Abstract
Gene editing technologies, particularly CRISPR/Cas9, have revolutionized livestock genetics. They enable precise, efficient, and inheritable genome modifications. This review summarizes recent advances in the application of gene editing in livestock. We focus on six key areas: enhancement of disease resistance, improvement of growth performance and meat production traits, modification of milk composition, regulation of reproductive traits, adaptation to environmental stress, and promotion of animal welfare. For example, they have played an important role in improving mastitis resistance in cows, enhancing meat production performance in pigs, increasing milk yield in goats, and producing polled cows. Despite rapid progress, practical implementation in animal breeding still faces challenges. These include off-target effects, low embryo editing efficiency, delivery limitations, and ethical as well as regulatory constraints. Future directions emphasize the development of advanced editing tools, multiplex trait integration, and harmonized public policy. With continued innovation and responsible oversight, gene editing holds great promise for sustainable animal agriculture and global food security.
1. Introduction
In the past decade, with the continuous growth of the global population, increased income, and the change of consumption concept, there has been a significant rise in the demand for livestock products, such as meat, dairy, and eggs [1]. From 2020 to 2050, the global demand for red meat (beef, sheep, goat, and pork), poultry, milk, and eggs is projected to increase by 8%, and per capita protein requirement will rise by 38% [2]. In the context of population growth and environmental challenges, accelerating the development of improved animal phenotypes has become essential for more efficient agricultural production [3]. Traditional breeding strategies, however, are constrained by long generation intervals and may inadvertently introduce unfavorable traits when selecting for beneficial ones [4]. Furthermore, sustained genetic improvement through conventional selection is heavily dependent on the availability of natural genetic variation; in its absence, the potential for trait enhancement is significantly limited [5]. Gene editing technologies offer a transformative solution by enabling the rapid creation of traits that are difficult or impossible to achieve through natural mutations. These technologies provide a precise, efficient, and rapid platform for genetic improvement, offering a promising pathway for the sustainable advancement of animal breeding [6].
Gene editing has already demonstrated its value in livestock across various domains, including disease resistance, trait enhancement, animal welfare, and productivity improvement [7]. Despite these promising applications, the widespread adoption of gene editing in livestock production remains limited, primarily due to challenges related to technological maturity, regulatory and ethical considerations, cost-effectiveness, and consumer acceptance. Nevertheless, numerous gene-edited animals have been successfully utilized as biomedical research models, such as cystic fibrosis sheep, Huntington’s disease pigs, and Prion disease cattle model [8,9].
With ongoing technological progress and evolving societal perspectives, gene editing is expected to play an increasingly vital role in both livestock improvement and human health. This review focuses on the current applications of gene editing technologies in livestock, and further discusses the associated technical challenges, ethical issues, and prospective future directions.
2. Summary of Gene Editing Technology
2.1. Mechanism of Gene Editing
Gene editing is a powerful genetic engineering technique that enables the precise modification of specific gene sequences, enabling gene knockout or knockin. At the core of gene editing technology is the use of engineered nucleases that introduce double-strand breaks (DSBs) at designated genomic loci [10]. These DSBs activate two primary DNA repair pathways in the cell: homology-directed repair (HDR) and non-homologous end joining (NHEJ) [11]. Homology-directed repair is a template-dependent repair mechanism that primarily occurs during the mitotic phase of the cell cycle, using exogenous DNA templates with homologous arms to accurately repair or replace the damaged region [12]. This enables precise gene insertion or point mutation correction. In contrast, NHEJ is a template-independent mechanism that repairs without a template, often introducing indels, which can disrupt gene function and thus achieve gene knockout [13]. Classical gene editing platforms such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR/Cas9 system rely on the induction of DSBs to facilitate gene modification. However, the generation of DSBs can lead to unintended genomic alterations and cellular toxicity. To overcome these limitations, novel systems such as base editors (BEs) and prime editors (PEs) have been developed based on the CRISPR/Cas9 platform (Figure 1). These technologies utilize single-strand breaks instead of DSBs, significantly reducing cytotoxicity and off-target effects [14,15], thereby representing a safer and more promising direction for the future development of gene editing.
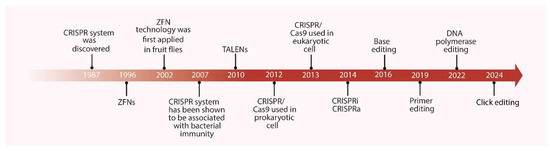
Figure 1.
Timeline of the Development of Gene Editing Technologies. ZFNs: zinc-finger nucleases; TALENs: transcription activator-like effector nucleases.
2.2. ZFNs Gene Editing Technology
Zinc-finger proteins (ZFPs) fused to the Fok I endonuclease [16]. Zinc-finger nucleases (ZFNs) represent the earliest form of gene editing [17]. They consist of zinc-finger proteins, built from Cys-Cys-His-His zinc-finger domains originally identified in eukaryotic transcription factors [18], can be rationally designed to recognize specific trinucleotide sequences [19]. Fok I cleaves DNA without sequence specificity but gains target specificity when coupled to ZFPs [20]. The first successful artificial fusion of ZFPs and Fok I was reported in 1996, laying the foundation for targeted gene editing [21]. Importantly, Fok I functions as an obligate dimer, necessitating the use of paired ZFP-Fok I constructs that bind to opposite strands of the target DNA in close proximity to enable nuclease activity [22]. Zinc-finger nucleases technology has been widely applied in livestock, for example, to enhance mastitis resistance in dairy cattle and to improve meat production traits in goats [23,24].
Although ZFN technology pioneered the gene editing field and offers advantages such as high targeting specificity and broad applicability, it has notable limitations. These include the complexity of zinc finger protein design and optimization, relatively low editing efficiency, and constraints related to intellectual property rights. Consequently, many researchers have shifted toward more user-friendly and efficient gene editing platforms [25].
2.3. TALENs Gene Editing Technology
Transcription activator-like effector nucleases utilize represent a gene editing technology developed after ZFNs, with a similar working principle and core components (Figure 2). Like ZFNs, TALENs consist of a DNA-binding domain and a Fok I endonuclease. The DNA-binding domain is composed of transcription activator-like effector (TALE) proteins, originally derived from Xanthomonas bacteria, which can be engineered to recognize specific DNA sequences [26]. TALEs are composed of tandem repeats of 33–35 amino acids, known as repeat-variable di-residues (RVDs), which determine DNA-binding specificity [27]. Within each RVD, the 12th and 13th amino acids are highly variable and play a key role in base recognition. For example, NI binds to adenine (A), NG to thymine (T), HD to cytosine (C), and NN to guanine (G) [28,29]. Each RVD specifically recognizes one nucleotide, and by assembling appropriate RVDs in a defined order, virtually any DNA sequence can be targeted. Once a pair of TALE arrays is designed to bind opposite strands flanking the target site, they are fused to Fok I nucleases. As Fok I requires dimerization for nuclease activity, the two TALE-Fok I constructs must bind in close proximity to induce a site-specific double-strand break, thereby enabling precise genome modification [30].
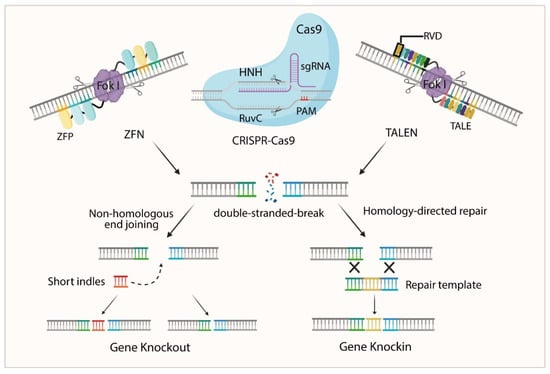
Figure 2.
Schematic Diagram of the Principles of ZFN, TALEN, and CRISPR/Cas9. ZFN consists of zinc finger proteins (ZFPs) that recognize specific DNA triplets and guide Fok I nuclease to induce double-strand breaks (DSBs) via dimerization. Transcription activator-like effector nucleases utilize transcription activator-like effector (TALE) proteins composed of repeat-variable di-residues (RVDs), each recognizing a single nucleotide, to direct Fok I-mediated DSBs. The CRISPR/Cas9 system employs a single-guide RNA (sgRNA) to target specific DNA sequences. Cas9 recognizes the PAM sequence and cleaves 3–4 base pairs upstream, generating a DSB. Subsequent gene modifications occur via non-homologous end joining (NHEJ) or homology-directed repair (HDR).
Compared to ZFNs, TALENs offer higher targeting specificity and are easier to design due to the straightforward one-to-one correspondence between RVDs and DNA bases. However, the construction of TALE arrays remains labor-intensive and costly, and their large size limits delivery efficiency, thereby restricting their broad application in certain settings [31]. In high-throughput or large-scale applications, the CRISPR/Cas9 system is often favored due to its simplicity, scalability, and efficiency.
2.4. CRISPR/Cas9 Gene Editing Technology
CRISPR/Cas9 is currently the most widely used and versatile gene editing technology, valued for its simplicity, efficiency, and broad applicability. The CRISPR system originated as an adaptive immune mechanism in bacteria and archaea, enabling them to defend against foreign genetic elements such as bacteriophages and plasmids [32]. CRISPR systems are classified into two main classes: Class 1 and Class 2, comprising six types and 19 subtypes [33,34]. The primary distinction between the two classes lies in their effector module composition: Class 1 systems utilize multi-protein effector complexes, while Class 2 systems employ a single, multidomain effector protein. Class 1 includes Types I, III, and IV, whereas Class 2 includes Types II, V, and VI [35,36].
The CRISPR/Cas9 system widely used in genome editing is derived from the Type II CRISPR system of Streptococcus pyogenes (spCas9) (Figure 2) [37]. Upon invasion by foreign DNA, the Cas1–Cas2 protein complex integrates short fragments of the invader’s DNA, known as spacers, into the CRISPR array in the host genome [38]. This spacer acquisition process depends on the presence of a protospacer adjacent motif (PAM) near the target DNA sequence. Different Cas proteins recognize different PAM sequences; for example, Cas9 typically recognizes 5′-NGG, while Cas12a (Cpf1) recognizes 5′-TTTV (where V represents A, C, or G) [39,40]. The CRISPR array is transcribed into a precursor CRISPR RNA (pre-crRNA), which pairs with a trans-activating crRNA (tracrRNA) to form a duplex that binds to Cas9. RNase III processes this complex into mature crRNA:tracrRNA units, which direct Cas9 to specific DNA sequences [36,39]. For genome editing applications, these two RNA components have been engineered into a single-guide RNA (sgRNA), which simplifies the system without compromising its targeting ability [39]. Guided by base pairing between the sgRNA and the target DNA, Cas9 introduces a DSB 3–4 base pairs downstream of the PAM site through the coordinated activity of its two nuclease domains—HNH and RuvC [41].
Due to its efficiency, programmability, and ease of use, CRISPR/Cas9 has become the preferred method for gene knockout, knockin, and high-throughput genome screening in diverse organisms. However, spCas9 has several limitations. First, its reliance on an NGG PAM sequence limits its application in AT-rich genomic regions [37]. Second, the large size of spCas9 (approximately 1368 amino acids) hinders its delivery using size-constrained vectors such as adeno-associated viruses (AAV) [42]. Third, spCas9 is prone to off-target activity, which may cause unintended DSBs and genomic instability.
To address these challenges, several strategies have been developed. To expand PAM compatibility, engineered Cas9 variants such as VQR, EQR, and VRER have been designed to recognize alternative PAM sequences [43]. To reduce size constraints, Cas9 orthologs from other species, such as Staphylococcus aureus Cas9 (saCas9), which is smaller and recognizes a different PAM, have been adopted [44]. To minimize off-target effects, high-fidelity Cas9 variants (e.g., Cas9HF) and Cas9 nickases (nCas9) have been developed. nCas9 carries a point mutation in either the HNH or RuvC domain, rendering it capable of inducing single-strand breaks (SSBs) instead of DSBs. When two nCas9 molecules are guided to adjacent sequences on opposite DNA strands by paired sgRNAs, a staggered DSB is generated with higher precision and lower off-target potential [45,46].
