Biomass and Nickel Tolerance: Canavalia ensiformis (L.) DC. as a Candidate Plant for Phytoremediation Applications
Abstract
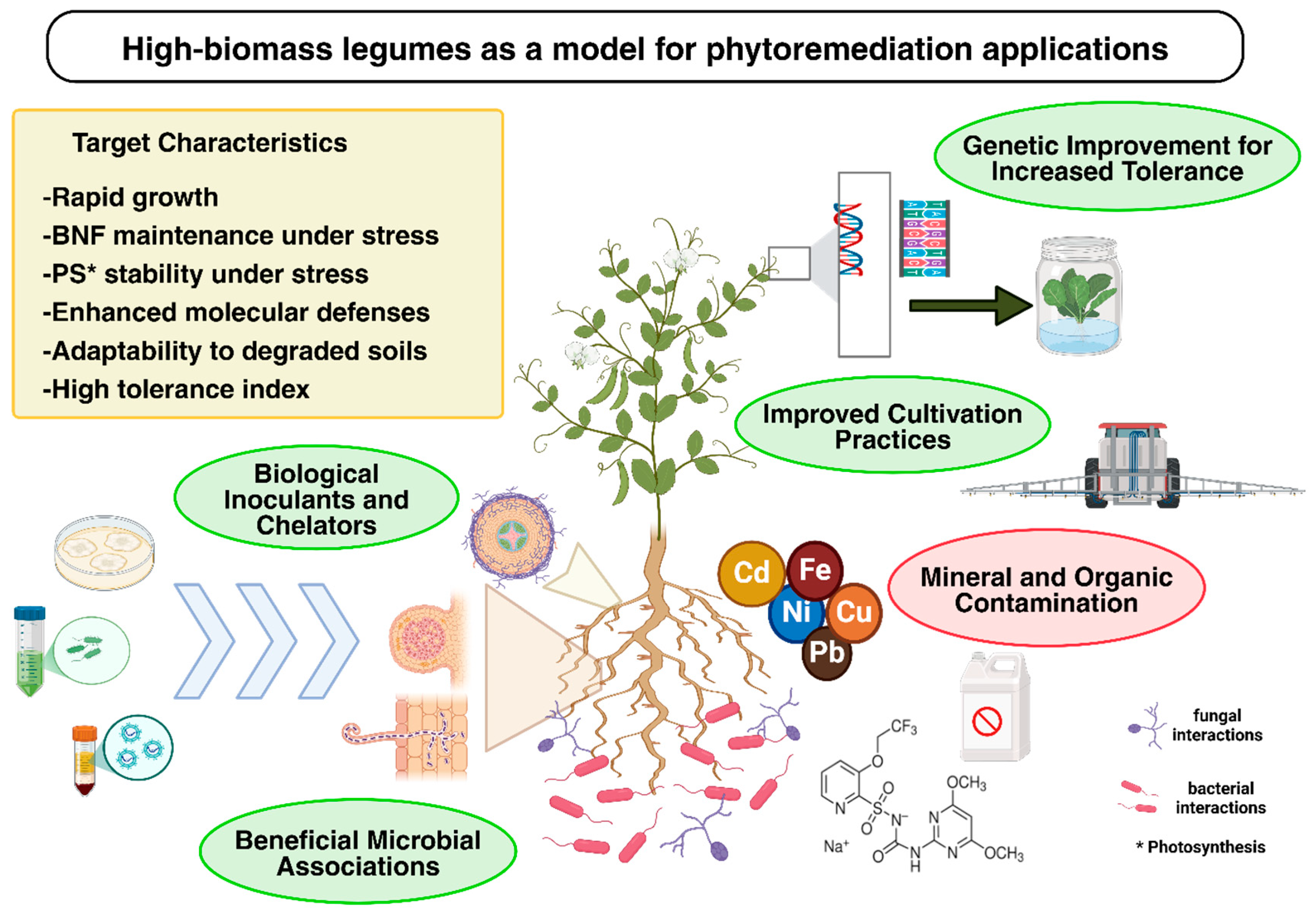
1. Introduction
2. Materials and Methods
2.1. Experimental Site
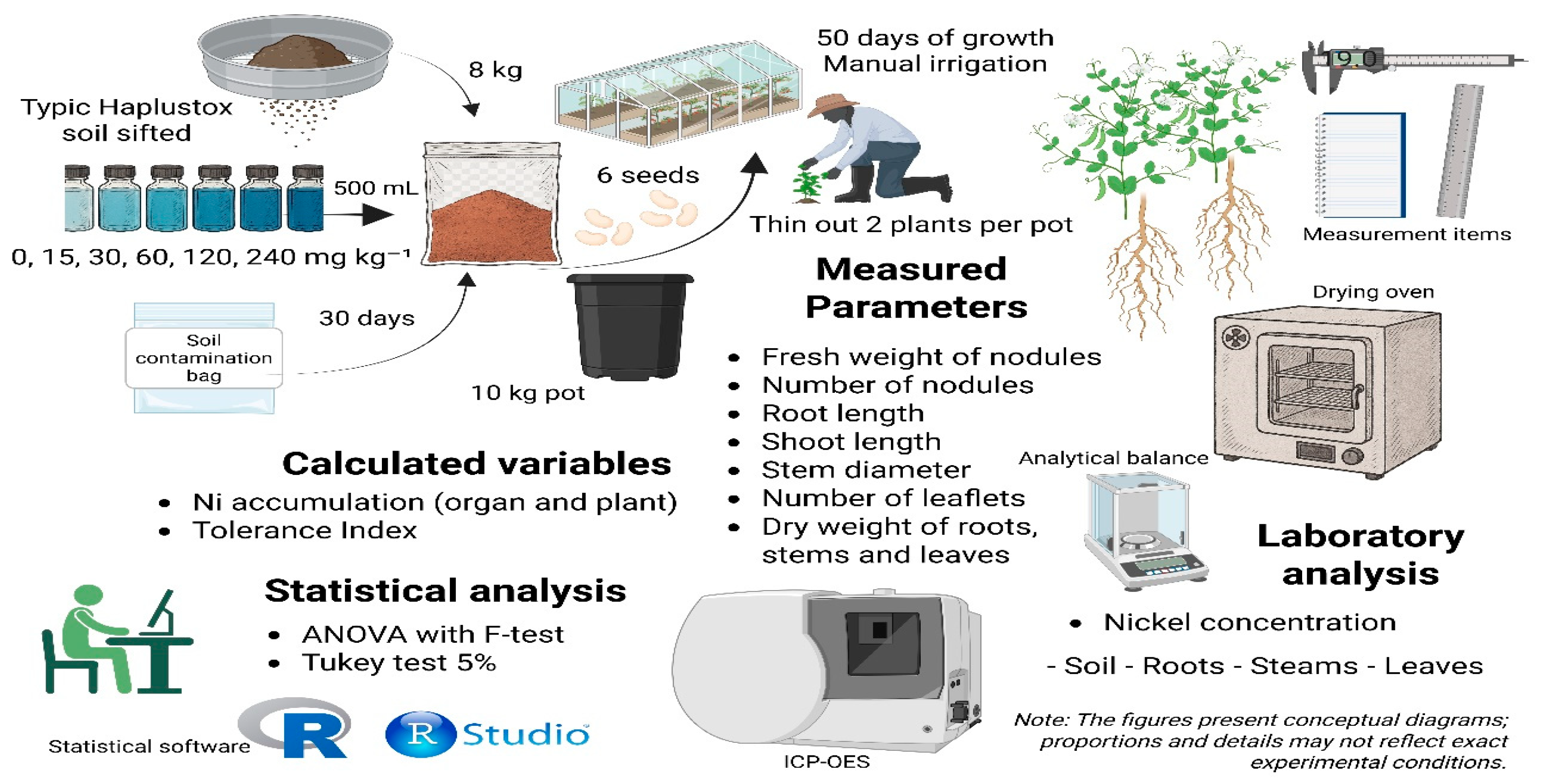
2.2. Growing Conditions, Experimental Design and Treatments
2.3. Ni Concentration in Soil Samples and Plant Tissues
2.4. Sample Collection, Measured and Calculated Parameters & Statistical Analysis
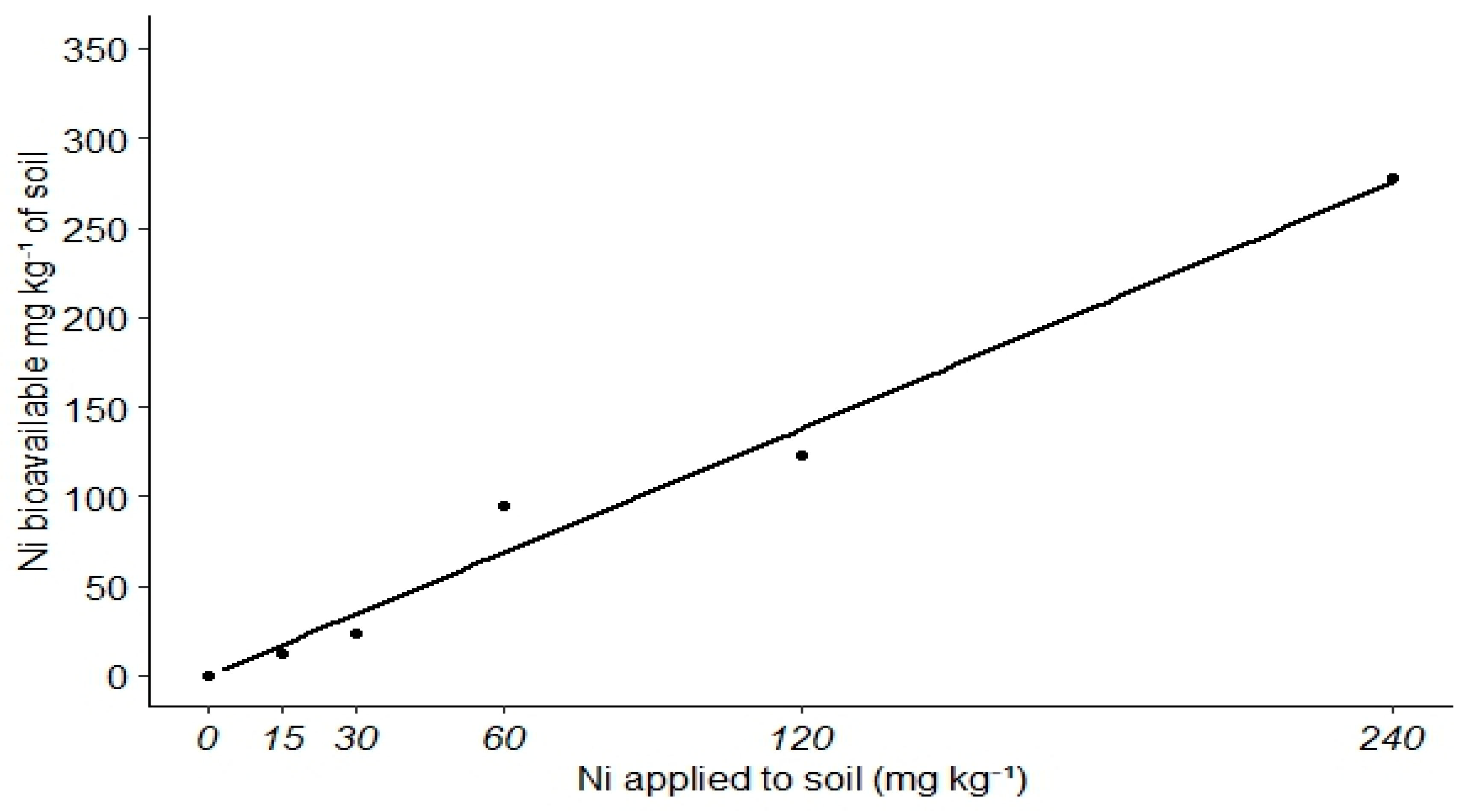
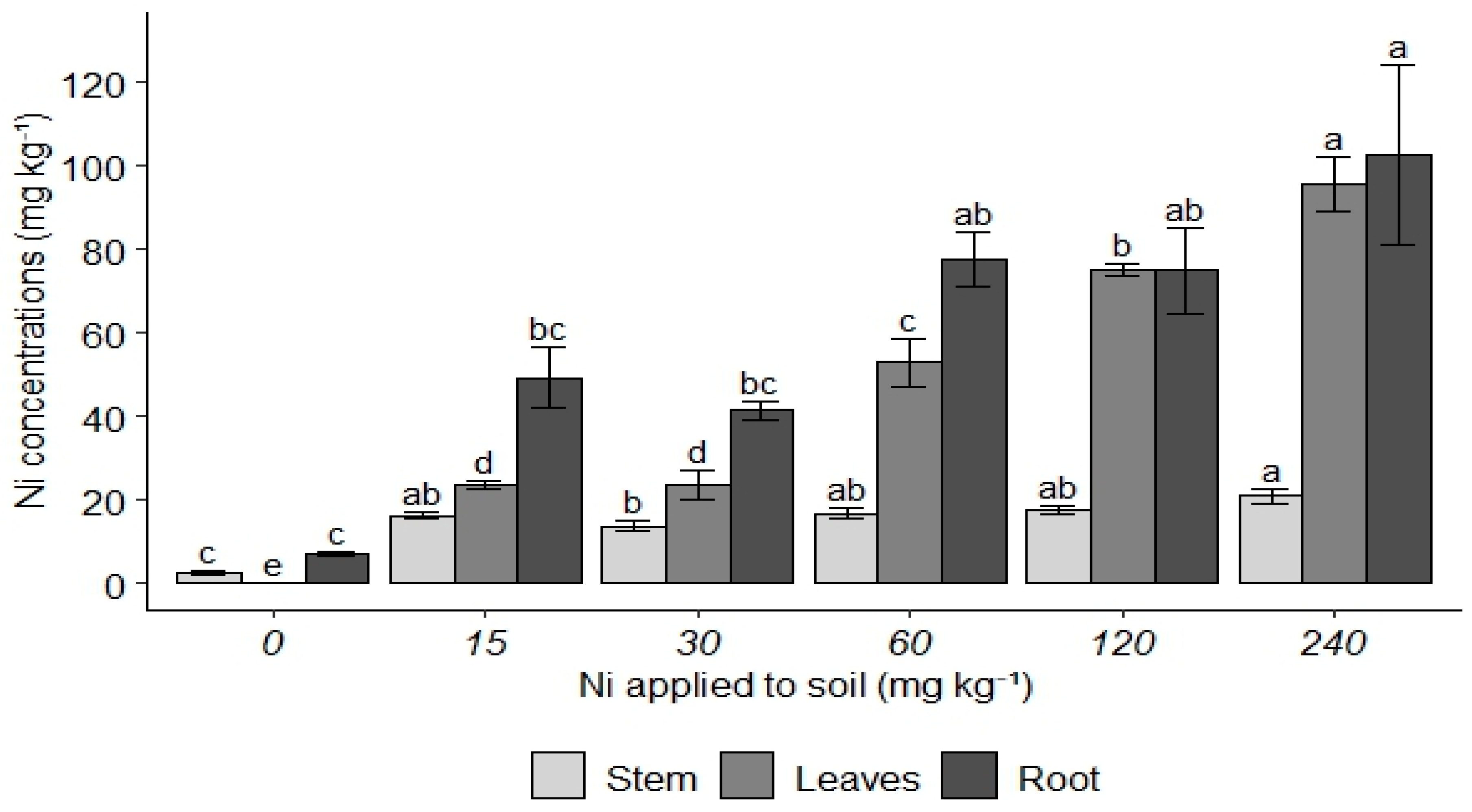
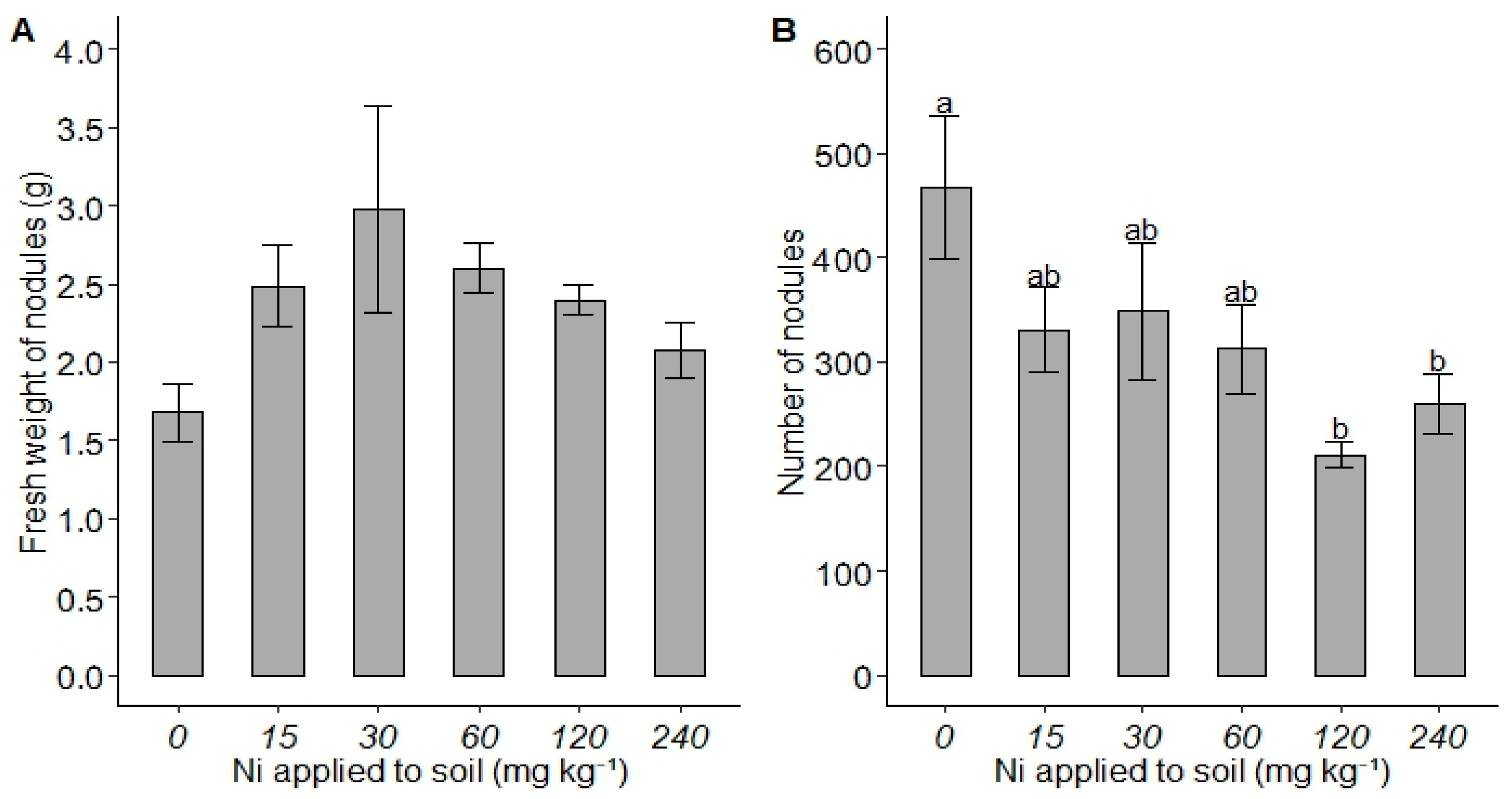
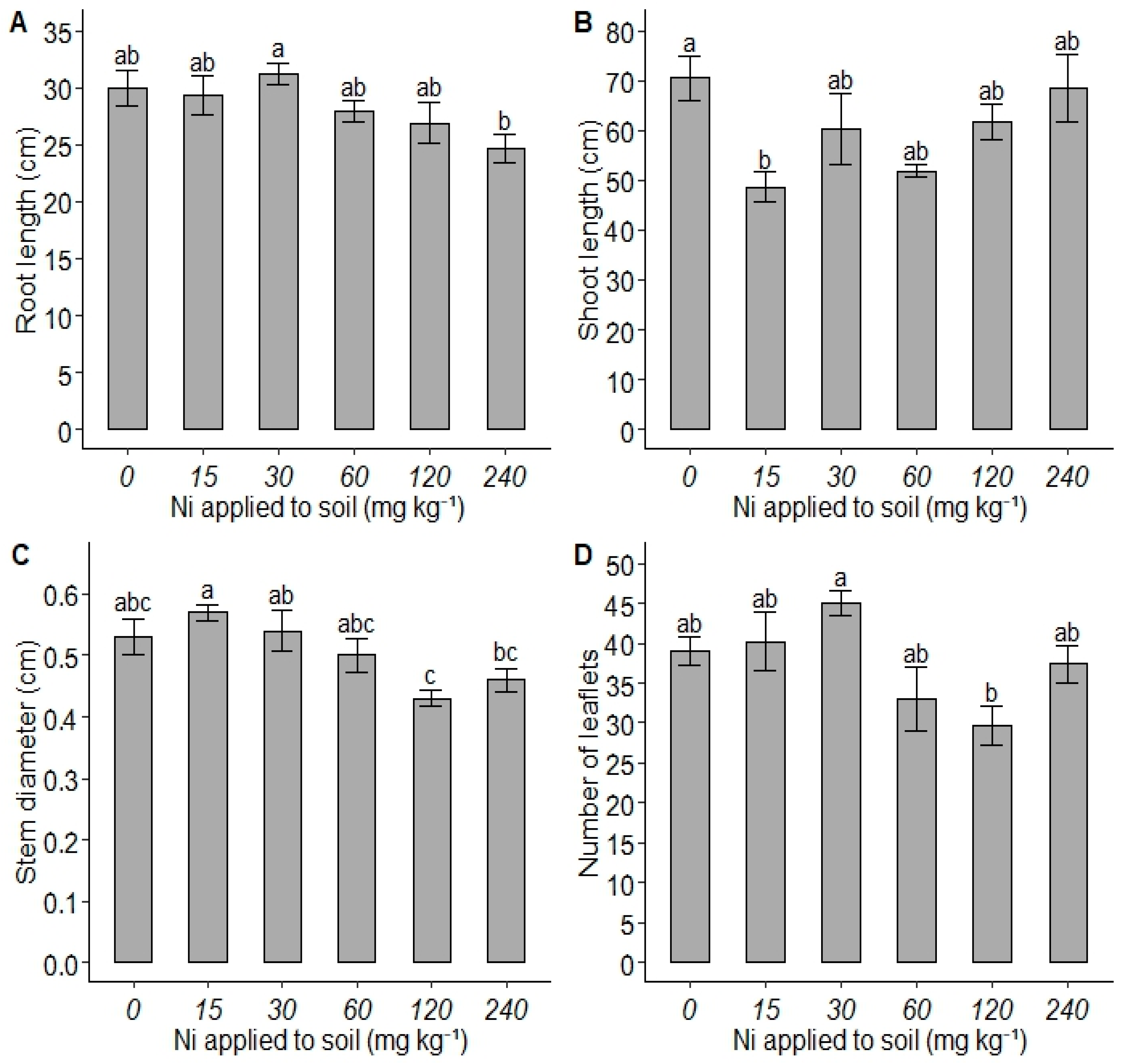
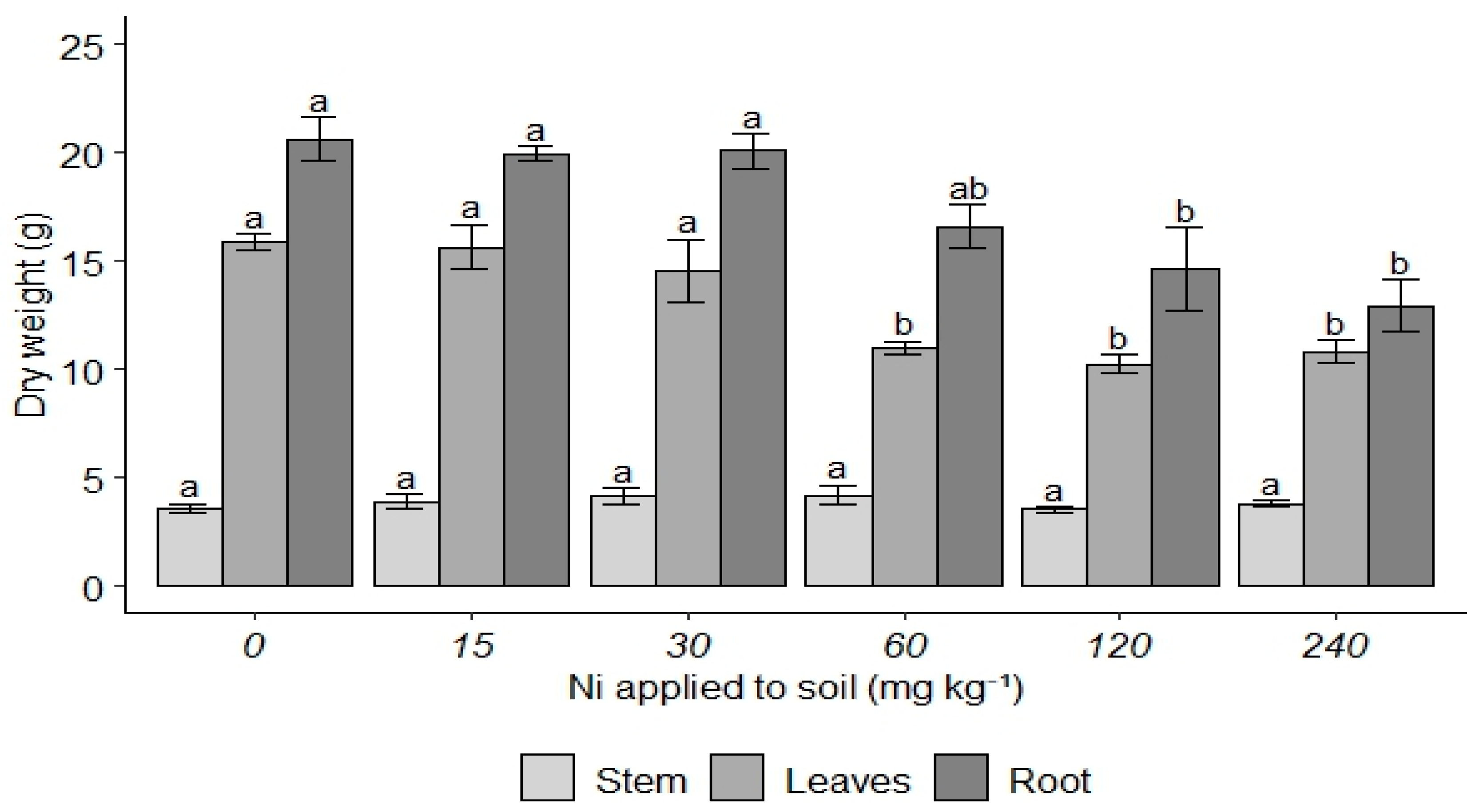
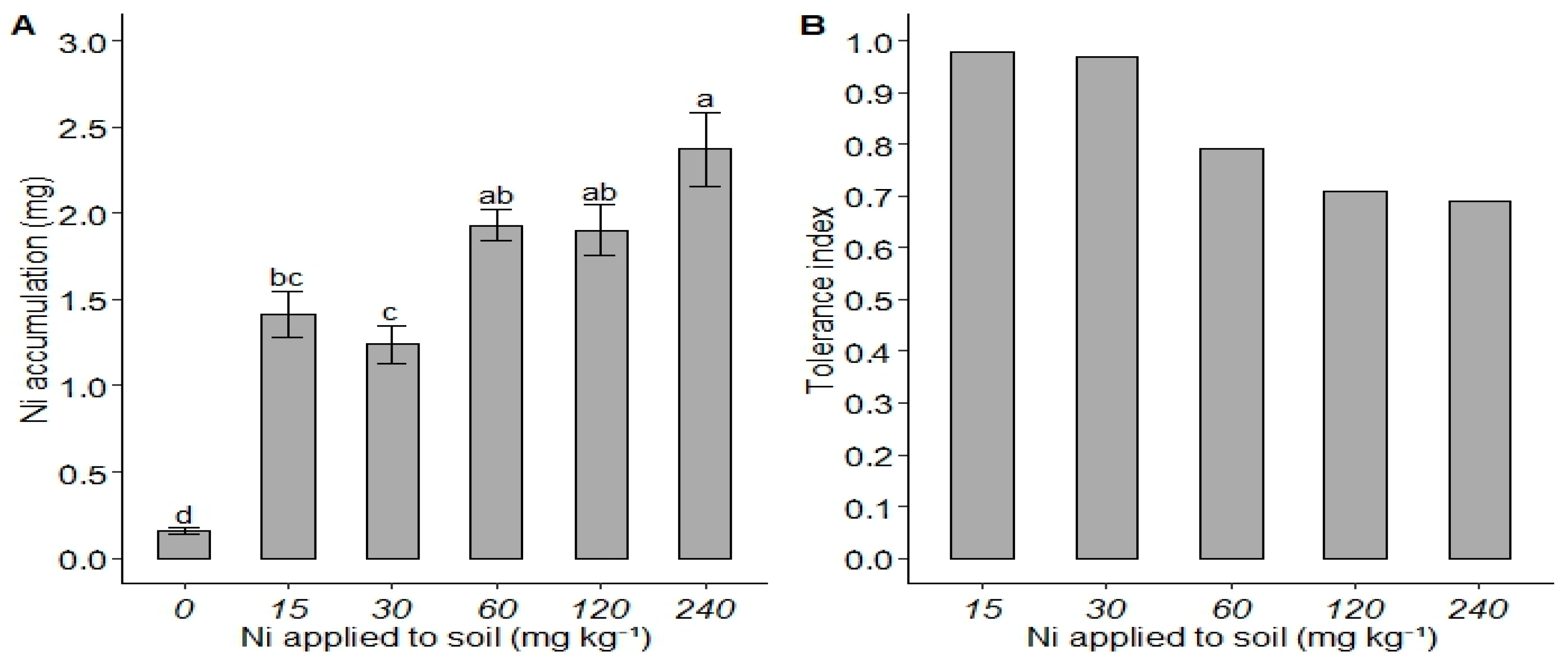
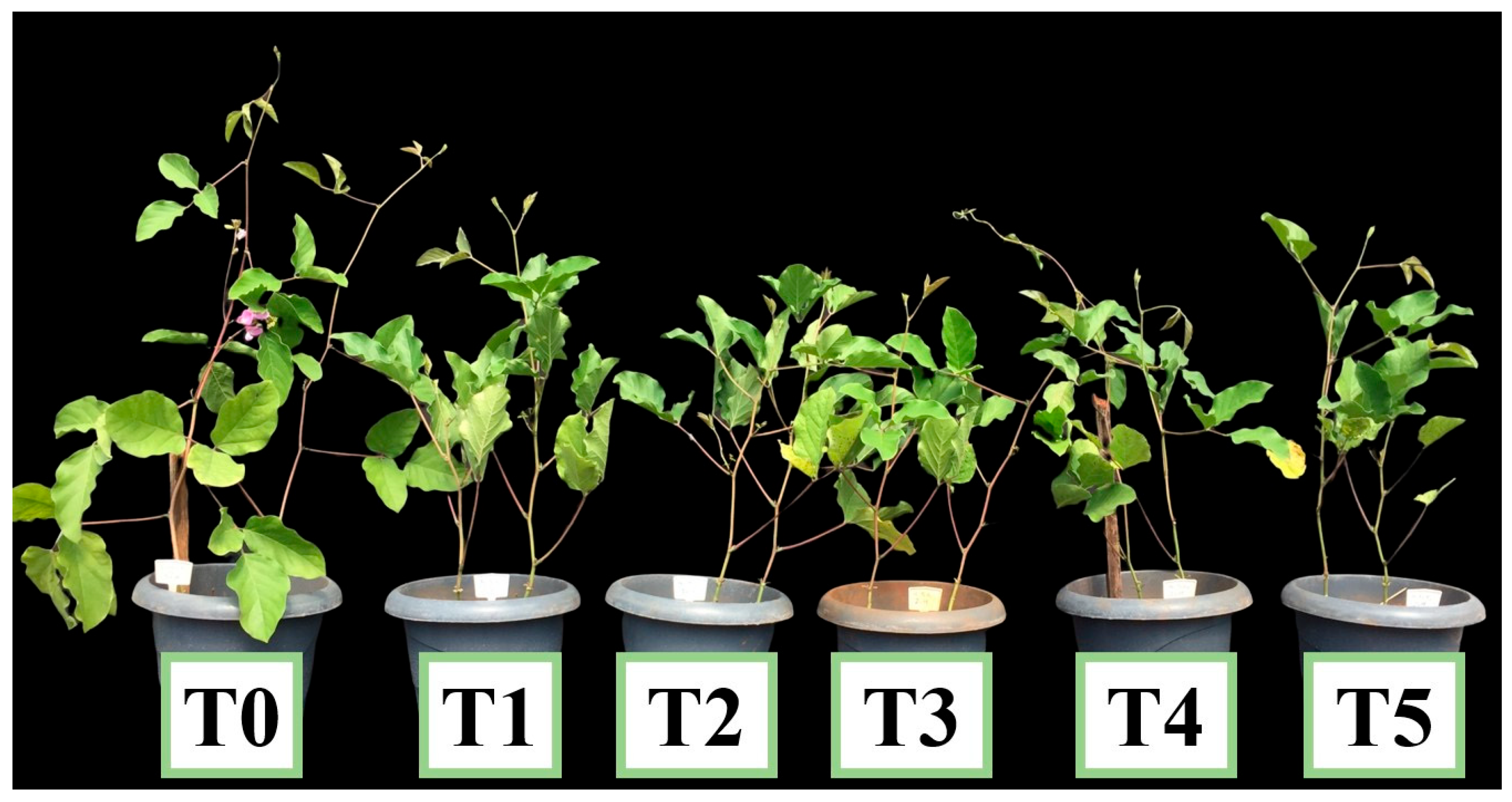
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasojo, Y.S.; Prasetyo, B.; Suwignyo, B. Morphology characteristic and biomass production of jack bean (Canavalia ensiformis) at different growth stages in Blora, Central Java, Indonesia. Aust. J. Crop Sci. 2025, 19, 84–88. [Google Scholar] [CrossRef]
- Lima Filho, O.F.D.; Ambrosano, E.J.; Wutke, E.B.; Ross, F.; Carlos, J.A.D. Adubação Verde e Plantas de Cobertura No Brasil: Fundamentos e Prática, 2nd ed.; rev. e atual.; Embrapa: Brasília, DF, Brazil, 2023; Volume 1. [Google Scholar]
- Sheahan, C.M. Plant Guide for Jack Bean (Canavalia ensiformis); USDA: Washington, DC, USA, 2023. [Google Scholar]
- Sumner, J.B. The isolation and crystallization of the enzyme urease: Preliminary paper. J. Biol. Chem. 1926, 69, 435–441. [Google Scholar] [CrossRef]
- Krajewska, B.; Ciurli, S. Jack bean (Canavalia ensiformis) urease. Probing acid–base groups of the active site by pH variation. Plant Physiol. Biochem. 2005, 43, 651–658. [Google Scholar] [CrossRef]
