From Contamination to Mitigation: Addressing Cadmium Pollution in Agricultural Soils
Abstract
1. Introduction
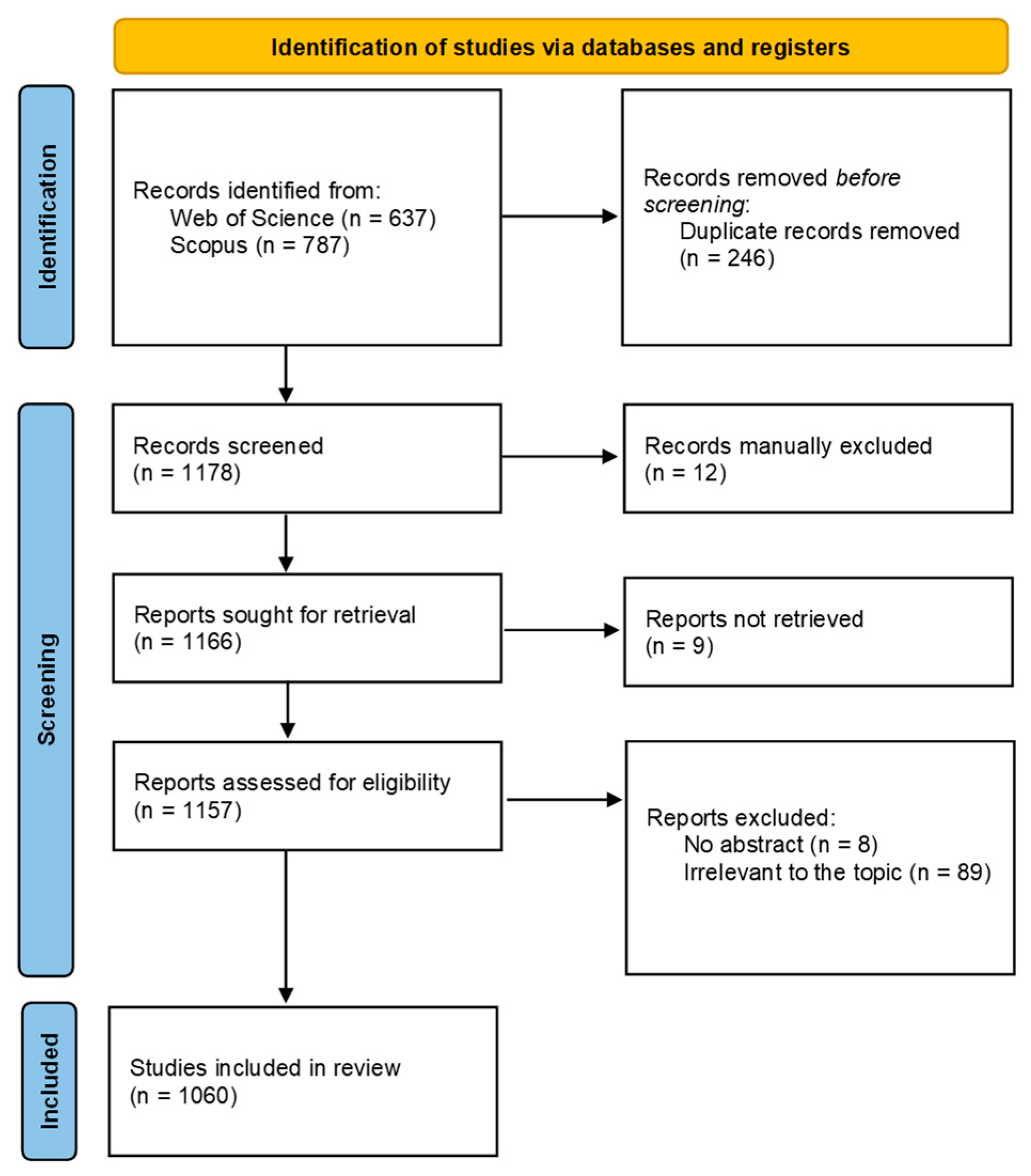
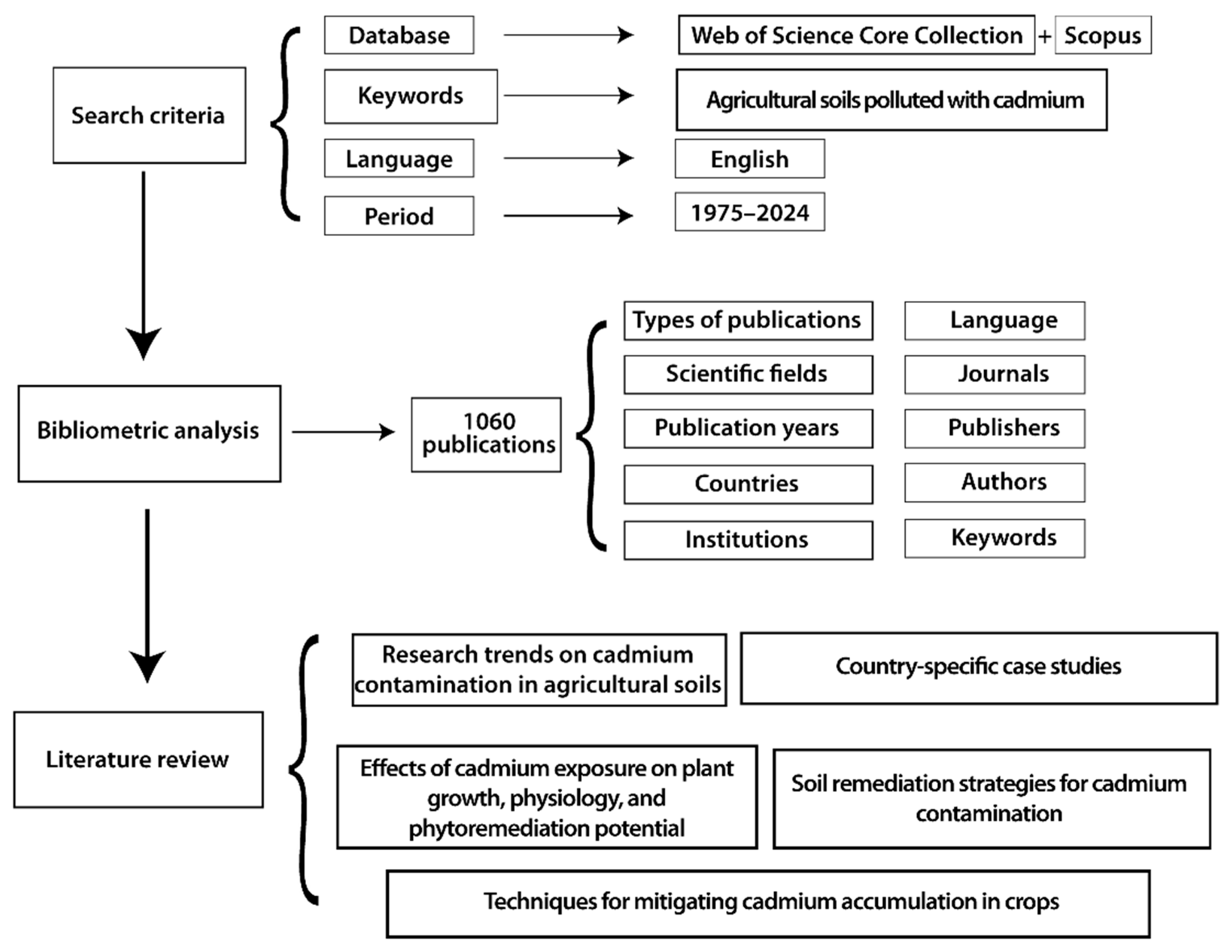
2. Materials and Methods
3. Results
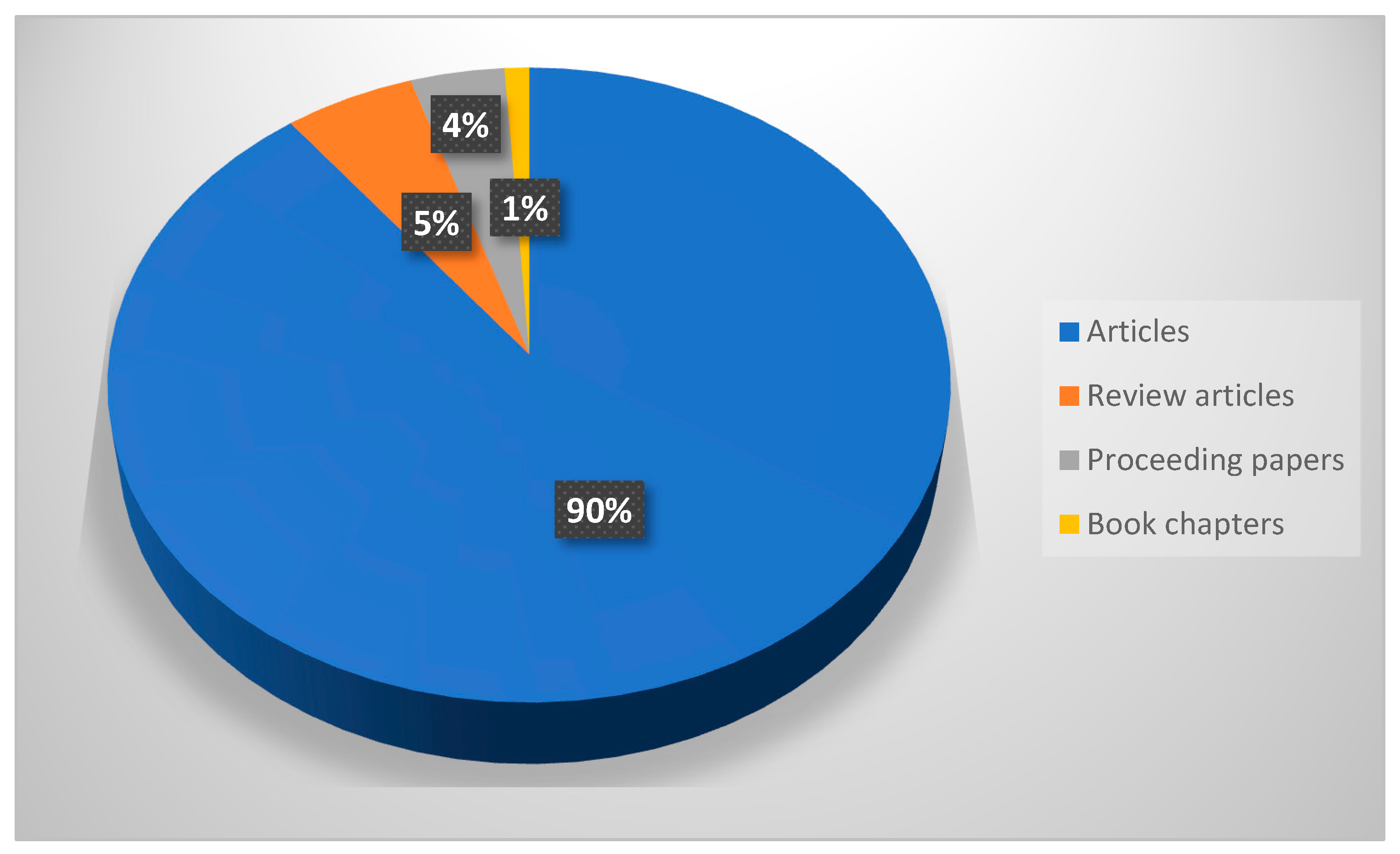
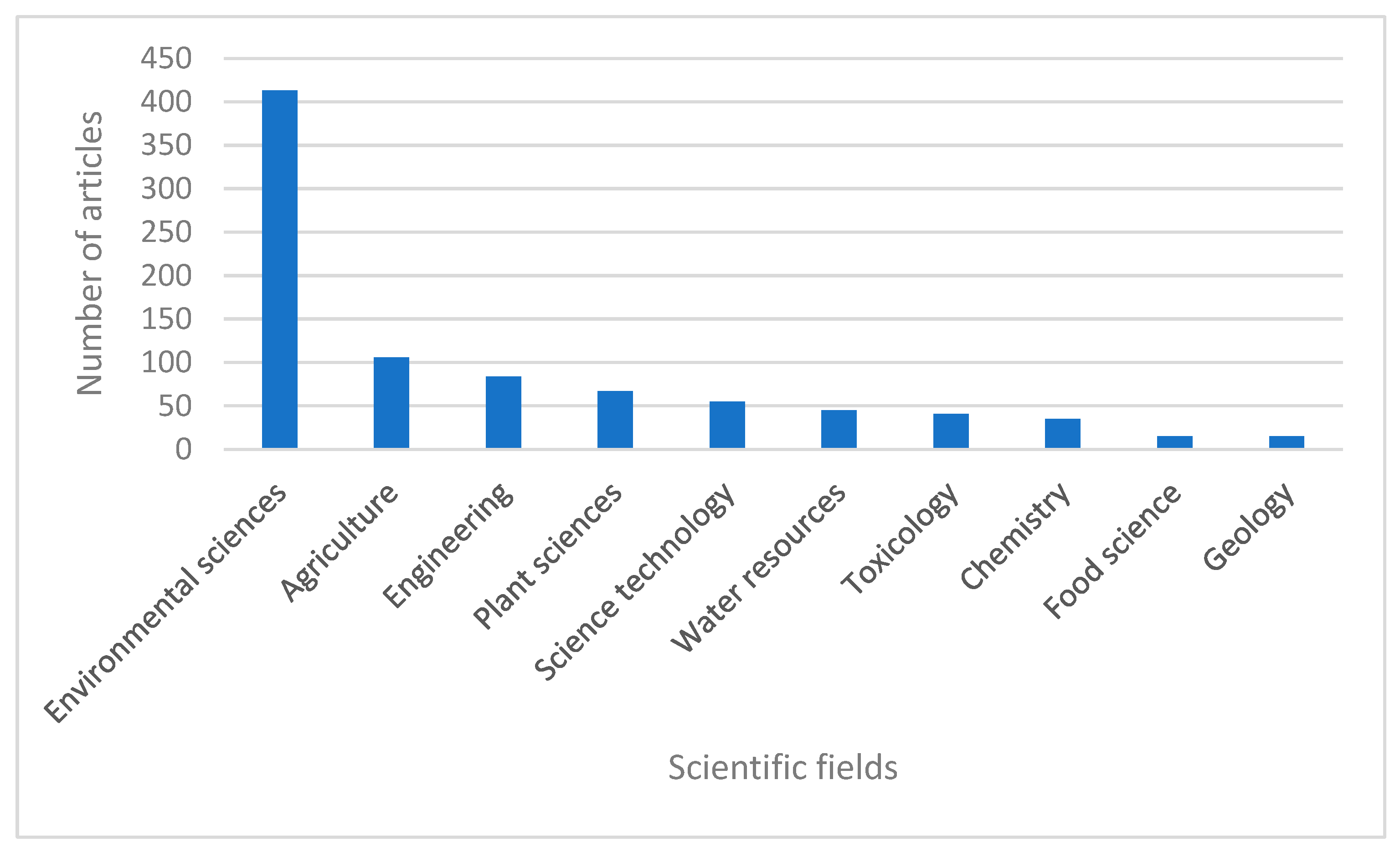
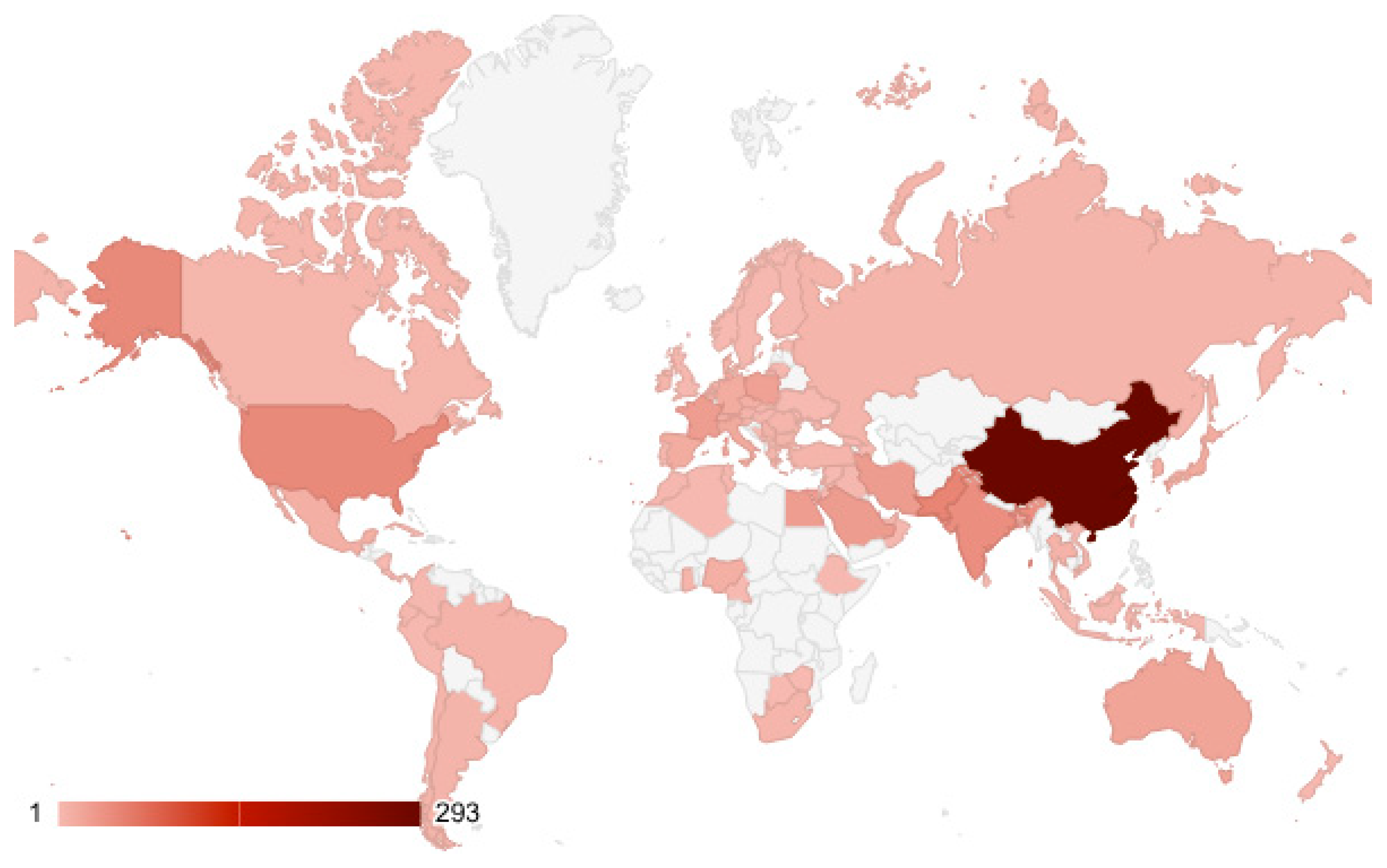
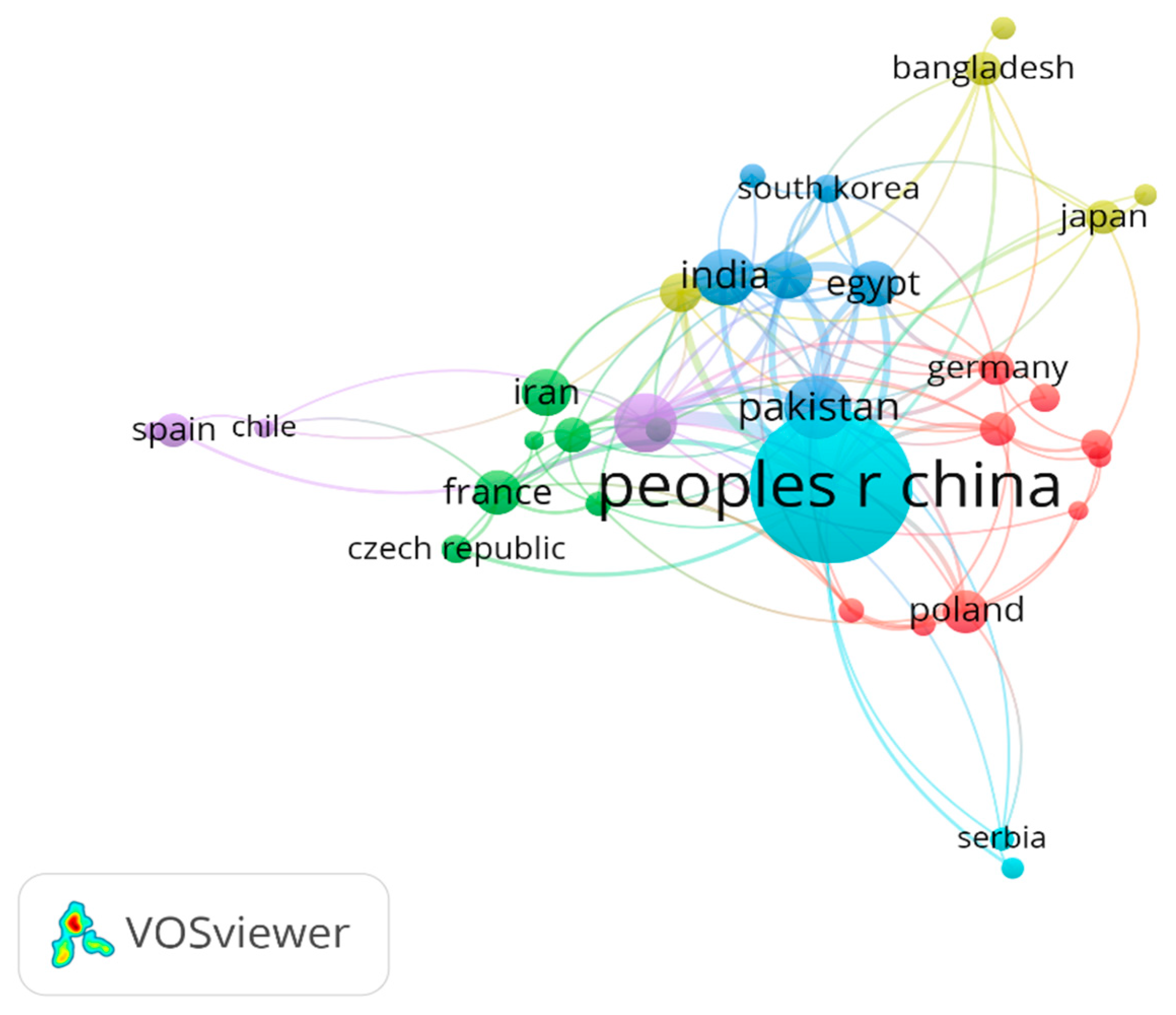
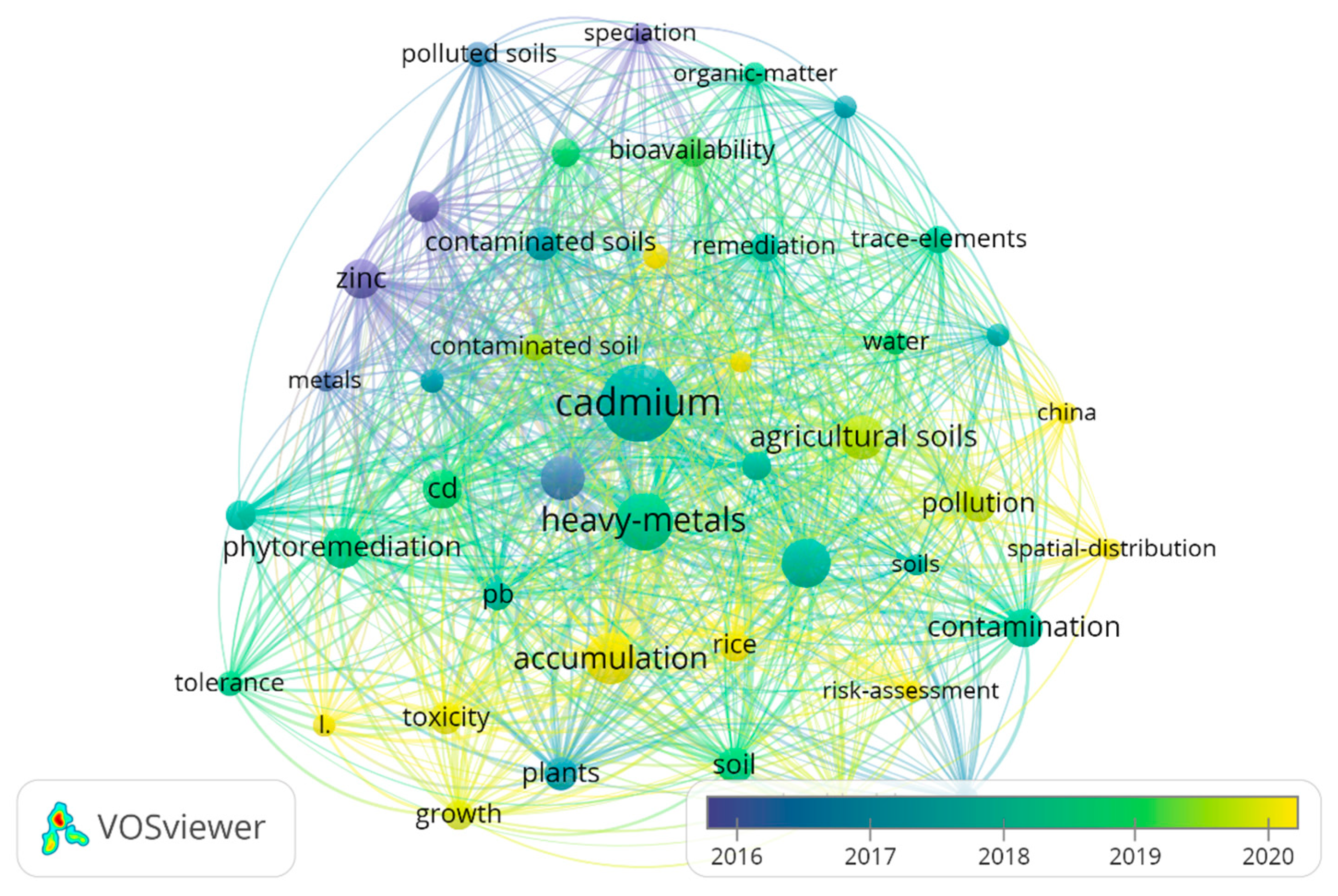
3.1. Qualitative Review of Literature
3.2. Literature Review
3.2.1. Research Trends on Cd Pollution in Agricultural Soils
3.2.2. Country Case Studies of Cd in Agricultural Soils
3.2.3. Effects of Cd Contamination on Plant Growth, Physiology, and Phytoremediation Potential
3.2.4. Remediation of Soils Contaminated with Cd
Phytoremediation
Microorganisms
Amendments
Organic Additives
Electrokinetic Remediation and Geo-Electrochemical Technology
Combined Methods
3.2.5. Techniques for Reducing Cd Accumulations in Plants
4. Discussion
4.1. Bibliometric Review
4.2. Research Trends on Cd Pollution in Agricultural Soils
4.3. Country Case Studies of Cd in Agricultural Soils
4.4. Plant Responses and Mitigation Strategies to Cd Contamination
4.5. Remediation of Soils Contaminated with Cd
4.6. Strategies for Mitigating Cd Accumulation in Food Crops
4.6.1. Nutrient and Chemical Amendments
4.6.2. Nanomaterials and Biostimulants
4.6.3. Genetic and Molecular Approaches
4.6.4. Agronomic Practices
4.6.5. Microbial Interventions
4.6.6. Towards Integrated Approaches
4.6.7. Implications for Sustainable Agriculture
4.7. Research Gaps and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samrane, K.; Bouhaouss, A. Cadmiumin Phosphorous Fertilizers: Balance and Trends. Rasayan J. Chem. 2022, 15, 2103–2117. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Dai, M.; Diao, X.; Dai, S.; Fang, T.; Dong, X. Input of Cd from agriculture phosphate fertilizer application in China during 2006–2016. Sci. Total. Environ. 2020, 698, 134149. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Lin, C. Response of toxic metal distributions and sources to anthropogenic activities and pedogenic processes in the Albic Luvisol profile of northeastern China. Environ. Adv. 2021, 6, 100142. [Google Scholar] [CrossRef]
- Shang, E.; Long, A.; Yang, J.; Ma, Y.; Yao, W.; Zhang, S. Dynamics of Cadmium Pollution Risk in Agricultural Land Soil of Tropical Islands in China from 2000 to 2024: A Case Study of Hainan Island. Appl. Sci. 2025, 15, 3817. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Zhou, L.; Liu, K.; You, A.; Zha, W. Cadmium Contamination in Asian Rice (Oryza sativa L.): Mechanistic Insights from Soil Sources to Grain Accumulation and Mitigation Strategies. Plants 2025, 14, 2844. [Google Scholar] [CrossRef] [PubMed]
- Mar, S.S.; Okazaki, M. Investigation of Cd contents in several phosphate rocks used for the production of fertilizer. Microchem. J. 2012, 104, 17–21. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Chen, Y.; Jing, H.; Wu, P.; Yang, W. Selection of rice and maize varieties with low Cadmium accumulation and derivation of soil environmental thresholds in karst. Ecotoxicol. Environ. Saf. 2022, 247, 114244. [Google Scholar] [CrossRef]
- Kayiranga, A.; Li, Z.; Isabwe, A.; Ke, X.; Simbi, C.H.; Ifon, B.E.; Yao, H.; Wang, B.; Sun, X. The effects of heavy metal pollution on Collembola in urban soils and associated recovery using biochar remediation: A review. Int. J. Environ. Res. Public Health 2023, 20, 3077. [Google Scholar] [CrossRef]
- Yang, Y.; Hassan, M.F.; Ali, W.; Zou, H.; Liu, Z.; Ma, Y. Effects of Cadmium Pollution on Human Health: A Narrative Review. Atmosphere 2025, 16, 225. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Tudose, N.; Crișan, V.; Georgescu, L.; Munteanu, D.; Mocanu, G.D. Structural characteristics of the main resinous stands from Southern Carpathians, Romania. Forests 2021, 12, 1029. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Topa, C.; Murariu, G.; Calmuc, V.; Calmuc, M.; Arseni, M.; Serban, C.; Chitescu, C.; Georgescu, L. A Spatial–Seasonal Study on the Danube River in the Adjacent Danube Delta Area: Case Study—Monitored Heavy Metals. Water 2024, 16, 2490. [Google Scholar] [CrossRef]
- Škrbić, B.D.; Živančev, J.; Antić, I.; Buljovčić, M. Pollution status and health risk caused by heavy elements in the flooded soil and vegetables from typical agricultural region in Vojvodina Province, Serbia. Environ. Sci. Pollut. Res. 2021, 28, 16065–16080. [Google Scholar] [CrossRef]
- Wall, W.A.; Busby, R.; Bosche, L. Vegetation predicts soil shear strength in Arctic Soils: Ground-based and remote sensing techniques. Ann. For. Res. 2024, 67, 155–166. [Google Scholar] [CrossRef]
- Vlad, R.; Constandache, C.; Dinca, L.; Tudose, N.C.; Sidor, C.G.; Popovici, L.; Ispravnic, A. Influence of climatic, site and stand characteristics on some structural parameters of Scots pine (Pinus sylvestris) forests situated on degraded lands from east Romania. Range Manag. Agrofor. 2019, 40, 40–48. [Google Scholar]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Dincă, L.; Murariu, G.; Enescu, C.M.; Achim, F.; Georgescu, L.; Murariu, A.; Holonec, L. Productivity differences between southern and northern slopes of Southern Carpathians (Romania) for Norway spruce, silver fir, birch and black alder. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1070–1084. [Google Scholar] [CrossRef]
- Raihan, F.; Shela, S.; Alam, M.; Haque, M.E.; Chen, T.W.; Horvatinec, J.; Ondrasek, G. Impact of Michelia champaca and Tectona grandis mono-species and their mixed plantation on chemical soil properties in a tropical semi-evergreen forest. Ann. For. Res. 2024, 67, 41–54. [Google Scholar] [CrossRef]
- Silvestru-Grigore, C.V.; Dinulică, F.; Spârchez, G.; Hălălișan, A.F.; Dincă, L.C.; Enescu, R.E.; Crișan, V.E. Radial growth behavior of pines on Romanian degraded lands. Forests 2018, 9, 213. [Google Scholar] [CrossRef]
- Marin, M.; Clinciu, I.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L.; Mărțoiu, N.E.; Tudose, O.N. Assessment of Seasonal Surface Runoff under Climate and Land Use Change Scenarios for a Small Forested Watershed: Upper Tarlung Watershed (Romania). Water 2022, 14, 2860. [Google Scholar] [CrossRef]