2.5. Extended Applications of CRISPR/Cas9
Beyond its genome-editing capabilities, the CRISPR/Cas9 system has been extensively adapted for regulating endogenous gene expression and labeling specific genomic loci in living cells or organisms. By introducing mutations into the two nuclease domains of the Cas9 protein, researchers have developed a catalytically inactive variant known as dead Cas9 (dCas9), which retains its ability to bind target DNA sequences without inducing DSBs [47]. This property enables dCas9 to serve as a programmable DNA-binding platform when fused to transcriptional regulators. For instance, fusion of dCas9 with transcriptional activation domains such as VP64 can upregulate gene expression, while fusion with repression domains such as KRAB can suppress transcription. These systems are referred to as CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi), respectively, and offer precise, sequence-specific control of gene expression in mammalian cells and model organisms [48].
In addition, dCas9 can be employed for epigenetic modifications. When fused with epigenetic effector proteins and directed to specific genomic loci via sgRNAs, dCas9 can modulate chromatin structure and gene activity. By fusing nuclease-deficient Cas9 (dCas9) with methyltransferases such as DNMT3A/3L, targeted DNA methylation can be induced at specific genomic loci, leading to stable and heritable gene silencing without altering the underlying DNA sequence [49]. Conversely, fusion with histone acetyltransferase p300 can increase histone acetylation, leading to chromatin relaxation and transcriptional activation [50]. Furthermore, dCas9 can be fused with fluorescent reporter proteins, such as enhanced green fluorescent protein (EGFP), allowing real-time visualization of specific chromosomal loci within living cells. This CRISPR-based live-cell imaging technique provides a powerful tool for studying chromatin dynamics and spatial genome organization [51,52]. And coupling dCas9 with the demethylase TET1 enables site-specific DNA demethylation and gene activation. These approaches provide powerful tools to dissect the causal role of epigenetic modifications in livestock traits and offer new opportunities for reversible and precise regulation of complex quantitative traits in breeding programs [53,54].
2.6. BE and PE Gene Editing Technology
The first-generation CRISPR gene editing technology, represented by Cas9 nuclease, induces double-strand breaks (DSBs) at specific genomic loci to enable insertions or deletions of gene segments. However, DSBs can trigger unwanted DNA damage responses and introduce imprecise mutations, particularly when homology-directed repair (HDR) is inefficient. To overcome these limitations, advanced editing tools such as base editors (BEs) and prime editors (PEs), often referred to as fourth-generation gene editing technologies, have been developed. These systems operate independently of DSBs and donor templates, offering enhanced precision and reduced off-target effects, thereby improving safety and expanding the scope of genome editing [55]. Base editing and PE technologies have been successfully applied in livestock. For instance, Wang et al. achieved efficient BE-mediated modification of the MSTN gene in Hu sheep, while Qi et al. demonstrated the feasibility of PE in pigs [56,57,58,59,60,61,62,63,64].
Base editing enables the direct, irreversible conversion of one DNA base to another without DSBs or HDR. It comprises two main types: cytosine base editing (CBE) and adenine base editing (ABE) (Figure 3). CBE is achieved by fusing a cytidine deaminase (e.g., rat APOBEC1) to a Cas9 nickase (nCas9) and an uracil DNA glycosylase inhibitor (UGI). Guided by sgRNA, the complex targets a specific DNA region, where cytosine is deaminated to uracil. UGI inhibits uracil DNA glycosylase (UDG) from excising uracil, allowing it to be interpreted as thymine during DNA replication or repair, resulting in a C to T substitution [65,66]. ABE follows a similar principle, using an engineered adenine deaminase fused to nCas9. At the target site, the adenine deaminase converts T to inosine (I), I is recognized as G, and through DNA repair, I is ultimately converted to G [67,68,69]. While BEs are well-suited for precise point mutation editing, they are limited in their ability to mediate insertions or deletions, motivating the development of prime editing technology.
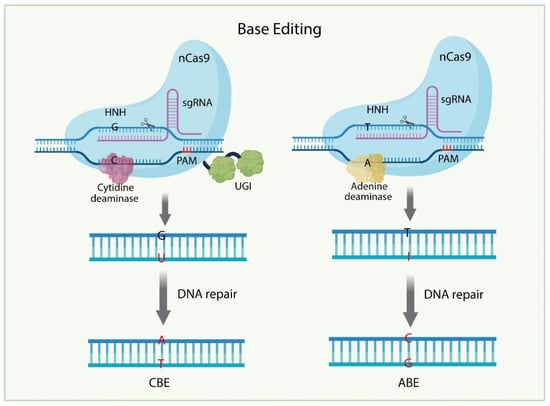
Figure 3.
Mechanisms of the Base Editing (BE) systems. In the Cytosine Base Editor (CBE) system, nCas9 is fused to a cytidine deaminase and a uracil DNA glycosylase inhibitor (UGI), and guided to the target site by sgRNA. The deaminase converts cytosine (C) to uracil (U), which is recognized as thymine (T) during DNA replication or repair, resulting in a C to T substitution. In the Adenine Base Editor (ABE) system, nCas9 is fused to an engineered adenine deaminase. The enzyme converts adenine (A) to inosine (I), which is interpreted as guanine (G), leading to an A to G substitution.
The PE system comprises a fusion protein (PE2) consisting of nCas9 and reverse transcriptase, along with a prime editing guide RNA (pegRNA). The pegRNA is an extended sgRNA containing a primer binding site (PBS) and a reverse transcription template (RTT) at its 3′ end. Upon recognition of the target site by pegRNA, nCas9 induces a nick in the non-target DNA strand, which then hybridizes with the PBS region of the pegRNA. Reverse transcriptase uses the RTT sequence to synthesize a DNA strand encoding the desired edit. This forms a 3′ DNA flap, which replaces the original sequence through flap resolution and DNA repair. The edited strand is then used as a template to correct the opposite strand, achieving precise, bidirectional editing without inducing DSBs [15,70,71].
Compared to BEs, PE supports a broader range of editing outcomes, including all 12 types of point mutations as well as short insertions or deletions of up to approximately 44 base pairs [70,72]. The high specificity of PE is attributed to its multi-step hybridization mechanism, which includes target DNA pairing with the pegRNA spacer, primer binding via the PBS region, and precise integration of the reverse transcription product. These coordinated steps enhance editing fidelity and reduce off-target activity (Figure 4). While PE provides a powerful tool for gene correction and short-fragment insertion, it currently exhibits low efficiency in large-fragment integration. Therefore, developing advanced systems capable of efficient long-fragment insertion remains a key focus for future gene editing innovations.
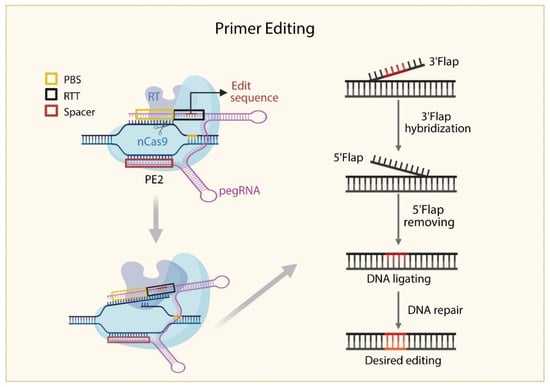
Figure 4.
Schematic Diagram of the Prime Editing (PE) Mechanism. The effector protein PE2, consisting of nCas9 fused to reverse transcriptase, is guided to the target site by a prime editing guide RNA (pegRNA). nCas9 nicks the non-target DNA strand, which pairs with the primer binding site (PBS) on the pegRNA. Reverse transcription is initiated using the reverse transcription template (RTT), generating a new DNA strand containing the desired edit. The 5′ flap is removed, and the 3′ flap is incorporated into the genome. DNA repair mechanisms then use the edited strand as a template to convert the opposite strand, resulting in precise, double-strand editing.
2.7. Delivery Methods for Gene-Editing Systems
Efficient delivery of gene-editing components is essential for achieving reliable modifications in animal cells and embryos. Microinjection remains the gold standard for introducing CRISPR/Cas9 plasmids or ribonucleoproteins into zygotes, providing high efficiency but low throughput and requiring technical expertise. Electroporation offers a higher-throughput alternative by transiently permeabilizing cell membranes with electrical pulses, though its efficiency is generally lower. Lipid-mediated transfection is simple and has low cytotoxicity, but its performance in large mammalian fibroblasts and embryos is poor, limiting its applicability in livestock. In contrast, somatic cell nuclear transfer (SCNT) is widely used in pigs, cattle, and sheep: gene editing is performed in donor somatic cells, which are then reprogrammed into embryos to generate cloned offspring carrying stable modifications. Each delivery strategy has specific strengths and weaknesses, and the optimal choice depends on species, cell type, and experimental goals [56,57,58,59,60,61,62,63,64].
3. Applications of Gene Editing Technology in Livestock
Gene editing has emerged as a powerful tool in livestock enabling precise and efficient modification of target genes to enhance traits such as disease resistance, productivity, product quality, and animal welfare (Table 1). With the development of advanced tools like CRISPR/Cas9, BE, and PE, gene editing offers faster and more targeted alternatives to traditional breeding, accelerating genetic improvement across various livestock species [73,74,75,76,77,78,79].

Table 1.
The application of gene editing technology in animals.
3.1. Improvement of Disease Resistance
Animal diseases pose significant threats to livestock productivity, economic efficiency, and animal health and welfare. They also increase the risk of zoonotic disease transmission. Therefore, breeding healthy, disease-resistant animals is essential for ensuring the safety of meat and dairy products [108]. Through gene editing technologies combined with molecular breeding, researchers have successfully developed livestock with enhanced disease resistance by targeting specific disease-related genes.
In cattle, mastitis is one of the most prevalent diseases, causing billions of dollars in annual losses. In 2013, Liu et al. employed ZFN technology to insert the human lysozyme (hLYZ) gene into the bovine β-casein locus, enabling expression of hLYZ in the mammary gland and generating cows resistant to mastitis [23]. Tuberculosis, caused by Mycobacterium bovis, is another major concern in cattle, leading to production losses and posing zoonotic risks [109]. In 2015, Wu, H., et al. used TALEN technology to insert the murine sp110 gene into the intergenic region between surfactant protein A1 (SFTPA1) and methionine adenosyltransferase I alpha (MAT1A) on chromosome 28, resulting in cattle with enhanced resistance to tuberculosis [80]. Mannheimia haemolytica, a major pathogen of bovine pneumonia, exerts its pathogenicity via leukotoxins that bind to the CD18 signal peptide. In 2016, Shanthalingam et al. disrupted the CD18 signal peptide using ZFNs, producing cattle resistant to leukocyte lysis and pneumonia [82]. Additionally, in 2017, Gao et al. inserted the NRAMP1 gene into a non-coding genomic region using CRISPR/nCas9, enhancing tuberculosis resistance while minimizing off-target effects [81].
In pigs, Porcine Reproductive and Respiratory Syndrome (PRRS), caused by PRRS virus (PRRSV), leads to serious reproductive and respiratory issues. Since its emergence in the late 1980s, it has spread globally, causing extensive economic losses. In 2014, Whitworth et al. used CRISPR/Cas9 to knock out the CD163 gene, a key receptor for PRRSV, generating pigs completely resistant to the virus [83,110]. Given the multiple biological roles of CD163, targeted editing strategies were later developed to retain its essential functions. These included deleting exon 7, which encodes the SRCR5 domain, or replacing it with the human CD163L1 exon to achieve both resistance and functional preservation [111,112,113]. For coronaviruses such as transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV), porcine aminopeptidase N (pAPN) serves as a receptor for TGEV entry. In 2020, Xu et al. generated double-knockout pigs lacking CD163 and pAPN using CRISPR/Cas9, which conferred resistance to both PRRSV and TGEV, and reduced susceptibility to PDCoV, without affecting production traits [84]. This study demonstrated that multiplex gene editing in the pig genome using CRISPR/Cas9 is feasible and revealed that editing cell surface receptors can produce individuals with multiple desirable traits.
In goats, transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative diseases caused by misfolded prion proteins (PrPSc) [114,115]. Using CRISPR/Cas9, researchers successfully knocked out the PRNP gene in goat fibroblast cells, which were then used in somatic cell nuclear transfer (SCNT) to generate TSE-resistant goats [85,115]. Similar strategies have also been applied in cattle [116,117].