- Gonçalves, R.N.; Gozzini Barbosa, S.D.; Silva-López, R.E.d. Corrigendum to “Proteases from Canavalia ensiformis: Active and Thermostable Enzymes with Potential of Application in Biotechnology”. Biotechnol. Res. Int. 2016, 2016, 9872540. [Google Scholar] [CrossRef]
- Raqueti, G.S.; Ferreira, T.C.; Olivio, M.L.G.; Morais, F.A.A.; Coscione, A.R.; Camargos, L.S. Emergence and N metabolism of Canavalia ensiformis (L.) DC. seedlings in soil contaminated by nickel. Not. Bot. Horti Agrobot. 2025, 53, 14214. [Google Scholar] [CrossRef]
- Tabaldi, L.A.; Vieira, M.d.C.; Zárate, N.A.H.; Silva, L.R.d.; Gonçalves, W.L.F.; Pilecco, M.; Formagio, A.S.N.; Gas-si, R.P.; Padovan, M.P. Cover crops and their effects on the biomass yield of Serjania marginata plants. Cienc. Rural 2012, 42, 614–620. [Google Scholar] [CrossRef][Green Version]
- Rayol, B.P.; Alvino-Rayol, F.d.O. Produção de Biomassa e Teor de Nutrientes do Feijão-de-Porco (Canavalia ensiformis (L.) DC.) em Reflorestamento No Estado do Pará. Rev. Agroecossist. 2013, 4, 85–90. [Google Scholar] [CrossRef]
- Guerra Sierra, B.E.; Muñoz Guerrero, J.; Sokolski, S. Phytoremediation of Heavy Metals in Tropical Soils an Overview. Sustainability 2021, 13, 2574. [Google Scholar] [CrossRef]
- Souza, L.A.; Piotto, F.A.; Nogueirol, R.C.; Azevedo, R.A. Use of non-hyperaccumulator plant species for the phytoextraction of heavy metals using chelating agents. Sci. Agric. 2013, 70, 290–295. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, X.; Liao, J.; Zheng, L.; Huang, X. Efficient phytoremediation of Cd-contaminated soils by Tagetes patula L.: Greenhouse experiment, field study and meta-analysis. Curr. Res. Biotechnol. 2024, 7, 100212. [Google Scholar] [CrossRef]
- Robinson, B.H.; Bañuelos, G.; Conesa, H.M.; Evangelou, M.W.H.; Schulin, R. The Phytomanagement of Trace Elements in Soil. Crit. Rev. Plant Sci. 2009, 28, 240–266. [Google Scholar] [CrossRef]
