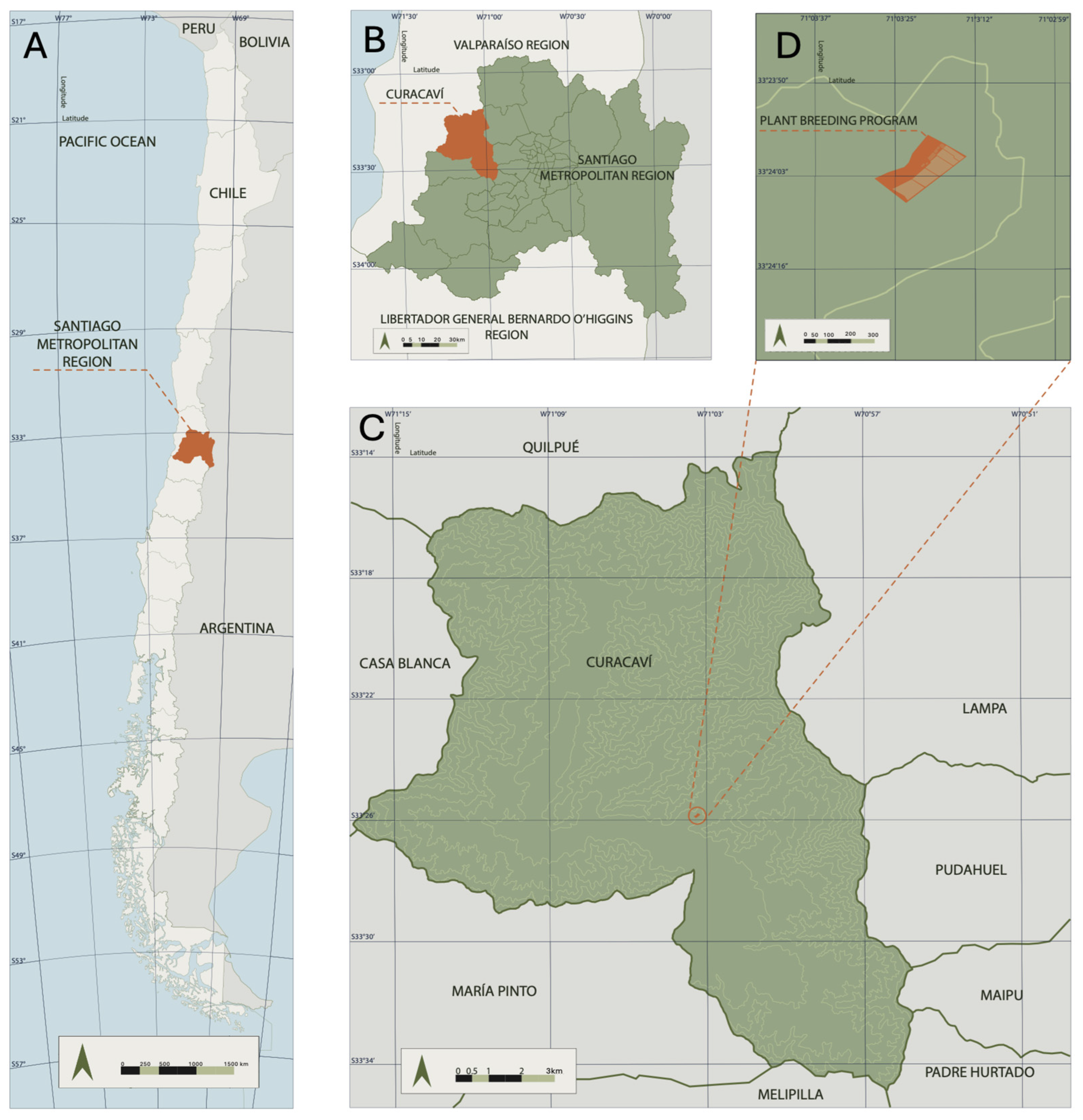
2.1. Plant Material and Experimental Site
2.2. Disease Resistance Evaluation
2.3. Harvest and Grape Analysis
2.4. Microvinification
2.5. Basic Oenological Analyses
2.6. Phenolic Composition Analysis
2.7. Color Parameters
2.8. HPLC Analysis of Individual Anthocyanins
2.9. Statistical Analysis
3. Results
3.1. Disease Resistance Performance
3.2. Ampelographic and Cluster Characteristics
3.3. Oenological Parameters and Wine Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OIV | Organisation Internationale de la Vigne et du Vin |
| ND | Not determined |
| SE | Standard error |
| GAE | Gallic acid equivalents |
| MV3G eq | Malvidin-3-glucoside equivalents |
| EC eq | Epicatechin equivalents |
| PCA | Principal components analysis |
| ANOVA | Analysis of variance |
| DNA | Deoxyribonucleic acid |
References
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Pedneault, K.; Provost, C. Fungus Resistant Grape Varieties as a Suitable Alternative for Organic Wine Production: Benefits, Limits, and Challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Barker, C.L.; Donald, T.; Pauquet, J.; Ratnaparkhe, M.B.; Bouquet, A.; Adam-Blondon, A.F.; Thomas, M.R.; Dry, I. Genetic and Physical Mapping of the Grapevine Powdery Mildew Resistance Gene, Run1, Using a Bacterial Artificial Chromosome Library. Theor. Appl. Genet. 2005, 111, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Agurto, M.; Schlechter, R.O.; Armijo, G.; Solano, E.; Serrano, C.; Contreras, R.A.; Zúñiga, G.E.; Arce-Johnson, P. RUN1 and REN1 Pyramiding in Grapevine (Vitis vinifera Cv. Crimson Seedless) Displays an Improved Defense Response Leading to Enhanced Resistance to Powdery Mildew (Erysiphe necator). Front. Plant Sci. 2017, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Di Gaspero, G.; Kovács, L.; Howard, S.; Kiss, E.; Galbács, Z.; Testolin, R.; Kozma, P. Resistance to Erysiphe necator in the Grapevine ‘Kishmish Vatkana’Is Controlled by a Single Locus through Restriction of Hyphal Growth. Theor. Appl. Genet. 2008, 116, 427–438. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Valenzuela, Á.V.; Barba, P.; Cancino, C.E.; Romero-Romero, J.L.; Arce-Johnson, P. Powdery Mildew Resistance Genes in Vines: An Opportunity to Achieve a More Sustainable Viticulture. Pathogens 2022, 11, 703. [Google Scholar] [CrossRef]