In poultry, avian leukosis virus (ALV) causes leukemia and other tumors, seriously affecting the poultry industry. Among the seven known ALV subgroups (A to E, J, and K), ALV-A and ALV-K utilize the Tva receptor, while ALV-J uses the chNHE1 receptor [86,118]. In 2020, Hellmich et al. precisely deleted the non-conserved tryptophan residue at position 38 (W38) in chNHE1, producing chickens resistant to ALV-J [86]. Separately, in 2021, Koslová et al. generated Tva-knockout chickens using CRISPR/Cas9, conferring resistance to ALV-A and ALV-K [87].
Beyond editing host genomes, CRISPR/Cas9 can be applied to directly inhibit viral replication, aligning with its prokaryotic immune origin [119]. For instance, Pseudorabies virus (PRV) remains a major threat to swine, with limited therapeutic options [120]. In 2017, Tang et al. used multiple sgRNAs targeting PRV DNA to completely suppress its replication in vitro [121]. Similarly, African swine fever virus (ASFV), a lethal DNA virus with no available vaccines, was targeted in 2018 by Hübner et al. using CRISPR/Cas9 against the CP204L gene encoding p30, effectively reducing viral replication in cell culture [122]. Though still in early stages, these approaches demonstrate the feasibility of CRISPR-based antiviral strategies. By designing specific sgRNAs, CRISPR systems can guide Cas9 or Cas13 proteins to degrade viral DNA or RNA, providing broad-spectrum antiviral potential. These components can be delivered as therapeutic agents or integrated into the genome of transgenic animals to generate virus-resistant livestock.
3.2. Improve Growth Performance and Meat Production Traits
Enhancing lean meat yield and growth performance has long been a primary goal in livestock breeding programs [123]. Among key regulatory genes, myostatin (MSTN) is a well-known negative regulator of muscle development and a member of the TGF-β superfamily. It inhibits myogenesis through multiple signaling pathways, thereby controlling muscle mass and metabolism [124,125,126,127,128]. Naturally occurring MSTN mutations have been identified in cattle, sheep, pigs, poultry, and dogs, resulting in a double-muscled phenotype with significantly increased muscle mass [127,129,130,131,132,133]. In 2015, Wang et al. designed two sgRNAs targeting exon 3 of the porcine MSTN gene, based on known mutations in Belgian Blue and Piedmontese cattle, and used CRISPR/Cas9 to produce biallelic MSTN-knockout pigs [88]. In 2016, Yu et al. applied TALENs to edit the MSTN gene in goats, generating MSTN-mutant individuals [24]. In another study Zhao et al. used TALENs to disrupt MSTN in sheep fibroblasts [134]. In 2022, Zhou, S., et al., used CRISPR/Cas9 technology to knock out the MSTN gene in sheep, obtaining MSTN double allele knockout sheep [89]. Similarly, Zhao et al. generated MSTN-edited Chinese Luxi Yellow cattle by targeting exon 1 with three CRISPR/Cas9-designed sgRNAs, producing individuals with improved growth and muscle mass [90]. These MSTN mutant individuals showed significantly improved growth rate and muscle mass.
Insulin-like growth factor 2 (IGF2) is another crucial gene regulating cell proliferation, differentiation, apoptosis, and metabolism. It is highly expressed during fetal development and markedly downregulated in adulthood [135]. A known mutation at nucleotide 3072 in intron 3 of IGF2 disrupts the ZBED6 transcriptional repressor binding site, resulting in increased IGF2 expression and enhanced muscle growth [136]. In 2018, Xiang et al. and Liu et al. independently used CRISPR/Cas9 to introduce this mutation in IGF2 intron 3 of Bama and Liang Guang Small Spotted pigs, respectively. The edited pigs showed significantly higher carcass weights than wild-type counterparts [91,92]. In 2022, Zou et al. targeted exon 3 of bovine IGF2 using CRISPR/Cas9 and generated IGF2-mutant embryos. Although live calves were not obtained due to SCNT failure, enhanced IGF2 promoter activity and muscle cell proliferation were observed in vitro, validating the gene’s functional role in bovine myogenesis [137].
The Myogenic differentiation 1 (MyoD1) gene plays a central role in muscle differentiation and fibrosis regulation. Although its function has been studied in pigs, mice, and chickens [138,139,140], its role in bovine muscle development remains unclear. To address this, Zhou et al. constructed a MyoD1-knockout bovine kidney cell line using CRISPR/Cas9 and found that CCND2 may serve as a downstream effector in bovine muscle development, providing new insight into MyoD1-regulated myogenic pathways [141].
The fat-1 gene, originally derived from Caenorhabditis elegans, encodes an omega-3 fatty acid desaturase that converts n-6 polyunsaturated fatty acids (PUFAs) into n-3 PUFAs, thus improving fatty acid balance in animals [142]. In 2018, Li et al. inserted the fat-1 gene into the Rosa26 locus of pigs using CRISPR/Cas9, significantly reducing the n-6/n-3 PUFA ratio in pork, from 9.36% to 2.12%, and enhancing its nutritional value [93]. That same year, Zhang et al. simultaneously inserted fat-1 into the MSTN locus of sheep, achieving dual-gene editing that yielded animals with both enriched n-3 PUFA content and hypertrophied muscle fibers [94]. In 2021, You et al. further advanced this approach by co-integrating fat-1 and IGF1 into the Rosa26 locus of pigs, generating dual-transgenic pigs with improved nutritional and growth-related traits [143]. This breakthrough lays the foundation for developing novel transgenic pig breeds with multiple advantageous phenotypes.
Uncoupling protein 1 (UCP1) is primarily expressed in brown adipose tissue and plays a pivotal role in non-shivering thermogenesis by dissipating the mitochondrial proton gradient to generate heat instead of ATP [144]. Modern pigs lack functional UCP1, making them sensitive to cold stress and prone to fat accumulation [145]. In 2017, Zheng et al. used CRISPR/Cas9 to insert the murine UCP1 gene into the porcine UCP1 locus, generating transgenic pigs with increased thermogenic capacity, reduced fat deposition, and higher lean meat yield [95].
3.3. Improve Milk Production Traits
Cow and goat milk are important sources of high-quality nutrition, yet they contain allergenic proteins such as α-lactalbumin, β-lactoglobulin (BLG), caseins, and immunoglobulins [146]. Among these, BLG, which is absent in human milk, is considered the primary allergen responsible for milk-induced hypersensitivity reactions [147]. Therefore, reducing or eliminating BLG content through gene editing is a promising strategy to enhance milk quality and reduce allergenicity. In 2013, Xiong et al. used ZFN technology to target the BLG gene in goats, successfully generating fibroblasts with BLG knockout [96]. In 2015, Cui et al. employed TALENs to insert the human lactoferrin (hLF) gene into the BLG locus in goats, resulting in transgenic animals that highly expressed hLF while showing low or undetectable levels of BLG. Kaiser et al. conducted a similar operation, but they accomplished it in cattle using conventional transgenic technology [148,149]. In 2018, Sun et al. applied ZFNs to disrupt BLG in cattle, producing double-allele BLG-knockout cows [97]. More recently, in 2024, Tara et al. used CRISPR/Cas9 to edit the BLG gene in water buffalo, creating BLG-knockout embryos intended to develop into animals capable of producing hypoallergenic milk [98]. These studies demonstrate that targeted disruption or replacement of the BLG gene effectively reduces milk immunogenicity and holds great potential for advancing the dairy industry.
3.4. Improve Reproductive Performance
Enhancing reproductive efficiency is a major goal in livestock breeding, encompassing traits such as increased litter size and sex-specific offspring production [150,151]. These traits are difficult to manipulate through conventional selection, but gene editing technologies offer a more direct and precise approach [152].
The Bone Morphogenetic Protein Receptor Type IB (BMPR-IB) gene in sheep plays an important role in skeletal and embryonic development and has been associated with increased ovulation rates and larger litter sizes when mutated [153,154]. In 2018, Zhou et al. used CRISPR/Cas9 to introduce a targeted point mutation into the BMPR-IB gene via HDR, successfully generating five sheep carrying the desired variant [99]. Similarly, growth Differentiation Factor 9 (GDF9), which is predominantly expressed in oocytes and granulosa cells, regulates follicular development and ovulation [155,156,157]. In the same year, Niu et al. introduced a point mutation into the GDF9 gene using CRISPR/Cas9, resulting in five mutant individuals. Although phenotypic effects on litter size were not immediately confirmed, the study validated the feasibility of precise genome editing for complex reproductive traits in livestock [100].
In addition to improving fertility, gene editing also holds promise for controlling offspring sex to meet production demands. For example, female calves are preferred in the dairy industry, while male calves are often favored in beef production. Sex selection via traditional methods is inefficient and leads to unnecessary culling and economic losses. The SRY gene, located on the Y chromosome, is essential for male sex determination [158,159]. In 2021, Wang et al. used TALENs to knock out the SRY gene in bovine fibroblasts, producing sex-reversed animals with single ovaries and infertility via SCNT [101]. This work not only provides a model for studying sex determination in mammals but also opens new avenues for sex-specific breeding. That same year, Douglas et al. reported a CRISPR-based method for producing sex-specific offspring in mice [102]. They inserted a sgRNA targeting the Topoisomerase 1 (TOP1) gene into the Hipp11 (H11) locus of mice, generating homozygous H11TOP1 females. The Cas9 gene was then integrated into either the X chromosome (XCas9Y) or the Y chromosome (XYCas9). Mating XCas9Y males with H11TOP1 females resulted in exclusively male offspring while mating XYCas9 males with H11TOP1 females produced only female offspring. Although not yet applied in livestock, this system is theoretically extendable to all species with heterogametic sex chromosomes, offering a novel tool for both genetic research and practical sex-specific breeding.
3.5. Enhancement of Environmental Adaptation
Environmental fluctuations, especially extreme temperatures, have a significant impact on livestock productivity, feed efficiency, reproductive performance, and immune function. Gene editing provides novel opportunities to enhance environmental resilience in livestock by improving thermotolerance and cryotolerance. For example, UCP1-knockin pigs not only exhibit reduced fat deposition but also demonstrate improved cold tolerance through enhanced non-shivering thermogenesis [95]. Members of the heat shock protein (HSP) family, such as HSP70 and HSF1, are key regulators of cellular response to heat stress in cattle [160,161]. In 2023, Shandilya et al. generated ATP1A1- and HSF1-knockout bovine fibroblast lines using CRISPR/Cas9 and demonstrated that both genes are crucial for heat shock resilience at the cellular level [103]. Moreover, editing the PRLR gene, naturally associated with slick coats and thermotolerance in certain cattle breeds, has shown promise for improving heat tolerance in non-slick breeds such as Angus and Jersey. Animals with biallelic PRLR edits exhibited improved body temperature regulation and growth under heat stress without adverse effects on fertility, offering a valuable genetic strategy for heat-resilient cattle breeding [104].
In addition to thermal stress adaptation, livestock contribute substantially to greenhouse gas emissions, especially methane (CH4), a potent contributor to global warming. Methane is primarily produced by methanogenic archaea in the rumen, which account for approximately 4% of the microbial population [162,163]. Recent advances have applied CRISPR/Cas9-based genome editing to methanogens as a strategy to reduce enteric methane emissions. For example, Methanosarcina acetivorans has been successfully edited using Streptococcus pyogenes Cas9 to introduce targeted insertions and deletions via homologous recombination without off-target effects [164]. Targeted disruption of essential methanogenesis genes such as mcr and hdrED significantly suppressed methane production. More sustainable strategies focus on non-essential genes like mtmCB1 and mtmCB2, which encode methylamine-specific methyltransferases involved in the methylotrophic pathway. Knockout of these genes reduced methane output while preserving microbial viability, offering a practical route to engineer low-emission rumen microbiota [105].
3.6. Enhancement of Animal Welfare
Improving animal welfare is an increasingly important consideration in modern livestock production. Gene editing technologies offer novel, non-invasive alternatives to traditional management practices such as dehorning and surgical castration, which often cause pain and stress.
Dehorning is commonly practiced in dairy and beef cattle systems to reduce injury, facilitate handling, and improve production safety [165]. While the polled (hornless) phenotype is naturally common in beef breeds, it is found in only about 6% of Holstein cattle [106]. The genetic basis for the polled trait is located in the centromeric region of bovine chromosome 1 and includes at least two well-characterized variants: the Celtic polled and Friesian polled alleles [166,167]. In 2016, Carlson et al. introduced the Celtic polled mutation into Holstein fibroblasts using TALENs, and subsequent SCNT yielded two gene-edited bulls with the congenital polled phenotype [106]. This landmark study established a precise genome editing strategy for breeding polled Holstein cattle without introducing exogenous DNA or requiring lengthy crossbreeding programs.