- Santos, J.B.d.; Procópio, S.d.O.; Pires, F.R.; Silva, A.A.d.; Santos, E.A.d. Fitorremediação de solo contaminado com trifloxysulfuron-sodium por diferentes densidades populacionais de feijão-de-porco (Canavalia ensiformis (L). DC.). Ciênc. Agrotec. 2006, 30, 444–449. [Google Scholar] [CrossRef]
- Araujo, G.R.; Augusto de Paiva Ferreira, G.; Vaz, V.; da Costa Lima, A.; Spolidorio, E.S.; Mendes, K.F. Canavalia ensiformis enhances the phytoremediation of remineralized and sulfentrazone-contaminated tropical soils. Chemosphere 2024, 348, 140725. [Google Scholar] [CrossRef]
- Pereira, B.F.F. Potencial Fitorremediador das Culturas de Feijão-de-Porco, Girassol E Milho Cultivadas em Latossolo Vermelho Contaminado com Chumbo. Master’s Thesis, Instituto Agronômico de Campinas (IAC), Campinas, Brazil, 2005. [Google Scholar]
- Savani, F.R. Avaliação de Feijão de Porco (Canavalia ensiformis) como Fitorremediador de Pb, Cu e Zn em Solos. Masther’s Thesis, Universidade Federal do ABC, Santo André, Brazil, 2016. [Google Scholar]
- Mendes, T.F.S.; Ferreira, T.C.; Bomfim, N.C.P.; Aguilar, J.V.; de Camargos, L.S. Differential Mechanisms of Tolerance and Accumulation in Cajanus cajan (L.) Millsp. and Canavalia ensiformis (L.) DC in Response to Soil Manganese Concentration. Soil Sediment Contam. Int. J. 2024, 34, 85–103. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Aguilar, J.V.; Bomfim, N.C.P.; Olivio, M.L.G.; dos Santos, B.S.; Rosalem, P.F.; de Camargos, L.S. The Fabaceae family in the face of the influence of the potentially toxic element nickel: A scoping review. Discov. Plants 2025, 2, 39. [Google Scholar] [CrossRef]
- Borah, P.; Rene, E.R.; Rangan, L.; Mitra, S. Phytoremediation of nickel and zinc using Jatropha curcas and Pongamia pinnata from the soils contaminated by municipal solid wastes and paper mill wastes. Environ. Res. 2023, 219, 115055. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.V.; Ferreira, T.C.; Bomfim, N.C.P.; Mendes, T.F.S.; de Marcos Lapaz, A.; Brambilla, M.R.; Coscione, A.R.; de Souza, L.A.; Junior, E.F.; de Camargos, L.S. Different responses to phenological stages: A role for nickel in growth and physiology of herbaceous cotton. Plant Growth Regul. 2023, 101, 663–678. [Google Scholar] [CrossRef]
- Pishchik, V.; Mirskaya, G.; Chizhevskaya, E.; Chebotar, V.; Chakrabarty, D. Nickel stress-tolerance in plant-bacterial associations. PeerJ 2021, 9, e12230. [Google Scholar] [CrossRef]
- Tsadilas, C.; Rinklebe, J.; Selim, M. (Eds.) Nickel in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-15466-4. [Google Scholar]
- CETESB (Companhia Ambiental do Estado de São Paulo). Valores Orientadores para o Solo e as Águas Subterrâneas no Estado de São Paulo [Guiding Values for Soil and Groundwater in the State of São Paulo]. 2021. Available online: https://cetesb.sp.gov.br/solo/valores-orientadores-para-solo-e-agua-subterranea/ (accessed on 24 January 2024).
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, DF, Brazil, 2017. [Google Scholar]
- Raij, B.v.; Andrade, J.C.d.; Cantarella, H.; Quaggio, J.A. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- United States Environmental Protection Agency. Guidance for Preparing Standard Operating Procedures (SOPs); EPA/600/B-07/001; U.S. EPA: Washington, DC, USA, 2007.
- United States Environmental Protection Agency. SW-846 Test Method 3051A: Microwave assisted acid digestion of sediments, sludges, soils, and oils. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods (SW-846); U.S. EPA: Washington, DC, USA, 2015. [Google Scholar]
- United States Environmental Protection Agency. Method 6010C (SW-846): Inductively Coupled Plasma-Atomic Emission Spectrometry; U.S. EPA: Washington, DC, USA, 2018.