- Vezzulli, S.; Zulini, L.; Stefanini, M. Genetics-Assisted Breeding for Downy/Powdery Mildew and Phylloxera Resistance at Fem. In Proceedings of the BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2019; Volume 12. [Google Scholar]
- Sun, Q.; Gates, M.J.; Lavin, E.H.; Acree, T.E.; Sacks, G.L. Comparison of Odor-Active Compounds in Grapes and Wines from Vitis vinifera and Non-Foxy American Grape Species. J. Agric. Food Chem. 2011, 59, 10657–10664. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybridgrape Cultivars Grown in Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef]
- Springer, L.F.; Sacks, G.L. Protein-Precipitable Tannin in Wines from Vitis vinifera and Interspecific Hybrid Grapes (Vitis Ssp.): Differences in Concentration, Extractability, and Cell Wall Binding. J. Agric. Food Chem. 2014, 62, 7515–7523. [Google Scholar] [CrossRef]
- Nicolle, P.; Marcotte, C.; Angers, P.; Pedneault, K. Pomace Limits Tannin Retention in Frontenac Wines. Food Chem. 2019, 277, 438–447. [Google Scholar] [CrossRef]
- Montaigne, E.; Coelho, A.; Khefifi, L. Economic Issues and Perspectives on Innovation in New Resistant Grapevine Varieties in France. Wine Econ. Policy 2016, 5, 73–77. [Google Scholar] [CrossRef]
- Liang, Z.; Owens, C.L.; Zhong, G.-Y.; Cheng, L. Polyphenolic Profiles Detected in the Ripe Berries of Vitis vinifera Germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, Y.; Romero-Cascales, I.; Gil-Munoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gomez-Plaza, E. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Scariolo, F.; Gabelli, G.; Magon, G.; Palumbo, F.; Pirrello, C.; Farinati, S.; Curioni, A.; Devillars, A.; Lucchin, M.; Barcaccia, G.; et al. The Transcriptional Landscape of Berry Skin in Red and White PIWI (“Pilzwiderstandsfähig”) Grapevines Possessing QTLs for Partial Resistance to Downy and Powdery Mildews. Plants 2024, 13, 2574. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New Horizons for Grapevine Breeding. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 79–100. [Google Scholar]
- Bois, B.; Zito, S.; Calonnec, A.; Ollat, N. Climate vs Grapevine Pests and Diseases Worldwide: The First Results of a Global Survey. J. Int. Des Sci. De La Vigne Et Du Vin 2017, 51, 133–139. [Google Scholar]
- Caffarra, A.; Rinaldi, M.; Eccel, E.; Rossi, V.; Pertot, I. Modelling the Impact of Climate Change on the Interaction between Grapevine and Its Pests and Pathogens: European Grapevine Moth and Powdery Mildew. Agric. Ecosyst. Environ. 2012, 148, 89–101. [Google Scholar] [CrossRef]