Castration of male livestock is widely used to reduce aggression, manage reproductive behavior, and prevent undesirable traits such as boar taint in pork [107,168,169]. However, surgical castration causes pain, violates animal welfare principles, and is increasingly restricted by ethical regulations. To address this, researchers have proposed “genetic castration” as a humane alternative. Kisspeptin-1 (KISS1), a key neuropeptide that activates the hypothalamic-pituitary-gonadal (HPG) axis through its receptor GPR54, plays a central role in regulating sexual maturation and reproductive hormone synthesis [170,171]. In 2022, Flórez et al. used CRISPR/Cas9 to disrupt the KISS1 gene in porcine zygotes, producing four KISS1-knockout pigs with hypoplastic gonads, low levels of sex hormones, and a complete failure to reach sexual maturity [107]. This study highlights the potential of genome editing to precisely target neuroendocrine regulators of reproduction, laying the foundation for developing non-surgical and welfare-friendly alternatives to castration. Table 2 compares the editing efficiency of different gene-editing technologies across animal species. Efficiency varies widely not only between systems but also within the same species and system. In general, knockouts are more efficient than knock-ins, and single-gene edits outperform multi-gene edits. Optimization, such as using improved nucleases and sgRNA design tools, is essential. Delivery method is another key factor: microinjection offers high efficiency but low throughput, electroporation allows higher throughput with lower efficiency, and liposome transfection has low toxicity but performs poorly in large mammals. Currently, SCNT remains the most commonly used and recommended approach in pigs, cattle, and sheep [56,57,58,59,60,61,62,63,64,73,74,75,76,77,78,79].

Table 2.
Editing Efficiencies of Different Gene Editing Platforms in Various Species.
4. Challenges and Future Directions
4.1. Technical Challenges
Although genome editing technologies offer promising tools for genetic improvement in livestock, their widespread application is currently constrained by several technical challenges.
One of the most pressing issues is the risk of off-target effects and biosafety concerns. Off-target mutations may lead to unintended protein alterations or transcriptional dysregulation, potentially impairing animal health, reproductive performance, and growth efficiency [5,42]. Several strategies have been proposed to mitigate these risks. For instance, high-fidelity Cas9 variants such as eSpCas9 and HypaCas9 have been developed to enhance editing specificity and minimize off-target activity [173]. Modifying the single-guide RNA (sgRNA) structure, such as by truncating 2–3 nucleotides at the 5′ end or adding two guanines at the 5′ terminus, can also improve targeting accuracy [174,175]. Additionally, using double-strand break (DSB)-independent systems like base editors (BEs) or prime editors (PEs) significantly reduces the risk of unpredictable genome damage. Optimizing delivery systems is equally crucial for safe and efficient genome editing. Compared to plasmid-based delivery, mRNA-based delivery via electroporation or lipid nanoparticles (LNPs) ensures transient Cas9 expression, thereby reducing the risk of genomic integration and enhancing editing precision [176].
A second major challenge lies in the low efficiency of embryo editing in livestock species, particularly in cattle and sheep [177]. Two main approaches are currently employed to generate gene-edited embryos: pronuclear microinjection and SCNT (Figure 5) [178,179]. Despite their widespread use, both techniques suffer from technical complexity and low success rates. The efficiency of the former in livestock ranges from 1% to 10%, while that of the latter is 5% to 20%, SCNT remains the method of choice for producing large animals [177,178]. Moreover, gene-edited animals produced via SCNT often exhibit developmental abnormalities, including mosaicism, obesity, immunodeficiency, and respiratory dysfunctions [180].
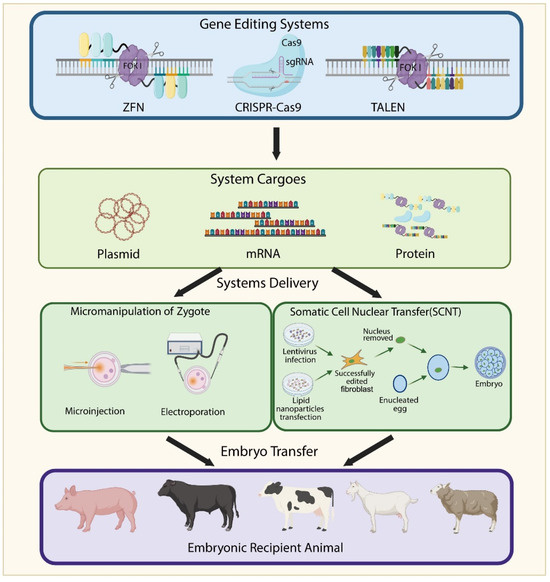
Figure 5.
Schematic Diagram of Generating Genetically Edited Animals. The gene editing system can be delivered in the form of plasmids, mRNA, or proteins. Two main approaches are used to obtain gene-edited embryos: zygote micromanipulation and somatic cell nuclear transfer (SCNT). In zygote micromanipulation, the gene editing system is introduced directly into fertilized eggs via microinjection or electroporation. In SCNT, the system is first delivered into fibroblasts using lentiviral vectors or lipid nanoparticles. Successfully edited cells are selected, and their nuclei are transferred into enucleated oocytes. The resulting embryos are implanted into recipient animals to produce genetically edited offspring.
To overcome these limitations, alternative strategies have been explored. Sperm-mediated gene transfer (SMGT) allows edited spermatozoa to fertilize oocytes, thereby delivering genetic modifications into the embryo. Testis-mediated gene transfer (TMGT) introduces exogenous DNA or gene-editing components directly into the testis using viral or non-viral vectors for in vivo genome editing. Additionally, spermatogonial stem cells can be isolated, genetically modified ex vivo, and then transplanted back into the testis to produce gene-edited spermatozoa [181,182]. Moradbeigi, P et al. transfected mouse sperm with a CRISPR/Cas9 system targeting the Gdf8 gene, and after in vitro fertilization and embryo transfer, they finally obtained a chimeric mouse [183]. Konoval, O et al. transfected duck sperm with the CRISPR/Cas9 system and a GFP gene sequence, and after artificial insemination, obtained 19 F1 offspring carrying the GFP gene [184]. Similarly, Cooper, C.A et al. used this method to generate chickens with a DFP gene knockout [185]. The cost and operational complexity of these two techniques are lower than those of the earlier methods. These emerging techniques offer the potential for more efficient and less invasive generation of gene-edited livestock.
4.2. Ethical and Regulatory Controversies
Despite the potential of Genome editing to improve animal health, productivity, and sustainability, their widespread application remains controversial due to ongoing ethical debates and regulatory disparities across regions [186].
Public perception of Genome editing animals, including genetically modified organisms (GMOs), varies significantly worldwide. In countries such as China and those within the European Union (EU), the general public tends to express skepticism or negative attitudes toward genetically modified foods, often driven by concerns over food safety and ecological impact. In contrast, consumers in the United States and Canada generally exhibit a higher level of acceptance, viewing GMOs more favorably as products of scientific progress [187]. Importantly, consumer acceptance is influenced not only by geographic and cultural contexts but also by specific factors such as trust in government oversight, perceived risks to human health and the environment, and the potential benefits of the technology; however, unfamiliarity with the technology and limited public engagement remain major barriers to broader acceptance. Studies have shown that individuals with higher levels of education are more likely to accept GMOs. At the same time, compared with genetically modified organisms used for food production, people are more inclined to accept those used for disease treatment. This is because most people regard Genome editing animals as genetically modified animals (GMAs) [188]. Furthermore, gene knockouts are generally perceived as more acceptable than gene knockin [189].
Regulatory frameworks also differ considerably among countries. In the United States, the Food and Drug Administration (FDA) and the Department of Agriculture (USDA) jointly oversee the regulation of GMOs and gene-edited animals, while the Environmental Protection Agency (EPA) addresses associated environmental risks. This framework adopts a relatively permissive stance, treating certified GMOs as safe and emphasizing innovation and the practical benefits of biotechnology. In contrast, the EU enforces stringent regulations under the guidance of the European Food Safety Authority (EFSA), grounded in the precautionary principle, and mandates comprehensive risk assessments for all activities involving genetic modification. It also prioritizes transparency and public awareness regarding potential health and environmental risks. China exercises strict oversight of GMO research and development. The Ministry of Agriculture and Rural Affairs (MARA), together with national food safety authorities, requires rigorous risk assessments and safety evaluations under the Administrative Measures for the Safety of Genetically Modified Food. Furthermore, such evaluations must be conducted in a scientifically sound, fair, and transparent manner to ensure public confidence and regulatory credibility [190].
Notably, some countries have taken a more differentiated approach. In Argentina, Australia, and Brazil, genome-edited animals that do not contain foreign DNA, such as those generated by site-specific base edits or indels, are exempt from GMO-specific regulations [191,192]. This distinction has prompted ongoing debates on whether genome editing should be categorized separately from traditional genetic modification in international regulatory contexts. Overall, ethical concerns, divergent consumer perceptions, and heterogeneous regulatory frameworks continue to shape the global debate on gene-edited animals, underscoring the need for transparent governance and sustained public engagement.
Currently, only a few gene-editing animals have been approved for commercial use. In the United States, the GalSafe pig, PRRS-resistant pig, and gene-edited short-haired, heat-tolerant beef cattle have received regulatory approval [193]. Meanwhile, Japan has allowed the sale of gene-edited tiger pufferfish and red sea bream [194]. These animals are theoretically expected to generate tremendous economic value; however, their actual impact will still be subject to market dynamics. The extent of the value they create can ultimately only be verified by the market and time.
4.3. Future Directions
Gene editing technologies have revolutionized the ability to precisely modify livestock genomes, offering transformative potential for advancing sustainable animal agriculture. As the field continues to evolve, several key directions are emerging that will shape its future development and application.
Among currently available tools, the CRISPR/Cas9 system has become the most widely adopted due to its flexibility, ease of use, and broad target applicability. However, its reliance on DSBs introduces potential risks, including off-target effects and genomic instability. In contrast, next-generation editing platforms such as BEs and PEs offer higher editing precision and reduced genotoxicity by avoiding DSBs. For example, while the editing efficiency of CRISPR/Cas9 for introducing point mutations at the porcine MSTN locus is approximately 10.3%, the optimized hA3A-BE3-NG base editor achieves an efficiency of 46.3% at the same site [88,195]. PE systems also hold great promise for versatile base conversion and sequence insertion. As such, BE and PE technologies are expected to become the dominant platforms for livestock genome editing, with PE showing particular potential for complex edits and precise corrections that can be used to treat point mutation–induced diseases such as Complex Vertebral Malformation and Porcine Stress Syndrome [70].
Another critical area for future advancement is the precise insertion of large DNA fragments. DNA polymerase-based editors and novel PE variants have begun to demonstrate feasibility in this regard, enabling the integration of entire functional gene cassettes. In parallel, the coordinated editing of multiple traits, such as combining disease resistance (e.g., NRAMP1) with growth-promoting genes (e.g., MSTN), is expected to become a standard strategy for genetic improvement in livestock [172]. This multi-trait optimization aligns with the goals of improving animal health, productivity, and welfare simultaneously.
Another important distinction lies between editing qualitative traits versus quantitative traits. Qualitative traits, such as disease resistance (e.g., CD163-mediated PRRSV resistance in pigs) or single-gene modifications affecting milk allergens (e.g., BLG knockout), are often monogenic and therefore more straightforward targets for gene editing [96,110]. In contrast, quantitative traits—including milk yield, fertility, feed efficiency, and heat tolerance—are regulated by complex polygenic networks and strongly influenced by environmental interactions [196,197,198]. Current editing tools are limited in their ability to precisely modify such traits, as we often do not know which genes, or how many, must be edited to produce the desired outcome. The recent debate surrounding the so-called “dire wolf resurrection” highlights this challenge: while it may be theoretically possible to edit isolated genes, recreating a polygenic phenotype is far beyond current capabilities [199]. In the recent widely publicized case of dire wolf “resurrection,” researchers merely applied CRISPR technology to edit 14 genes in gray wolves to match those of the dire wolf, and the changes involved only qualitative traits [200]. In contrast, the more complex quantitative traits controlled by multiple genes were not edited. Therefore, how to precisely and efficiently edit complex quantitative traits remains a major challenge for gene-editing technologies in the future. Thus, future progress will require integration of genome-wide association studies, multi-omics data, and genomic selection to identify key contributors before targeted editing of quantitative traits becomes feasible.
