Generally Recognized as Safe Salts for a Natural Strategy to Managing Fungicide-Resistant Penicillium Strains in the Moroccan Citrus Packinghouse
Abstract
1. Introduction
2. Materials and Methods
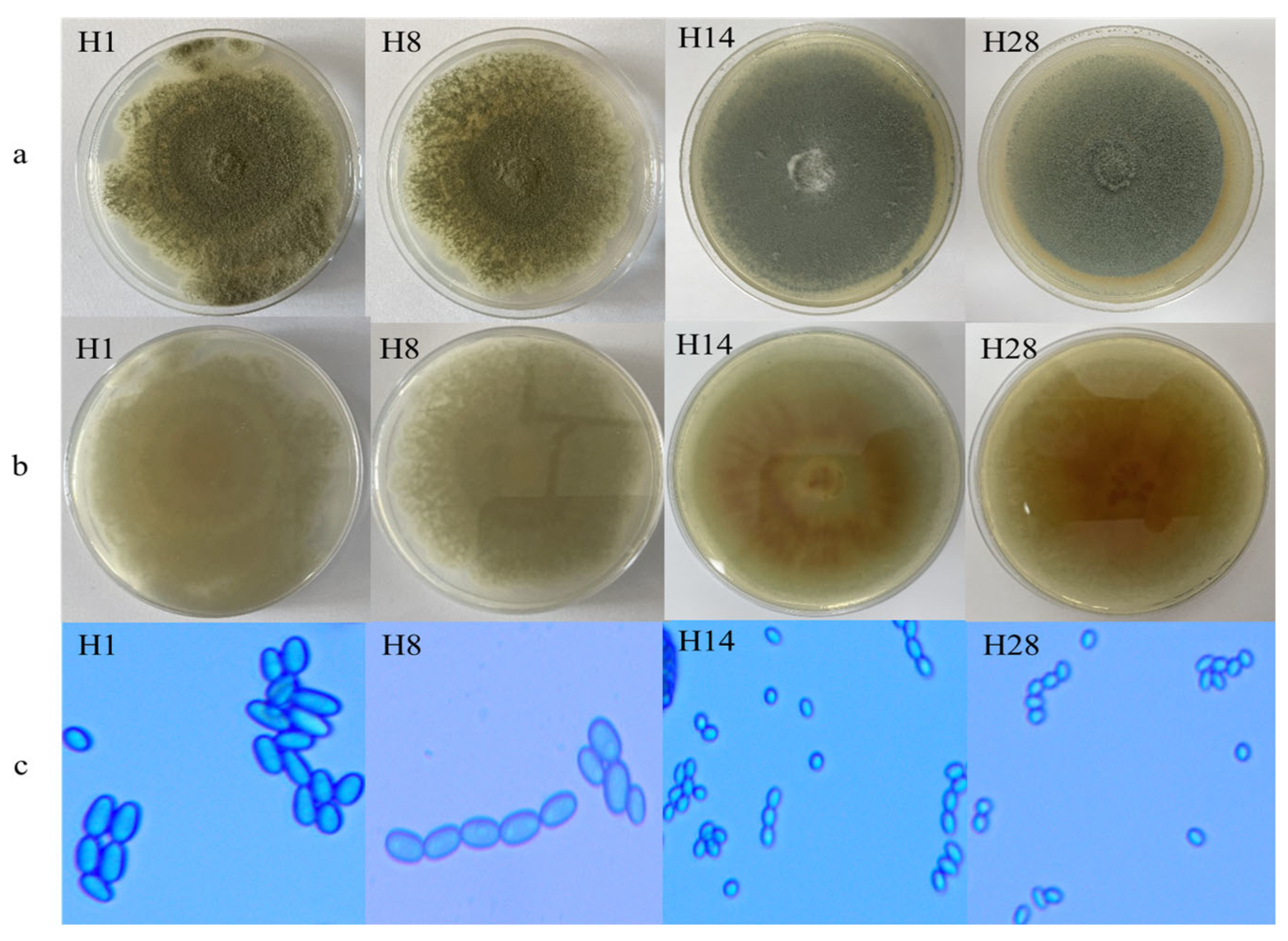
2.1. Collection and Identification of Isolates from the Packinghouse
2.2. Characterization of Fungal Sensitivity to Fungicides
2.3. Performance of GRAS Salts in Preventing Penicillium spp. Strains Resistant to Fungicides
2.3.1. In Vitro Antifungal Assay
2.3.2. Preventive and Curative Antifungal Assessments
2.3.3. Assessment of Total Phenolic and Flavonoid Contents
2.3.4. Assessment of Salt-Induced Defense Enzyme Activities: Chitinase and Peroxidase
