An Integrated Strategy of Nitrogen Reduction, Microbial Amendment, and Straw Incorporation Mitigates Soil Degradation and Enhances Cucumber Yield in Northern Chinese Greenhouses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Sampling and Physicochemical Analyses
2.3. Microbial Community Profiling
2.4. Greenhouse Gas Measurements
2.5. Plant Sampling and Analyses
2.6. Statistical Analyses
3. Results
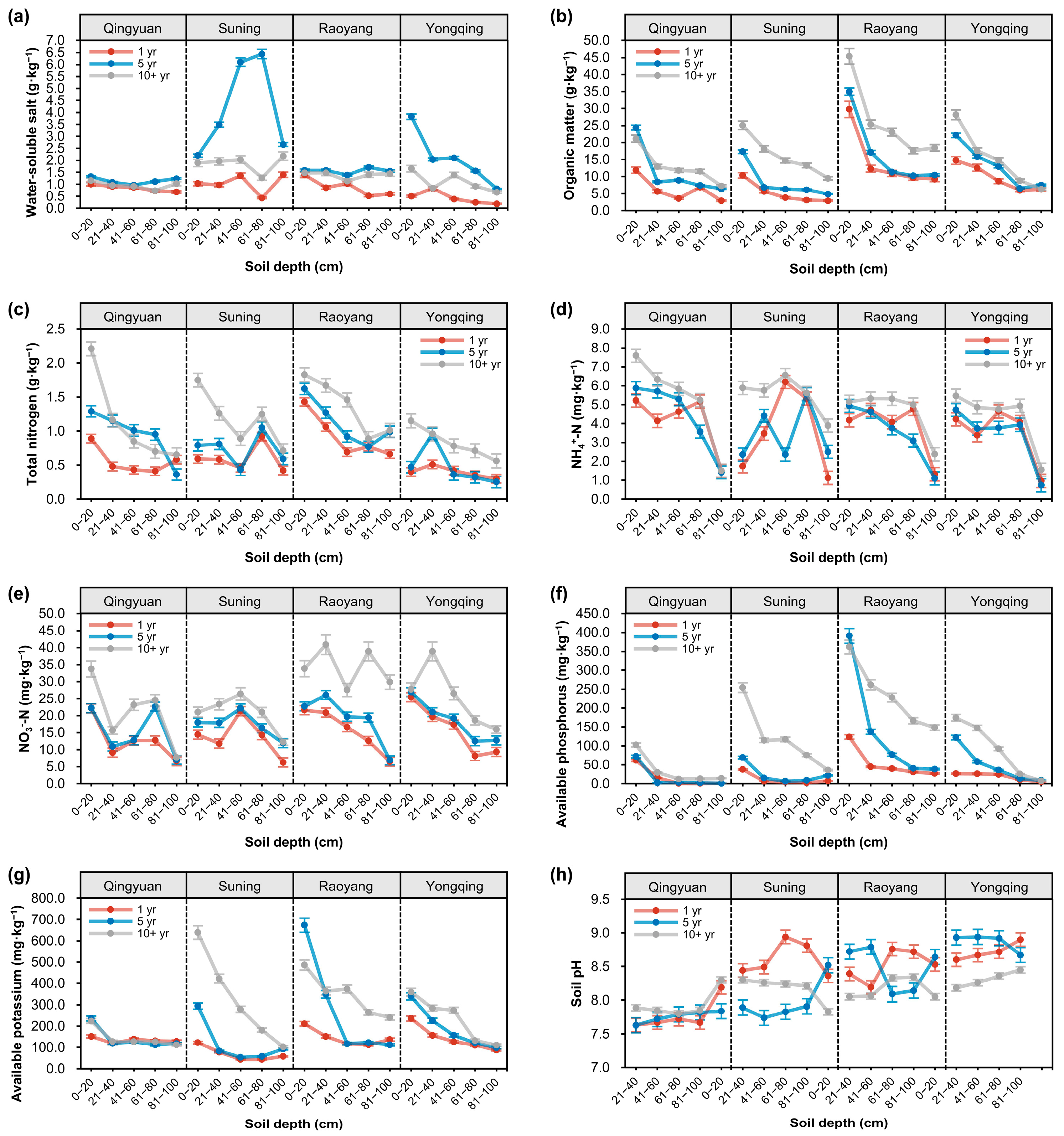
3.1. Excessive Fertilization Drives Pronounced Salt and Nutrient Buildup in Topsoil of Hebei Greenhouse Vegetable Fields
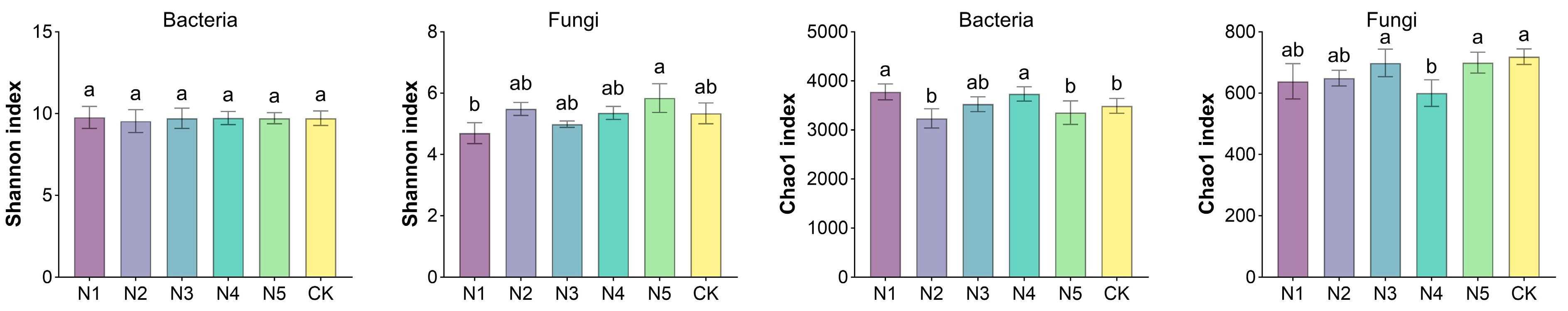
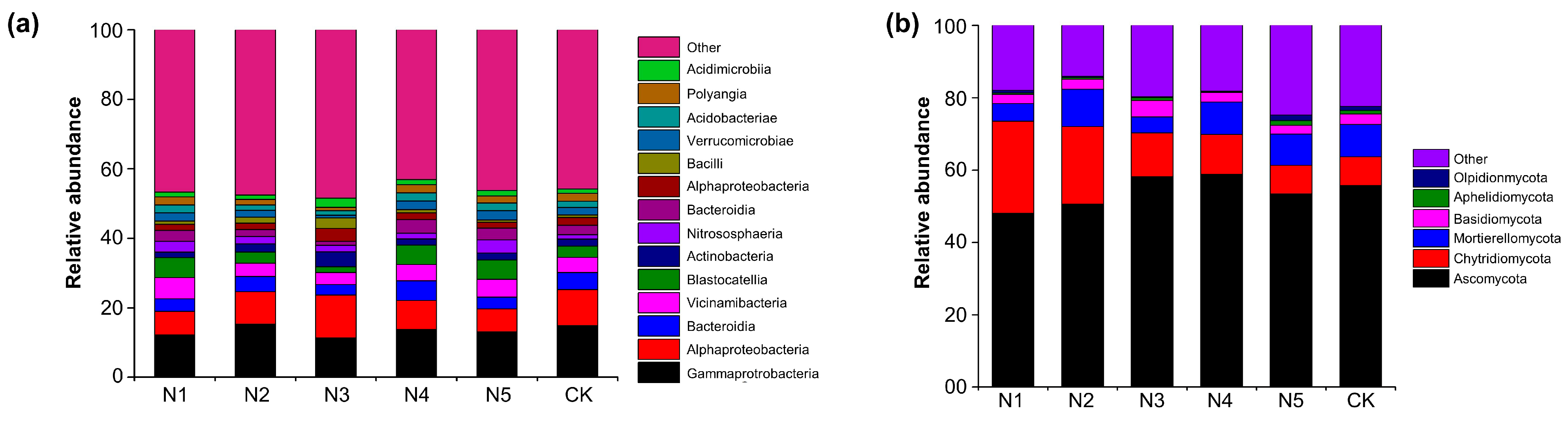
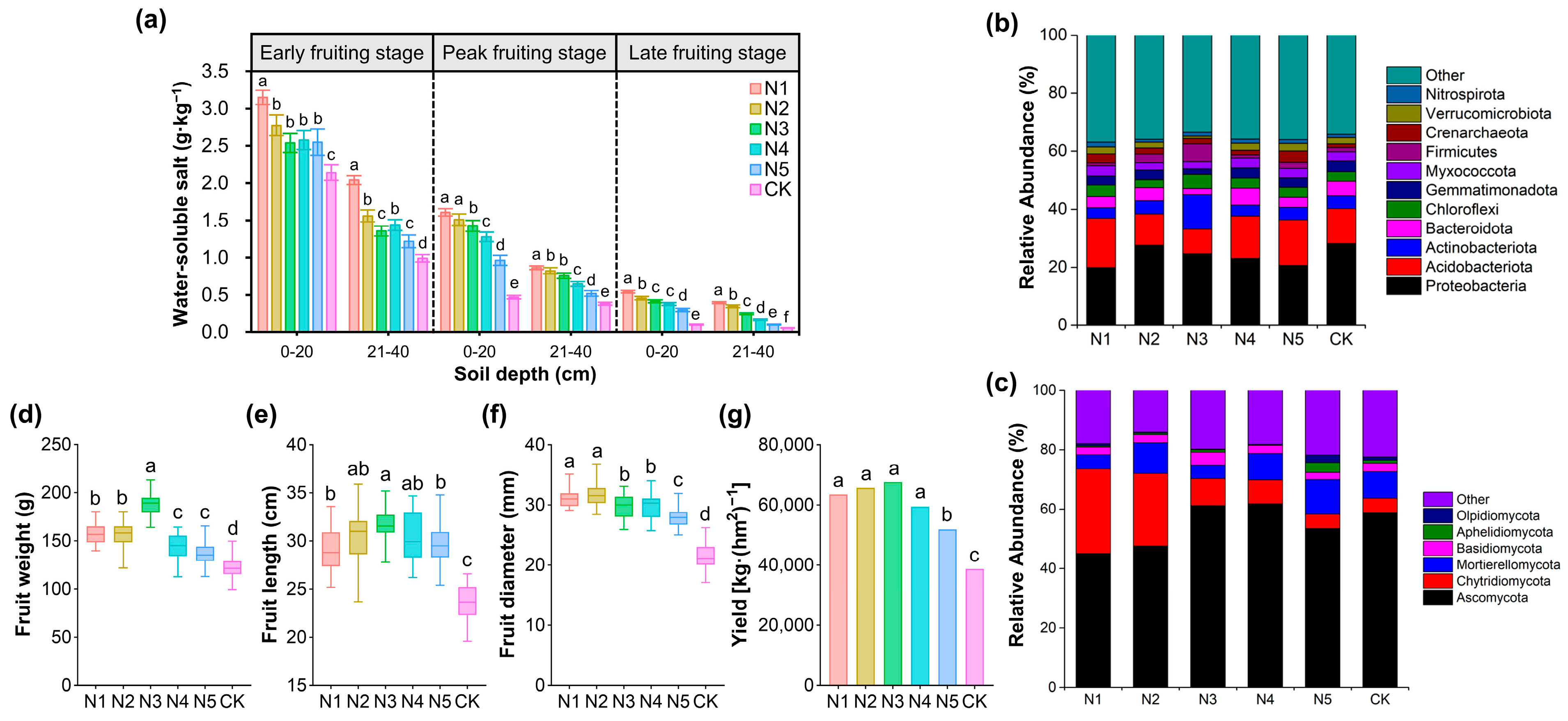
3.2. Sustained Cucumber Yield Achieved with 20% Nitrogen Reduction Through Improved Soil and Microbial Conditions
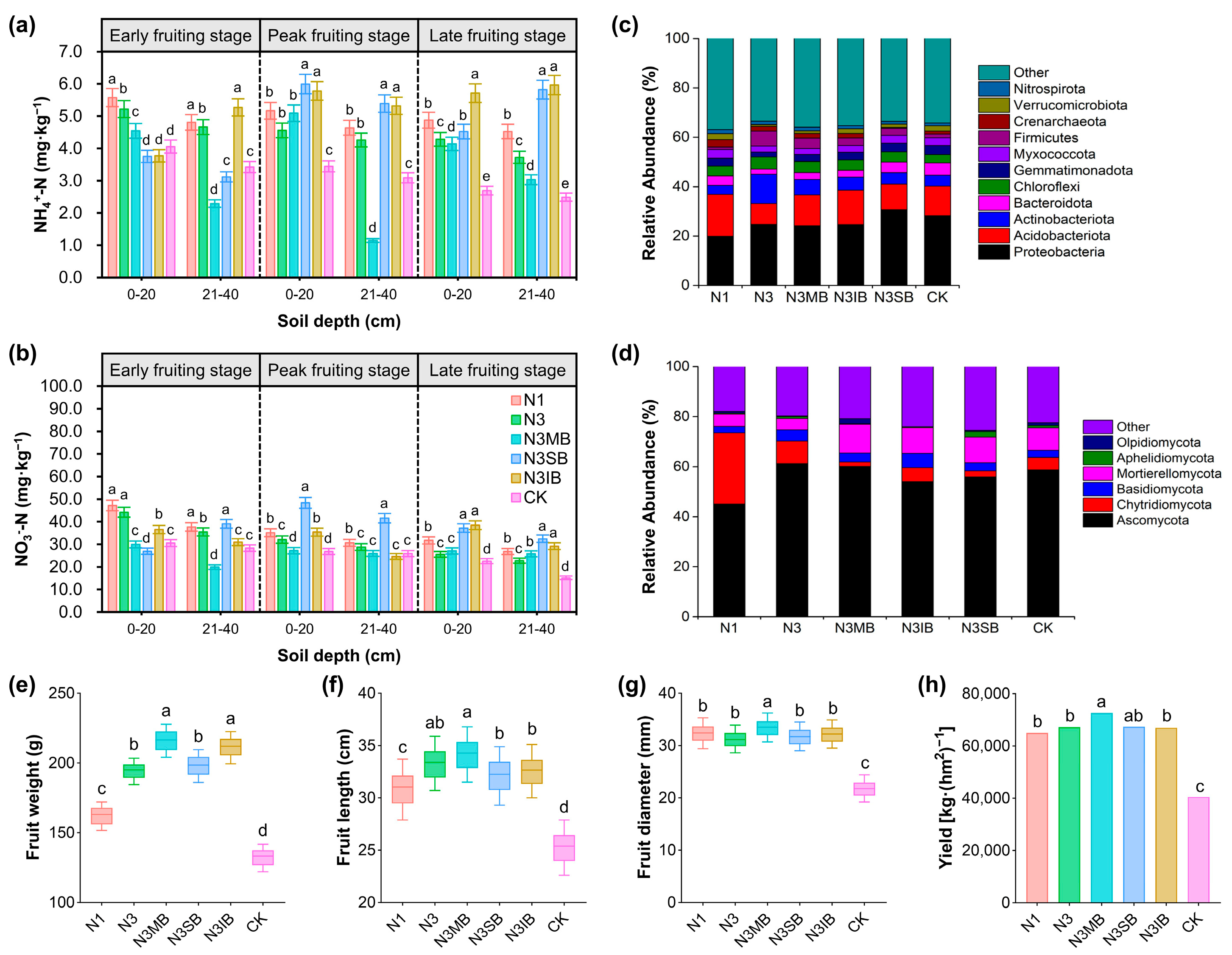
3.3. Applying Bacillus Inoculants with 20% Less Nitrogen Enhances Soil Fertility, Reshapes Microbial Communities, Lowers GWP, and Boosts Cucumber Production