Despite these technical advances, public awareness and acceptance of gene-edited animals remain limited. Misinformation, ethical concerns, and regulatory uncertainty have hindered the adoption of gene-edited products. Therefore, it is crucial that governments, scientific institutions, and regulatory bodies actively promote public education on gene editing, establish transparent legislation, and ensure ethical oversight. Moreover, whether Genome editing animals should be regulated separately from GMAs is also an issue that governments need to address [188]. Such efforts will help build trust and support the responsible development of the gene editing industry [201].
Finally, the integration of artificial intelligence (AI) is poised to accelerate progress in this field. AI algorithms are increasingly applied to design sgRNAs and pegRNAs, optimize Cas protein engineering, predict off-target sites, and simulate editing outcomes, thereby improving both efficiency and precision [55]. The synergy between AI and gene editing is expected to drive innovation across all stages of genome engineering, from design to validation. Meanwhile, the future of animal breeding will rely on interdisciplinary integration, particularly the combination of genomics and reproductive technologies. Genomic selection and multi-omics approaches can help identify candidate genes and regulatory variants underlying economically important traits, while advanced reproductive techniques such as in vitro fertilization, embryo transfer, and somatic cell nuclear transfer provide practical means to introduce these edits into breeding populations. The real challenge—and opportunity—lies in evaluating the feasibility of incorporating such genome-editing pipelines into large-scale breeding programs, balancing technical efficiency with ethical, regulatory, and societal considerations [188,202].
5. Conclusions
Gene editing technologies, including ZFNs, TALENs, and CRISPR-based systems, have transformed the landscape of animal breeding by enabling precise and inheritable genetic modifications. Beyond their technical advances, these tools present new opportunities to promote sustainability and global food security. For example, gene editing can be used to enhance feed efficiency, reduce methane emissions, and improve resistance to endemic diseases, thereby supporting more resource-efficient livestock production systems and contributing to climate change mitigation. In parallel, the ability to tailor traits such as milk composition or animal welfare characteristics provides further avenues for meeting the nutritional and ethical demands of a growing population.
Despite these opportunities, significant barriers remain before gene-edited animals can be widely integrated into agricultural practice. Regulatory frameworks differ substantially among countries, ranging from permissive to highly restrictive approaches, and such disparities complicate global trade and adoption. In addition, consumer acceptance continues to pose a challenge, with attitudes influenced by cultural context, perceived risks to food safety and the environment, and trust in regulatory institutions. These persistent issues highlight the need for harmonized policies and transparent communication strategies to build societal trust.
Looking ahead, the future of gene editing in livestock will depend not only on technical progress but also on the principles of responsible innovation. This includes ensuring rigorous risk assessment, safeguarding animal welfare, and fostering dialogue among scientists, policymakers, farmers, and consumers. By aligning innovation with ethical considerations and public values, gene editing can move beyond laboratory success to become a reliable tool for advancing sustainable agriculture and strengthening global food security.
Author Contributions
J.W. and L.Z. contributed equally to this work and share first authorship. They were responsible for the conceptualization, literature collection, and writing of the original draft. C.P. and X.L. participated in data analysis, figure preparation, and manuscript editing. M.L. and B.X. conceived the project, reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFF1001200, 2023Y12143, 2021YFD1301200), National Natural Science Foundation of China (32172686), Key Research and Development Program of Henan Province (251111110200).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no competing interests in this section.
References
- Clonan, A.; Roberts, K.E.; Holdsworth, M. Socioeconomic and demographic drivers of red and processed meat consumption: Implications for health and environmental sustainability. Proc. Nutr. Soc. 2016, 75, 367–373. [Google Scholar] [CrossRef]
- Komarek, A.M.; Dunston, S.; Enahoro, D.; Godfray, H.C.J.; Herrero, M.; Mason-D’Croz, D.; Rich, K.M.; Scarborough, P.; Springmann, M.; Sulser, T.B.; et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob. Environ. Change Hum. Policy Dimens. 2021, 70, 102343. [Google Scholar] [CrossRef]
- Gonen, S.; Jenko, J.; Gorjanc, G.; Mileham, A.J.; Whitelaw, C.B.; Hickey, J.M. Potential of gene drives with genome editing to increase genetic gain in livestock breeding programs. Genet. Sel. Evol. GSE 2017, 49, 3. [Google Scholar] [CrossRef] [PubMed]
- Bishop, T.F.; Van Eenennaam, A.L. Genome editing approaches to augment livestock breeding programs. J. Exp. Biol. 2020, 223, jeb207159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Xu, J.; Chen-Tsai, R.Y.; Li, K. Genome editing in livestock: Are we ready for a revolution in animal breeding industry? Transgenic Res. 2017, 26, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, S.C.; Blake, A.; Carlson, D.F.; Doran, T.; Van Eenennaam, A.; Faber, D.; Galli, C.; Gao, Q.; Hackett, P.B.; Li, N.; et al. Precision genetics for complex objectives in animal agriculture. J. Anim. Sci. 2010, 88, 2530–2539. [Google Scholar] [CrossRef]
- Mueller, M.L.; Van Eenennaam, A.L. Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agric. Biosci. 2022, 3, 13. [Google Scholar] [CrossRef]
- Mariano, C.G.; de Oliveira, V.C.; Ambrósio, C.E. Gene editing in small and large animals for translational medicine: A review. Anim. Reprod. 2024, 21, e20230089. [Google Scholar] [CrossRef]
- Fan, Z.; Perisse, I.V.; Cotton, C.U.; Regouski, M.; Meng, Q.; Domb, C.; Van Wettere, A.J.; Wang, Z.; Harris, A.; White, K.L.; et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight 2018, 3, e123529. [Google Scholar] [CrossRef]
- Shamshirgaran, Y.; Liu, J.; Sumer, H.; Verma, P.J.; Taheri-Ghahfarokhi, A. Tools for Efficient Genome Editing; ZFN, TALEN, and CRISPR. Methods Mol. Biol. 2022, 2495, 29–46. [Google Scholar] [CrossRef]
- Sargent, R.G.; Brenneman, M.A.; Wilson, J.H. Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol. Cell. Biol. 1997, 17, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.; Klein, H. Mechanism of homologous recombination: Mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [CrossRef]
- Hu, S.; Gan, M.; Wei, Z.; Shang, P.; Song, L.; Feng, J.; Chen, L.; Niu, L.; Wang, Y.; Zhang, S.; et al. Identification of host factors for livestock and poultry viruses: Genome-wide screening technology based on the CRISPR system. Front. Microbiol. 2024, 15, 1498641. [Google Scholar] [CrossRef]
- Diakun, G.P.; Fairall, L.; Klug, A. EXAFS study of the zinc-binding sites in the protein transcription factor IIIA. Nature 1986, 324, 698–699. [Google Scholar] [CrossRef]
- Wolfe, S.A.; Nekludova, L.; Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 183–212. [Google Scholar] [CrossRef]
- Rémy, S.; Tesson, L.; Ménoret, S.; Usal, C.; Scharenberg, A.M.; Anegon, I. Zinc-finger nucleases: A powerful tool for genetic engineering of animals. Transgenic Res. 2010, 19, 363–371. [Google Scholar] [CrossRef]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef]
- Bitinaite, J.; Wah, D.A.; Aggarwal, A.K.; Schildkraut, I. FokI dimerization is required for DNA cleavage. Proc. Natl. Acad. Sci. USA 1998, 95, 10570–10575. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Guo, W.; Chang, B.; Liu, J.; Guo, Z.; Quan, F.; Zhang, Y. Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nat. Commun. 2013, 4, 2565. [Google Scholar] [CrossRef]
- Yu, B.; Lu, R.; Yuan, Y.; Zhang, T.; Song, S.; Qi, Z.; Shao, B.; Zhu, M.; Mi, F.; Cheng, Y. Efficient TALEN-mediated myostatin gene editing in goats. BMC Dev. Biol. 2016, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Tan, S.; Qiao, G.; Barlow, K.A.; Wang, J.; Xia, D.F.; Meng, X.; Paschon, D.E.; Leung, E.; Hinkley, S.J.; et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011, 29, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Atef, A.; Piatek, A.; Ali, Z.; Piatek, M.; Aouida, M.; Sharakuu, A.; Mahjoub, A.; Wang, G.; Khan, S.; et al. Characterization and DNA-binding specificities of Ralstonia TAL-like effectors. Mol. Plant 2013, 6, 1318–1330. [Google Scholar] [CrossRef]
- Moscou, M.J.; Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 2009, 326, 1501. [Google Scholar] [CrossRef]
- Boch, J.; Scholze, H.; Schornack, S.; Landgraf, A.; Hahn, S.; Kay, S.; Lahaye, T.; Nickstadt, A.; Bonas, U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 2009, 326, 1509–1512. [Google Scholar] [CrossRef]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef]
- Mussolino, C.; Alzubi, J.; Fine, E.J.; Morbitzer, R.; Cradick, T.J.; Lahaye, T.; Bao, G.; Cathomen, T. TALENs facilitate targeted genome editing in human cells with high specificity and low cytotoxicity. Nucleic Acids Res. 2014, 42, 6762–6773. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Mohanraju, P.; Makarova, K.S.; Zetsche, B.; Zhang, F.; Koonin, E.V.; van der Oost, J. Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science 2016, 353, aad5147. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.A.; McKenzie, R.E.; Fagerlund, R.D.; Kieper, S.N.; Fineran, P.C.; Brouns, S.J. CRISPR-Cas: Adapting to change. Science 2017, 356, eaal5056. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Hu, D.; Li, X.; Guo, Y.; Guo, H.; Zhang, L.; Ding, X. Research Progress on the Mechanism and Application of the Type I CRISPR-Cas System. Int. J. Mol. Sci. 2024, 25, 12544. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Z.; Xu, K.; Li, Y.; Lu, S.; Guan, L. CRISPR-Cpf1 system and its applications in animal genome editing. Mol. Genet. Genom. MGG 2024, 299, 75. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Fu, Y.; Foden, J.A.; Khayter, C.; Maeder, M.L.; Reyon, D.; Joung, J.K.; Sander, J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013, 31, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.; Li, Z.; Peterson, R.T.; Yeh, J.R.; et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Lo, A.; Qi, L. Genetic and epigenetic control of gene expression by CRISPR-Cas systems. F1000Research 2017, 6, 747. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e217. [Google Scholar] [CrossRef]
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 2013, 155, 1479–1491. [Google Scholar] [CrossRef]