2.4. Data Analysis
3. Results
3.1. Fungal Population and Sensitivity to Fungicides
3.2. Imazalil and Thiabendazole Sensitivity of the Isolates
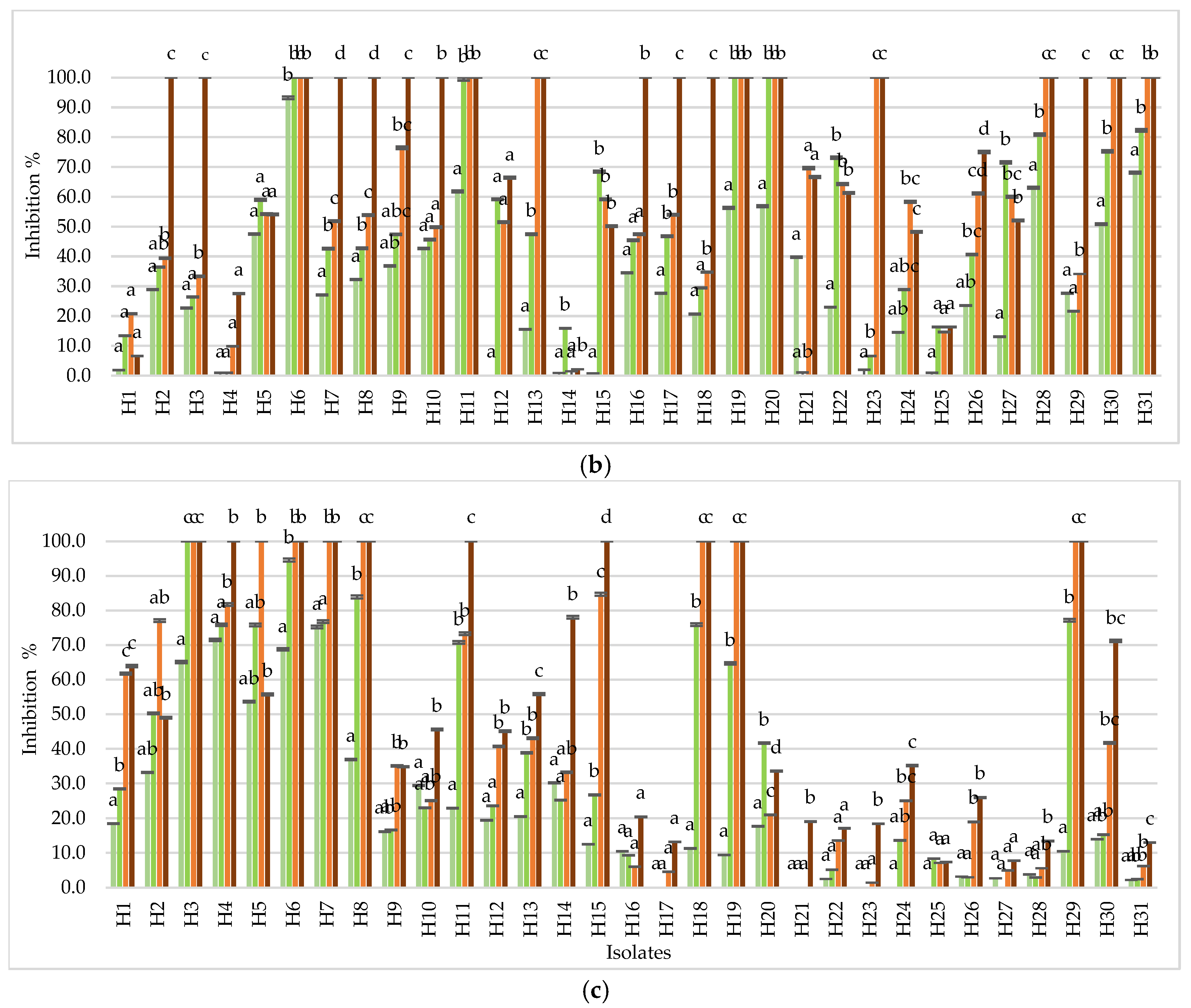
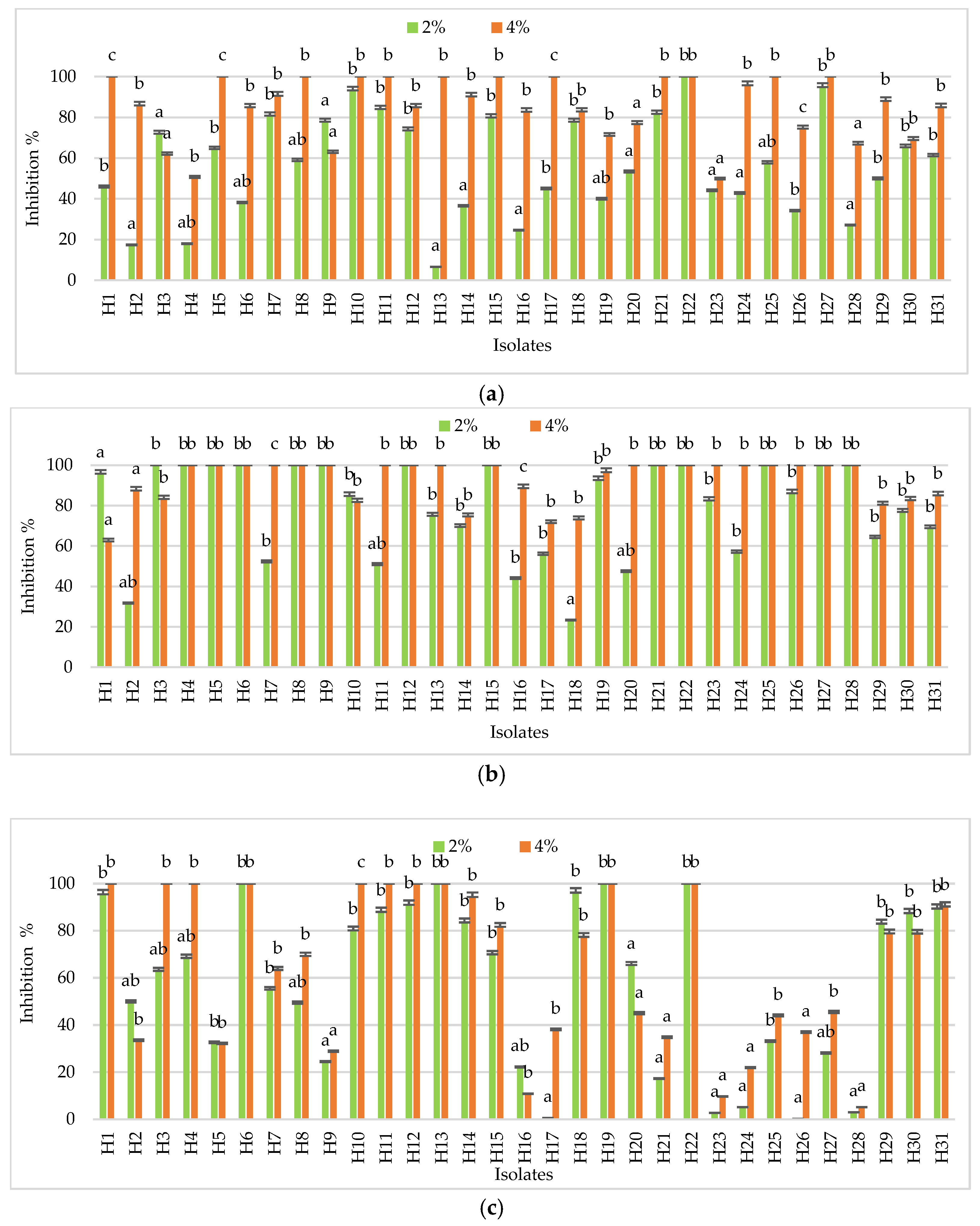
3.3. Assessment of GRAS Salts Efficacy on Penicillium spp. Isolates
3.3.1. Antifungal Activity of Salts in In Vitro Assays
3.3.2. Antifungal Activity of Salts in Preventive and Curative Assays
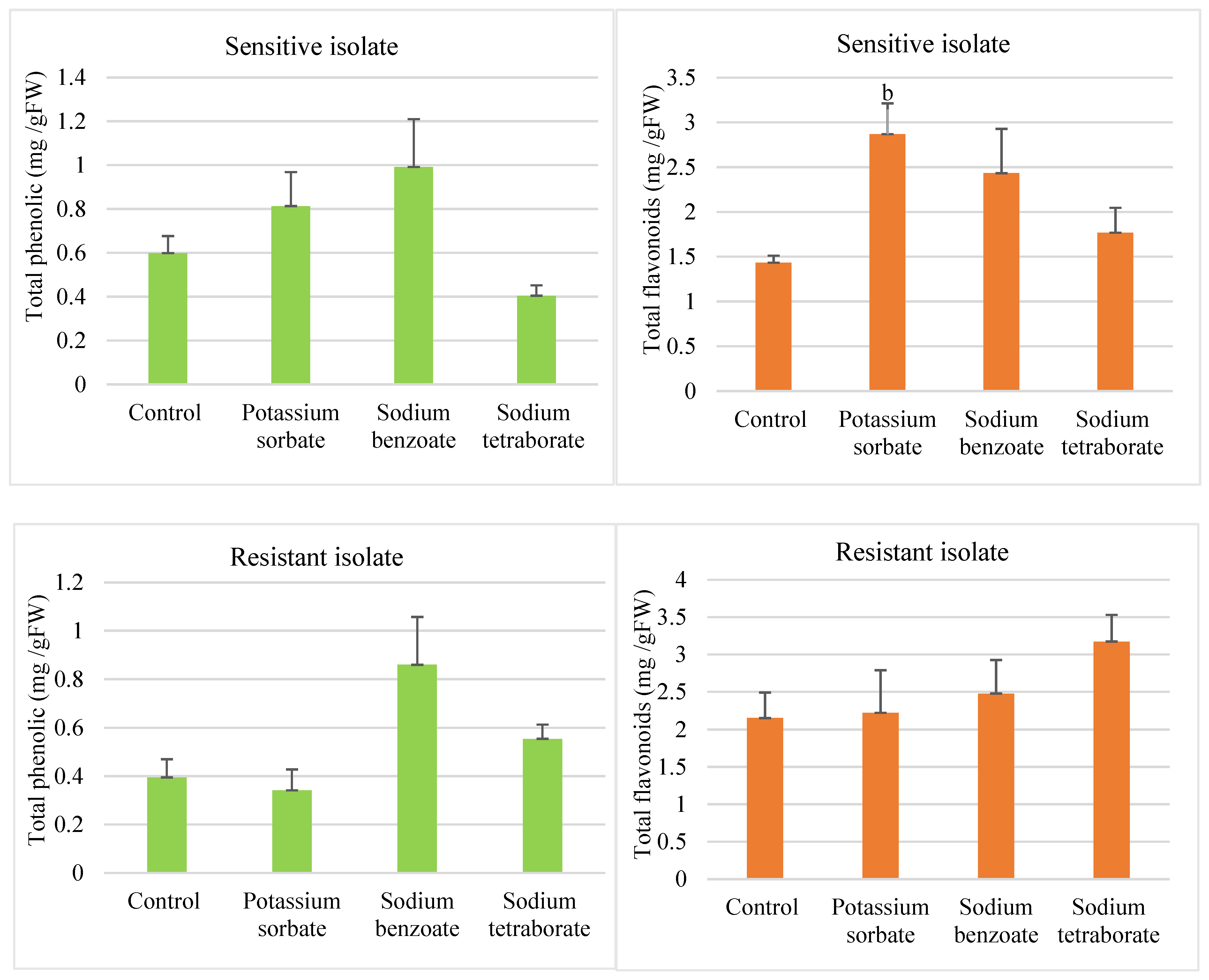
3.3.3. Total Phenolic and Total Flavonoid Contents
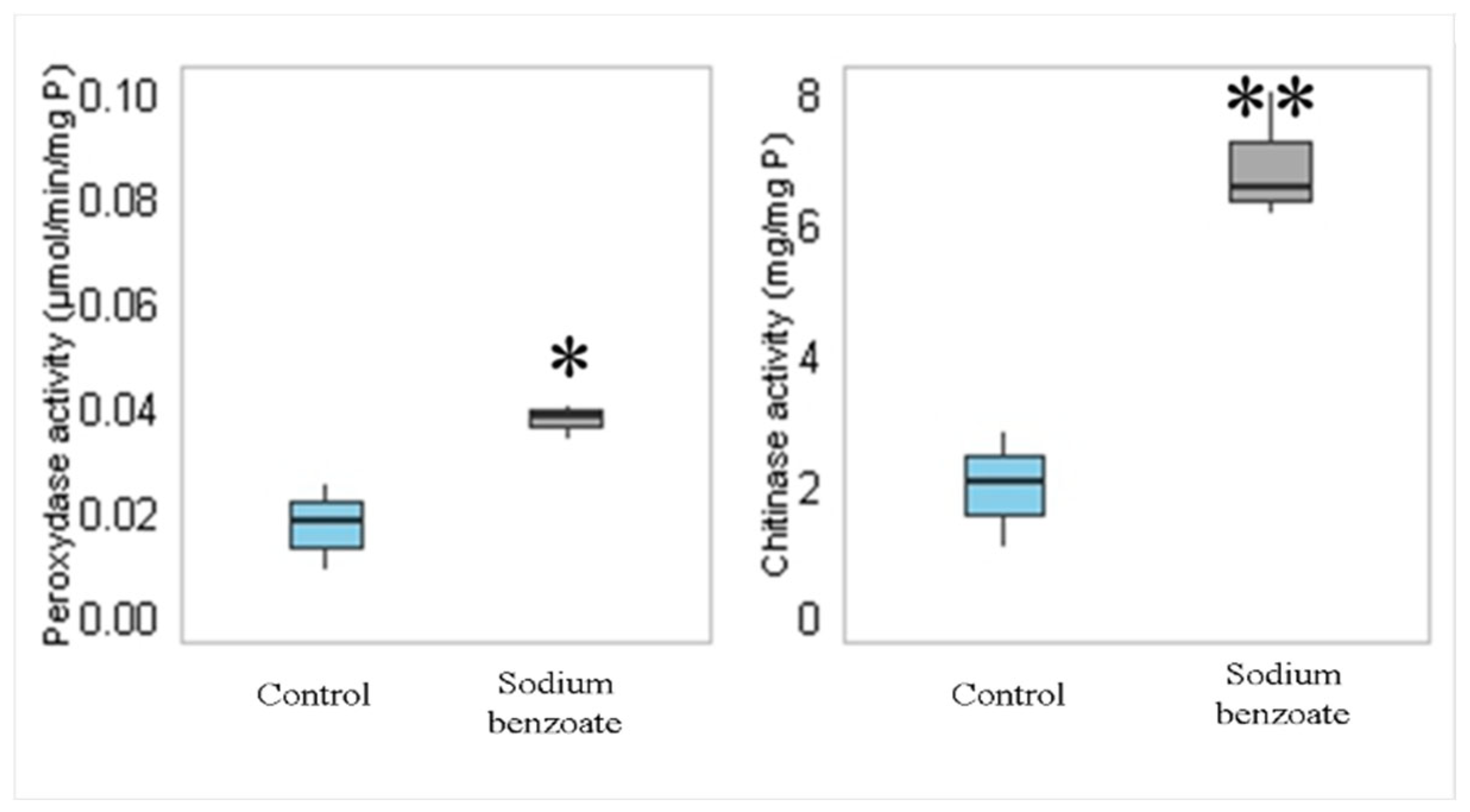
3.3.4. Chitinase and Peroxidase Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC50 | Effective concentrations 50 |