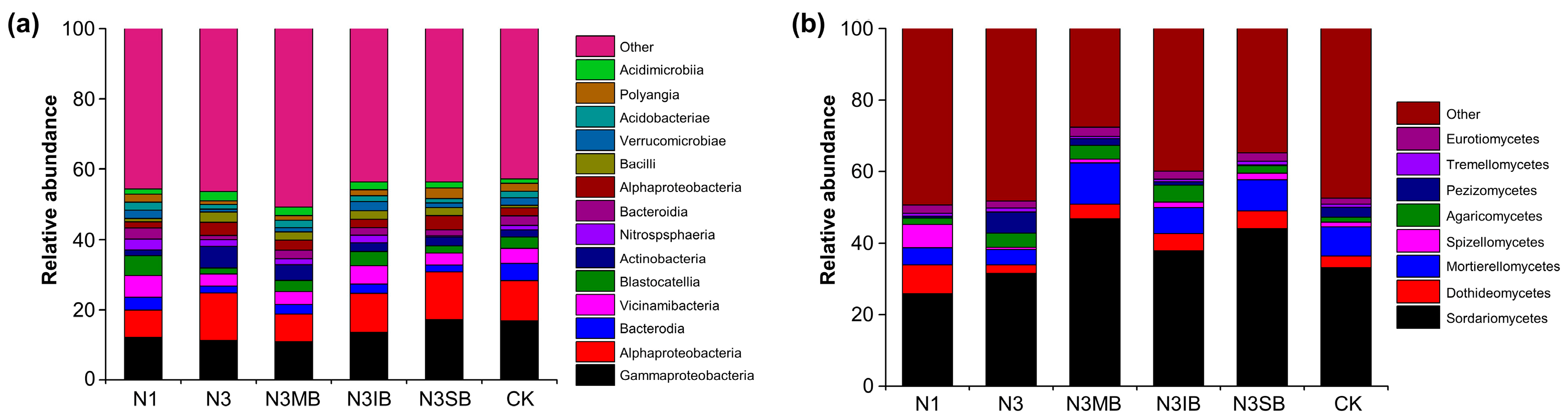
3.4. Optimal Soil Remediation and Crop Performance Achieved via Deep Tillage Implementing 20% N Reduction, B. megaterium Inoculation, and Soybean Straw Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kabir, M.S.N.; Reza, M.N.; Chowdhury, M.; Ali, M.; Samsuzzaman; Ali, M.R.; Lee, K.Y.; Chung, S.O. Technological Trends and Engineering Issues on Vertical Farms: A Review. Horticulturae 2023, 9, 1229. [Google Scholar] [CrossRef]
- Rayhana, R.; Xiao, G.; Liu, Z. Internet of Things Empowered Smart Greenhouse. IEEE J. Radio Freq. Identif. 2020, 4, 195–211. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; You, K.; Zhu, C.; Wang, K.; Gan, M.; Zhang, J. Trends, Drivers, and Land Use Strategies for Facility Agricultural Land during the Agricultural Modernization Process: Evidence from Huzhou City, China. Land 2024, 13, 543. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, Z.; Lu, H. Impact of agricultural production outsourcing service and land fragmentation on agricultural non-point source pollution in China: Evidence from Jiangxi Province. Front. Environ. Sci. 2023, 10, 1079709. [Google Scholar] [CrossRef]
- Song, Q.; Fu, H.; Shi, Q.; Shan, X.; Wang, Z.; Sun, Z.; Li, T. Overfertilization reduces tomato yield under long-term continuous cropping system via regulation of soil microbial community composition. Front. Microbiol. 2022, 13, 952021. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.; Park, K.-C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Wei, B.; Yu, J.; Cao, Z.; Meng, M.; Yang, L.; Chen, Q. The availability and accumulation of heavy metals in greenhouse soils associated with intensive fertilizer application. Int. J. Environ. Res. Public Health 2020, 17, 5359. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, N.; Bao, L. Research progress on soil degradation and regulation of facility agriculture in China. Chin. J. Eco-Agric. 2013, 21, 787–794. [Google Scholar] [CrossRef]
- Ma, J.; Qin, J.; Ma, H.; Zhou, Y.; Shen, Y.; Xie, Y.; Xu, D. Soil characteristic changes and quality evaluation of degraded desert steppe in arid windy sandy areas. PeerJ 2022, 10, e13100. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; Van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Jiang, Y. Response of Nitrogen Losses to Excessive Nitrogen Fertilizer Application in Intensive Greenhouse Vegetable Production. Sustainability 2019, 11, 1513. [Google Scholar] [CrossRef]
- Hellerstein, D.; Vilorio, D.; Ribaudo, M. Agricultural Resources and Environmental Indicators, 2019; Department of Agriculture, Economic Research Service: Washington, DC, USA, 2019. [Google Scholar]
- Ganguly, K.; Gulati, A.; Von Braun, J. Innovations Spearheading the Next Transformations in India’s Agriculture; ZEF Working Paper: Bonn, Germany, 2017. [Google Scholar]
- Good, A.G.; Beatty, P.H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Agriculture. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Pat, Z.H.; Casas-García, R.A.; Meneses, B.U.-L.d. Economic and environmental fertilization optimization on sugar beet farms in a European region. Rev. Chapingo Ser. Hortic. 2014, 20, 117–129. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Chen, J.; Liu, P.; Hou, X.; Yang, L.; Zhang, L. Progress in microbial fertilizer regulation of crop growth and soil remediation research. Plants 2024, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng.-Ground Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Roy, S.; Chowdhury, N. A novel approach toward better use of saline soils vis-á-vis the evaluation of microbial responses. Soil Sci. Annu. 2023, 74, 183656. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2022: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps (4th ed.). 2022. Available online: https://files.isric.org/public/documents/WRB_fourth_edition_2022-12-18.pdf (accessed on 31 August 2024).
