Magnetized and Aerated Irrigation Promotes Nitrogen Dynamics and Metabolite Accumulation in Salvia miltiorrhiza
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Field Experiment Design
2.2. Methods of Soil and Plant Sampling
2.3. Soil Nutrient Status
2.4. Metabolites of Salvia miltiorrhiza
2.5. Plant Dry Matter Weight and Leaf Chlorophyll Index
2.6. Statistical Analyses
3. Results
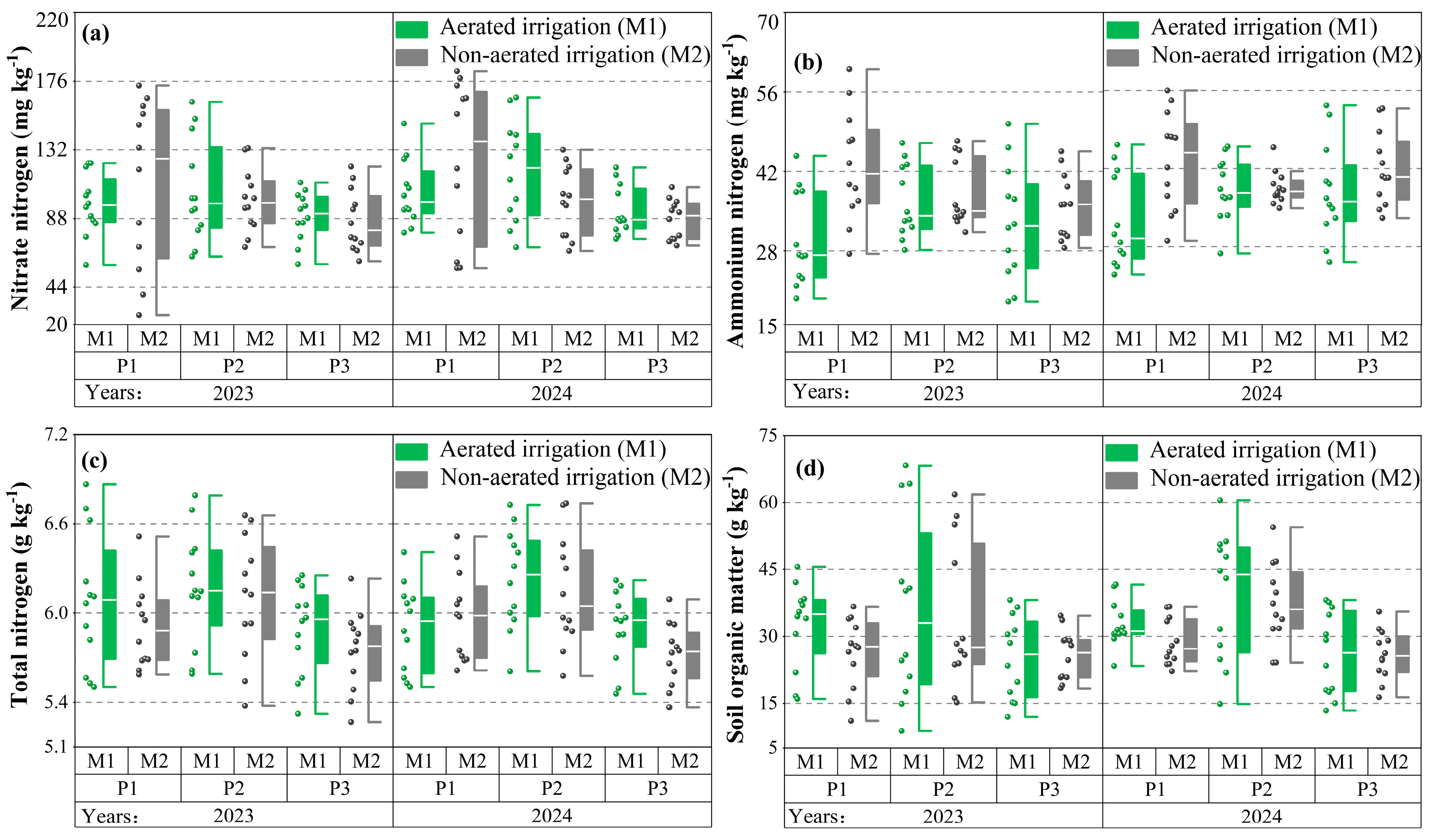
3.1. Soil Nutrient Dynamic
3.1.1. Soil Available Nitrogen
3.1.2. Soil Organic Matter and Total Nitrogen
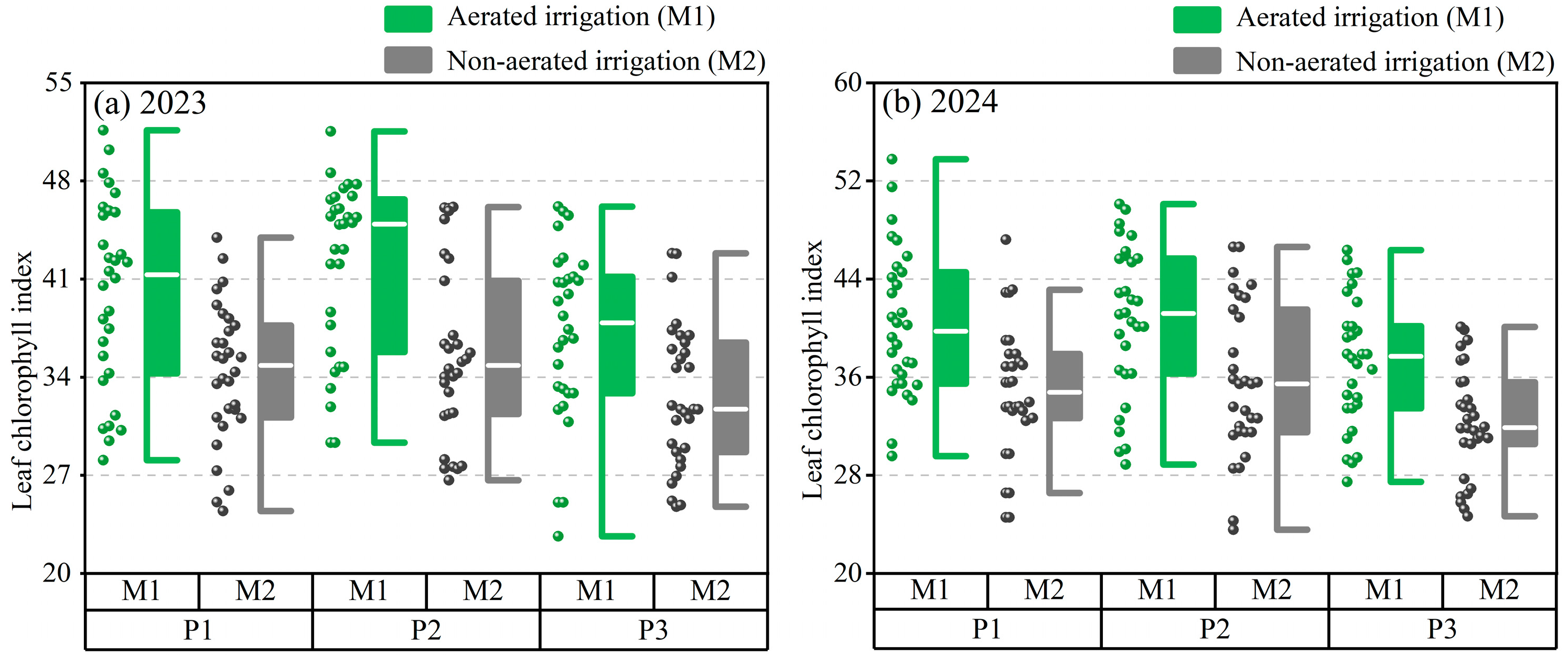
3.2. Growth Performance of Salvia miltiorrhiza
3.2.1. Dry Matter Weight
3.2.2. Leaf Chlorophyll Index
3.3. Bioactive Compound of Salvia miltiorrhiza and Their Correlations
3.3.1. Tanshinone Composition
3.3.2. Salvianolic Acids and Metabolite Intercorrelations
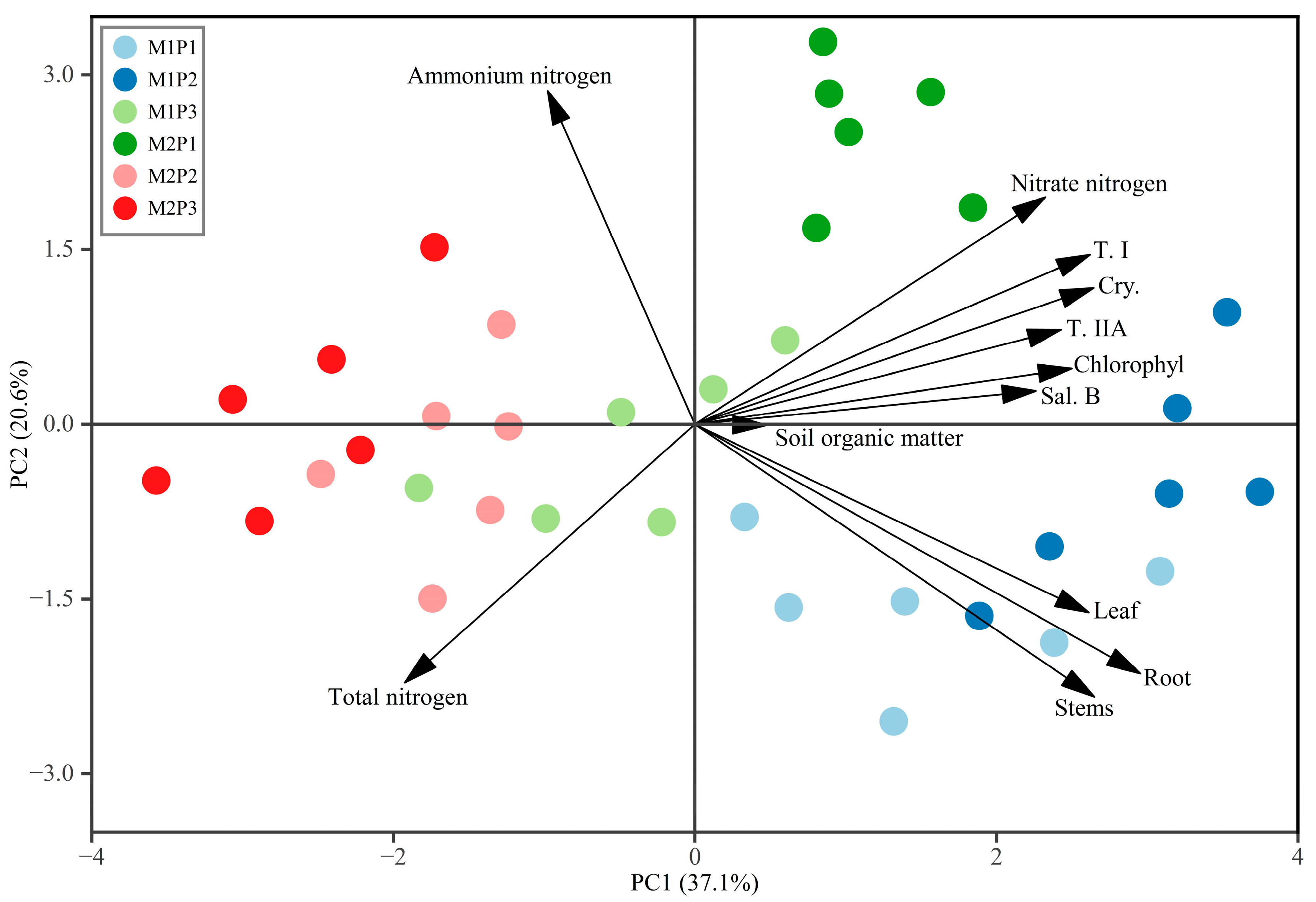
3.4. Principal Component Analysis of Growth-Metabolite Relationships
4. Discussion
4.1. Soil Nitrogen Availability and SOM Dynamics Under Irrigation Measures
4.2. Effects of Magnetized and Aerated Irrigation on S. miltiorrhiza Growth
4.3. Interactions Among Metabolites of S. miltiorrhiza Revealed by Multivariate Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.L.; Zhang, P.; Deng, X.Y.; Wang, S.; Li, H. Assessment of Crop Water Footprint and Actual Agricultural Water Consumption in Arid Inland Regions: A Case Study of Aksu Region. Sustainability 2024, 16, 2911. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.P.; Sun, J.Y.; Chen, W.; Liu, X. Optimizing water resources allocation and soil salinity control for supporting agricultural and environmental sustainable development in Central Asia. Sci. Total Environ. 2020, 704, 135281. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Costa Crusciol, C.A.; Portugal, J.R.; Moretti, L.G.; Garcia, A. Long-Term Liming Improves Soil Fertility and Soybean Root Growth, Reflecting Improvements in Leaf Gas Exchange and Grain Yield. Eur. J. Agron. 2021, 128, 126308. [Google Scholar] [CrossRef]