- Marin, M.; Tudose, N.C.; Ungurean, C.; Mihalache, A.L. Application of Life Cycle Assessment for Torrent Control Structures: A Review. Land 2024, 13, 1956. [Google Scholar] [CrossRef]
- Davidescu, S.O.; Clinciu, I.; Tudose, N.C.; Ungurean, C. An evaluating methodology for hydrotechnical torrent-control structures condition. Ann. For. Res. 2012, 55, 125–143. [Google Scholar] [CrossRef]
- Mihalache, A.L.; Marin, M.; Davidescu, Ș.O.; Ungurean, C.; Adorjani, A.; Tudose, N.C.; Davidescu, A.A.; Clinciu, I. Physical status of torrent control structures in Romania. Environ. Eng. Manag. J. 2020, 19, 861–872. [Google Scholar] [CrossRef]
- Vasile, D.; Petritan, A.-M.; Tudose, N.C.; Toiu, F.L.; Scarlatescu, V.; Petritan, I.C. Structure and Spatial Distribution of Dead Wood in Two Temperate Old-Growth Mixed European Beech Forests. Not. Bot. Horti. Agrobot 2017, 45, 639–645. [Google Scholar] [CrossRef]
- Mustățea, M.; Clius, M.; Tudose, N.C.; Cheval, S. An enhanced Machado Index of naturalness. Catena 2022, 212, 106091. [Google Scholar] [CrossRef]
- Oprică, R.; Tudose, N.C.; Davidescu, S.O.; Zup, M.; Marin, M.; Comanici, A.N.; Crit, M.N.; Pitar, D. Gender inequalities in Transylvania’s largest peri-urban forest usage. Ann. For. Res. 2022, 65, 57–69. [Google Scholar] [CrossRef]
- Tudose, N.C.; Petritan, I.C.; Toiu, F.L.; Petritan, A.-M.; Marin, M. Relation between Topography and Gap Characteristics in a Mixed Sessile Oak–Beech Old-Growth Forest. Forests 2023, 14, 188. [Google Scholar] [CrossRef]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Tillage Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Freibauer, A.; Rounsevell, M.D.; Smith, P.; Verhagen, J. Carbon sequestration in the agricultural soils of Europe. Geoderma 2004, 122, 1–23. [Google Scholar] [CrossRef]
- Lal, R. Soils and sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 57–64. [Google Scholar] [CrossRef]
- Vig, K.; Megharaj, M.; Sethunathan, N.; Naidu, R. Bioavailability and toxicity of Cadmium to microorganisms and their activities in soil: A review. Adv. Environ. Res. 2003, 8, 121–135. [Google Scholar] [CrossRef]
- Singh, B.R.; McLaughlin, M.J. Cd in soils and plants: Summary and research perspectives. In Cadmium in Soils and Plants; Springer: Dordrecht, The Netherlands, 1999; pp. 257–267. [Google Scholar]
- Nungula, E.Z.; Raza, M.A.; Nasar, J.; Maitra, S.; Seleiman, M.F.; Ranjan, S.; Gitari, H.H. Cadmium in soil and plants: A review. In Cadmium Toxicity in Water: Challenges and Solutions; Springer Nature: Cham, Switzerland, 2024; pp. 21–43. [Google Scholar]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clarivate.com. Web of Science Core Collection. Available online: https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/webofscience-platform/web-of-science-core-collection/ (accessed on 20 May 2025).
- Elsevier. Scopus. Available online: https://www.elsevier.com/products/scopus (accessed on 20 May 2025).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel?legRedir=true&CorrelationId=3bb60ab0-fe13-41a4-812b-2627667cf346 (accessed on 26 May 2025).
- Google. Geochart. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 23 May 2025).
- VOSviewer. Available online: https://www.vosviewer.com/ (accessed on 22 May 2025).
- Chen, Z.; Zhao, Y.; Gu, L.; Wang, S.; Li, Y.; Dong, F. Accumulation and localization of Cd in potato (Solanum tuberosum) under different soil Cd levels. Bull. Environ. Contam. Toxicol. 2014, 92, 745–751. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Twardowska, I.; Zhang, Q. In search of the exclusion/low-accumulation mechanisms: Cadmium uptake and accumulation from soil by cultivated (Solanum melongena L.) and wild eggplants (Solanum torvum L.). J. Clean. Prod. 2021, 323, 129141. [Google Scholar] [CrossRef]
- Chen, J.; Guo, J.; Li, Z.; Liang, X.; You, Y.; Li, M.; He, Y.; Zhan, F. Effects of an arbuscular mycorrhizal fungus on the growth of and Cadmium uptake in maize grown on polluted wasteland, farmland and slopeland soils in a lead-zinc mining area. Toxics 2022, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Biswash, M.R.; Li, K.W.; Lu, H.L.; Shi, Y.X.X.; Uwiringiyimana, E.; Guo, L.; Xu, R.K. Effect of Cd(II) adsorption onto rice roots on its uptake by different indica and japonica rice varieties and toxicity effect of Cd(II) under acidic conditions. Environ. Sci. Pollut. Res. 2024, 31, 30399–30414. [Google Scholar] [CrossRef]
- Guo, T.; He, D.; Liu, Y.; Li, J.; Wang, F. Lanthanum promotes Solanum nigrum L. growth and phytoremediation of Cadmium and lead through endocytosis: Physiological and biochemical response, heavy metal uptake and visualization. Sci. Total Environ. 2024, 912, 168915. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Dou, W.; Lin, D.; Qin, L.; An, Y.; Chen, H.; Wu, L.; Mou, L. Do tillage systems affect the Cadmium threshold in farmland soil for environmental quality standard setting? Sci. Total Environ. 2023, 862, 160816. [Google Scholar] [CrossRef]
- Avkopashvili, G.; Avkopashvili, M.; Gongadze, A.; Gakhokidze, R.; Avkopashvili, I.; Asanidze, L. Influence of biostimulants on the Cadmium, zinc and copper accumulation potential of the sugar beet (Beta vulgaris) and analysis ANOVA and accumulation coefficient. Carpathian J. Earth Environ. Sci. 2022, 17, 149–157. [Google Scholar] [CrossRef]
- Beri, W.T.; Gesessew, W.S.; Tian, S. Maize cultivars relieve health risks of Cd-Polluted Soils: In vitro Cd bioaccessibility and bioavailability. Sci. Total Environ. 2020, 703, 134852. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Zhao, Z.; Ma, C.; Chen, S. Adsorption and desorption of Cd by soil amendment: Mechanisms and environmental implications in field-soil remediation. Sustainability 2018, 10, 2337. [Google Scholar] [CrossRef]
- Dai, Y.; Song, M.; Liu, Y.; Zhang, Y.; Zhu, J.; Peng, H. Effect of Stubble Height on Cadmium Removal Potential of Removed Straw. Sustainability 2025, 17, 7123. [Google Scholar] [CrossRef]
- Sui FengFeng, S.F.; Wang JingBo, W.J.; Wu Hao, W.H.; Li LianQing, L.L.; Pan GenXing, P.G. Several research progresses in Cd inactivation by biochar application in agricultural soil. J. Agro-Environ. Sci. 2018, 37, 1468–1474. [Google Scholar]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of zeolite on aggregation, nutrient availability, and growth characteristics of corn (Zea mays L.) in Cadmium-contaminated soils. Water Air Soil Pollut. 2022, 233, 436. [Google Scholar] [CrossRef]