- Yang, Y.; Guo, R.-z.; Wang, Y.-q.; Fu, X.; Wang, Y.-l.; Li, X.-g.; Men, M.-x. Effects of three nitrogen control supplements on yield and quality traits of protected cucumber (Cucumis sativus L.). J. Hebei Agric. Univ. 2023, 46, 45–50. [Google Scholar] [CrossRef]
- Gu, J.; Wu, Y.; Tian, Z.; Xu, H. Nitrogen use efficiency, crop water productivity and nitrous oxide emissions from Chinese greenhouse vegetables: A meta-analysis. Sci. Total Environ. 2020, 743, 140696. [Google Scholar] [CrossRef]
- Bao, S. Soil Agricultural Chemistry Analysis; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Li, J.; Yang, X.; Hou, R.; Ma, Y.; Wang, Y.; Ma, Y.; Zhen, W.; Huang, Y.; Fu, X.; Peng, Z. Effects of nitrogen fertilizer basal-to-top-dressing ratios on maize straw decomposition, soil carbon and nitrogen, and bacterial community structure in different soil textures on the north china plain. Front. Microbiol. 2025, 16, 1506155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Dai, W.; Feng, G.; Huang, Y.; Adeli, A.; Jenkins, J.N. Influence of cover crops on soil aggregate stability, size distribution and related factors in a no-till field. Soil Tillage Res. 2024, 244, 106197. [Google Scholar] [CrossRef]
- Liu, M.; Gan, B.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen and phosphorus addition on soil extracellular enzyme activity and stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, L.; Liu, L.; Sheng, F.; Cao, C.; Li, C. Effects of long-term no tillage and straw return on greenhouse gas emissions and crop yields from a rice-wheat system in central China. Agric. Ecosyst. Environ. 2021, 322, 107650. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Climate change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Guo, R.; Yang, Y.; Wang, Y.; Sun, K.; Peng, Z.; Yao, P.; Xie, B.; Men, M. Effects of nitrogen reduction on yield, quality of greenhouse cucumber and soil nitrogen balance. J. Hebei Agric. Univ. 2022, 45, 44–50. [Google Scholar] [CrossRef]
- Balleux, G.; Höfte, M.; Arguelles-Arias, A.; Deleu, M.; Ongena, M. Bacillus lipopeptides as key players in rhizosphere chemical ecology. Trends Microbiol. 2025, 33, 80–95. [Google Scholar] [CrossRef]
- Ninkuu, V.; Liu, Z.; Qin, A.; Xie, Y.; Song, X.; Sun, X. Impact of straw returning on soil ecology and crop yield: A review. Heliyon 2025, 11, e41651. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Bian, T.; Song, Q.; Wu, G.; Awais, M.; Liu, Y.; Fu, H.; Sun, Z. Effects of nitrogen addition on soil microbial functional diversity and extracellular enzyme activities in greenhouse cucumber cultivation. Agriculture 2022, 12, 1366. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Bian, T.; Wang, T.; Fu, H.; Sun, Z. Revealing the Response of Cucumber Soil Microbial Community Composition and Function to Nitrogen Addition in Northern Chinese Greenhouses. Horticulturae 2024, 10, 1090. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, C.-W.; Song, Y.-S.; Lee, Y.-J. Varying nitrogen fertigation for cucumbers grown in greenhouses with soil of optimal or high nutrient status. Korean J. Soil Sci. Fertil. 2022, 55, 27–37. [Google Scholar] [CrossRef]
- Steponavičienė, V.; Rudinskienė, A.; Žiūraitis, G.; Bogužas, V. The impact of tillage and crop residue incorporation systems on agrophysical soil properties. Plants 2023, 12, 3386. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of Northeast China. PLoS ONE 2018, 13, e0199523. [Google Scholar] [CrossRef]
