Abstract
The present study aimed to determine the Genotype × Environment interaction (GEI), yield stability, and agronomic performance of 24 “Pinto” bean lines under semi-arid conditions in Central-West Mexico. All the lines possess a slow-darkening seed coat, a trait that prolongs visual quality and increases market value. The lines, which exhibit an indeterminate prostrate growth habit, were evaluated in three contrasting environments: irrigated, rainfed, and drought-stressed. A combined analysis of variance, Tukey’s test, and the additive main effects and multiplicative interaction (AMMI 2) model were applied to assess seed yield and agronomic traits. Average seed yield declined markedly across environments, from 2279 kg ha−1 under irrigation to 593 kg ha−1 under drought stress, with different lines performing best in each environment. AMMI 2 biplot analysis showed that the first two principal components explained 100% of GEI variability for seed yield, dry shoot biomass, total biomass, harvest index, pods per plant, and seeds per pod. Both genetic and environmental effects were significant, with notable GEI patterns. Despite pronounced environmental influence, several lines exhibited stable performance across environments. Line 11 consistently combined high yield and stability, positioning it as a strong candidate for cultivar registration and as a parent in breeding programs targeting semiarid regions. These results underscore the importance of multi-environment evaluation for identifying genotypes with broad or specific adaptation, contributing to genetic improvement and sustainable bean production under variable moisture regimes.
1. Introduction
In emerging economies, common beans (Phaseolus vulgaris L.) play a critical role in food security [1,2] due to their high nutritional value, including substantial levels of protein, dietary fiber, iron, magnesium, zinc, and potassium [3]. As a plant-based protein source, beans provide a cost-effective means of meeting daily protein requirements, thereby reducing reliance on animal protein, which is often less accessible due to its higher market price [4]. Furthermore, the capacity of common beans for fixing atmospheric nitrogen in association with Rhizobium spp. is highly valued in regions where the crop is a subsistence foodstuff in marginal lands [2].
In Mexico, the common bean is a strategic crop for the socioeconomic development of producers and serves as an important source of protein for the population [5]. It is cultivated across all agricultural regions in two different growing seasons, Spring–Summer, and Fall–Winter, and constitutes a fundamental component of the national diet. In 2022, the total area planted with beans reached 1,472,462 ha, of which 87% was grown under rainfed conditions, with an average yield of 510 kg ha−1. In the semi-arid highlands of north-central Mexico, 1,034,084 ha were sown, representing 70% of the national area, with a corresponding yield of 434 kg ha−1 [6].
In this semi-arid region, limited water-holding capacity of the soils, mostly due to low organic content and scarce rainfall during the crop cycle, are the primary causes of the low yield per unit area. For decades, the development of drought-resistant cultivars has been the primary strategy to increase and stabilize bean yields under rainfed conditions [7]. In addition, agronomic practices directed to water catchment and moisture retention are also implemented, i.e., tied ridging, with the furrows blocked by small mounds of soil made at regular intervals during crop mechanic cultivation for weed control. In this region, “Durango” race genotypes have shown superior adaptation to abiotic stresses compared to other races within the Mesoamerican gene pool. Consequently, “Pinto-type” beans have become predominant in recent decades [8]. In the more favorable environments within the region, although not locally consumed, shiny black-seeded cultivars from the Jalisco race are also grown.
Miklas et al. [9] reported that a new “Pinto” bean phenotype—characterized by slow-darkening and brighter seed coat—is gaining popularity in the United States, where 500,000 t were produced in 2022. In Mexico, this phenotype emerged with the release of the cultivar “Pinto Saltillo” [10], which rapidly displaced all previously improved cultivars and native varieties due to its market appeal and wide adaptation. All cultivars of the Pinto type released after this cultivar are also of slow darkening seed coat [11,12].
In addition to their short biological cycle, “Pinto” bean cultivars exhibit a rapid transition from vegetative to reproductive stages and possess a high capacity to allocate both assimilated and photosynthetically fixed carbon compounds to developing pods and seeds—which is reflected in a high harvest index [13,14]. This early and strong sink demand enhances escape from terminal drought, particularly when rainfall ceases during the grain-filling stage [15,16]. Two other traits are important for adaptation to the erratic rainfall pattern of the region, the short-day response that allows for phenological plasticity [16,17] and the staggered flowering associated with the indeterminate prostrated growth habit.
Beyond overall seed yield, yield components such as pods per plant (PP), seeds per pod (SP), and pod length (PL) are critical agronomic traits for evaluating genotype performance under contrasting water regimes. These variables provide valuable insights into the reproductive efficiency and adaptive capacity of bean genotypes, helping breeders identify lines that maintain productivity under drought, rainfed, and irrigated conditions [15].