- Karaca, U.Ç.; Chalabee, O.A.H. The effect of increasing dose of vermicompost application on Cadmium contaminated soils on some soil enzyme activities. Carpathian J. Earth Environ. Sci. 2023, 18, 79–88. [Google Scholar] [CrossRef]
- Badora, A.; Furrer, G.; Grünwald, A.; Schulin, R. Immobilization of zinc and Cadmium in polluted soils by polynuclear Al13 and Al-montmorillonite. J. Soil Contam. 1998, 7, 573–588. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of Cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.G.; Garrido, F.; Illera, V.; García-González, M.T. Transport of Cd, Cu and Pb in an acid soil amended with phosphogypsum, sugar foam and phosphoric rock. Appl. Geochem. 2006, 21, 1030–1043. [Google Scholar] [CrossRef]
- Cui, J.; Li, P.; Qi, X.; Rahman, S.U.; Zhang, Z. Changes of Microbial Diversity in Rhizosphere of Different Cadmium-Gradients Soil under Irrigation with Reclaimed Water. Sustainability 2022, 14, 8891. [Google Scholar] [CrossRef]
- Beduk, F. Combined effects of Cadmium and azithromycin on soil nitrification process. Water 2023, 15, 881. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Okubo, H.; Murakami, K.; Miyamoto, K.; Hosoi, Y.; Murata, K.; Kayama, F. Age-relevant renal effects of Cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ. Int. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Cai, S.; Yue, L.; Hu, Z.; Zhong, X.; Ye, Z.; Xu, H.; Zhang, F. Cadmium exposure and health effects among residents in an irrigation area with ore dressing wastewater. Sci. Total Environ. 1990, 90, 67–73. [Google Scholar] [PubMed]
- Lalor, G.C. Review of Cadmium transfers from soil to humans and its health effects in the Jamaican environment. Sci. Total Environ. 2008, 400, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Bazoobandi, A.; Emamgholizadeh, S.; Ghorbani, H. Estimating the amount of Cadmium and lead in the polluted soil using artificial intelligence models. Eur. J. Environ. Civ. Eng. 2022, 26, 933–951. [Google Scholar] [CrossRef]
- De Vries, W.; McLaughlin, M.J.; Groenenberg, J.E. Transfer functions for solid–solution partitioning of Cadmium for Australian soils. Environ. Pollut. 2011, 159, 3583–3594. [Google Scholar] [CrossRef]
- Degryse, F.; Vlassak, V.; Smolders, E.; Merckx, R. Mobilization of Cd upon acidification of agricultural soils: Column study and field modelling. Eur. J. Soil Sci. 2007, 58, 152–165. [Google Scholar] [CrossRef]
- Deng, Y.; Fu, S.; Sarkodie, E.K.; Zhang, S.; Jiang, L.; Liang, Y.; Yin, H.; Bai, L.; Liu, X.; Liu, H.; et al. Ecological responses of bacterial assembly and functions to steep Cd gradient in a typical Cd-contaminated farmland ecosystem. Ecotoxicol. Environ. Saf. 2022, 229, 113067. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Okal, E.J.; Waseem, M. Cadmium toxicity impacts plant growth and plant remediation strategies. Plant Growth Regul. 2023, 99, 397–412. [Google Scholar] [CrossRef]
- Granel, T.; Robinson, B.; Mills, T.; Clothier, B.; Green, S.; Fung, L. Cadmium accumulation by willow clones used for soil conservation, stock fodder, and phytoremediation. Soil Res. 2002, 40, 1331–1337. [Google Scholar] [CrossRef]
- Sattar, S.; Yahya, M.; Aslam, S.; Hussain, R.; Shah, S.M.M.; Rauf, Z.; Shahzad, A. Environmental occurrence, hazards, and remediation strategies for the removal of Cadmium from the polluted environment. Results Eng. 2025, 25, 104322. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Guo, S.; Zhao, M. Cadmium concentration and its typical input and output fluxes in agricultural soil downstream of a heavy metal sewage irrigation area. J. Hazard. Mater. 2021, 412, 125203. [Google Scholar] [CrossRef]
- Guan, M.; Xia, Y.; Zhang, W.; Chen, M.; Cao, Z. A Review of Reducing Cadmium Pollution in the Rice–Soil System in China. Foods 2025, 14, 1747. [Google Scholar] [CrossRef]
- Lu, L.T.; Chang, I.C.; Hsiao, T.Y.; Yu, Y.H.; Ma, H.W. Identification of pollution source of Cadmium in soil: Application of material flow analysis and a case study in Taiwan. Environ. Sci. Pollut. Res. Int. 2007, 14, 49–59. [Google Scholar]
- Chiang, P.H.; Musa, G.J.; Hsieh, D.P.; Liou, D.M.; Wen, C.P.; Chan, T.C.; Chen, H.L. Spatial interpolation of Cadmium contamination of agricultural soils in Changhua County, Taiwan. Int. J. Environ. Pollut. 2010, 40, 322–336. [Google Scholar] [CrossRef]
- Garg, A. Level of Cd in different types of soil of Rohtak district and its bioremediation. J. Environ. Chem. Eng. 2016, 4, 3797–3802. [Google Scholar] [CrossRef]
- Hussain, A.; Murtaza, G.; Ghafoor, A.; Basra, S.M.A.; Qadir, M.; Sabir, M. Cadmium contamination of soils and crops by long term use of raw effluent, ground and canal waters in agricultural lands. Int. J. Agric. Biol. 2010, 12, 851–856. [Google Scholar]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Búllon Castillo, A.; Muñoz-Rojas, M. Soil, site, and management factors affecting Cadmium concentrations in cacao-growing soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil properties and agronomic factors affecting Cadmium concentrations in cacao beans: A nationwide survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, V.; Tandon, V.; Kumar, S.; Roohi. Effect and Responses of Cadmium in Plants. In Cadmium Toxicity in Water: Challenges and Solutions; Springer Nature: Cham, Switzerland, 2024; pp. 327–347. [Google Scholar]
- Akoury, E.; El Kantar, S.; Abdallah, H.; Al Timani, D.; Daher, Z. Evaluation of Cadmium uptake and consumption of parsley in Lebanese diet. Int. J. Environ. Sci. Technol. 2023, 20, 6079–6090. [Google Scholar] [CrossRef]
- Arsenov, D.; Župunski, M.; Pajević, S.; Borišev, M.; Nikolić, N.; Mimica-Dukić, N. Health assessment of medicinal herbs, celery and parsley related to Cadmium soil pollution—Potentially toxic elements (PTEs) accumulation, tolerance capacity and antioxidative response. Environ. Geochem. Health 2021, 43, 2927–2943. [Google Scholar] [CrossRef]
- Al Obaid, S.; Ansari, M.J. Enhancing maize resilience to microplastics and Cadmium through nanoparticles intervention. S. Afr. J. Bot. 2024, 171, 106–119. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Mahar, A.; Wang, P.; Ma, F.; Shen, F.; Li, R.; Zhang, Z. Phytoextraction of toxic trace elements by Sorghum bicolor inoculated with Streptomyces pactum (Act12) in contaminated soils. Ecotoxicol. Environ. Saf. 2017, 139, 202–209. [Google Scholar] [CrossRef]