- Ma, H.; Naseri, A.; Reyes-Gutierrez, P.; Wolfe, S.A.; Zhang, S.; Pederson, T. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc. Natl. Acad. Sci. USA 2015, 112, 3002–3007. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, S.X. CRISPR/dCas9-Tet1-Mediated DNA Methylation Editing. Bio-Protocol 2024, 14, e4976. [Google Scholar] [CrossRef]
- Park, H.; Shin, J.; Kim, Y.; Saito, T.; Saido, T.C.; Kim, J. CRISPR/dCas9-Dnmt3a-mediated targeted DNA methylation of APP rescues brain pathology in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, present, and future of CRISPR genome editing technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, Y.; Tian, S.; Zong, R.; Yan, X.; Wang, Y.; Wang, Y.; Zhao, J. An optimized prime editing system for efficient modification of the pig genome. Sci. China Life Sci. 2023, 66, 2851–2861. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.J.; Meng, C.H.; Wang, H.L.; Cui, Z.K.; Zhang, J.; Zhang, J.L.; Qian, Y.; Li, Y.X.; Cao, S.X. Cytosine base editor-mediated high-efficiency myostatin editing in Hu sheep. Funct. Integr. Genom. 2025, 25, 176. [Google Scholar] [CrossRef]
- Ding, Q.; Cui, Z.; Shi, Q.; Zhang, Y.; He, N.; Guo, R.; Tian, Y.; Cao, S.; Zhong, J.; Wang, H. An advanced cytosine base editor enabled the generation of cattle with a stop codon in the β-lactoglobulin gene. Transgenic Res. 2025, 34, 14. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, D.; Sui, T.; Chen, M.; Liu, Z.; Liu, H.; Zhang, T.; Chen, S.; Lai, L.; Li, Z. Efficient and precise generation of Tay-Sachs disease model in rabbit by prime editing system. Cell Discov. 2021, 7, 50. [Google Scholar] [CrossRef]
- Song, R.; Wang, Y.; Zheng, Q.; Yao, J.; Cao, C.; Wang, Y.; Zhao, J. One-step base editing in multiple genes by direct embryo injection for pig trait improvement. Sci. China Life Sci. 2022, 65, 739–752. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, T.; Wang, L.; Yang, L.; Zhang, Y.; Liu, H.; Li, M.; Tang, X.; Liu, Z.; Li, Z.; et al. Efficient base editing by RNA-guided cytidine base editors (CBEs) in pigs. Cell. Mol. Life Sci. CMLS 2020, 77, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Shi, Y.; Wang, H.; Wang, Z.; Dang, Y.; Li, S.; Wang, S.; Zhang, K. Base editing in bovine embryos reveals a species-specific role of SOX2 in regulation of pluripotency. PLoS Genet. 2022, 18, e1010307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Wu, Y.; Li, W.; An, J.; Zhang, F.; Liu, M. Knockout of Myostatin by Zinc-finger Nuclease in Sheep Fibroblasts and Embryos. Asian-Australas. J. Anim. Sci. 2016, 29, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cui, C.; Zhu, H.; Li, Q.; Zhao, F.; Jin, Y. Expression, purification and characterization of zinc-finger nuclease to knockout the goat beta-lactoglobulin gene. Protein Expr. Purif. 2015, 112, 1–7. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef]
- Li, J.; Yu, W.; Huang, S.; Wu, S.; Li, L.; Zhou, J.; Cao, Y.; Huang, X.; Qiao, Y. Structure-guided engineering of adenine base editor with minimized RNA off-targeting activity. Nat. Commun. 2021, 12, 2287. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Wang, G.; Zhang, R.; Duan, J.; Gao, P.; Lei, X.; Qiu, H.; Zhang, C.; Zhang, Y.; et al. Efficient targeted insertion of large DNA fragments without DNA donors. Nat. Methods 2022, 19, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, H.; Jong, U.; Rim, C.; Li, W.; Lin, X.; Zhang, D.; Luo, Q.; Cui, C.; Huang, H.; et al. Generation of GGTA1 biallelic knockout pigs via zinc-finger nucleases and somatic cell nuclear transfer. Sci. China Life Sci. 2014, 57, 263–268. [Google Scholar] [CrossRef]
- Whyte, J.J.; Zhao, J.; Wells, K.D.; Samuel, M.S.; Whitworth, K.M.; Walters, E.M.; Laughlin, M.H.; Prather, R.S. Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs. Mol. Reprod. Dev. 2011, 78, 2. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.T.; Kwon, D.K.; Park, A.R.; Lee, E.J.; Yun, Y.J.; Ji, D.Y.; Lee, K.; Park, K.W. Production of α1,3-galactosyltransferase targeted pigs using transcription activator-like effector nuclease-mediated genome editing technology. J. Vet. Sci. 2016, 17, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bondareva, A.; González, R.; Rodriguez-Sosa, J.R.; Carlson, D.F.; Webster, D.; Fahrenkrug, S.; Dobrinski, I. TALEN-mediated gene targeting in porcine spermatogonia. Mol. Reprod. Dev. 2018, 85, 250–261. [Google Scholar] [CrossRef]
- Crispo, M.; Mulet, A.P.; Tesson, L.; Barrera, N.; Cuadro, F.; dos Santos-Neto, P.C.; Nguyen, T.H.; Crénéguy, A.; Brusselle, L.; Anegón, I.; et al. Efficient Generation of Myostatin Knock-Out Sheep Using CRISPR/Cas9 Technology and Microinjection into Zygotes. PLoS ONE 2015, 10, e0136690. [Google Scholar] [CrossRef]
- Li, G.; Zhou, S.; Li, C.; Cai, B.; Yu, H.; Ma, B.; Huang, Y.; Ding, Y.; Liu, Y.; Ding, Q.; et al. Base pair editing in goat: Nonsense codon introgression into FGF5 results in longer hair. FEBS J. 2019, 286, 4675–4692. [Google Scholar] [CrossRef]
- Zhou, S.; Ding, Y.; Liu, J.; Liu, Y.; Zhao, X.; Li, G.; Zhang, C.; Li, C.; Wang, Y.; Kalds, P.; et al. Highly efficient generation of sheep with a defined FecB(B) mutation via adenine base editing. Genet. Sel. Evol. GSE 2020, 52, 35. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, Y.; Yang, M.; Lv, J.; Liu, J.; Zhang, Y. TALE nickase-mediated SP110 knockin endows cattle with increased resistance to tuberculosis. Proc. Natl. Acad. Sci. USA 2015, 112, E1530–E1539. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, H.; Wang, Y.; Liu, X.; Chen, L.; Li, Q.; Cui, C.; Liu, X.; Zhang, J.; Zhang, Y. Single Cas9 nickase induced generation of NRAMP1 knockin cattle with reduced off-target effects. Genome Biol. 2017, 18, 13. [Google Scholar] [CrossRef]
- Shanthalingam, S.; Tibary, A.; Beever, J.E.; Kasinathan, P.; Brown, W.C.; Srikumaran, S. Precise gene editing paves the way for derivation of Mannheimia haemolytica leukotoxin-resistant cattle. Proc. Natl. Acad. Sci. USA 2016, 113, 13186–13190. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Lee, K.; Benne, J.A.; Beaton, B.P.; Spate, L.D.; Murphy, S.L.; Samuel, M.S.; Mao, J.; O’Gorman, C.; Walters, E.M.; et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol. Reprod. 2014, 91, 78. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhou, Y.; Mu, Y.; Liu, Z.; Hou, S.; Xiong, Y.; Fang, L.; Ge, C.; Wei, Y.; Zhang, X.; et al. CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance. eLife 2020, 9, e57132. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Qiao, J.; Hu, S.; Zhao, X.; Regouski, M.; Yang, M.; Polejaeva, I.A.; Chen, C. Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS ONE 2014, 9, e106718. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, R.; Sid, H.; Lengyel, K.; Flisikowski, K.; Schlickenrieder, A.; Bartsch, D.; Thoma, T.; Bertzbach, L.D.; Kaufer, B.B.; Nair, V.; et al. Acquiring Resistance Against a Retroviral Infection via CRISPR/Cas9 Targeted Genome Editing in a Commercial Chicken Line. Front. Genome Ed. 2020, 2, 3. [Google Scholar] [CrossRef]
- Koslová, A.; Trefil, P.; Mucksová, J.; Krchlíková, V.; Plachý, J.; Krijt, J.; Reinišová, M.; Kučerová, D.; Geryk, J.; Kalina, J.; et al. Knock-Out of Retrovirus Receptor Gene Tva in the Chicken Confers Resistance to Avian Leukosis Virus Subgroups A and K and Affects Cobalamin (Vitamin B(12))-Dependent Level of Methylmalonic Acid. Viruses 2021, 13, 2504. [Google Scholar] [CrossRef]
- Wang, K.; Ouyang, H.; Xie, Z.; Yao, C.; Guo, N.; Li, M.; Jiao, H.; Pang, D. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 2015, 5, 16623. [Google Scholar] [CrossRef]
- Zhou, S.; Kalds, P.; Luo, Q.; Sun, K.; Zhao, X.; Gao, Y.; Cai, B.; Huang, S.; Kou, Q.; Petersen, B.; et al. Optimized Cas9:sgRNA delivery efficiently generates biallelic MSTN knockout sheep without affecting meat quality. BMC Genom. 2022, 23, 348. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Su, G.; Wei, Z.; Liu, X.; Song, L.; Hai, C.; Wu, D.; Hao, Z.; Wu, Y.; et al. Growth Traits and Sperm Proteomics Analyses of Myostatin Gene-Edited Chinese Yellow Cattle. Life 2022, 12, 627. [Google Scholar] [CrossRef]
- Xiang, G.; Ren, J.; Hai, T.; Fu, R.; Yu, D.; Wang, J.; Li, W.; Wang, H.; Zhou, Q. Editing porcine IGF2 regulatory element improved meat production in Chinese Bama pigs. Cell. Mol. Life Sci. CMLS 2018, 75, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Wang, M.; Li, R.; Zeng, J.; Mo, D.; Cong, P.; Liu, X.; Chen, Y.; He, Z. Disruption of the ZBED6 binding site in intron 3 of IGF2 by CRISPR/Cas9 leads to enhanced muscle development in Liang Guang Small Spotted pigs. Transgenic Res. 2019, 28, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ouyang, H.; Yuan, H.; Li, J.; Xie, Z.; Wang, K.; Yu, T.; Liu, M.; Chen, X.; Tang, X.; et al. Site-Specific Fat-1 Knock-In Enables Significant Decrease of n-6PUFAs/n-3PUFAs Ratio in Pigs. G3 Genes Genomes Genet. 2018, 8, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, M.L.; Nie, Y.W.; Dai, B.; Li, F.R.; Liu, D.J.; Liang, H.; Cang, M. CRISPR/Cas9-mediated specific integration of fat-1 at the goat MSTN locus. FEBS J. 2018, 285, 2828–2839. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, J.; Huang, J.; Zhang, H.; Zhang, R.; Zhang, X.; Cao, C.; Hambly, C.; Qin, G.; Yao, J.; et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl. Acad. Sci. USA 2017, 114, E9474–E9482. [Google Scholar] [CrossRef]
- Xiong, K.; Li, S.; Zhang, H.; Cui, Y.; Yu, D.; Li, Y.; Sun, W.; Fu, Y.; Teng, Y.; Liu, Z.; et al. Targeted editing of goat genome with modular-assembly zinc finger nucleases based on activity prediction by computational molecular modeling. Mol. Biol. Rep. 2013, 40, 4251–4256. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Han, S.; Ma, S.; Zou, Z.; Ding, F.; Li, X.; Li, L.; Tang, B.; Wang, H.; et al. Production of hypoallergenic milk from DNA-free beta-lactoglobulin (BLG) gene knockout cow using zinc-finger nucleases mRNA. Sci. Rep. 2018, 8, 15430. [Google Scholar] [CrossRef]
- Tara, A.; Singh, P.; Gautam, D.; Tripathi, G.; Uppal, C.; Malhotra, S.; De, S.; Singh, M.K.; Telugu, B.P.; Selokar, N.L. CRISPR-mediated editing of β-lactoglobulin (BLG) gene in buffalo. Sci. Rep. 2024, 14, 14822. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, H.; Zhao, X.; Cai, B.; Ding, Q.; Huang, Y.; Li, Y.; Li, Y.; Niu, Y.; Lei, A.; et al. Generation of gene-edited sheep with a defined Booroola fecundity gene (FecB(B)) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9. Reprod. Fertil. Dev. 2018, 30, 1616–1621. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, X.; Zhou, J.; Li, Y.; Huang, Y.; Cai, B.; Liu, Y.; Ding, Q.; Zhou, S.; Zhao, J.; et al. Efficient generation of goats with defined point mutation (I397V) in GDF9 through CRISPR/Cas9. Reprod. Fertil. Dev. 2018, 30, 307–312. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Z.; Ding, F.; Wang, H.; Li, L.; Li, X.; Zheng, X.; Li, N.; Dai, Y.; Wu, C. Efficient TALEN-mediated gene knockin at the bovine Y chromosome and generation of a sex-reversal bovine. Cell. Mol. Life Sci. 2021, 78, 5415–5425. [Google Scholar] [CrossRef]