- Ibañez, T.B.; Santos, L.F.d.M.; Lapaz, A.d.M.; Ribeiro, I.V.; Ribeiro, F.V.; Reis, A.R.d; Moreira, A.; Heinrichs, R. Sulfur modulates yield and storage proteins in soybean grains. Sci. Agric. 2021, 78, e20190020. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Jürgen Pohlan, H.A.; Janssens, M.J.J.; Eversbusch, B.G. Impact of Canavalia Cover Crop Management in Coffea arabica L. on Plant-Invertebrate Associations. Open Agric. J. 2008, 2, 84–89. [Google Scholar] [CrossRef][Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-19203-6. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments; Springer: New York, NY, USA, 2001; ISBN 978-1-4684-9505-8. [Google Scholar]
- Lancheros, A.; Cajamarca, F.; Guedes, C.; Brito, O.; Guimarães, M.d.F. Exploring the potential of Canavalia ensiformis for phytoremediation of B10 biodiesel-contaminated soil: Insights on aromatic compound degradation and soil fertility. Int. J. Phytoremediation 2024, 26, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.F.d.; Alves, P.B.; Ferreira, T.C.; Santos, B.S.d.; Cozin, B.B.; Souza, R.P.d.; Camargos, L.S. Effects of iron and copper on emergence and physiology of Canavalia ensiformis (L.) DC. Rev. Caatinga 2025, 38, e12686. [Google Scholar] [CrossRef]
- de Paula Correia, D.V.; Rodak, B.W.; Machado, H.A.; Lopes, G.; Freitas, D.S. Beneficial or detrimental? How nickel application alters the ionome of soybean plants. Plant Sci. 2024, 349, 112274. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Yáñez, A.; Caldera, J.; Oropeza, C.; Robert, M.L.; Quiroz, J.; Scorer, K.N. Nitrogen Metabolism in Canavalia ensiformis (L.) DC. J. Plant Physiol. 1988, 132, 289–293. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes During Seed Germination. In Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen: Rijeka, Croatia, 2017; ISBN 978-953-51-3622-4. [Google Scholar]