- Davis, L.M.M.; Hidayati, N.; Firdaus, A.M.; Talib, C.; Rini, D.S.; Juhaeti, T.; Syarif, F.; Gunawan, I. Uptake and translocation of lead and Cadmium in wild-found plant species from Bekasi and Karawang, West Java, for phytoremediation. IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012070. [Google Scholar] [CrossRef]
- Hayat, R.; Khan, I.; Chattha, M.U.; Hassan, M.U.; Jian, W.; Al-Khayri, J.M.; Aldaej, M.I.; Sattar, M.N.; Rezk, A.A.-S.; Almaghasla, M.I. Growth, physiological and biochemical responses of mung bean (Vigna radiata L.) to Cadmium polluted soil. J. Ecol. Eng. 2024, 25, 75–84. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Z.; Hou, Y.; Li, J.; Shen, Z.; Chen, Y. The original polyethylene microplastics inhibit the growth of sweet potatoes and increase the safety risk of Cadmium. Front. Plant Sci. 2023, 14, 1138281. [Google Scholar] [CrossRef]
- Al-Hassoon, S.N.H.; Al-Hayani, A.S.J.Z.; Al-Obaidi, M.A.J. Evaluation of the Biological Phenomenon of Cd Accumulation of Wheat Crop in Salt-Affected Soil. IOP Conf. Ser. Earth Environ. Sci. 2024, 1325, 012010. [Google Scholar] [CrossRef]
- Kazemi, F.; Jozay, M. Developing a sustainable nature-based agricultural vertical system in Cadmium polluted urban environments. Ecol. Eng. 2024, 208, 107385. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Seleiman, M.F.; Aljabri, M.; Ahmad, A.; Alotaibi, M.; Battaglia, M.L. Phytoremediation: A promising approach for re-vegetation of Cadmium-polluted land. In Cadmium Toxicity Mitigation; Springer Nature: Cham, Switzerland, 2024; pp. 215–242. [Google Scholar]
- Chen, L.; Yang, W.; Yang, Y.; Tu, P.; Hu, S.; Zeng, Q. Three-season rotation of chicory–tobacco–peanut with high biomass and bioconcentration factors effectively remediates Cadmium-contaminated farmland. Environ. Sci. Pollut. Res. 2022, 29, 64822–64831. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Z.; Wang, J.; Liu, H.; Li, N.; Wu, L.; Hu, P.; Luo, Y.; Christie, P. Long-term field phytoextraction of zinc/Cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int. J. Phytoremediat. 2016, 18, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Dai, H.; Twardowska, I.; Wei, S. Hyperaccumulation of Cd by Rorippa globosa (Turcz.) Thell. from soil enriched with different Cd compounds, and impact of soil amendment with glutathione (GSH) on the hyperaccumulation efficiency. Environ. Pollut. 2019, 255, 113270. [Google Scholar] [CrossRef]
- Fei, L.; Xie, S.; Wu, Y. Preliminary Screening of Cadmium Hyperaccumulators. In International Conference on Environmental Science and Development; Springer Nature: Cham, Switzerland, 2024; pp. 17–25. [Google Scholar]
- Chen, Z.Q.; Liu, Q.Z.; Chen, D.; Wu, Y.J.; Hamid, Y.; Lin, Q.; Zhang, S.J.; Feng, Y.; He, Z.L.; Yin, X.Y.; et al. Enhancing the phytoextraction efficiency of heavy metals in acidic and alkaline soils by Sedum alfredii Hance: A study on the synergistic effect of plant growth regulator and plant growth-promoting bacteria. Sci. Total Environ. 2024, 932, 173029. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Cao, X.; Dong, Q.; Mao, L.; Yang, X.; Wang, X.; Zou, Q. Enhanced Phytoextraction Technologies for the Sustainable Remediation of Cadmium-Contaminated Soil Based on Hyperaccumulators—A Review. Plants 2025, 14, 115. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Qin, Y.; Cui, S.; Wang, G. Dual tolerance of ageratum (Ageratum conyzoides L.) to combined pollution of acid and Cadmium: Field survey and pot experiment. J. Environ. Manag. 2023, 326, 116677. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Song, Z.; Wang, D.; Qiu, W. Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2019, 237, 124480. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environ. Sci. Pollut. Res. 2009, 16, 844–854. [Google Scholar] [CrossRef]
- Tan, K.; Tu, P.; Yang, Y.; Yuan, J.; Chen, L.; Zeng, Q. Phytoextraction of Cadmium contaminated agricultural soil by tobacco and swiss chard rotation systems. J. Environ. Eng. Technol. 2020, 10, 440–448. [Google Scholar]
- Wang, Q.; Zhou, Q.; Huang, L.; Xu, S.A.; Fu, Y.; Hou, D.; Feng, Y.; Yang, X. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol. Environ. Saf. 2022, 237, 113541. [Google Scholar] [CrossRef]
- Sultana, S.; Chakma, K.; Bhuiyan, M.S.U. Development of transgenic Brassica napus plants with AtATM3 gene to enhance Cadmium and lead tolerance. J. Hortic. Sci. 2024, 19, 1–9. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Wu, Y.; Wang, Q.; Sahito, Z.A.; Zhou, Q.; Feng, Y. The effects and health risk assessment of cauliflower co-cropping with Sedum alfredii in Cadmium contaminated vegetable field. Environ. Pollut. 2021, 268, 115869. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, Y.; Yang, Z.; Xin, J.; Yuan, J.; Wang, J.; Xin, G. Responses of different Chinese flowering cabbage (Brassica parachinensis L.) cultivars to Cadmium and lead exposure: Screening for Cd + Pb pollution-safe cultivars. CLEAN—Soil Air Water 2011, 39, 925–932. [Google Scholar] [CrossRef]
- Yu, G.; Ullah, H.; Lin, H.; Sunahara, G.I.; Zhang, X.; Chen, B.; Yu, H.; Shahab, A.; Liu, L.; Liu, J. Long-term phytoextraction potential and mechanism of Celosia argentea on soils with different levels of Cd and Mn co-contamination. J. Environ. Chem. Eng. 2024, 12, 112125. [Google Scholar] [CrossRef]
- Kumar, H.; Ishtiyaq, S.; Ponia, V.; Favas, P.J.; D’Souza, R.J.; Varun, M.; Paul, M.S. Cadmium toxicity and role of plant growth promoting bacteria in phytoremediation. In Cadmium Toxicity Mitigation; Springer Nature: Cham, Switzerland, 2024; pp. 169–194. [Google Scholar]
- Lu, X.; Chen, Y.; Song, J.; Bao, J.; Dai, C.; Sun, R.; Oh, K. Screening of Profitable Chrysanthemums for the Phytoremediation of Cadmium-Contaminated Soils. Toxics 2025, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Saran, A.; Fernandez, L.; Cora, F.; Savio, M.; Thijs, S.; Vangronsveld, J.; Merini, L.J. Phytostabilization of Pb and Cd polluted soils using Helianthus petiolaris as pioneer aromatic plant species. Int. J. Phytoremediat. 2020, 22, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, S.; Li, Z.; Yan, X.; Qin, Z.; Huang, R. Field scale remediation of Cd and Pb contaminated paddy soil using three mulberry (Morus alba L.) cultivars. Ecol. Eng. 2019, 129, 38–44. [Google Scholar] [CrossRef]