- Merli, R.; Preziosi, M.; Acampora, A. Sustainability Experiences in the Wine Sector: Toward the Development of an International Indicators System. J. Clean Prod. 2018, 172, 3791–3805. [Google Scholar] [CrossRef]
- Matus, J.T. Development and Status of the Chilean Wine Industry. In Grapevine Breeding Programs for the Wine Industry; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 385–409. [Google Scholar]
- Agrometeorología INIA Red Agrometeorológica INIA. Available online: https://agrometeorologia.cl (accessed on 17 October 2025).
- Ciubotaru, R.M.; Franceschi, P.; Vezzulli, S.; Zulini, L.; Stefanini, M.; Oberhuber, M.; Robatscher, P.; Chitarrini, G.; Vrhovsek, U. Secondary and Primary Metabolites Reveal Putative Resistance-Associated Biomarkers against Erysiphe Necator in Resistant Grapevine Genotypes. Front. Plant Sci. 2023, 14, 1112157. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 111958776X. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A New Simplified Method for Measuring the Color of Wines. I. Red and Rosé Wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar] [CrossRef]
- Casanova-Gascón, J.; Ferrer-Martín, C.; Bernad-Eustaquio, A.; Elbaile-Mur, A.; Ayuso-Rodríguez, J.M.; Torres-Sánchez, S.; Jarne-Casasús, A.; Martín-Ramos, P. Behavior of Vine Varieties Resistant to Fungal Diseases in the Somontano Region. Agronomy 2019, 9, 738. [Google Scholar] [CrossRef]
- Van Heerden, C.J.; Burger, P.; Vermeulen, A.; Prins, R. Detection of Downy and Powdery Mildew Resistance QTL in a ‘Regent’ × ‘RedGlobe’ Population. Euphytica 2014, 200, 281–295. [Google Scholar] [CrossRef]
- Serrano, A.; Espinoza, C.; Armijo, G.; Inostroza-Blancheteau, C.; Poblete, E.; Meyer-Regueiro, C.; Arce, A.; Parada, F.; Santibáñez, C.; Arce-Johnson, P. Omics Approaches for Understanding Grapevine Berry Development: Regulatory Networks Associated with Endogenous Processes and Environmental Responses. Front. Plant Sci. 2017, 8, 1486. [Google Scholar] [CrossRef]
- Sunitha, S.; Loyola, R.; Alcalde, J.A.; Arce-Johnson, P.; Matus, J.T.; Rock, C.D. The Role of UV-B Light on Small RNA Activity During Grapevine Berry Development. G3 Genes Genomes Genet. 2019, 9, 769–787. [Google Scholar] [CrossRef]
- Verdugo-Vásquez, N.; Orrego, R.; Gutiérrez-Gamboa, G.; Reyes, M.; Zurita-Silva, A.; Balbontín, C.; Gaete, N.; Salazar-Parra, C. Climate Trends and Variability in the Chilean Viticultural Production Zones during 1985–2015. OENO One 2023, 57, 345–362. [Google Scholar] [CrossRef]