- Parwez, R.; Nabi, A.; Mukarram, M.; Aftab, T.; Khan, M.M.A.; Naeem, M. Chapter 17—Role of nickel in regulation of nitrogen metabolism in legume–rhizobium symbiosis under critical conditions. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 495–522. ISBN 978-0-323-90943-3. [Google Scholar]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.M.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Amari, T.; Lutts, S.; Taamali, M.; Lucchini, G.; Sacchi, G.A.; Abdelly, C.; Ghnaya, T. Implication of citrate, malate and histidine in the accumulation and transport of nickel in Mesembryanthemum crystallinum and Brassica juncea. Ecotoxicol. Environ. Saf. 2016, 126, 122–128. [Google Scholar] [CrossRef]
- Smith, S.C.; Johnson, S.; Andrews, J.; McPherson, A. Biochemical Characterization of Canavalin, the Major Storage Protein of Jack Bean 1. Plant Physiol. 1982, 70, 1199–1209. [Google Scholar] [CrossRef]
- Bomfim, N.C.P.; Aguilar, J.V.; Ferreira, T.C.; de Souza, L.A.; Camargos, L.S. Could nitrogen compounds be indicators of tolerance to high doses of Cu and Fe in the cultivation of Leucaena leucocephala? Plant Physiol. Biochem. 2023, 194, 489–498. [Google Scholar] [CrossRef]
- Mendes, T.F.S.; Ferreira, T.C.; Bomfim, N.C.P.; Aguilar, J.V.; de Camargos, L.S. Nitrogen metabolism and P-Cu interaction in Cajanus cajan (L.) Millsp contributes to the removal of excess copper in viticulture soil. Int. J. Environ. Stud. 2024, 81, 1884–1902. [Google Scholar] [CrossRef]
- dos Santos, B.S.; Mendonça, G.W.; Ferreira, T.C.; Bomfim, N.C.P.; de Carvalho, I.F.; Aguilar, J.V.; Camargos, L.S. Exploring the Potential of Crotalaria juncea L. for Phytoremediation: Insights from Gas Exchange, Pigment Quantification, and Growth Measurements under Copper Stress. Horticulturae 2024, 10, 746. [Google Scholar] [CrossRef]
- Mendes, T.F.S.; Ferreira, T.C.; Bomfim, N.C.P.; Aguilar, J.V.; de Camargos, L.S. Metabolic Responses to Excess Manganese in Legumes: Variations in Nitrogen Compounds in Canavalia ensiformis (L.) DC and Cajanus cajan (L.) Millsp. Legume Sci. 2024, 6, e70003. [Google Scholar] [CrossRef]