- Prapagdee, B.; Khonsue, N. Bacterial-assisted Cadmium phytoremediation by Ocimum gratissimum L. in polluted agricultural soil: A field trial experiment. Int. J. Environ. Sci. Technol. 2015, 12, 3843–3852. [Google Scholar] [CrossRef]
- Nezhad, M.Y.; Hemmati, F. Survey of phragmites australis for phytoextraction of Cadmium in International Shadegan wetland. Adv. Environ. Biol. 2012, 4045–4050. [Google Scholar]
- Van Nevel, L.; Mertens, J.; Staelens, J.; De Schrijver, A.; Tack, F.M.; De Neve, S.; Meers, E.; Verheyen, K. Elevated Cd and Zn uptake by aspen limits the phytostabilization potential compared to five other tree species. Ecol. Eng. 2011, 37, 1072–1080. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, R.P. Ricinus communis L. a value added crop for remediation of Cadmium contaminated soil. Bull. Environ. Contam. Toxicol. 2016, 96, 265–269. [Google Scholar] [PubMed]
- Sun, Y.; Wen, C.; Liang, X.; He, C. Determination of the phytoremediation efficiency of Ricinus communis L. and methane uptake from Cadmium and nickel-contaminated soil using spent mushroom substrate. Environ. Sci. Pollut. Res. 2018, 25, 32603–32616. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Yin, Y.; Ji, R.; Wu, J.; Wang, X.; Guo, H. Ethyl lactate-EDTA composite system enhances the remediation of the Cadmium-contaminated soil by autochthonous willow (Salix × Aureo-pendula CL ‘J1011’) in the lower reaches of the Yangtze River. J. Hazard. Mater. 2010, 181, 673–678. [Google Scholar] [CrossRef]
- Wieshammer, G.; Unterbrunner, R.; García, T.B.; Zivkovic, M.F.; Puschenreiter, M.; Wenzel, W.W. Phytoextraction of Cd and Zn from agricultural soils by Salix spp. and intercropping of Salix caprea and Arabidopsis halleri. Plant Soil 2007, 298, 255–264. [Google Scholar] [CrossRef]
- Papadimou, S.G.; Barbayiannis, Ν.; Golia, E.E. Preliminary investigation of the use of Silybum marianum (L.) Gaertn. as a Cd accumulator in contaminated Mediterranean soils: The relationships among Cadmium (Cd) soil fractions and plant Cd content. Euro-Mediterr. J. Environ. Integr. 2024, 9, 405–417. [Google Scholar]
- Alamer, K.H.; Attia, H.; Alrobaie, H.S.; Hamad, A.A. Biocompatibility of Solanum lycopersicum and Solanum melongena which developed in heavy metal polluted soils. S. Afr. J. Bot. 2022, 147, 24–34. [Google Scholar] [CrossRef]
- Ji, P.; Song, Y.; Sun, T.; Liu, Y.; Cao, X.; Xu, D.; Yang, X.; McRae, T. In-situ Cadmium phytoremediation using Solanum nigrum L.: The bio-accumulation characteristics trail. Int. J. Phytoremediat. 2011, 13, 1014–1023. [Google Scholar]
- Yin, Y.C.; Ji, P.H.; Song, X.Y.; Zhang, W.; Dong, X.X.; Cao, X.F.; Song, Y.F. Field experiment on phytoremediation of Cadmium contaminated soils using Solanum nigrum L. Chin. J. Ecol. 2014, 33, 3060. [Google Scholar]
- Xiao, M.Z.; Sun, R.; Du, Z.Y.; Yang, W.B.; Sun, Z.; Yuan, T.Q. A sustainable agricultural strategy integrating Cd-contaminated soils remediation and bioethanol production using sorghum cultivars. Ind. Crops Prod. 2021, 162, 113299. [Google Scholar]
- Saison, C.; Schwartz, C.; Morel, J.L. Hyperaccumulation of metals by Thlaspi caerulescens as affected by root development and Cd–Zn/Ca–Mg interactions. Int. J. Phytoremediat. 2004, 6, 49–61. [Google Scholar] [CrossRef]
- Bagot, D.; Lebeau, T.; Jezequel, K.; Fabre, B. Selection of microorganisms for bioremediation of agricultural soils contaminated by Cadmium. In Environmental Chemistry: Green Chemistry and Pollutants in Ecosystems; Springer: Berlin/Heidelberg, Germany, 2005; pp. 215–222. [Google Scholar]
- Bhardwaj, T.; Singh, R.; Singh, H.; Bhanwaria, R.; Gandhi, S.G.; Bhardwaj, R.; Ahmad, A.; Ahmad, P. Pseudomonas consortium improves soil health and alleviates Cadmium (Cd) toxicity in Brassica juncea L. via biochemical and in silico approaches. Plant Stress 2024, 14, 100611. [Google Scholar] [CrossRef]
- Cáceres, P.F.F.; Vélez, L.P.; Junca, H.; Moreno-Herrera, C.X. Theobroma cacao L. agricultural soils with natural low and high Cadmium (Cd) in Santander (Colombia), contain a persistent shared bacterial composition shaped by multiple soil variables and bacterial isolates highly resistant to Cd concentrations. Curr. Res. Microb. Sci. 2021, 2, 100086. [Google Scholar] [CrossRef]
- Taghavi Ghasemkheili, F.; Ekelund, F.; Johansen, J.L.; Pirdashti, H.; Ghadirnezhad Shiade, S.R.; Fathi, A.; Kjøller, R. Ameliorative effects of Trichoderma harzianum and rhizosphere soil microbes on Cadmium biosorption of barley (Hordeum vulgare L.) in Cd-polluted soil. J. Soil Sci. Plant Nutr. 2022, 22, 527–539. [Google Scholar] [CrossRef]
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; et al. Role of sepiolite for Cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manag. 2020, 258, 110020. [Google Scholar] [CrossRef]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Cattani, I.; Romani, M.; Boccelli, R. Effect of cultivation practices on Cadmium concentration in rice grain. Agron. Sustain. Dev. 2008, 28, 265–271. [Google Scholar] [CrossRef]
- He, S.; Song, H.; Peng, L.; Kuang, X.; Zeng, Q.; Yin, M.; Deng, F. Sunflower bottom ash improve soil properties and microbial community in Cadmium-polluted acid farmland. Water Air Soil Pollut. 2024, 235, 786. [Google Scholar] [CrossRef]
- Huang, Z.; Niu, S.; Li, X.; Guo, J.; Yang, Z.; Zhou, J.; Cheng, Y.; Zhang, Y.; Jiang, L.; Yu, J.; et al. Biochar immobilized Proteus mirabilis Ch8 to enhance the Cd phytoremediation potential of woody plant Robinia pseudoacacia L. J. Environ. Manag. 2025, 377, 124620. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of Cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Duwiejuah, A.B. Biochar-Based Adsorptive Materials for the Efficient Immobilisation of Cadmium in Contaminated Soils. In Cadmium Toxicity: Challenges and Solutions; Springer Nature: Cham, Switzerland, 2024; pp. 223–241. [Google Scholar]