| FW | Fresh Weight |
| GAE | Gallic acid equivalents |
| GRAS | Generally Recognized As Safe |
| IMZ | Imazalil |
| MRLs | Maximum residue limits |
| PDA | Potato dextrose agar |
| P. digitatum | Penicillium digitatum |
| P. italicum | Penicillium italicum |
| QE | Quercitin equivalents |
| TBZ | Thiabendazole |
References
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Barzee, T.J.; Zhang, R.; Pan, Z. Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–242. [Google Scholar] [CrossRef]
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2020; FAO: Rome, Italy, 2021; Available online: https://coilink.org/20.500.12592/bsg3dv (accessed on 29 April 2025).
- Fédération Interprofessionnelle Marocaine des Agrumes—Recherche Google n.d. Available online: https://www.google.com/search?q=F%C3%A9d%C3%A9ration+Interprofessionnelle+Marocaine+des+Agrumes&oq=F%C3%A9d%C3%A9ration+Interprofessionnelle+Marocaine+des+Agrumes&gs_lcrp=EgZjaHJvbWUqBggAEEUYOzIGCAAQRRg7MggIARAAGBYYHjIHCAIQABjvBTIHCAMQABjvBdIBBzk1MWowajeoAgiwAgHxBQbqkEqpPS2i&sourceid=chrome&ie=UTF-8 (accessed on 29 April 2025).
- USDA. Navigo 2024. Available online: https://voyager.fas.usda.gov/voyager/navigo/show?id=2c144d95-62c1-593c-82b5-a31e44bd0885&disp=default (accessed on 23 February 2024).