- Naresh, R.K.; Singh, P.K.; Bhatt, R.; Chandra, M.S.; Kumar, Y.; Mahajan, N.C.; Gupta, S.K.; Al-Ansari, N.; Mattar, M.A. Long-term application of agronomic management strategies effects on soil organic carbon, energy budgeting, and carbon footprint under rice-wheat cropping system. Sci. Rep. 2024, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Hu, H.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of deep tillage and straw returning on soil microorganism and enzyme activities. Sci. World J. 2014, 2014, 451493. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Yao, B.; Peng, Y.; Gao, C.; Qin, T.; Zhou, Y.; Sun, C.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Zhang, H.; Li, S.; Zheng, X.; Zhang, J.; Zhang, H.; Zhou, S.; Sun, H.; Lv, W. Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice-wheat rotation system. PeerJ 2019, 6, e6171. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, P.; Wang, K.; Ding, R.; Yang, B.; Nie, J.; Jia, Z.; Han, Q. Effects of wheat straw incorporation on the availability of soil nutrients and enzyme activities in semiarid areas. PLoS ONE 2015, 10, e0120994. [Google Scholar] [CrossRef]
- Sui, P.; Tian, P.; Wang, Z.; Lian, H.; Yang, Y.; Ma, Z.; Jiang, Y.; Zheng, J.; Qi, H. Soil properties and microbial communities of spring maize filed in response to tillage with straw incorporation and nitrogen fertilization in northeast China. PeerJ 2022, 10, e13462. [Google Scholar] [CrossRef]
- Galindo, F.S.; Strock, J.S.; Pagliari, P.H. Impacts of corn stover management and fertilizer application on soil nutrient availability and enzymatic activity. Sci. Rep. 2022, 12, 1985. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Zhang, Z.; Duan, X.; et al. Straw addition enhances crop yield, soil aggregation, and soil microorganisms in a 14-year wheat-rice rotation system in central China. Plants 2024, 13, 985. [Google Scholar] [CrossRef]
- Zhang, H.; Batchelor, W.D.; Hu, K.; Han, H.; Li, J. Modeling nitrogen fate and water and nitrogen use efficiencies under different greenhouse vegetable production systems using the WHCNS-Veg model. Plants 2024, 13, 1384. [Google Scholar] [CrossRef]
- Wang, J.; Hussain, S.; Sun, X.; Zhang, P.; Javed, T.; Dessoky, E.S.; Ren, X.; Chen, X. Effects of Nitrogen Application Rate Under Straw Incorporation on Photosynthesis, Productivity and Nitrogen Use Efficiency in Winter Wheat. Front. Plant Sci. 2022, 13, 862088. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Yao, H.; Zhang, B.; Li, Y.; Jin, S.; Cao, H. Reducing Application of Nitrogen Fertilizer Increases Soil Bacterial Diversity and Drives Co-Occurrence Networks. Microorganisms 2024, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Leite, M.F.; Pan, Y.; Bloem, J.; Berge, H.T.; Kuramae, E.E. Organic nitrogen rearranges both structure and activity of the soil-borne microbial seedbank. Sci. Rep. 2017, 7, 42634. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Muhammad, I.; Chi, Y.X.; Wang, D.; Zhou, X.B. Straw Return and Nitrogen Fertilization to Maize Regulate Soil Properties, Microbial Community, and Enzyme Activities Under a Dual Cropping System. Front. Microbiol. 2022, 13, 823963. [Google Scholar] [CrossRef]
- Wang, B.; Chu, S.; Liu, X.; Zhang, D.; Chai, X.; Yang, X.; Zhi, Y.; Chi, Y.; Zhou, P. Changes in soil bacterial and fungal communities in response to Bacillus megaterium NCT-2 inoculation in secondary salinized soil. PeerJ 2021, 9, e12309. [Google Scholar] [CrossRef]