- El-Tohory, S.; Zeng, W.; Huang, J.; Moussa, M.G.; Dong, L.; Mohamed, A.; Khalifa, O.; Saleem, M.H.; Zhran, M.; Salama, M.; et al. Effect of intercropping and biochar application on Cadmium removal capacity by Corchorus olitorius and Zea mays. Environ. Technol. Innov. 2023, 29, 103033. [Google Scholar] [CrossRef]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A comprehensive literature review on Cadmium (Cd) status in the soil environment and its immobilization by biochar-based materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced Cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Hafeez, F.; Rizwan, M.; Saqib, M.; Yasmeen, T.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Qayyum, M.F. Residual effect of biochar on growth, antioxidant defence and Cadmium (Cd) accumulation in rice in a Cd contaminated saline soil. Pak. J. Agric. Sci. 2019, 56, 197–204. [Google Scholar]
- ur Rehman, M.Z.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of Cadmium (Cd) toxicity and minimizing its uptake in wheat (Triticum aestivum) by using organic carbon sources in Cd-spiked soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Meng, Y.; Pan, B.; Pan, B.; Wei, J.; Ding, J.; Deng, Y.; Su, X.; Yuan, Z.; Zhang, M. Multidimensional effects of green waste vermicomposting on Cadmium contaminated soil ecosystems: From physicochemical properties to microbial communities. J. Hazard. Mater. 2024, 480, 136429. [Google Scholar] [CrossRef]
- Raiesi, F.; Dayani, L. Compost application increases the ecological dose values in a non-calcareous agricultural soil contaminated with Cadmium. Ecotoxicology 2021, 30, 17–30. [Google Scholar] [CrossRef]
- Heydari, R.; Kolahi, M.; Mohajel Kazemi, E.; Nosrati, H.; Movafeghi, A. The role of nano-chelated iron on anatomical and biochemical characteristics and concentration of mineral nutrients in lettuce (Lactuca sativa L.) under Cadmium toxicity. Physiol. Mol. Biol. Plants 2024, 30, 1383–1400. [Google Scholar] [CrossRef]
- Chang, J.H.; Wang, Y.L.; Shen, S.Y. A specific configuration of circulation-enhanced electrokinetics (CEEK) to remediate real-site Cd and Pb contaminated soils. J. Hazard. Mater. 2018, 359, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wen, M.; Liu, P.; Jiang, Y. Removal of Heavy Metal Cd Element from Paddy Soil by Geo-Electrochemical Technology. Appl. Sci. 2023, 13, 11685. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cheema, S.A.; Farooq, M.; Wu, J.; Zhang, R.; Shuaijie, G.; Liqun, C. Co-application of biochar and microorganisms improves soybean performance and remediate Cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 214, 112112. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Khan, I.; Farooq, M.; Cai, L.; Li, Y. Co-application of biochar and plant growth regulators improves maize growth and decreases Cd accumulation in Cadmium-contaminated soil. J. Clean. Prod. 2024, 440, 140515. [Google Scholar] [CrossRef]
- Halim, M.A.; Rahman, M.M.; Megharaj, M.; Naidu, R. Cadmium immobilization in the rhizosphere and plant cellular detoxification: Role of plant-growth-promoting rhizobacteria as a sustainable solution. J. Agric. Food Chem. 2020, 68, 13497–13529. [Google Scholar] [CrossRef]
- Han, H.; Wu, X.; Yao, L.; Chen, Z. Heavy metal-immobilizing bacteria combined with calcium polypeptides reduced the uptake of Cd in wheat and shifted the rhizosphere bacterial communities. Environ. Pollut. 2020, 267, 115432. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Wu, X.W.; Li, Y.L.; Fang, Y.Y.; Wang, M.; Zhao, H.W. Nanohydroxyapatite combined with white rot fungus can effectively reduce Cadmium activity and change the soil microbial population structure. Fresenius Environ. Bull. 2020, 29, 9644–9653. [Google Scholar]
- Chen, Z.; Lu, Z.; Zhang, Y.; Li, B.; Chen, C.; Shen, K. Effects of biochars combined with ferrous sulfate and pig manure on the bioavailability of Cd and potential phytotoxicity for wheat in an alkaline contaminated soil. Sci. Total Environ. 2021, 753, 141832. [Google Scholar] [CrossRef]
- Gao, W.K.; Yang, Y.Y.; Zong, H.Y.; Liu, J.; Song, N.N.; Wang, F.L. Simultaneously sorptive reduction in Cadmium and glyphosate diffuse loss by biochar-amended soil. Fresenius Environ. Bull. 2020, 29, 4545–4555. [Google Scholar]
- Rahimi, M.; Bertalan-Balázs, B.; Adelinia, A.; Ebrahimi, E.; Ojani, M. Impact assessment of Zeolite, Ca-bentonite and Biochar amendments on Cd bioavailability and fractions in polluted calcareous soils. Environ. Earth Sci. 2024, 83, 506. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, J.; Cai, P.; Chen, W.; Huang, Q. Bio-organic stabilizing agent shows promising prospect for the stabilization of Cadmium in contaminated farmland soil. Environ. Sci. Pollut. Res. 2019, 26, 23399–23406. [Google Scholar] [CrossRef]
- Barman, F.; Guha, T.; Kundu, R. Exogenous Selenium Supplements Reduce Cd Accumulation and Restore Micronutrient Content in Rice Grains. J. Soil Sci. Plant Nutr. 2025, 25, 2275–2293. [Google Scholar] [CrossRef]
- Bao, Q.; Bao, W.; Li, Y.; Zhang, S.; Lian, F.; Huang, Y. Silicon combined with foliar melatonin for reducing the absorption and translocation of Cd and As by Oryza sativa L. in two contaminated soils. J. Environ. Manag. 2021, 287, 112343. [Google Scholar] [CrossRef]
- Bao, Q.; Bao, Y.; Shi, J.; Sun, Y. Nano zero-valent iron and melatonin synergistically alters uptake and translocation of Cd and As in soil–rice system and mechanism in soil chemistry and microbiology. Environ. Int. 2024, 185, 108550. [Google Scholar] [CrossRef]
- Cao, H.W.; Zhao, Y.N.; Liu, X.S.; Rono, J.K.; Yang, Z.M. A metal chaperone OsHIPP16 detoxifies Cadmium by repressing its accumulation in rice crops. Environ. Pollut. 2022, 311, 120058. [Google Scholar] [CrossRef]
- Peng, C.; Song, H.; Zhao, Z.; Kuang, X.; Wang, Y.; Chen, S.; Peng, L. Foliar spraying with a mixture of transpiration inhibitor-rhamnolipid reduces the Cd content in rice grains. Sci. Total Environ. 2023, 885, 163844. [Google Scholar] [CrossRef]
- Duan, G.L.; Wang, F.; Cen, K.; Wang, B.X.; Cheng, W.D.; Liu, Y.C.; Zhang, H.M. Effects of straw incorporation on Cadmium accumulation and subcellular distribution in rice. Huan Jing Ke Xue = Huanjing Kexue 2017, 38, 3927–3936. [Google Scholar]
- Li, S.; Fu, W.; Li, B.; Wang, Y.; Cheng, Y.; Kang, H.; Zeng, J. Insight into Cd Detoxification and Accumulation in Wheat by Foliar Application of Ferulic Acid. Plants 2025, 14, 1436. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; He, L.; Xu, Y.; Huang, Q.; Xie, Z.; Yang, F. Inhibition of Cd accumulation in winter wheat (Triticum aestivum L.) grown in alkaline soil using mercapto-modified attapulgite. Sci. Total Environ. 2019, 688, 818–826. [Google Scholar] [CrossRef]