- Mbukwa, D.; Gui, R.; Deng, S.; Li, X.; Wang, Y. Effects of Aeration Treatments on Root and Rhizome Growth Parameters of Phyllostachys violascens (Lei Bamboo) under Intensive Cultivation: A Field Study. Sci. Total Environ. 2023, 900, 165738. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Rukh, G.; Ruan, Z.; Chen, L.; Zhang, H. Effects of Hypoxia Stress on Growth, Root Respiration, and Metabolism of Phyllostachys praecox. Life 2022, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Brtnický, M.; Hammerschmiedt, T.; Kintl, A.; Holatko, J. Food and Agricultural Wastes-Derived Biochars in Combination with Mineral Fertilizer as Sustainable Soil Amendments to Enhance Soil Microbiological Activity, Nutrient Cycling and Crop Production. Front. Plant Sci. 2022, 13, 1028101. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Shaukat, K.; Batool, S.; Wahid, A. Hypoxia and Anoxia Stress: Plant Responses and Tolerance Mechanisms. J. Agron. Crop Sci. 2021, 207, 249–284. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Huang, C.Y.; Lee, S.D.; Chen, Y.; Lin, H. Review of Danshen: From Its Metabolism to Possible Mechanisms of Its Biological Activities. J. Funct. Foods 2021, 85, 104613. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.N.; Liu, C.; Zhang, Y.; Zhao, X. Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef]
- He, C.; Lu, L.; Jin, Y.; Zhang, X.; Wang, S. Effects of Nitrogen on Root Development and Contents of Bioactive Compounds in Salvia miltiorrhiza Bunge. Crop Sci. 2013, 53, 2028–2039. [Google Scholar] [CrossRef]
- Li, J.; Mei, X.; Zhang, J.; Wang, Y.; Liu, H. Effects of Potassium Application on Growth and Root Metabolism of Salvia miltiorrhiza under Drought Stress. Agronomy 2023, 13, 2796. [Google Scholar] [CrossRef]