- Gratl, V.; Sturm, S.; Zini, E.; Letschka, T.; Stefanini, M.; Vezzulli, S.; Stuppner, H. Comprehensive Polyphenolic Profiling in Promising Resistant Grapevine Hybrids Including 17 Novel Breeds in Northern Italy. J. Sci. Food Agric. 2021, 101, 2380–2388. [Google Scholar] [CrossRef]
- Feechan, A.; Anderson, C.; Torregrosa, L.; Jermakow, A.; Mestre, P.; Wiedemann-Merdinoglu, S.; Merdinoglu, D.; Walker, A.R.; Cadle-Davidson, L.; Reisch, B. Genetic Dissection of a TIR-NB-LRR Locus from the Wild N Orth A Merican Grapevine Species M Uscadinia Rotundifolia Identifies Paralogous Genes Conferring Resistance to Major Fungal and Oomycete Pathogens in Cultivated Grapevine. Plant J. 2013, 76, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, V.; Crespan, M.; Maddalena, G.; Migliaro, D.; Brancadoro, L.; Maghradze, D.; Failla, O.; Toffolatti, S.L.; De Lorenzis, G. Novel Loci Associated with Resistance to Downy and Powdery Mildew in Grapevine. Front. Plant Sci. 2024, 15, 1386225. [Google Scholar] [CrossRef] [PubMed]
- Mundt, C.C. Pyramiding for Resistance Durability: Theory and Practice. Phytopathology 2018, 108, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Fresnedo-Ramírez, J.; Yang, S.; Sun, Q.; Cote, L.M.; Schweitzer, P.A.; Reisch, B.I.; Ledbetter, C.A.; Luby, J.J.; Clark, M.D.; Londo, J.P.; et al. An Integrative AmpSeq Platform for Highly Multiplexed Marker-Assisted Pyramiding of Grapevine Powdery Mildew Resistance Loci. Mol. Breed. 2017, 37, 145. [Google Scholar] [CrossRef]
- Qiu, W.; Feechan, A.; Dry, I. Current Understanding of Grapevine Defense Mechanisms against the Biotrophic Fungus (Erysiphe necator), the Causal Agent of Powdery Mildew Disease. Hortic. Res. 2015, 2, 15020. [Google Scholar] [CrossRef]
- Cadle-Davidson, L.; Chicoine, D.R.; Consolie, N.H. Variation Within and Among Vitis Spp. for Foliar Resistance to the Powdery Mildew Pathogen Erysiphe Necator. Plant Dis. 2011, 95, 202–211. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Schneider, C.; Prado, E.; Wiedemann-Merdinoglu, S.; Mestre, P. Breeding for Durable Resistance to Downy and Powdery Mildew in Grapevine. OENO One 2018, 52, 203–209. [Google Scholar] [CrossRef]
- Trapp, O.; Avia, K.; Borrelli, C.; Eibach, R.; Merdinoglu, D.; Töpfer, R. More Sustainability in Europe’s Vineyards—Using Resistant Grapevine Varieties to Reduce the Input of Pesticides. Plants People Planet 2025, 7, 1621–1628. [Google Scholar] [CrossRef]