- Douglas, C.; Maciulyte, V.; Zohren, J.; Snell, D.M.; Mahadevaiah, S.K.; Ojarikre, O.A.; Ellis, P.J.I.; Turner, J.M.A. CRISPR-Cas9 effectors facilitate generation of single-sex litters and sex-specific phenotypes. Nat. Commun. 2021, 12, 6926. [Google Scholar] [CrossRef]
- Shandilya, U.K.; Sharma, A.; Sodhi, M.; Mukesh, M. Editing of HSF-1 and Na/K-ATPase α1 subunit by CRISPR/Cas9 reduces thermal tolerance of bovine skin fibroblasts to heat shock in vitro. Anim. Biotechnol. 2023, 34, 3626–3636. [Google Scholar] [CrossRef]
- Cuellar, C.J.; Amaral, T.F.; Rodriguez-Villamil, P.; Ongaratto, F.; Martinez, D.O.; Labrecque, R.; Losano, J.D.A.; Estrada-Cortés, E.; Bostrom, J.R.; Martins, K.; et al. Consequences of gene editing of PRLR on thermotolerance, growth, and male reproduction in cattle. FASEB BioAdvances 2024, 6, 223–234. [Google Scholar] [CrossRef]
- Nayak, D.D.; Metcalf, W.W. Cas9-mediated genome editing in the methanogenic archaeon Methanosarcina acetivorans. Proc. Natl. Acad. Sci. USA 2017, 114, 2976–2981. [Google Scholar] [CrossRef]
- Carlson, D.F.; Lancto, C.A.; Zang, B.; Kim, E.-S.; Walton, M.; Oldeschulte, D.; Seabury, C.; Sonstegard, T.S.; Fahrenkrug, S.C. Production of hornless dairy cattle from genome-edited cell lines. Nat. Biotechnol. 2016, 34, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Flórez, J.M.; Martins, K.; Solin, S.; Bostrom, J.R.; Rodríguez-Villamil, P.; Ongaratto, F.; Larson, S.A.; Ganbaatar, U.; Coutts, A.W.; Kern, D.; et al. CRISPR/Cas9-editing of KISS1 to generate pigs with hypogonadotropic hypogonadism as a castration free trait. Front. Genet. 2022, 13, 1078991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, T.; Xiang, G.; Wang, H.; Wang, B.; Feng, Z.; Mu, Y.; Li, K. Enhancing Animal Disease Resistance, Production Efficiency, and Welfare through Precise Genome Editing. Int. J. Mol. Sci. 2022, 23, 7331. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yan, B.S.; Rojas, M.; Shebzukhov, Y.V.; Zhou, H.; Kobzik, L.; Higgins, D.E.; Daly, M.J.; Bloom, B.R.; Kramnik, I. Ipr1 gene mediates innate immunity to tuberculosis. Nature 2005, 434, 767–772. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef]
- Burkard, C.; Opriessnig, T.; Mileham, A.J.; Stadejek, T.; Ait-Ali, T.; Lillico, S.G.; Whitelaw, C.B.A.; Archibald, A.L. Pigs Lacking the Scavenger Receptor Cysteine-Rich Domain 5 of CD163 Are Resistant to Porcine Reproductive and Respiratory Syndrome Virus 1 Infection. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Bai, J.; Liu, W.; Liu, X.; Yu, D.; Feng, T.; Sun, Z.; Zhang, L.; Ma, L.; et al. Generation of Pigs Resistant to Highly Pathogenic-Porcine Reproductive and Respiratory Syndrome Virus through Gene Editing of CD163. Int. J. Biol. Sci. 2019, 15, 481–492. [Google Scholar] [CrossRef]
- Wang, H.; Shen, L.; Chen, J.; Liu, X.; Tan, T.; Hu, Y.; Bai, X.; Li, Y.; Tian, K.; Li, N.; et al. Deletion of CD163 Exon 7 Confers Resistance to Highly Pathogenic Porcine Reproductive and Respiratory Viruses on Pigs. Int. J. Biol. Sci. 2019, 15, 1993–2005. [Google Scholar] [CrossRef]
- Yu, G.; Chen, J.; Yu, H.; Liu, S.; Chen, J.; Xu, X.; Sha, H.; Zhang, X.; Wu, G.; Xu, S.; et al. Functional disruption of the prion protein gene in cloned goats. J. Gen. Virol. 2006, 87, 1019–1027. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, M.; Regouski, M.; Polejaeva, I.A. Gene Knockouts in Goats Using CRISPR/Cas9 System and Somatic Cell Nuclear Transfer. Methods Mol. Biol. 2019, 1874, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Kasinathan, P.; Hamir, A.N.; Castilla, J.; Sathiyaseelan, T.; Vargas, F.; Sathiyaseelan, J.; Wu, H.; Matsushita, H.; Koster, J.; et al. Production of cattle lacking prion protein. Nat. Biotechnol. 2007, 25, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, R.J.; Fernandez-Martín, R.; Savy, V.; Canel, N.G.; Gismondi, M.I.; Kues, W.A.; Carlson, D.F.; Fahrenkrug, S.C.; Niemann, H.; Taboga, O.A.; et al. Efficient edition of the bovine PRNP prion gene in somatic cells and IVF embryos using the CRISPR/Cas9 system. Theriogenology 2016, 86, 1886–1896.e1. [Google Scholar] [CrossRef] [PubMed]
- Federspiel, M.J. Reverse Engineering Provides Insights on the Evolution of Subgroups A to E Avian Sarcoma and Leukosis Virus Receptor Specificity. Viruses 2019, 11, 497. [Google Scholar] [CrossRef]
- Niu, D.; Wei, H.J.; Lin, L.; George, H.; Wang, T.; Lee, I.H.; Zhao, H.Y.; Wang, Y.; Kan, Y.; Shrock, E.; et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017, 357, 1303–1307. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Molecular biology of pseudorabies (Aujeszky’s disease) virus. Comp. Immunol. Microbiol. Infect. Dis. 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Tang, Y.-D.; Liu, J.-T.; Wang, T.-Y.; Sun, M.-X.; Tian, Z.-J.; Cai, X.-H. CRISPR/Cas9-mediated multiple single guide RNAs potently abrogate pseudorabies virus replication. Arch. Virol. 2017, 162, 3881–3886. [Google Scholar] [CrossRef]
- Hübner, A.; Petersen, B.; Keil, G.M.; Niemann, H.; Mettenleiter, T.C.; Fuchs, W. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci. Rep. 2018, 8, 1449. [Google Scholar] [CrossRef]
- Li, X.; He, S.G.; Li, W.R.; Luo, L.Y.; Yan, Z.; Mo, D.X.; Wan, X.; Lv, F.H.; Yang, J.; Xu, Y.X.; et al. Genomic analyses of wild argali, domestic sheep, and their hybrids provide insights into chromosome evolution, phenotypic variation, and germplasm innovation. Genome Res. 2022, 32, 1669–1684. [Google Scholar] [CrossRef]
- Ayuti, S.R.; Lamid, M.; Warsito, S.H.; Al-Arif, M.A.; Lokapirnasari, W.P.; Rosyada, Z.N.A.; Sugito, S.; Akmal, M.; Rimayanti, R.; Gangil, R.; et al. A review of myostatin gene mutations: Enhancing meat production and potential in livestock genetic selection. Open Vet. J. 2024, 14, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mohan, B.; Natarajan, A.; Murali, N.; Selvaraj, P.; Vasanthakumar, P. Effect on growth performance, carcass traits, and myostatin gene expression in Aseel chicken fed varied levels of dietary protein in isocaloric energy diets. Trop. Anim. Health Prod. 2023, 55, 82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Asiamah, C.A.; Ye, R.; Pan, Y.; Lu, L.-l.; Zhao, Z.; Jiang, P.; Su, Y. Decorin regulates myostatin and enhances proliferation and differentiation of embryonic myoblasts in Leizhou black duck. Gene 2021, 804, 145884. [Google Scholar] [CrossRef] [PubMed]
- Malila, Y.; Thanatsang, K.V.; Sanpinit, P.; Arayamethakorn, S.; Soglia, F.; Zappaterra, M.; Bordini, M.; Sirri, F.; Rungrassamee, W.; Davoli, R.; et al. Differential expression patterns of genes associated with metabolisms, muscle growth and repair in Pectoralis major muscles of fast- and medium-growing chickens. PLoS ONE 2022, 17, e0275160. [Google Scholar] [CrossRef]
- Matika, O.; Robledo, D.; Pong-Wong, R.; Bishop, S.C.; Riggio, V.; Finlayson, H.; Lowe, N.R.; Hoste, A.E.; Walling, G.A.; Del-Pozo, J.; et al. Balancing selection at a premature stop mutation in the myostatin gene underlies a recessive leg weakness syndrome in pigs. PLoS Genet. 2019, 15, e1007759. [Google Scholar] [CrossRef]
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef]
- Boman, I.A.; Klemetsdal, G.; Blichfeldt, T.; Nafstad, O.; Våge, D.I. A frameshift mutation in the coding region of the myostatin gene (MSTN) affects carcass conformation and fatness in Norwegian White Sheep (Ovis aries). Anim. Genet. 2009, 40, 418–422. [Google Scholar] [CrossRef]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007, 3, e79. [Google Scholar] [CrossRef]
- Stinckens, A.; Luyten, T.; Bijttebier, J.; Van den Maagdenberg, K.; Dieltiens, D.; Janssens, S.; De Smet, S.; Georges, M.; Buys, N. Characterization of the complete porcine MSTN gene and expression levels in pig breeds differing in muscularity. Anim. Genet. 2008, 39, 586–596. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.M.; Suh, Y.; Shin, S.; Lee, J.; Hwang, S.; Lee, K. Research Note: Association of temporal expression of myostatin with hypertrophic muscle growth in different Japanese quail lines. Poult. Sci. 2020, 99, 2926–2930. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, W.; Chen, C.; Sai, W.; Qiao, J.; Sheng, J.; Zhang, H.; Li, G.; Wang, D.; Hu, S. Targeted Editing of Myostatin Gene in Sheep by Transcription Activator-like Effector Nucleases. Asian-Australas. J. Anim. Sci. 2016, 29, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.; Schönke, M.; Massart, J.; Hjortebjerg, R.; Sundström, E.; Gustafson, U.; Björnholm, M.; Krook, A.; Frystyk, J.; Zierath, J.R.; et al. The ZBED6-IGF2 axis has a major effect on growth of skeletal muscle and internal organs in placental mammals. Proc. Natl. Acad. Sci. USA 2018, 115, E2048–E2057. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, M.; Liu, S.; Tang, X.; Yi, X.; Li, Q.; Wang, S.; Sun, X. Liver Expression of IGF2 and Related Proteins in ZBED6 Gene-Edited Pig by RNA-Seq. Animals 2020, 10, 2184. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yu, D.; Yao, S.; Ding, F.; Li, J.; Li, L.; Li, X.; Zhao, S.; Pang, Y.; Hao, H.; et al. Efficient Editing of the ZBED6-Binding Site in Intron 3 of IGF2 in a Bovine Model Using the CRISPR/Cas9 System. Genes 2022, 13, 1132. [Google Scholar] [CrossRef]
- Shi, X.; Garry, D.J. Myogenic regulatory factors transactivate the Tceal7 gene and modulate muscle differentiation. Biochem. J. 2010, 428, 213–221. [Google Scholar] [CrossRef]
- Fan, C.M.; Li, L.; Rozo, M.E.; Lepper, C. Making skeletal muscle from progenitor and stem cells: Development versus regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 315–327. [Google Scholar] [CrossRef]
- Montarras, D.; Pinset, C.; Chelly, J.; Kahn, A.; Gros, F. Expression of MyoD1 coincides with terminal differentiation in determined but inducible muscle cells. EMBO J. 1989, 8, 2203–2207. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Y.; Yang, R.; Wang, F.; Zhao, Z.; Wang, X.; Xie, L.; Tian, X.; Wang, G.; Li, B.; et al. The MyoD1 Promoted Muscle Differentiation and Generation by Activating CCND2 in Guanling Cattle. Animals 2022, 12, 2571. [Google Scholar] [CrossRef]
- Kang, J.X. From fat to fat-1: A tale of omega-3 fatty acids. J. Membr. Biol. 2005, 206, 165–172. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Li, M.; Qi, Y.; Wang, Y.; Chen, Y.; Liu, Y.; Li, L.; Ouyang, H.; Pang, D. CRISPR/Cas9-Mediated Specific Integration of Fat-1 and IGF-1 at the pRosa26 Locus. Genes 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, J.; Clarke, G.; Enerbäck, S.; Spiegelman, B.; Kozak, L.P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Investig. 1995, 96, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, S.; Schwartz, T.S. Here and there, but not everywhere: Repeated loss of uncoupling protein 1 in amniotes. Biol. Lett. 2017, 13, 20160749. [Google Scholar] [CrossRef]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
- El-Agamy, E.I. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Cui, C.; Song, Y.; Liu, J.; Ge, H.; Li, Q.; Huang, H.; Hu, L.; Zhu, H.; Jin, Y.; Zhang, Y. Gene targeting by TALEN-induced homologous recombination in goats directs production of β-lactoglobulin-free, high-human lactoferrin milk. Sci. Rep. 2015, 5, 10482. [Google Scholar] [CrossRef]