- Salvatierra, A.; Toro, G.; Mateluna, P.; Pimentel, P.; Gonzalez, W. Keep Calm and Survive: Adaptation Strategies to Energy Crisis in Fruit Trees under Root Hypoxia. Plants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Xing, Z.; Bi, G.; Li, T.; Zhang, Y.; Wang, L. Nitrogen Fertilization Improves Growth and Bioactive Compound Content for Salvia miltiorrhiza Bunge. Horticulturae 2023, 9, 254. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. Characteristics and Applications of Magnetized Water as a Green Technology. J. Clean. Prod. 2017, 161, 908–921. [Google Scholar] [CrossRef]
- Ma, C.; Li, Q.; Song, Z.; Zhang, X.; Wang, Y. Irrigation with Magnetized Water Alleviates the Harmful Effect of Saline-Alkaline Stress on Rice Seedlings. Int. J. Mol. Sci. 2022, 23, 10048. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Hajar, A.S.; Alzahrani, Y.; Alghamdi, S. The Effect of Magnetized Water on the Growth and Physiological Conditions of Moringa Species under Drought Stress. Pol. J. Environ. Stud. 2019, 28, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Alattar, E.; Radwan, E.; Elwasife, K.Y.; Mohamed, A.; El-Sayed, M. Improvement in Growth of Plants under the Effect of Magnetized Water. AIMS Biophys. 2022, 9, 346–387. [Google Scholar] [CrossRef]
- Massah, J.; Dousti, A.; Khazaei, J.; Moradi, S.; Azizi, M. Effects of Water Magnetic Treatment on Seed Germination and Seedling Growth of Wheat. J. Plant Nutr. 2019, 42, 1283–1289. [Google Scholar] [CrossRef]
- El-Zawily, A.E.-S.; Meleha, M.; El-Sawy, M.; Alshaal, T.; El-Ramady, H. Application of Magnetic Field Improves Growth, Yield and Fruit Quality of Tomato Irrigated Alternatively by Fresh and Agricultural Drainage Water. Ecotoxicol. Environ. Saf. 2019, 181, 248–254. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Sun, Y.; Li, X.; Zhang, H. Effects of Oxygenated Brackish Water on Germination and Growth Characteristics of Wheat. Agric. Water Manag. 2021, 245, 106520. [Google Scholar] [CrossRef]
- Li, J.; He, P.; Jin, Q.; Wang, S.; Chen, W. Aeration Alleviated the Adverse Effects of Nitrogen Topdressing Reduction on Tomato Root Vigor, Photosynthetic Performance, and Fruit Development. Plants 2024, 13, 1378. [Google Scholar] [CrossRef]