- Talibi, I.; Boubaker, H.; Boudyach, E.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Louw, J.P.; Korsten, L. Pathogenicity and Host Susceptibility of Penicillium spp. on Citrus. Plant Dis. 2015, 99, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yazid, J.B.; Chafik, Z.; Chedadi, T. Assessment of the main causes of sorting gaps in the Citrus packinghouses of Berkane, Morocco. Moroc. J. Agric. Sci. 2020, 1, 264–273. [Google Scholar]
- Tuset, J. Tuset: Podredumbres de los Frutos Cítricos—Google Scholar 1987. Available online: https://scholar.google.com/scholar_lookup?title=Podredumbres%20de%20los%20Frutos%20C%C3%ADtricos&author=J.J.%20Tuset&publication_year=1988 (accessed on 23 February 2024).
- Costa, J.H.; Bazioli, J.M.; de Moraes Pontes, J.G.; Fill, T.P. Penicillium digitatum infection mechanisms in citrus: What do we know so far? Fungal Biol. 2019, 123, 584–593. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef]
- Ladaniya, M.S. Commercial Fresh Citrus Cultivars and Producing Countries: Citrus Fruit; Elsevier: Amsterdam, The Netherlands, 2008; pp. 13–65. [Google Scholar] [CrossRef]
- Saito, S.; Xiao, C.L. Prevalence of Postharvest Diseases of Mandarin Fruit in California. Plant Health Prog. 2017, 18, 204–210. [Google Scholar] [CrossRef]
- Hamrani, M.; Baiz, Z.; Samedi, A.; Iacoboni, D.; Abdelhak, H.; El Guilli, M. Effectiveness of the ANECO® as a new disinfectant in packinghouse against the main citrus postharvest pathogens. Acta Hortic. 2025, 1422, 431–442. [Google Scholar] [CrossRef]
- Kanetis, L.; Förster, H.; Adaskaveg, J.E. Baseline Sensitivities for New Postharvest Fungicides Against Penicillium spp. on Citrus and Multiple Resistance Evaluations in P. digitatum. Plant Dis. 2008, 92, 301–310. [Google Scholar] [CrossRef]
- Kellerman, M.; Liebenberg, E.; Njombolwana, N.; Erasmus, A.; Fourie, P.H. Postharvest dip, drench and wax coating application of pyrimethanil on citrus fruit: Residue loading and green mould control. Crop Prot. 2018, 103, 115–129. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium italicum in Citrus Fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Ireland, K.B.; Beard, C.; Cameron, J.; Chang, S.; Davidson, J.; Dodhia, K.N.; Garrard, T.A.; Hills, A.L.; Holloway, G.; Jayasena, K.W.; et al. Fungicide Resistance Management in Australian Grain Crops; Grains Research and Development Corporation: Barton, Australia, 2021. [Google Scholar]
- Zhu, H.; Zhao, L.; Zhang, X.; Foku, J.M.; Li, J.; Hu, W.; Zhang, H. Efficacy of Yarrowia lipolytica in the biocontrol of green mold and blue mold in Citrus reticulata and the mechanisms involved. Biol. Control 2019, 139, 104096. [Google Scholar] [CrossRef]
- El-Goorani, M.A.; El-Kasheir, H.M.; Kabeel, M.T.; Shoeib, A.A. Resistance to benzimidazole fungicides of Penicillium italicum and P. digitatum isolated from packinghouses and orchards in Egypt. Plant Dis. 1984, 68, 100–102. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Tuset, J.J. Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Postharvest Biol. Technol. 2011, 59, 159–165. [Google Scholar] [CrossRef]
- Carter, H.E.; Fraaije, B.A.; West, J.S.; Kelly, S.L.; Mehl, A.; Shaw, M.W.; Cools, H.J. Alterations in the predicted regulatory and coding regions of the sterol 14α-demethylase gene (CYP51) confer decreased azole sensitivity in the oilseed rape pathogen Pyrenopeziza brassicae. Mol. Plant Pathol. 2014, 15, 513–522. [Google Scholar] [CrossRef]
- CBI. What Requirements Must Fresh Fruit or Vegetables Comply with to Be Allowed on the European Market?|CBI 2024. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/buyer-requirements (accessed on 30 September 2024).
- Hamrani, M.; Lahlali, R.; Ziri, R.; Ezzouggari, R.; Brhadda, N.; Lauri, F.; Mokrini, F.; Barka, E.A.; El Guilli, M. Citrus bliss: Potassium, sodium, and calcium silicates secrets for post-harvest diseases of fruit defense. Ital. J. Food Saf. 2024, 13, 12714. [Google Scholar] [CrossRef]
- Palou, L.; Pérez-Gago, M.B. GRAS Salts as Alternative Low-Toxicity Chemicals for Postharvest Preservation of Fresh Horticultural Products; Spadaro, D., Droby, S., Gullino, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 11, pp. 163–479. [Google Scholar] [CrossRef]
- Soto-Muñoz, L.; Taberner, V.; de la Fuente, B.; Jerbi, N.; Palou, L. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int. J. Food Microbiol. 2020, 335, 108860. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. Phylogeny in the genus Penicillium: A morphologist’s perspective. Can. J. Bot. 1995, 73, 768–777. [Google Scholar] [CrossRef]
- Fischer, I.H.; Lourenço, S.A.; Spósito, M.B.; Amorim, L. Characterisation of the fungal population in citrus packing houses. Eur. J. Plant Pathol. 2009, 123, 449–460. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, Q.; Li, H. Occurrence of imazalil-resistant biotype of Penicillium digitatum in China and the resistant molecular mechanism. J. Zhejiang Univ. Sci. A 2006, 7, 362–365. [Google Scholar] [CrossRef]
- Holmes, G.J.; Eckert, J.W. Sensitivity of Penicillium digitatum and P. italicum to Postharvest Citrus Fungicides in California. Phytopathology 1999, 89, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.; Panozzo, M.; Almirón, N.; Bello, F.; Burdyn, L.; Garrán, S. Characterization of sensitivity of grove and packing house isolates of Penicillium digitatum to pyrimethanil. Postharvest Biol. Technol. 2014, 98, 1–6. [Google Scholar] [CrossRef]
- EU. Regulation-231/2012-EN-EUR-Lex 2024. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012R0231 (accessed on 3 March 2024).