- Eisenmann, B.; Wingerter, C.; Dressler, M.; Freund, C.; Kortekamp, A.; Bogs, J. Fungicide-Saving Potential and Economic Advantages of Fungus-Resistant Grapevine Cultivars. Plants 2023, 12, 3120. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Viganò, F. Unveiling the Motivations Behind Cultivating Fungus-Resistant Wine Varieties: Insights from Wine Growers in South Tyrol, Italy. Sustainability 2025, 17, 2615. [Google Scholar] [CrossRef]
- Mian, G.; Nassivera, F.; Sillani, S.; Iseppi, L. Grapevine Resistant Cultivars: A Story Review and the Importance on the Related Wine Consumption Inclination. Sustainability 2023, 15, 390. [Google Scholar] [CrossRef]
- Warneke, B.W.; Nackley, L.L.; Pscheidt, J.W. Management of Grape Powdery Mildew with an Intelligent Sprayer and Sulfur. Plant Dis. 2022, 106, 1837–1844. [Google Scholar] [CrossRef]
- Brault, C.; Segura, V.; Roques, M.; Lamblin, P.; Bouckenooghe, V.; Pouzalgues, N.; Cunty, C.; Breil, M.; Frouin, M.; Garcin, L.; et al. Enhancing Grapevine Breeding Efficiency through Genomic Prediction and Selection Index. G3 Genes Genomes Genet. 2024, 14, jkae038. [Google Scholar] [CrossRef]
- Tourrette, E.; Falque, M.; Martin, O.C. Enhancing Backcross Programs through Increased Recombination. Genet. Sel. Evol. 2021, 53, 25. [Google Scholar] [CrossRef]
- Duley, G.; Ceci, A.T.; Longo, E.; Boselli, E. Oenological Potential of Wines Produced from Disease-Resistant Grape Cultivars. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2591–2610. [Google Scholar] [CrossRef]
- Vecchio, R.; Pomarici, E.; Giampietri, E.; Borrello, M. Consumer Acceptance of Fungus-Resistant Grape Wines: Evidence from Italy, the UK, and the USA. PLoS ONE 2022, 17, e0267198. [Google Scholar] [CrossRef]
- Kiefer, C.; Szolnoki, G. Consumer Acceptance of Fungus-Resistant Grape Varieties—An Exploratory Study Using Sensory Evaluation Tests among Consumers in Germany. Sustainability 2023, 15, 10664. [Google Scholar] [CrossRef]

| ID | Genotype | Resistance Locus/Loci | Female Parent | Male Parent | Backcross Number | Estimated % of V. vinifera Genome | OIV Resistance |
|---|---|---|---|---|---|---|---|
| AJ-T6 | P09-CAR | Run1Ren1 | BC5(02-2) × 91-4/27 | Carménère | BC7 | 99.6% | 9 |
| AJ-T2 | P09-107-113 | Run1 | BC5(02-2) | 91-4/27 | BC6 | 99.2% | 9 |
| AJ-B1 | P09-107-147 | Run1 | BC5(02-2) | 91-4/27 | BC6 | 99.2% | 9 |
| AJ-T3 | P09-105-81 | Run1 | BC5(02-2) | 91-4/27 | BC6 | 99.2% | 9 |