- Dong, H.; Fan, S.; Sun, H.; Chen, C.; Wang, A.; Jiang, L.; Ma, D. Rhizosphere-Associated Microbiomes of Rice (Oryza sativa L.) Under the Effect of Increased Nitrogen Fertilization. Front. Microbiol. 2021, 12, 730506. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Zeng, X.B.; Bai, L.Y.; Mei, X.R.; Yang, J.B.; Hu, L.J. Cadmium accumulation in vegetable plantation land soils under protected cultivation: A case study. Commun. Soil Sci. Plant Anal. 2009, 40, 2169–2184. [Google Scholar] [CrossRef]
- Pu, J.; Li, Z.; Tang, H.; Zhou, G.; Wei, C.; Dong, W.; Jin, Z.; He, T. Response of soil microbial communities and rice yield to nitrogen reduction with green manure application in karst paddy areas. Front. Microbiol. 2023, 13, 1070876. [Google Scholar] [CrossRef] [PubMed]

| Treatment | N2O GWP | CO2 GWP | CH4 GWP | Total GWP |
|---|---|---|---|---|
| N3 | 128.46 a | 5185.51 a | −10.41 c | 5303.56 a |
| N3IB | 76.85 c | 3816.00 c | −5.78 a | 3887.07 c |
| N3SB | 100.17 b | 4348.80 b | −7.73 b | 4441.24 b |
| N3MB | 125.34 a | 5123.15 a | −10.74 c | 5237.75 a |
| Management | Fertilizer Use Efficiency - Nitrogen | Fertilizer Use Efficiency - Phosphorus | Fertilizer Use Efficiency - Potassium |
|---|---|---|---|
| SCK | 96.76 b | 154.81 b | 58.05 b |
| SG1 | 98.14 b | 157.02 b | 58.88 b |
| SG2 | 106.30 a | 170.08 a | 63.78 a |
| SG3 | 102.02 ab | 163.23 ab | 61.21 ab |
| DCK | 100.20 c | 160.33 c | 60.12 c |
| DG1 | 104.86 b | 167.77 b | 62.91 b |
| DG2 | 105.12 b | 168.19 b | 63.07 b |
| DG3 | 113.69 a | 181.91 a | 68.22 a |
| Significance | |||
| T | ** | ** | *** |
| G | ** | *** | *** |
| T × G | * | * | ** |
| Management | N2O GWP | CO2 GWP | CH4 GWP | Total GWP |
|---|---|---|---|---|
| SCK | 1435.09 a | 6323.32 a | −16.66 c | 7741.74 a |
| SG1 | 1059.85 b | 5331.99 ab | −13.49 bc | 6378.35 ab |
| SG2 | 800.90 c | 3803.96 c | −6.95 a | 4597.91 c |
| SG3 | 978.19 b | 5203.47 b | −11.32 b | 6170.35 b |
| DCK | 1024.08 a | 4525.70 ab | −12.97 b | 5536.81 a |
| DG1 | 869.02 ab | 3816.21 b | −8.41 a | 4676.82 b |
| DG2 | 786.77 bc | 4039.84 b | −7.48 a | 4819.12 ab |
| DG3 | 678.96 c | 3722.48 b | −5.91 a | 4395.53 b |
| Significance | ||||
| T | *** | *** | ** | ** |
| G | *** | *** | ** | ** |
| T × G | * | ** | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Guo, R.; Fu, X.; Sun, T.; Wang, Y.; Peng, Z. An Integrated Strategy of Nitrogen Reduction, Microbial Amendment, and Straw Incorporation Mitigates Soil Degradation and Enhances Cucumber Yield in Northern Chinese Greenhouses. Agriculture 2025, 15, 2231. https://doi.org/10.3390/agriculture15212231
Yang Y, Guo R, Fu X, Sun T, Wang Y, Peng Z. An Integrated Strategy of Nitrogen Reduction, Microbial Amendment, and Straw Incorporation Mitigates Soil Degradation and Enhances Cucumber Yield in Northern Chinese Greenhouses. Agriculture. 2025; 15(21):2231. https://doi.org/10.3390/agriculture15212231
Chicago/Turabian StyleYang, Yang, Runze Guo, Xin Fu, Tianjie Sun, Yanqun Wang, and Zhengping Peng. 2025. "An Integrated Strategy of Nitrogen Reduction, Microbial Amendment, and Straw Incorporation Mitigates Soil Degradation and Enhances Cucumber Yield in Northern Chinese Greenhouses" Agriculture 15, no. 21: 2231. https://doi.org/10.3390/agriculture15212231
APA StyleYang, Y., Guo, R., Fu, X., Sun, T., Wang, Y., & Peng, Z. (2025). An Integrated Strategy of Nitrogen Reduction, Microbial Amendment, and Straw Incorporation Mitigates Soil Degradation and Enhances Cucumber Yield in Northern Chinese Greenhouses. Agriculture, 15(21), 2231. https://doi.org/10.3390/agriculture15212231