- Martínez-Blay, V.; Pérez-Gago, M.B.; de la Fuente, B.; Carbó, R.; Palou, L. Edible coatings formulated with antifungal GRAS salts to control citrus anthracnose caused by Colletotrichum gloeosporioides and preserve postharvest fruit quality. Coatings 2020, 10, 730. [Google Scholar] [CrossRef]
- Youssef, K.; Ligorio, A.; Sanzani, S.M.; Nigro, F.; Ippolito, A. Control of storage diseases of citrus by pre-and postharvest application of salts. Postharvest Biol. Technol. 2012, 72, 57–63. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, L.; Ai, J.; Ji, R.; He, L.; Liu, C. Effect of heat shock and potassium sorbate treatments on gray mold and postharvest quality of ‘XuXiang’ kiwifruit. Food Chem. 2020, 324, 126891. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; El-Gamal, N.G.; Abd-El-Kareem, F. Use of organic acids and salts to control postharvest diseases of lemon fruits in Egypt. Arch. Phytopathol. Plant Prot. 2008, 41, 467–476. [Google Scholar] [CrossRef]
- Abeysinghe, D.C.; Li, X.; Sun, C.; Zhang, W.; Zhou, C.; Chen, K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007, 104, 1338–1344. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Ballester, A.R.; Lafuente, M.T.; González-Candelas, L. Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit–Penicillium digitatum interaction. Postharvest Biol. Technol. 2006, 39, 115–124. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Fallanaj, F.; Nigro, F.; Ippolito, A. Biochemical and transcriptomic changes associated with induced resistance in citrus fruits treated with sodium salts. Acta Hortic. 2015, 1065, 1627–1632. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El Guilli, M.; Hamza, A.; Clément, C.; Ibriz, M.; Ait Barka, E. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 2016, 6, 12. [Google Scholar] [CrossRef]
- Fallanaj, F.; Ippolito, A.; Ligorio, A.; Garganese, F.; Zavanella, C.; Sanzani, S.M. Electrolyzed sodium bicarbonate inhibits Penicillium digitatum and induces defence responses against green mould in citrus fruit. Postharvest Biol. Technol. 2016, 115, 18–29. [Google Scholar] [CrossRef]
- Costa, J.H.; Fernandes, L.S.; Akiyama, D.Y.; Fill, T.P. Exploring the interaction between citrus flavonoids and phytopathogenic fungi through enzymatic activities. Bioorganic Chem. 2020, 102, 104126. [Google Scholar] [CrossRef]
- Ortuño, A.; Díaz, L.; Alvarez, N.; Porras, I.; García-Lidón, A.; Del Río, J.A. Comparative study of flavonoid and scoparone accumulation in different Citrus species and their susceptibility to Penicillium digitatum. Food Chem. 2011, 125, 232–239. [Google Scholar] [CrossRef]
- Ismail, M.; Zhang, J. Post-harvest citrus diseases and their control. Outlooks Pest Manag. 2004, 15, 29–35. [Google Scholar] [CrossRef]
- FRAC, Pathogen Resk List. 2019. Available online: https://www.frac.info/publications/all-downloads/#open-tour (accessed on 29 April 2025).
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G. Baseline sensitivity of Florida isolates of Penicillium digitatum to imazalil. Plant Dis. 1989, 73, 773–774. [Google Scholar] [CrossRef]
- Kinay, P.; Mansour, M.F.; Gabler, F.M.; Margosan, D.A.; Smilanick, J.L. Characterization of fungicide-resistant isolates of Penicillium digitatum collected in California. Crop Prot. 2007, 26, 647–656. [Google Scholar] [CrossRef]
- Pérez, E.; Blanco, O.; Berreta, C.; Dol, I.; Lado, J. Imazalil concentration for in vitro monitoring of imazalil resistant isolates of Penicillium digitatum in citrus packinghouses. Postharvest Biol. Technol. 2011, 60, 258–262. [Google Scholar] [CrossRef]
- Boubaker, H.; Saadi, B.; Boudyach, E.H.; Benaoumar, A.A. Sensitivity of Penicillium digitatum and P. italicum to Imazalil and Thiabendazole in Morocco. Plant Pathol. J. 2009, 8, 152–158. [Google Scholar] [CrossRef]
- Lee, M.-H.; Pan, S.-M.; Ng, T.-W.; Chen, P.-S.; Wang, L.-Y.; Chung, K.-R. Mutations of β-tubulin codon 198 or 200 indicate thiabendazole resistance among isolates of Penicillium digitatum collected from citrus in Taiwan. Int. J. Food Microbiol. 2011, 150, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Usall, J.; Pons, J.; Cerdà, M.C.; Viñas, I. Micoflora em centrales citrícolas de Tarragona. Rev. Investig. Agrar. Prod. Protección Veg. 2001, 16, 447–462. [Google Scholar]
- Paluh, J.L.; Killilea, A.N.; Detrich, H.W.; Downing, K.H. Meiosis-specific Failure of Cell Cycle Progression in Fission Yeast by Mutation of a Conserved β-Tubulin Residue. Mol. Biol. Cell. 2004, 15, 1160–1171. [Google Scholar] [CrossRef]