| AJ-T5 | P09-EN69 | Run1 | BC5(02-2) × 91-4/27 | Crimson Seedless | BC7 | 99.6% | 9 |
| Genotype | Year | Type | 100-Berry Mass (g) | 100-Berry Volume (mL) | °Brix | pH | Titratable Acidity (g/L) | % Healthy Berries (Count) | % Healthy Berries (Mass) | % Rachis Mas |
|---|---|---|---|---|---|---|---|---|---|---|
| AJ-T6 | 2024 | Red | 139.9 ± 5.1 | 116 ± 4 | 22.9 | ND | ND | 97.3 ± 0.1 | 93.8 ± 0.1 | 5.75 ± 0.3 |
| AJ-T6 | 2025 | Red | 178.1 ± 4.2 | 161.7 ± 3.3 | 22.0 | 3.5 | 5.2 | ND | ND | ND |
| AJ-T2 | 2024 | Red | 307.6 ± 10.3 | 286.7 ± 6.6 | 24.9 | 3.5 | 7.1 | 92 ± 4.7 | 95.7 ± 4.7 | 2.31 ± 0.7 |
| AJ-T2 | 2025 | Red | 396.6 ± 11.5 | 368 ± 6.1 | 22.3 | 3.6 | 4.8 | ND | ND | ND |
| AJ-B1 | 2024 | White | 265.4 ± 5.3 | 273.3 ± 23.4 | 21.0 | 3.4 | 5.0 | 94.9 ± 1.7 | 91.5 ± 1.7 | 2.64 ± 0.2 |
| AJ-B1 | 2025 | White | 353.2 ± 13.3 | 323.3 ± 13.3 | 21.1 | 3.7 | 4.1 | ND | ND | ND |
| AJ-T3 | 2024 | Red | 99.7 ± 2.1 | 92.7 ± 1.8 | 24.7 | 3.4 | 7.1 | 82.1 ± 3.2 | 80.7 ± 3.2 | 6.87 ± 0.3 |
| AJ-T5 | 2024 | Red | 235.8 ± 1.5 | 222 ± 1.2 | 17.2 | ND | ND | 93.9 ± 1.6 | 95.4 ± 1.6 | 1.99 ± 0.1 |
| Anthocyanin | AJ-T6 | AJ-T2 |
|---|---|---|
| delp-3-gluc | 3.15 ± 0.54 | 1.24 ± 0.09 |
| cyan-3-gluc | 0.27 ± 0.09 | 1.06 ± 0.1 |
| petun-3-gluc | 9.48 ± 1.42 | 4.66 ± 0.85 |
| peon-3-gluc | 3.16 ± 1.21 | 16.99 ± 2.25 |
| malv-3-gluc | 120.47 ± 16.45 | 128.85 ± 30.29 |
| delp-3-gluc Ac | 0.94 ± 0.08 | 0.57 ± 0.05 |
| cyan-3-gluc Ac | 1.26 ± 0.25 | - |
| petun-3-gluc Ac | 2.6 ± 0.39 | - |
| peon-3-gluc Ac | 2.03 ± 0.45 | 2.58 ± 0.29 |
| malv-3-gluc Ac | 55.01 ± 7.87 | 3.78 ± 0.3 |
| delp-3-gluc coum | 1.64 ± 0.05 | 1.59 ± 0.1 |
| cyan-3-gluc coum | 1.81 ± 0.4 | 1.57 ± 0.15 |
| petun-3-gluc coum | 0.94 ± 0.15 | 0.35 ± 0.04 |
| peon-3-gluc coum | 1.63 ± 0.31 | 17.53 ± 2.1 |
| malv-3-gluc coum | 23.69 ± 4.59 | 25.86 ± 2.37 |
| TOTAL | 229.57 ± 32.52 | 206.63 ± 15.65 |
| Genotype | Year | Type | Winemaking Type | Titratable Acidity (g/L) | pH | Total Phenolics (GA eq.) | Total Anthocyanins (mg/L MV3G eq.) | Total Tannins (g/L EC eq.) | CI | Tone | % Yellow | % Red | % Blue |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJ-T6 | 2024 | Red | Red | 5.8 ± 0.3 | 3.60 ± 0.02 | 1091.4 ± 20.8 | 84.2 ± 6.2 | 712.6 ± 8.5 | 7.3 ± 0.3 | 0.7 ± 0.0 | 36.2 ± 5.3 | 36.2 ± 6.9 | 10.3 ± 0.1 |
| AJ-T6 | 2025 | Red | Red | 5.5 ± 0.1 | 3.70 ± 0.07 | 231 ± 20.8 | 231 ± 40.5 | - | 8.8 ± 0.4 | 0.6 ± 0.0 | 34.3 ± 0.2 | 53.3 ± 0.4 | 12.4 ± 0.1 |