- Chen, H.; Shang, Z.; Cai, H.; Liu, Y.; Wang, X. Irrigation Combined with Aeration Promoted Soil Respiration through Increasing Soil Microbes, Enzymes, and Crop Growth in Tomato Fields. Catalysts 2019, 9, 945. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Cao, X.; Zhang, J.; Li, H. Growth Response of Greenhouse-Produced Muskmelon and Tomato to Sub-Surface Drip Irrigation and Soil Aeration Management Factors. BMC Plant Biol. 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, S.; Zou, H.; Zhang, Y.; Liu, X. R2R3-MYB Transcription Factor SmMYB52Positively Regulates Biosynthesis of Salvianolic Acid B and Inhibits Root Growth in Salvia miltiorrhiza. Int. J. Mol. Sci. 2021, 22, 9538. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Li, Y.; Wang, J.; Liu, H. Overexpression of AtMYB2Promotes Tolerance to Salt Stress and Accumulations of Tanshinones and Phenolic Acid in Salvia miltiorrhiza. Int. J. Mol. Sci. 2024, 25, 4111. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Y.; Xia, P.; Zhang, L.; Chen, W. Diverse Responses of Tanshinone Biosynthesis to Biotic and Abiotic Elicitors in Hairy Root Cultures of Salvia miltiorrhiza and Salvia castanea Diels f. tomentosa. Gene 2018, 643, 61–67. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Piccolo, M.; Maione, F.; Ferrara, N.; Roviezzo, F. Tanshinones from Salvia miltiorrhiza Bunge Revert Chemotherapy-Induced Neuropathic Pain and Reduce Glioblastoma Cells Malignancy. Biomed. Pharmacother. 2018, 105, 1042–1049. [Google Scholar] [CrossRef]
- Hatfield, M.J.; Binder, R.J.; Gannon, R.; Tsurkan, L.; Hyatt, J.L. Potent, Irreversible Inhibition of Human Carboxylesterases by Tanshinone Anhydrides Isolated from Salvia miltiorrhiza (“Danshen”). J. Nat. Prod. 2018, 81, 2410–2418. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, B.; Qin, L.; Zhang, X.; Wang, Y. Transcription Factor: A Powerful Tool to Regulate Biosynthesis of Active Ingredients in Salvia miltiorrhiza. Front. Plant Sci. 2021, 12, 622011. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, G.; Su, Y.; Zhang, L.; Chen, W. Effect of CeO2, TiO2 and SiO2 Nanoparticles on the Growth and Quality of Model Medicinal Plant Salvia miltiorrhiza by Acting on Soil Microenvironment. Ecotoxicol. Environ. Saf. 2024, 280, 116552. [Google Scholar] [CrossRef]
- Liu, S.; Yang, G.; Wu, F.; Zhang, H.; Li, J. Traditional Chinese Medicine Residues Promote the Growth and Quality of Salvia miltiorrhiza Bunge by Improving Soil Health under Continuous Monoculture. Front. Plant Sci. 2023, 14, 1112382. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Gao, D.; Chen, W.; Zhang, Y.; Wang, X. UHPLC-QTOF-MS-Based Targeted Metabolomics Provides Novel Insights into the Accumulative Mechanism of Soil Types on the Bioactive Components of Salvia miltiorrhiza. Molecules 2024, 29, 4016. [Google Scholar] [CrossRef]
- Wu, D.; He, X.; Jiang, L.; Zhang, Y.; Wang, S. Root Exudates Facilitate the Regulation of Soil Microbial Community Function in the Genus Haloxylon. Front. Plant Sci. 2024, 15, 1461893. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, F.; Min, J.; Zhang, X.; Chen, W. Biological Mitigation of Soil Nitrous Oxide Emissions by Plant Metabolites. Glob. Change Biol. 2024, 30, e17333. [Google Scholar] [CrossRef]
- Liu, Z.; Long, K.; Zeng, J.; Zhang, H.; Li, J. Effects of Cyclic Aeration Subsurface Drip Irrigation on Greenhouse Tomato Quality and Water and Fertilizer Use Efficiency. Plants 2024, 13, 3559. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Q.; Deng, M.; Zhang, Y.; Liu, X. The Mechanism of Using Magnetized-Ionized Water in Combination with Organic Fertilizer to Enhance Soil Health and Cotton Yield. Sci. Total Environ. 2024, 941, 173781. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Zhong, H.; Wang, S.; Li, X. Heterogeneous Condensation of Magnetized Water Vapor on Fine SiO2 Particles. Environ. Res. 2019, 169, 173–179. [Google Scholar] [CrossRef]