- Hollomon, D.W.; Butters, J.A.; Barker, H.; Hall, L. Fungal β-Tubulin, Expressed as a Fusion Protein, Binds Benzimidazole and Phenylcarbamate Fungicides. Antimicrob. Agents Chemother. 1998, 42, 2171–2173. [Google Scholar] [CrossRef]
- Baraldi, E.; Mari, M.; Chierici, E.; Pondrelli, M.; Bertolini, P.; Pratella, G.C. Studies on thiabendazole resistance of Penicillium expansum of pears: Pathogenic fitness and genetic characterization. Plant Pathol. 2003, 52, 362–370. [Google Scholar] [CrossRef]
- van Gestel, J. Imazalil-sensitive and less sensitive strains of Penicillium digitatum: Competition experiments on oranges. CABI Databases 1988, 3, 1511–1514. [Google Scholar]
- Waard, M.A.; Groeneweg, H.; Nistelrooy, J.G.M. Laboratory resistance to fungicides which inhibit ergosterol biosynthesis in Penicillium italicum. Neth. J. Plant Pathol. 1982, 88, 99–112. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, J.; Fan, B.; Sun, M.; Cao, W.; Liu, X.; Gai, Y.; Shen, C.; Wang, H.; Wang, M. Transcriptome Analysis Reveals Potential Regulators of DMI Fungicide Resistance in the Citrus Postharvest Pathogen Penicillium digitatum. J. Fungi 2024, 10, 360. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Feng, D.; Ma, Z.; Li, H. PdCYP51B, a new putative sterol 14α-demethylase gene of Penicillium digitatum involved in resistance to imazalil and other fungicides inhibiting ergosterol synthesis. Appl. Microbiol. Biotechnol. 2011, 91, 1107–1119. [Google Scholar] [CrossRef]
- Ghosoph, J.M.; Schmidt, L.S.; Margosan, D.A.; Smilanick, J.L. Imazalil resistance linked to a unique insertion sequence in the PdCYP51 promoter region of Penicillium digitatum. Postharvest Biol. Technol. 2007, 44, 9–18. [Google Scholar] [CrossRef]
- Hamamoto, H.; Hasegawa, K.; Nakaune, R.; Lee, Y.J.; Makizumi, Y.; Akutsu, K.; Hibi, T. Tandem Repeat of a Transcriptional Enhancer Upstream of the Sterol 14α-Demethylase Gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 2000, 66, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- de Waard, M.A.; van Nistelrooy, J.G.M. An energy-dependent efflux mechanism for fenarimol in a wild-type strain and fenarimol-resistant mutants of Aspergillus nidulans. Pestic. Biochem. Physiol. 1980, 13, 255–266. [Google Scholar] [CrossRef]
- El Ammari, M.; Ziri, R.; El Bahja, F.; Hamrani, M.; Boukita, H.; Brhadda, N.; Bouzakraoui, S.; Fahad, K. Biological control of citrus pests: A systematic bibliometric analysis 2000–2023. J. Agric. Food Res. 2025, 19, 101492. [Google Scholar] [CrossRef]
- Aarrouf, J.; Urban, L. Flashes of UV-C light: An innovative method for stimulating plant defences. PLoS ONE 2020, 15, e0235918. [Google Scholar] [CrossRef]
- Palou, L. Control of citrus postharvest diseases by physical means. Tree For. Sci. Biotechnol. 2009, 3, 127–142. [Google Scholar]
- Zhao, J.; Wang, Y.; Liu, Q.; Liu, S.; Pan, H.; Cheng, Y.; Long, C. The GRAS Salts of Na2SiO3 and EDTA-Na2 Control Citrus Postharvest Pathogens by Disrupting the Cell Membrane. Foods 2023, 12, 2368. [Google Scholar] [CrossRef]
- Dehghan, P.; Mohammadi, A.; Mohammadzadeh-Aghdash, H.; Ezzati Nazhad Dolatabadi, J. Pharmacokinetic and toxicological aspects of potassium sorbate food additive and its constituents. Trends Food Sci. Technol. 2018, 80, 123–130. [Google Scholar] [CrossRef]
- Montesinos-Herrero, C.; Moscoso-Ramírez, P.A.; Palou, L. Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 2016, 115, 72–80. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods: Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh-Aghdash, H.; Sohrabi, Y.; Mohammadi, A.; Shanehbandi, D.; Dehghan, P.; Ezzati Nazhad Dolatabadi, J. Safety assessment of sodium acetate, sodium diacetate and potassium sorbate food additives. Food Chem. 2018, 257, 211–215. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Technol. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Debes, M.A.; Sepúlveda, M.; Ramallo, J.; Rapisarda, V.A.; Cerioni, L.; Volentini, S.I. Overcoming lemon postharvest molds caused by Penicillium spp. multiresistant isolates by the application of potassium sorbate in aqueous and wax treatments. J. Food Sci. 2023, 88, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Romli, N.F.A.; Sukor, R.; Rukayadi, Y.; Mohsin, A.Z. The efficacy of sodium benzoate and potassium sorbate in inhibiting the growth of food fungi and bacteria. Songklanakarin J. Sci. Technol. 2023, 45, 138–145. [Google Scholar]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Krebs, H.A.; Wiggins, D.; Stubbs, M.; Sols, A.; Bedoya, F. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 1983, 214, 657–663. [Google Scholar] [CrossRef]