- Ge, C.; Wang, Y.; Ma, W.; Ahmad, H.A.; Cheng, C.; Li, H.B.; Zhou, D. The potential ability of fungi in preventing Cadmium accumulation in wheat seedlings grown in weakly alkaline soils: Evidence from the application of fungicide. Appl. Soil Ecol. 2024, 198, 105359. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Li, W.; Xie, Y.; Wang, T.; Liu, J. Endophytic bacteria-assisted Cadmium removal in sunflower stalks: Towards safe biomass recycling. Environ. Technol. 2025, 46, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yuan, K.; Peng, Q.; Lv, R.; Zheng, Y. Stenotrophomonas strain CD2 reduces Cadmium accumulation in Brassica rapa L. Front. Sustain. Food Syst. 2024, 8, 1362265. [Google Scholar] [CrossRef]
- Shahid, S.; Dar, A.; Hussain, A.; Khalid, I.; Latif, M.; Ahmad, H.T.; Aloud, S.S. Enhancing cauliflower growth under Cadmium stress: Synergistic effects of Cd-tolerant Klebsiella strains and jasmonic acid foliar application. Front. Microbiol. 2024, 15, 1444374. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Majumdar, S. Cadmium stress management in plants: Prospects of plant growth-promoting rhizobacteria. In Plant Stress: Challenges and Management in the New Decade; Springer International Publishing: Cham, Switzerland, 2022; pp. 235–249. [Google Scholar]
- Bratu, I.; Dinca, L.; Constandache, C.; Murariu, G. Resilience and decline: The impact of climatic variability on temperate oak forests. Climate 2025, 13, 119. [Google Scholar] [CrossRef]
- Dincă, L.; Crisan, V.; Ienaşoiu, G.; Murariu, G.; Drăşovean, R. Environmental Indicator Plants in Mountain Forests: A Review. Plants 2024, 13, 3358. [Google Scholar] [CrossRef] [PubMed]
- Slepetiene, A.; Belova, O.; Fastovetska, K.; Dinca, L.; Murariu, G. Managing Boreal Birch Forests for Climate Change Mitigation. Land 2025, 14, 1909. [Google Scholar] [CrossRef]
- Dincă, L.; Murariu, G.; Lupoae, M. Understanding the ecosystem services of riparian forests: Patterns, gaps, and global trends. Forests 2025, 16, 947. [Google Scholar] [CrossRef]
- Budău, R.; Timofte, C.S.C.; Mirisan, L.V.; Bei, M.; Dinca, L.; Murariu, G.; Racz, K.A. Living Landmarks: A Review of Monumental Trees and Their Role in Ecosystems. Plants 2025, 14, 2075. [Google Scholar] [CrossRef]
- Bratu, I.; Dinca, L.; Schiteanu, I.; Mocanu, G.; Murariu, G.; Stanciu, M.; Zhiyanski, M. Sports in Natural Forests: A Systematic Review of Environmental Impact and Compatibility for Readability. Sports 2025, 13, 250. [Google Scholar] [CrossRef]
- Dincă, L.; Constandache, C.; Postolache, R.; Murariu, G.; Tupu, E. Timber Harvesting in Mountainous Regions: A Comprehensive Review. Forests 2025, 16, 495. [Google Scholar] [CrossRef]
- Murariu, G.; Dinca, L.; Munteanu, D. Trends and Applications of Principal Component Analysis in Forestry Research: A Literature and Bibliometric Review. Forests 2025, 16, 1155. [Google Scholar] [CrossRef]
- Dinca, L.; Coca, A.; Tudose, N.C.; Marin, M.; Murariu, G.; Munteanu, D. The Role of Trees in Sand Dune Rehabilitation: Insights from Global Experiences. Appl. Sci. 2025, 15, 7358. [Google Scholar] [CrossRef]
- Achim, F.; Dinca, L.; Chira, D.; Raducu, R.; Chirca, A.; Murariu, G. Sustainable Management of Willow Forest Landscapes: A Review of Ecosystem Functions and Conservation Strategies. Land 2025, 14, 1593. [Google Scholar] [CrossRef]
- Enescu, C.M.; Mihalache, M.; Ilie, L.; Dinca, L.; Constandache, C.; Murariu, G. Agricultural benefits of shelterbelts and windbreaks: A bibliometric analysis. Agriculture 2025, 15, 1204. [Google Scholar] [CrossRef]
- Liu, X.; Tian, G.; Jiang, D.; Zhang, C.; Kong, L. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. 2016, 23, 17941–17952. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A review of soil Cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Zhong, T.; Zhang, X.; Cheng, M.; Li, X. Assessment of Cadmium (Cd) concentration in arable soil in China. Environ. Sci. Pollut. Res. 2015, 22, 4932–4941. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Shi, Y.; Feng, Y.L.; Li, Q.; Chen, W.Q.; Zhang, W.J.; Li, H.Q. Anthropogenic cadmium cycles and emissions in Mainland China 1990–2015. J. Clean. Prod. 2019, 230, 1256–1265. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, F.M. Accumulation of lead and cadmium in soil and vegetable crops along major highways in Agra (India). J. Chem. 2010, 7, 1174–1183. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Khan, S.; Brusseau, M.L.; Shah, M.T. Lead and cadmium contamination and exposure risk assessment via consumption of vegetables grown in agricultural soils of five-selected regions of Pakistan. Chemosphere 2017, 168, 1589–1596. [Google Scholar] [CrossRef]
- Thomas, E.; Atkinson, R.; Zavaleta, D.; Rodriguez, C.; Lastra, S.; Yovera, F.; Arango, K.; Pezo, A.; Aguilar, J.; Tames, M.; et al. The distribution of cadmium in soil and cacao beans in Peru. Sci. Total Environ. 2023, 881, 163372. [Google Scholar] [CrossRef]
- Ochoa, M.; Tierra, W.; Tupuna-Yerovi, D.S.; Guanoluisa, D.; Otero, X.L.; Ruales, J. Assessment of cadmium and lead contamination in rice farming soils and rice (Oryza sativa L.) from Guayas province in Ecuador. Environ. Pollut. 2020, 260, 114050. [Google Scholar] [CrossRef]
- Goel, S. Phytoremediation of Cadmium Toxicity in Water and Soil: A Sustainable, Multidisciplinary and Eco-Friendly Approach. In Cadmium Toxicity: Challenges and Solutions; Springer Nature: Cham, Switzerland, 2024; pp. 181–207. [Google Scholar]
- Xue, H.; Liu, Z.X.; Yan, M.L. Advances in physiological mechanisms of heavy metal hyperaccumulation by plants. Biot. Resour. 2019, 41, 289–297. [Google Scholar]
- Chuaphasuk, C.; Prapagdee, B. Effects of biochar-immobilized bacteria on phytoremediation of Cadmium-polluted soil. Environ. Sci. Pollut. Res. 2019, 26, 23679–23688. [Google Scholar] [CrossRef]
- Apostol, E.N.; Stuparu, E.; Scarlatescu, V.; Budeanu, M. Testing Hungarian oak (Quercus frainetto Ten.) provenances in Romania. iForest 2020, 13, 9–15. [Google Scholar] [CrossRef]