- Cui, M.; Zhan, G.; Zhang, L.; Wang, Y.; Liu, H. Micro-Oxygen Supply Combined with Applied Voltage Enhance Ammonium Oxidation and Denitrification in a Single-Chamber Microbial Electrolysis Cell: Performance and Mechanism. Chem. Eng. J. 2024, 487, 150494. [Google Scholar] [CrossRef]
- Linam, F.; Limmer, M.A.; Ebling, A.M.; Thompson, A.; Seyfferth, A.L. Rice Husk and Husk Biochar Soil Amendments Store Soil Carbon While Water Management Controls Dissolved Organic Matter Chemistry in Well-Weathered Soil. J. Environ. Manag. 2023, 339, 117936. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Z.; Wang, H.X.; Yu, D.S.; Zhang, Y.; Liu, X. The Effect of Aeration and Irrigation on the Improvement of Soil Environment and Yield in Dryland Maize. Front. Plant Sci. 2024, 15, 1464624. [Google Scholar] [CrossRef]
- Ke, W.; Liu, Z.; Zhu, F.; Zhang, X.; Wang, Y. Remediation Potential of Magnetic Biochar in Lead Smelting Sites: Insight from the Complexation of Dissolved Organic Matter with Potentially Toxic Elements. J. Environ. Manag. 2023, 344, 118556. [Google Scholar] [CrossRef]
- Chang, R.; Li, Y.; Chen, Q.; Zhang, H.; Wang, S. Effects of Carbon-Based Additives and Ventilation Rate on Nitrogen Loss and Microbial Community during Chicken Manure Composting. PLoS ONE 2020, 15, e0229880. [Google Scholar] [CrossRef]
- Khosrojerdi, M.; Moghaddam, M.; Farhadi, N.; Azizi, M.; Moradi, S. Magnetic Water Irrigation Changes Physiological Traits and Stress Tolerance of Salvia virgata under Saline Conditions. Sci. Hortic. 2023, 314, 111935. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Awad, M.; Sorour, O.; Mohamed, A.; El-Sayed, M. Synergistic Effects of Magnetic Water Treatment and Mulching on Crop and Soil Moisture-Salinity Distribution. Sci. Rep. 2025, 15, 15741. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, H.; Song, L.; Chen, H.; Zhang, X. Oxygation Improving Soil Aeration around Tomato Root Zone in Greenhouse. Trans. Chin. Soc. Agric. Eng. 2017, 33, 163–172. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.; Wang, K.; Zhang, Y.; Liu, X. Spring Irrigation with Magnetized Water Affects Soil Water-Salt Distribution, Emergence, Growth, and Photosynthetic Characteristics of Cotton Seedlings in Southern Xinjiang, China. BMC Plant Biol. 2023, 23, 41. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, D.; Zhou, J.; Wang, S.; Chen, W. Oxygenated Compound Fertilizer Improved Rice Yield by Empowering Soil Aeration. Commun. Soil Sci. Plant Anal. 2021, 52, 1900219. [Google Scholar] [CrossRef]
- Souza-Torres, A.D.; Sueiro-Pelegrín, L.; Zambrano-Reyes, M.B.; Gonzalez, W.; Pimentel, P. Extremely Low Frequency Non-Uniform Magnetic Fields Induce Changes in Water Relations, Photosynthesis and Tomato Plant Growth. Int. J. Radiat. Biol. 2020, 96, 1748912. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Khan, M.; Al-Mutairi, N. Silicon Alleviate Hypoxia Stress by Improving Enzymatic and Non-Enzymatic Antioxidants and Regulating Nutrient Uptake in Muscadine Grape (Muscadinia rotundifolia Michx.). Front. Plant Sci. 2021, 12, 618873. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Gonnelli, C.; Loppi, S. Impact of Microplastics on Growth, Photosynthesis and Essential Elements in Cucurbita pepoL. J. Hazard. Mater. 2021, 423, 127238. [Google Scholar] [CrossRef] [PubMed]
- Podleśna, A.; Bojarszczuk, J.; Podleśny, J. Effect of Pre-sowing Magnetic Field Treatment on Some Biochemical and Physiological Processes in Faba Bean (Vicia faba L. spp. Minor). J. Plant Growth Regul. 2019, 38, 1153–1160. [Google Scholar] [CrossRef]
- Lu, L.; Fu, S.; Feng, J.; Wei, J. Growth and Accumulation of Five Main Bioactive Components in the Roots of Salvia miltiorrhiza at Different Growth Stages and Using Different Culture Systems. J. Dis. Med. Plants 2018, 4, 59–68. [Google Scholar] [CrossRef][Green Version]
- Xu, C.; Lv, Y.; Liu, D.; Gong, W. Exploring cellular biological effect of short-term stimulation of different high-intensity static magnetic fields on fresh-cut young ginger based on metabolome analysis. Food Res. Int. 2025, 213, 116618. [Google Scholar] [CrossRef]