- Kaiser, G.G.; Mucci, N.C.; González, V.; Sánchez, L.; Parrón, J.A.; Pérez, M.D.; Calvo, M.; Aller, J.F.; Hozbor, F.A.; Mutto, A.A. Detection of recombinant human lactoferrin and lysozyme produced in a bitransgenic cow. J. Dairy Sci. 2017, 100, 1605–1617. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; De Figueiredo Silva, F.; Trott, J.F.; Zilberman, D. Genetic Engineering of Livestock: The Opportunity Cost of Regulatory Delay. Annu. Rev. Anim. Biosci. 2021, 9, 453–478. [Google Scholar] [CrossRef]
- Park, T.S. —Invited Review—Gene-editingGene-editing techniques and their applications in livestock and beyond. Anim. Biosci. 2023, 36, 333–338. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Liu, S.-Z.; Farhab, M.; Lv, M.-Y.; Zhang, T.; Cao, S.-X. Genome editing: An insight into disease resistance, production efficiency, and biomedical applications in livestock. Funct. Integr. Genom. 2024, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Mulsant, P.; Lecerf, F.; Fabre, S.; Schibler, L.; Monget, P.; Lanneluc, I.; Pisselet, C.; Riquet, J.; Monniaux, D.; Callebaut, I.; et al. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino ewes. Proc. Natl. Acad. Sci. USA 2001, 98, 5104–5109. [Google Scholar] [CrossRef]
- McNatty, K.P.; Henderson, K.M. Gonadotrophins, fecundity genes and ovarian follicular function. J. Steroid Biochem. 1987, 27, 365–373. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the Genes for Oocyte-Derived Growth Factors GDF9 and BMP15 Are Associated with Both Increased Ovulation Rate and Sterility in Cambridge and Belclare Sheep (Ovis aries)1. Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Souza, C.J.; McNeilly, A.S.; Benavides, M.V.; Melo, E.O.; Moraes, J.C. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim. Genet. 2014, 45, 732–739. [Google Scholar] [CrossRef]
- Berta, P.; Hawkins, J.R.; Sinclair, A.H.; Taylor, A.; Griffiths, B.L.; Goodfellow, P.N.; Fellous, M. Genetic evidence equating SRY and the testis-determining factor. Nature 1990, 348, 448–450. [Google Scholar] [CrossRef]
- Daneau, I.; Houde, A.; Ethier, J.F.; Lussier, J.G.; Silversides, D.W. Bovine SRY gene locus: Cloning and testicular expression. Biol. Reprod. 1995, 52, 591–599. [Google Scholar] [CrossRef]
- Liu, S.; Yue, T.; Ahmad, M.J.; Hu, X.; Zhang, X.; Deng, T.; Hu, Y.; He, C.; Zhou, Y.; Yang, L. Transcriptome Analysis Reveals Potential Regulatory Genes Related to Heat Tolerance in Holstein Dairy Cattle. Genes 2020, 11, 68. [Google Scholar] [CrossRef]
- Guzmán, L.F.; Martínez-Velázquez, G.; Villaseñor-González, F.; Vega-Murillo, V.E.; Palacios-Fránquez, J.A.; Ríos-Utrera, Á.; Montaño-Bermúdez, M. Expression of heat shock protein genes in Simmental cattle exposed to heat stress. Anim. Biosci. 2023, 36, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Tseten, T.; Sanjorjo, R.A.; Kwon, M.; Kim, S.-W. Strategies to Mitigate Enteric Methane Emissions from Ruminant Animals. J. Microbiol. Biotechnol. 2022, 32, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H.; Kirs, M. Structure of the archaeal community of the rumen. Appl. Environ. Microbiol. 2008, 74, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Subedi, U.; Kader, K.; Jayawardhane, K.N.; Poudel, H.; Chen, G.; Acharya, S.; Camargo, L.S.A.; Bittencourt, D.M.d.C.; Singer, S.D. The Potential of Novel Gene Editing-Based Approaches in Forages and Rumen Archaea for Reducing Livestock Methane Emissions. Agriculture 2022, 12, 1780. [Google Scholar] [CrossRef]
- Spurlock, D.M.; Stock, M.L.; Coetzee, J.F. The impact of 3 strategies for incorporating polled genetics into a dairy cattle breeding program on the overall herd genetic merit. J. Dairy Sci. 2014, 97, 5265–5274. [Google Scholar] [CrossRef]
- Georges, M.; Drinkwater, R.; King, T.; Mishra, A.; Moore, S.S.; Nielsen, D.; Sargeant, L.S.; Sorensen, A.; Steele, M.R.; Zhao, X.; et al. Microsatellite mapping of a gene affecting horn development in Bos taurus. Nat. Genet. 1993, 4, 206–210. [Google Scholar] [CrossRef]
- Medugorac, I.; Seichter, D.; Graf, A.; Russ, I.; Blum, H.; Göpel, K.H.; Rothammer, S.; Förster, M.; Krebs, S. Bovine polledness--an autosomal dominant trait with allelic heterogeneity. PLoS ONE 2012, 7, e39477. [Google Scholar] [CrossRef]
- Hess, R.A.; Park, C.J.; Soto, S.; Reinacher, L.; Oh, J.E.; Bunnell, M.; Ko, C.J. Male animal sterilization: History, current practices, and potential methods for replacing castration. Front. Vet. Sci. 2024, 11, 1409386. [Google Scholar] [CrossRef]
- Duarte, D.A.S.; Schroyen, M.; Mota, R.R.; Vanderick, S.; Gengler, N. Recent genetic advances on boar taint reduction as an alternative to castration: A review. J. Appl. Genet. 2021, 62, 137–150. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.-M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-coupled Receptor GPR54 *. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Yeo, S.H.; Colledge, W.H. The Role of Kiss1 Neurons As Integrators of Endocrine, Metabolic, and Environmental Factors in the Hypothalamic-Pituitary-Gonadal Axis. Front. Endocrinol. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hai, T.; Chen, Y.; Sun, K.; Han, Z.; Wang, J.; Li, C.; Wang, Q.; Wang, L.; Zhu, H.; et al. Improve meat production and virus resistance by simultaneously editing multiple genes in livestock using Cas12i(Max). Sci. China Life Sci. 2024, 67, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Dagdas, Y.S.; Kleinstiver, B.P.; Welch, M.M.; Sousa, A.A.; Harrington, L.B.; Sternberg, S.H.; Joung, J.K.; Yildiz, A.; Doudna, J.A. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 2017, 550, 407–410. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, Y.; Kweon, J.; Kim, H.S.; Bae, S.; Kim, J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 2014, 32, 279–284. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2013, 368, 20110329. [Google Scholar] [CrossRef]
- Tang, L.; González, R.; Dobrinski, I. Germline modification of domestic animals. Anim. Reprod. 2015, 12, 93–104. [Google Scholar]
- Hamze, J.G.; Cambra, J.M.; Navarro-Serna, S.; Martinez-Serrano, C.A. Navigating gene editing in porcine embryos: Methods, challenges, and future perspectives. Genomics 2025, 117, 111014. [Google Scholar] [CrossRef]
- Loi, P.; Iuso, D.; Czernik, M.; Ogura, A. A New, Dynamic Era for Somatic Cell Nuclear Transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar] [CrossRef]
- Parrington, J.; Coward, K.; Gadea, J. Sperm and testis mediated DNA transfer as a means of gene therapy. Syst. Biol. Reprod. Med. 2011, 57, 35–42. [Google Scholar] [CrossRef][Green Version]
- Magnano, A.R.; Giordano, R.; Moscufo, N.; Baccetti, B.; Spadafora, C. Sperm/DNA interaction: Integration of foreign DNA sequences in the mouse sperm genome. J. Reprod. Immunol. 1998, 41, 187–196. [Google Scholar] [CrossRef]
- Moradbeigi, P.; Hosseini, S.; Salehi, M.; Mogheiseh, A. Methyl β-Cyclodextrin-sperm-mediated gene editing (MBCD-SMGE): A simple and efficient method for targeted mutant mouse production. Biol. Proced. Online 2024, 26, 3. [Google Scholar] [CrossRef]
- Konoval, O.; Korol, P.; Tabaka, P.; Kostenko, S.; Lizhi, L.; Chepiha, A.; Doroshenko, M.; Drahulian, M.; Xingchen, B.; Xuetao, H.; et al. Generation of transgenic ducks by crispr/CAS9-mediated gene inser-tion combined with the sperm-mediated gene transfer (SMGT). Biopolym. Cell 2019, 35, 427–436. [Google Scholar] [CrossRef]
- Cooper, C.A.; Challagulla, A.; Jenkins, K.A.; Wise, T.G.; O’Neil, T.E.; Morris, K.R.; Tizard, M.L.; Doran, T.J. Generation of gene edited birds in one generation using sperm transfection assisted gene editing (STAGE). Transgenic Res. 2017, 26, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, L. Ethical aspects associated with genome alteration techniques applied to animal reproduction research. Reprod. Domest. Anim. = Zuchthyg. 2024, 59 (Suppl. 3), e14670. [Google Scholar] [CrossRef]
- Cui, K.; Shoemaker, S.P. Public perception of genetically-modified (GM) food: A Nationwide Chinese Consumer Study. NPJ Sci. Food 2018, 2, 10. [Google Scholar] [CrossRef]
- Bruce, A. Genome edited animals: Learning from GM crops? Transgenic Res. 2017, 26, 385–398. [Google Scholar] [CrossRef]
- Caplan, A.L.; Parent, B.; Shen, M.; Plunkett, C. No time to waste—The ethical challenges created by CRISPR: CRISPR/Cas, being an efficient, simple, and cheap technology to edit the genome of any organism, raises many ethical and regulatory issues beyond the use to manipulate human germ line cells. EMBO Rep. 2015, 16, 1421–1426. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- Thygesen, P. Clarifying the regulation of genome editing in Australia: Situation for genetically modified organisms. Transgenic Res. 2019, 28, 151–159. [Google Scholar] [CrossRef]
- Lema, M.A. Regulatory aspects of gene editing in Argentina. Transgenic Res. 2019, 28, 147–150. [Google Scholar] [CrossRef]
- Ledesma, A.V.; Van Eenennaam, A.L. Global status of gene edited animals for agricultural applications. Vet. J. 2024, 305, 106142. [Google Scholar] [CrossRef]
- Nature Biotechnology apan embraces CRISPR-edited fish. Nat. Biotechnol. 2022, 40, 10. [CrossRef]
- Wang, Y.; Bi, D.; Qin, G.; Song, R.; Yao, J.; Cao, C.; Zheng, Q.; Hou, N.; Wang, Y.; Zhao, J. Cytosine Base Editor (hA3A-BE3-NG)-Mediated Multiple Gene Editing for Pyramid Breeding in Pigs. Front. Genet. 2020, 11, 592623. [Google Scholar] [CrossRef]
- Lopdell, T.J.; Tiplady, K.; Couldrey, C.; Johnson, T.J.J.; Keehan, M.; Davis, S.R.; Harris, B.L.; Spelman, R.J.; Snell, R.G.; Littlejohn, M.D. Multiple QTL underlie milk phenotypes at the CSF2RB locus. Genet. Sel. Evol. GSE 2019, 51, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Boschiero, C.; Li, C.J.; Connor, E.E.; Baldwin, R.L.t.; Liu, G.E. Unraveling the Genetic Basis of Feed Efficiency in Cattle through Integrated DNA Methylation and CattleGTEx Analysis. Genes 2023, 14, 2121. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Anholt, R.R.H. Pleiotropy, epistasis and the genetic architecture of quantitative traits. Nat. Rev. Genet. 2024, 25, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.R.; Mitchell, K.J.; Mouton, A.; Álvarez-Carretero, S.; Hulme-Beaman, A.; Haile, J.; Jamieson, A.; Meachen, J.; Lin, A.T.; Schubert, B.W.; et al. Dire wolves were the last of an ancient New World canid lineage. Nature 2021, 591, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kluger, J. The Return of the Dire Wolf. Available online: https://time.com/7274542/colossal-dire-wolf/ (accessed on 8 October 2025).
- Resnik, D.B.; Medina, R.F.; Gould, F.; Church, G.; Kuzma, J. Genes drive organisms and slippery slopes. Pathog. Glob. Health 2024, 118, 348–357. [Google Scholar] [CrossRef]
- Li, A.; Zhu, Z.; Yang, J.; Liu, Y.; Zhang, Y.; Liu, J. Precise Insertion of AttB Sequences in Goat Genome Using Enhanced Prime Editor. Int. J. Mol. Sci. 2024, 25, 9486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).