When genotypes are cultivated across multiple environments, their yield tends to vary due to differences in environmental conditions. This variation in relative performance is referred to as Genotype × Environment interaction (GEI) [18]. From a grower’s perspective, cultivars that consistently produce high yields across diverse and even specific environments across years are more desirable. Therefore, plant breeders typically evaluate genotypes across multiple locations to identify superior and stable lines adapted to varying agroecological conditions [19]. Seed yield, size, and quality, as well as resistance to prevalent diseases in the test environments, are useful indicators of plant performance and stability. The additive main effects and multiplicative interaction (AMMI) models are widely used to identify genotypes that combine disease resistance with high yield potential under diverse environmental conditions [20], including those characterized by low and erratic rainfall patterns. As such, AMMI is considered a valuable tool in crop genetic improvement programs [21]. Moreover, the model facilitates the identification of genotypes with low GEI and stable performance across testing environments [22].
The objective of this study was to analyze Genotype × Environment interactions, seed yield stability, and key agronomic traits, including the major yield components, of 24 “Pinto” bean lines to identify those with high seed yield, stable performance, and low GEI in a semi-arid region of north-central Mexico.
2. Materials and Methods
2.1. Study Location
Field trials were conducted at two sites, one site was the National Center for Disciplinary Research in Family Agriculture (CENID-AF) of INIFAP, located at 21°33′00″ N, 101°02′30″ W, at an elevation of 2100 m.a.s.l. The second site was a commercial plot belonging to a cooperating farmer in “La Blanquita”, Ojuelos de Jalisco, Jalisco, Mexico (21°54′19″ N, 101°43′34″ W; 2129 m.a.s.l.). The region has a semi-arid climate (BSw according to Köppen classification climate [23]), with an average annual precipitation of 422 mm and a mean annual temperature of 17.5 ± 0.5 °C. The topography consists of valleys and gently rolling hills. The predominant soils are classified as Haplic Xerosols (associated with Lithosols and Eutric Planosols) and Haplic Phaeozems [24].
2.2. Soil Sampling and Chemical Analysis
Soils at the experimental sites were randomly sampled, collecting five individual soil cores per site, mixed thoroughly to create a composite sample for analyzing their physical and chemical characteristics according to the Official Mexican Norms 021-RECNAT-2000 [25]. Soil texture was determined using the Bouyoucos method, while soil organic matter (SOM) was quantified by the oxidation of organic carbon [26]. Nitrogen (N) content was measured using the Micro-Kjeldahl method [27], and the available phosphorus (P) content was analyzed using the Bray 1 method [28]. The concentration of calcium (Ca), magnesium (Mg), potassium (K), and sodium (Na) was determined by saturating the cation exchange sites with ammonium ions from a 1 N ammonium acetate solution (C2H7NO2; pH~7) [29]. Iron (Fe), manganese (Mn), and copper (Cu) contents were quantified using the DTPA (diethylene triamine pentacetic acid) micronutrient extraction method [30]. Boron (B) and sulfur (S) contents were analyzed through the azomethine-H method [31,32]. Finally, the cation exchange capacity (CEC) was calculated as the sum of the extracted cations (Ca, Mg, K, Na) [33].
2.3. Bean Germplasm
Twenty-five “Pinto” bean genotypes were evaluated, including twenty-four experimental lines developed by the Bean Genetic Improvement Program of the National Institute of Forestry, Agricultural, and Livestock Research and the commercial cultivar “Pinto Saltillo” (25, Table 1). This cultivar was included as a reference due to its high social and economic importance in the semi-arid region of Mexico. All the genotypes possess a slow-darkening seed coat (sd sd recessive trait) and an indeterminate prostrate growth habit (Fin Fin dominant trait) (Table 1).

Table 1.
Genealogy of 24 experimental “Pinto” bean lines evaluated in drought, rainfed, and irrigated environments in the semi-arid highlands of north-central Mexico.
This Pinto bean type, belonging to the Durango race, is generally best adapted to the semi-arid highlands and exhibits a short-day photoperiod response (Ppd Ppd photoperiod sensitive genotype). Due to the short-day photoperiod response, evaluated lines display varying degrees of phenological and morphological plasticity [17]. Although the most evident response to photoperiod sensitivity occurs at the onset of flowering, it also influences the duration of the reproductive stages [16].