- Khan, P.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.-M. Proline Promotes Drought Tolerance in Maize. Biology 2025, 14, 41. [Google Scholar] [CrossRef] [PubMed]
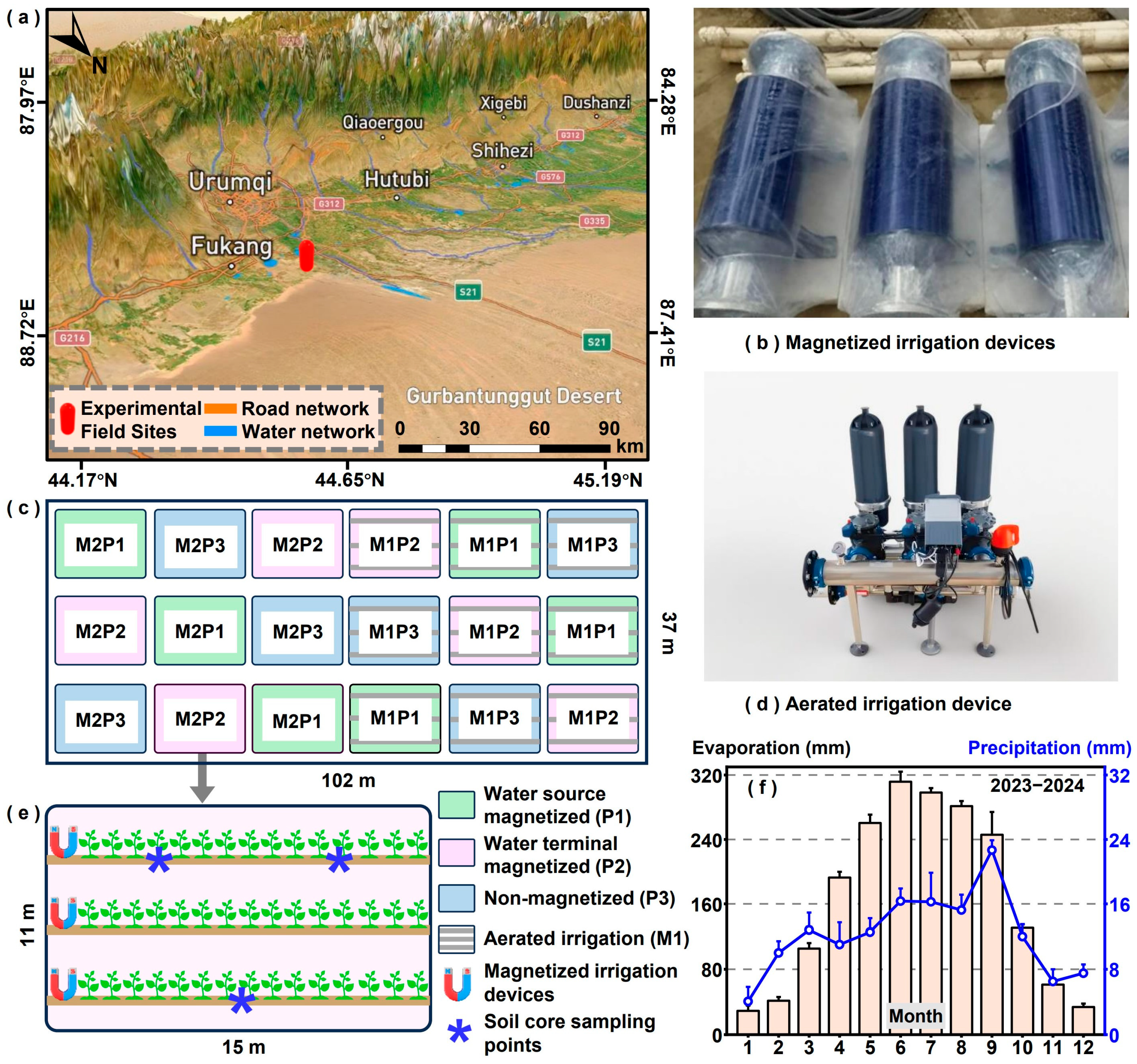


| Growth Period | Irrigation Date | Irrigation Amount (m3 ha−1) | Fertilizer a (kg ha−1) |
|---|---|---|---|
| Seedling | 15 April | 320 | 15 |
| 25 April | 320 | 16 | |
| 5 May | 400 | 20 | |
| 15 May | 400 | 15 | |
| Stem elongation | 25 May | 540 | 30 |
| 5 June | 500 | 39 | |
| 15 June | 500 | 30 | |
| Peak flowering | 25 June | 600 | 25 |
| 5 July | 600 | 25 | |
| 20 July | 550 | 25 | |
| Root thickening | 15 August | 550 | 30 |
| 30 August | 500 | 30 | |
| 15 September | 450 | 20 | |
| 30 September | 400 | 28 | |
| Total quota | 6630 | 348 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhao, W.; Zhang, Y.; Zhu, X.; Aerdake, A.-B.; Heng, T. Magnetized and Aerated Irrigation Promotes Nitrogen Dynamics and Metabolite Accumulation in Salvia miltiorrhiza. Agriculture 2025, 15, 2243. https://doi.org/10.3390/agriculture15212243
Fan Y, Zhao W, Zhang Y, Zhu X, Aerdake A-B, Heng T. Magnetized and Aerated Irrigation Promotes Nitrogen Dynamics and Metabolite Accumulation in Salvia miltiorrhiza. Agriculture. 2025; 15(21):2243. https://doi.org/10.3390/agriculture15212243
Chicago/Turabian StyleFan, Yaofang, Weixin Zhao, Yixin Zhang, Xiangnian Zhu, Ai-Bosheng Aerdake, and Tong Heng. 2025. "Magnetized and Aerated Irrigation Promotes Nitrogen Dynamics and Metabolite Accumulation in Salvia miltiorrhiza" Agriculture 15, no. 21: 2243. https://doi.org/10.3390/agriculture15212243
APA StyleFan, Y., Zhao, W., Zhang, Y., Zhu, X., Aerdake, A.-B., & Heng, T. (2025). Magnetized and Aerated Irrigation Promotes Nitrogen Dynamics and Metabolite Accumulation in Salvia miltiorrhiza. Agriculture, 15(21), 2243. https://doi.org/10.3390/agriculture15212243