- Besliu, E.; Curtu, A.L.; Apostol, E.N.; Budeanu, M. Using adapted and productive European beech (Fagus sylvatica L.) provenances as future solutions for sustainable forest management in Romania. Land 2024, 13, 183. [Google Scholar] [CrossRef]
- Budeanu, M.; Şofletea, N.; Petriţan, I.C. Among-population variation in quality traits in two Romanian provenance trials with Picea abies L. Balt. For. 2014, 20, 37–47. [Google Scholar]
- Budeanu, M.; Besliu, E.; Pepelea, D. Testing the radial increment and climate–growth relationship between Swiss stone pine European provenances in the Romanian Carpathians. Forests 2025, 16, 391. [Google Scholar] [CrossRef]
- Younas, H.; Nazir, A.; Bareen, F.E. From sources to solutions: Integrated approaches for Cd, Hg, and Pb remediation-a comprehensive review. Plant Soil 2025, 510, 1–47. [Google Scholar]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K. Integrated remediation processes toward heavy metal removal/recovery from various environments—A review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar]
- Martin, T.A.; Ruby, M.V. Review of in situ remediation technologies for lead, zinc, and cadmium in soil. Remediat. J. J. Environ. Cleanup Costs Technol. Tech. 2004, 14, 35–53. [Google Scholar]

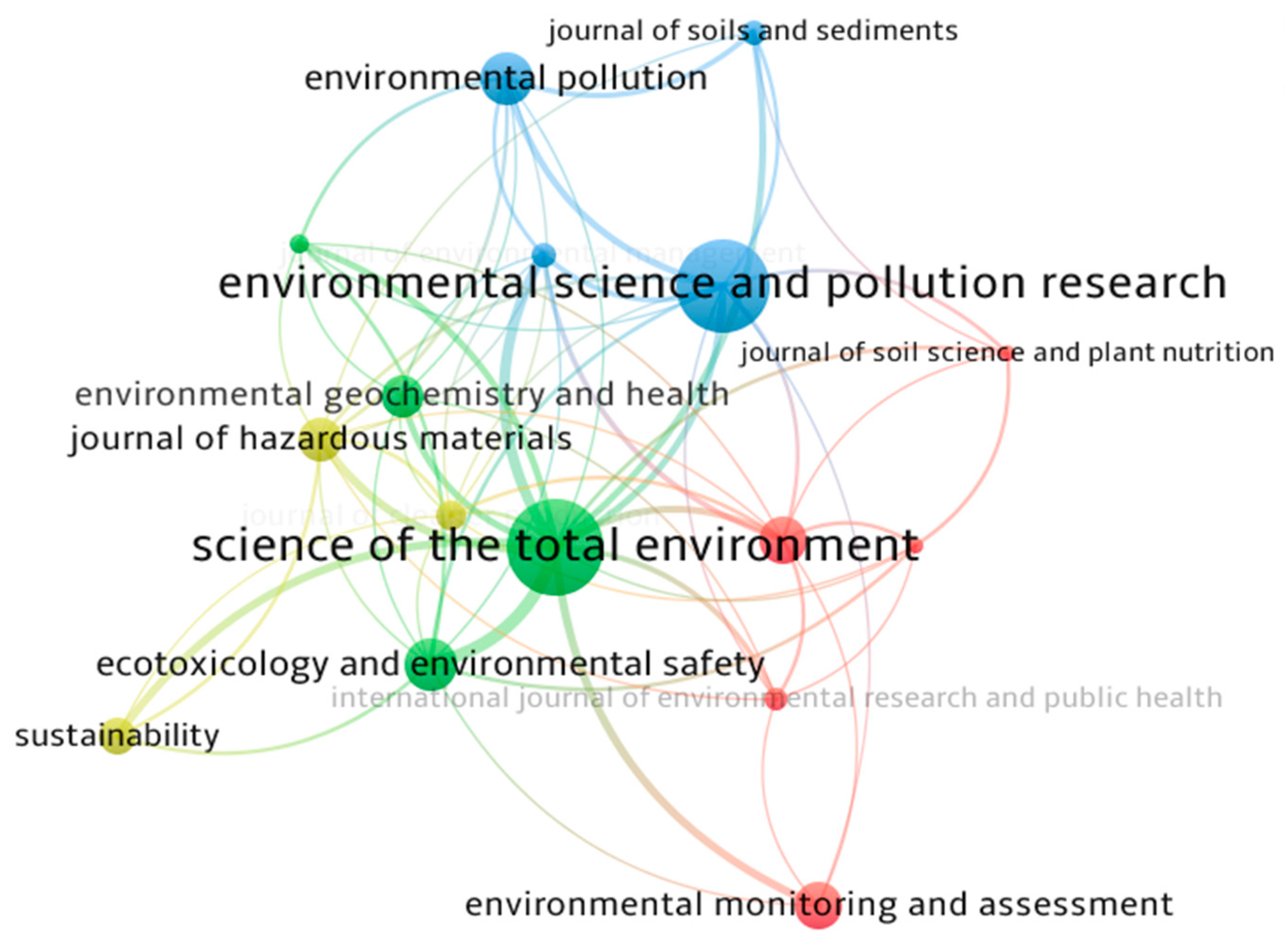

| Serial Number | Journal | Documents | Citations | Total Link Strength * |
|---|---|---|---|---|
| 1 | Science of the Total Environment | 41 | 4513 | 74 |
| 2 | Environment Science and Pollution research | 39 | 1152 | 34 |
| 3 | Ecotoxicology and Environmental Safety | 20 | 1052 | 27 |
| 4 | Journal of Hazardous Material | 16 | 930 | 25 |
| 5 | International Journal of Phytoremediation | 17 | 320 | 23 |
| 6 | Environmental Pollution | 20 | 1404 | 22 |
| 7 | Chemosphere | 13 | 1169 | 12 |
| 8 | Environmental Geochemistry and Health | 15 | 278 | 12 |
| 9 | Environmental Monitoring and Assessment | 17 | 452 | 10 |
| 10 | Sustainability | 13 | 95 | 10 |
| 11 | Agronomy | 8 | 217 | 7 |
| 12 | Plant and Soil | 7 | 551 | 7 |
| Serial Number | Keyword | Occurrences | Total Link Strength |
|---|---|---|---|
| 1 | Cd | 338 | 1496 |
| 2 | heavy metals | 324 | 1385 |
| 3 | accumulation | 148 | 714 |
| 4 | lead | 110 | 548 |
| 5 | agricultural soils | 112 | 527 |
| 6 | zinc | 87 | 450 |
| 7 | phytoremediation | 96 | 438 |
| 8 | contamination | 85 | 377 |
| 9 | plants | 67 | 332 |
| 10 | pollution | 76 | 323 |
| 11 | contaminated soils | 63 | 319 |
| 12 | bioavailability | 55 | 297 |
| 13 | toxicity | 59 | 277 |
| 14 | copper | 56 | 264 |
| 15 | phytoextraction | 51 | 256 |
| 16 | remediation | 46 | 218 |
| Current Number | Generic Research Aspect | Representative Studies (Country, Citation) | Number of Studies |
|---|---|---|---|
| 1 | Cadmium accumulation and plant response | China [43,44,45,46,47,48]; Georgia [49]; Ethiopia [50] | 8 |
| 2 | Soil amendment and immobilization techniques | China [47,48,51,52,53]; Czech Republic [54]; Turkey [55]; Switzerland [56,57]; USA [58] | 6 |
| 3 | Microbial and biochemical responses | China [59]; Turkey [55,60] | 3 |
| 4 | Human exposure and health effects | Japan [61]; China [62]; Jamaica [63] | 3 |
| 5 | Modeling and environmental assessment | Iran [64]; Australia [65]; Belgium [66]; USA [58]; China [67]; general [68]; New Zealand [69] | 7 |
| Cur. № | Plants Species | Country | Cited by |
|---|---|---|---|
| 1 | Ageratum conyzoides L. | China | Wang et al., 2023 [97] |
| 2 | Amaranthus hypochondriacus L. | China | Wang et al., 2019 [98] |
| 3 | Atriplex halimus L. | Greece | Manousaki et al., 2009 [99] |
| 4 | Beta vulgaris var. cicla L. | China | Tan et al., 2020 [100] |
| 5 | Brassica juncea L. | China | Wang et al., 2022 [101] |
| 6 | Brassica napus L. | Bangladesh | Sultana et al., 2024 [102] |
| 7 | Brassica oleracea L. | China | Ma et al., 2021 [103] |
| 8 | Brassica parachinensis L. | China | Qiu et al., 2011 [104] |
| 9 | Celosia argentea L. | China | Yu et al., 2024 [105] |
| 10 | Chenopodium album L. | India | Kumar et al., 2024 [106] |
| 11 | Chrysanthemum indicum L. | China | Lu et al., 2025 [107] |
| 12 | Helianthus petiolaris Nutt. | Belgium | Saran et al., 2020 [108] |
| 13 | Morus alba L. | China | Jiang et al., 2019 [109] |
| 14 | Ocimum gratissimum L. | Thailand | Prapagdee and Khonsue, 2015 [110] |
| 15 | Phragmites australis (Cav.) Trin. Ex Steud. | Iran | Nezhad et al., 2012 [111] |
| 16 | Populus tremula L. | Belgium | Van Nevel et al., 2011 [112] |
| 17 | Ricinus communis L. | India; China | Bauddh et al., 2016; Sun et al., 2018 [113,114] |
| 18 | Rorippa globosa Turcz. | China | Dou et al., 2019 [92] |
| 19 | Salix × aureo-pendula CL ‘J1011’ | China | Li et al., 2010 [115] |
| 20 | Salix caprea L. | Austria | Wieshammer et al., 2007 [116] |
| 21 | Sedum plumbizincicola | China | Deng et al., 2016 [91] |
| 22 | Silybum marianum (L.) Gaertn. | Greece | Papadimou et al., 2024 [117] |
| 23 | Solanum lycopersicum L. | Saudi Arabia | Alamer et al., 2022 [118] |
| 24 | Solanum melongena L. | Saudi Arabia | Alamer et al., 2022 [118] |
| 25 | Solanum nigrum L. | China | Ji et al., 2011; Yin et al., 2014 [119,120] |
| 26 | Sorghum bicolor (L.) Moench | China | Xiao et al., 2021 [121] |
| 27 | Thlaspi caerulescens (J.Presl & C.Presl) F.K. Mey. | France | Saison et al., 2004 [122] |
| 28 | Nicotiana tobaccum L. | China | Tan et al., 2020 [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chețan, F.; Moraru, P.I.; Rusu, T.; Șimon, A.; Dinca, L.; Murariu, G. From Contamination to Mitigation: Addressing Cadmium Pollution in Agricultural Soils. Agriculture 2025, 15, 2179. https://doi.org/10.3390/agriculture15202179
Chețan F, Moraru PI, Rusu T, Șimon A, Dinca L, Murariu G. From Contamination to Mitigation: Addressing Cadmium Pollution in Agricultural Soils. Agriculture. 2025; 15(20):2179. https://doi.org/10.3390/agriculture15202179
Chicago/Turabian StyleChețan, Felicia, Paula Ioana Moraru, Teodor Rusu, Alina Șimon, Lucian Dinca, and Gabriel Murariu. 2025. "From Contamination to Mitigation: Addressing Cadmium Pollution in Agricultural Soils" Agriculture 15, no. 20: 2179. https://doi.org/10.3390/agriculture15202179
APA StyleChețan, F., Moraru, P. I., Rusu, T., Șimon, A., Dinca, L., & Murariu, G. (2025). From Contamination to Mitigation: Addressing Cadmium Pollution in Agricultural Soils. Agriculture, 15(20), 2179. https://doi.org/10.3390/agriculture15202179