- Iqbal, N.; Sadras, V.O.; Denison, R.F.; Zhou, Y.; Denton, M.D. Clade-dependent effects of drought on nitrogen fixation and its components—Number, size, and activity of nodules in legumes. Field Crops Res. 2022, 284, 108586. [Google Scholar] [CrossRef]
- Peer, W.A.; Baxter, I.R.; Richards, E.L.; Freeman, J.L.; Murphy, A.S. Phytoremediation and hyperaccumulator plants. In Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man; Tamas, M.J., Martinoia, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 299–340. ISBN 978-3-540-31719-7. [Google Scholar]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- da Silva, M.B.; Bomfim, N.C.P.; da Silva, V.N.; de Lima Frachia, C.; de Souza, L.A.; Justino, G.C.; de Camargos, L.S. Response of Cajanus cajan to excess copper in the soil: Tolerance and biomass production. Physiol. Mol. Biol. Plants 2022, 28, 1335–1345. [Google Scholar] [CrossRef]
- Ortúzar, M.; Trujillo, M.E.; Román-Ponce, B.; Carro, L. Micromonospora metallophores: A plant growth promotion trait useful for bacterial-assisted phytoremediation? Sci. Total Environ. 2020, 739, 139850. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Yasmin, A.; Fariq, A. Metabolites produced by inoculated Vigna radiata during bacterial assisted phytoremediation of Pb, Ni and Cr polluted soil. PLoS ONE 2022, 17, e0277101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ciric, L.; Bhatti, M. Phytoremediation of organic pollution using leguminous plants and auxiliary additives: Principles and advantages. Chem. Biol. Technol. Agric. 2025, 12, 98. [Google Scholar] [CrossRef]
- Magsayo, B.M.T.; Aggangan, N.S.; Gilbero, D.M.; Amparado, R.F. Evaluating Microbial Biofertilizers for Root Colonization Potential in Narra (Pterocarpus indicus Willd.) and Their Efficacy in Heavy Metal Remediation. Forests 2024, 15, 180. [Google Scholar] [CrossRef]