| AJ-T2 | 2024 | Red | Red | 6.5 ± 0.1 | 3.70 ± 0.00 | 1356.1 ± 62.3 | 261 ± 16.0 | 815.7 ± 40.7 | 12.4 ± 0.6 | 0.6 ± 0.0 | 34 ± 3.0 | 54.1 ± 2.1 | 11.9 ± 0.1 |
| AJ-T2 | 2025 | Red | Red | 4.5 ± 0.1 | 3.90 ± 0.02 | 1005.8 ± 4.5 | 248.7 ± 16.0 | - | 3.5 ± 0.2 | 0.6 ± 0.0 | 33.5 ± 1.2 | 55.2 ± 1.8 | 11.3 ± 0.0 |
| AJ-B1 | 2024 | White | White | 6.2 ± 0.1 | 3.40 ± 0.01 | 229.2 ± 4 | - | - | 0.2 ± 0.0 | 2.4 ± 0.1 | 61.1 ± 1.0 | 25.6 ± 0.6 | 13.3 ± 0.2 |
| AJ-T3 | 2024 | Red | Red | 6.5 ± 0.0 | 3.50 ± 0.00 | 632 ± 31.5 | 130.9 ± 1.0 | 188.1 ± 16.8 | 6.5 ± 0.6 | 0.7 ± 0.0 | 37.1 ± 0.1 | 49.7 ± 0.2 | 13.2 ± 0.3 |
| AJ-T5 | 2024 | Red | Red | 7.8 ± 0.8 | 3.40 ± 0.06 | 420.8 ± 6.1 | 15.6 ± 3.6 | 69.3 ± 40.8 | 4.0 ± 0.1 | 0.8 ± 0.0 | 38.8 ± 5.5 | 53.5 ± 7.2 | 10.3 ± 0.1 |
| AJ-T6 | 2024 | Red | Red | 5.8 ± 0.3 | 3.60 ± 0.02 | 1091.4 ± 20.8 | 84.2 ± 6.2 | 712.6 ± 8.5 | 7.3 ± 0.3 | 0.7 ± 0.0 | 36.2 ± 5.3 | 36.2 ± 6.9 | 10.3 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormeño-Vásquez, P.; Sosa-Zuniga, V.; Gil-Cortiella, M.; Morales-Poblete, R.; Vallejos, C.; Medina, C.; Meneses, C.; Arce-Johnson, P. Biotechnological and Oenological Potential of Advanced Genetic Lines of Grapevine Resistant to Powdery Mildew (Erysiphe necator). Agriculture 2025, 15, 2267. https://doi.org/10.3390/agriculture15212267
Ormeño-Vásquez P, Sosa-Zuniga V, Gil-Cortiella M, Morales-Poblete R, Vallejos C, Medina C, Meneses C, Arce-Johnson P. Biotechnological and Oenological Potential of Advanced Genetic Lines of Grapevine Resistant to Powdery Mildew (Erysiphe necator). Agriculture. 2025; 15(21):2267. https://doi.org/10.3390/agriculture15212267
Chicago/Turabian StyleOrmeño-Vásquez, Phillip, Viviana Sosa-Zuniga, Mariona Gil-Cortiella, Rene Morales-Poblete, Carolina Vallejos, Consuelo Medina, Claudio Meneses, and Patricio Arce-Johnson. 2025. "Biotechnological and Oenological Potential of Advanced Genetic Lines of Grapevine Resistant to Powdery Mildew (Erysiphe necator)" Agriculture 15, no. 21: 2267. https://doi.org/10.3390/agriculture15212267
APA StyleOrmeño-Vásquez, P., Sosa-Zuniga, V., Gil-Cortiella, M., Morales-Poblete, R., Vallejos, C., Medina, C., Meneses, C., & Arce-Johnson, P. (2025). Biotechnological and Oenological Potential of Advanced Genetic Lines of Grapevine Resistant to Powdery Mildew (Erysiphe necator). Agriculture, 15(21), 2267. https://doi.org/10.3390/agriculture15212267


Arif_ATAK.jpg)