- Qin, G.; Zong, Y.; Chen, Q.; Hua, D.; Tian, S. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int. J. Food Microbiol. 2010, 138, 145–150. [Google Scholar] [CrossRef]
- Allagui, M.B.; Amara, M.B. Effectiveness of Sodium Metabisulfite against Fungal Rot of Fruit after Harvest and Assessment of the Phytotoxicity Induced in Treated Fruit. Preprint 2024. [Google Scholar] [CrossRef]
- Allagui, M.B.; Ben Amara, M. Effectiveness of Several GRAS Salts against Fungal Rot of Fruit after Harvest and Assessment of the Phytotoxicity of Sodium Metabisufite in Treated Fruit. J. Fungi 2024, 10, 359. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Zhang, D.; Yang, F.; He, X.; Long, C. Aluminum sulfate inhibits green mold by inducing chitinase activity of Penicillium digitatum and enzyme activity of citrus fruit. Food Control 2022, 136, 108854. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.-Q. Chitin Synthesis and Degradation in Fungi: Biology and Enzymes; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; Volume 1142, pp. 153–167. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Induction of lignin and pathogenesis related proteins in dragon fruit plants in response to submicron chitosan dispersions. Crop Prot. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Soliman, M.H.; El-Mohamedy, R.S.R. Induction of Defense-Related Physiological and Antioxidant Enzyme Response against Powdery Mildew Disease in Okra (Abelmoschus esculentus L.) Plant by Using Chitosan and Potassium Salts. Mycobiology 2017, 45, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ballester, A.R.; Izquierdo, A.; Lafuente, M.T.; González-Candelas, L. Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest Biol. Technol. 2010, 56, 31–38. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; Tiepo, A.N.; Constantino, L.V.; de Resende, J.T.V.; Abo-Elyousr, K.A. Salt solution treatments trigger antioxidant defense response against gray mold disease in table grapes. J. Fungi 2020, 6, 179. [Google Scholar] [CrossRef]


| Code Number | Type of Fungus | Resistance | Fungicide EC50 (µg/mL) | ||
|---|---|---|---|---|---|
| IMZ | TBZ | IMZ | TBZ | ||
| H1 | P. digitatum | None | None | 0.04 | 2.90 |
| H2 | None | None | 0.01 | 0.53 | |
| H3 | None | None | 0.01 | 0.48 | |
| H4 | R | R | 2.25 | 89.03 | |
| H5 | R | R | 2.74 | 83.18 | |
| H6 | R | R | 0.28 | 25.40 | |
| H7 | R | R | 2.11 | 35.04 | |
| H8 | R | R | 12.90 | 55.50 | |
| H9 | R | R | 0.21 | 72.63 | |
| H10 | R | R | 0.60 | 15.38 | |
| H11 | P. italicum | None | None | 0.03 | 0.50 |
| H12 | None | None | 0.02 | 0.48 | |
| H13 | None | None | 0.04 | 0.48 | |
| H14 | None | None | 0.04 | 0.48 | |
| H15 | None | None | 0.01 | 4.35 | |
| H16 | None | None | 0.02 | 0.52 | |
| H17 | None | None | 0.02 | 0.52 | |
| H18 | None | None | 0.04 | 0.47 | |
| H19 | None | R | 0.04 | 9.46 | |
| H20 | None | R | 0.05 | 19.61 | |
| H21 | R | None | 10.39 | 4.88 | |
| H22 | R | R | 7.47 | 42.28 | |
| H23 | R | R | 6.65 | 35.64 | |
| H24 | R | R | 7.39 | 26.43 | |
| H25 | R | R | 3.94 | 22.33 | |
| H26 | R | R | 14.00 | 26.61 | |
| H27 | R | R | 7.86 | 37.48 | |
| H28 | R | R | 7.99 | 35.54 | |
| H29 | R | R | 4.58 | 18.58 | |
| H30 | R | R | 0.51 | 48.35 | |
| H31 | R | R | 1.09 | 26.68 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamrani, M.; Zelmat, L.; Jazayeri, S.M.; El Ammari, M.; Brhadda, N.; Ziri, R.; Aarrouf, J.; El Guilli, M. Generally Recognized as Safe Salts for a Natural Strategy to Managing Fungicide-Resistant Penicillium Strains in the Moroccan Citrus Packinghouse. Agriculture 2025, 15, 2184. https://doi.org/10.3390/agriculture15212184
Hamrani M, Zelmat L, Jazayeri SM, El Ammari M, Brhadda N, Ziri R, Aarrouf J, El Guilli M. Generally Recognized as Safe Salts for a Natural Strategy to Managing Fungicide-Resistant Penicillium Strains in the Moroccan Citrus Packinghouse. Agriculture. 2025; 15(21):2184. https://doi.org/10.3390/agriculture15212184
Chicago/Turabian StyleHamrani, Meriem, Lamyaa Zelmat, Seyed Mehdi Jazayeri, Mohamed El Ammari, Najiba Brhadda, Rabea Ziri, Jawad Aarrouf, and Mohammed El Guilli. 2025. "Generally Recognized as Safe Salts for a Natural Strategy to Managing Fungicide-Resistant Penicillium Strains in the Moroccan Citrus Packinghouse" Agriculture 15, no. 21: 2184. https://doi.org/10.3390/agriculture15212184
APA StyleHamrani, M., Zelmat, L., Jazayeri, S. M., El Ammari, M., Brhadda, N., Ziri, R., Aarrouf, J., & El Guilli, M. (2025). Generally Recognized as Safe Salts for a Natural Strategy to Managing Fungicide-Resistant Penicillium Strains in the Moroccan Citrus Packinghouse. Agriculture, 15(21), 2184. https://doi.org/10.3390/agriculture15212184