- Saad, R.F.; Kobaissi, A.; Goux, X.; Calusinska, M.; Echevarria, G.; Kidd, P.; Benizri, E. Soil microbial and Niagronomic responses to Alyssum murale interplanted with a legume. Appl. Soil Ecol. 2018, 132, 60–73. [Google Scholar] [CrossRef]
- Thomas, G.; Sheridan, C.; Holm, P.E. Co-cropping vetiver grass and legume for the phytoremediation of an acid mine drainage (AMD) impacted soil. Environ. Pollut. 2024, 341, 122873. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, J.V.; Mendes, T.F.S.; Bomfim, N.C.P.; Brambilla, M.R.; Alves, P.B.; Petreca, J.A.; Coscione, A.R.; Camargos, L.S. Biomass and Nickel Tolerance: Canavalia ensiformis (L.) DC. as a Candidate Plant for Phytoremediation Applications. Agriculture 2025, 15, 2200. https://doi.org/10.3390/agriculture15212200
Aguilar JV, Mendes TFS, Bomfim NCP, Brambilla MR, Alves PB, Petreca JA, Coscione AR, Camargos LS. Biomass and Nickel Tolerance: Canavalia ensiformis (L.) DC. as a Candidate Plant for Phytoremediation Applications. Agriculture. 2025; 15(21):2200. https://doi.org/10.3390/agriculture15212200
Chicago/Turabian StyleAguilar, Jailson Vieira, Thalita Fischer Santini Mendes, Nayane Cristina Pires Bomfim, Matheus Ribeiro Brambilla, Patrícia Borges Alves, Julia Araujo Petreca, Aline Renee Coscione, and Liliane Santos Camargos. 2025. "Biomass and Nickel Tolerance: Canavalia ensiformis (L.) DC. as a Candidate Plant for Phytoremediation Applications" Agriculture 15, no. 21: 2200. https://doi.org/10.3390/agriculture15212200
APA StyleAguilar, J. V., Mendes, T. F. S., Bomfim, N. C. P., Brambilla, M. R., Alves, P. B., Petreca, J. A., Coscione, A. R., & Camargos, L. S. (2025). Biomass and Nickel Tolerance: Canavalia ensiformis (L.) DC. as a Candidate Plant for Phytoremediation Applications. Agriculture, 15(21), 2200. https://doi.org/10.3390/agriculture15212200