2.4. Agronomic Cultivation Practices
Soil preparation included subsoiling, fallowing, and harrowing to ensure optimal conditions for seed germination. Prior to establishing the experiment, soil samples were collected for physical and chemical characterization. Field sowing was performed manually in all three evaluation environments, according primarily to soil moisture availability (drought, rainfed, and irrigated). The experiments were conducted during the 2023 and 2024 cropping seasons, with the same layout applied each year. In the drought environment (irrigation withheld after flowering simulates terminal drought conditions), sowing took place on 20 July 2023, and a total accumulated rainfall of 148.6 mm was recorded during the July–September crop cycle. For the 2024 season, for both the rainfed and irrigated conditions, sowing was conducted on 4 July 2024. Under rainfed conditions, the crop relied exclusively on natural precipitation, with 271.8 mm of rainfall during the crop cycle (Figure 1). In the irrigated environment, supplemental irrigation was provided in addition to the 271.8 mm of rainfall; two supplementary irrigations of 40 mm each were applied during the grain-filling stage in August, for a total water input of 351.8 mm. The values of precipitation and temperature were compared to the historical average (1991–2020) to assess deviations from long-term climate patterns (Figure 1).

Figure 1.
Monthly accumulated rainfall (bars) and maximum (blue dotted lines) and minimum (orange dotted lines) average temperatures during the bean growth cycles in 2023 and 2024 at CENID-AF, INIFAP. Historical climate averages for the period 1991–2020 are shown for comparison.
In all three environments, 100 kg ha−1 of diammonium phosphate (18–46–00) was applied at sowing. During flowering, a foliar spray of 4 kg of urea and 2 L of phosphoric acid diluted in 200 L of water was applied. Weed control was carried out with a post-emergence application of the herbicide FLEX BIW® at a rate of 1 L ha−1, when weeds had developed 2–4 leaves and reached a height of 5–7 cm.
2.5. Measured Traits
At physiological maturity (90 days after sowing), whole plants were harvested from two 5 m rows per genotype after trimming half a meter at each row end (7 m2 per experimental unit). The following variables were recorded: number of pods per plant (PP), which was counted on five plants randomly taken; pod length (PL), measured in millimeters (mm) using a Truper® stainless-steel digital vernier caliper (Mexico City, Mexico); number of seeds per pod (SP); seed yield (SY) and aboveground dry biomass (ADB), determined after manual threshing of all plants corresponding to each experimental unit and adjusting their weights to a moisture content of 12%, were expressed in kg ha−1; 100-seed weight (W100S), measured in grams (g) from a subsample of 100 seeds randomly taken per genotype; total dry biomass (TDB), obtained by summing the ADB and dry grain biomass (DGB); and harvest index (HI), calculated by dividing the DGB by the TDB.
2.6. Experimental Design
In all three evaluation environments, the 25 bean genotypes were established using a randomized complete block design (RCBD) with four replications, implemented independently within each environment. Each experimental unit consisted of two 6 m rows, with 70 cm between rows and 10 cm between plants for a total of 80 plants per experimental unit and a density of 190,000 plants per hectare. The total trial area was 700 m2.
2.7. Statistical Analysis
A pooled analysis of variance (ANOVA) was conducted to estimate differences among genotypes, environments, and their interaction (GEI), as well as the variability among replicates within environments. Tukey’s multiple range test (p ≤ 0.05) was applied for mean comparisons. In this statistical model, genotypes were considered fixed effects because the 24 pinto bean lines included in this research were selected from a larger set of 81 lines evaluated in 2022. These 24 lines represent the superior materials identified for potential release and use in our breeding program. Environments were treated as random effects to allow the results to be generalized to a broader range of similar conditions beyond those tested in this study. The model is represented as follows:
where Yijk = phenotypic value of the j-th replication within the i-th environment for the k-th genotype; μ = overall mean; Ai = effect of the i-th environment; Rj(Ai) = effect of the j-th replication nested within the i-th environment; Gk = effect of k-th genotype; AiGk = interaction effect between the i-th environment and the k-th genotype; and Eijk = experimental error.
Likewise, the AMMI biplot was generated using the first two principal components from the following model:
where Yij = phenotypic value of the i-th genotype in the j-th environment; μ = overall mean; gi = deviation of the i-th genotype from the overall mean; ej = deviation of the j-th environment from the overall mean; λk = singular value of the k-th Interaction Principal Component (IPC); αik = singular value for the i-th genotype in the k-th IPC; Υjk = singular value of the j-th environment in the k-th IPC; and Rij = residual.
Additionally, Pearson correlation analyses were performed to assess the relationships between yield and agronomic traits. All the statistical analyses were conducted using R (version 4.5) with the agricolae package [34,35].
3. Results
3.1. Soil Composition
The analyses of the soil composition at the three experimental sites (droughted, rainfed, and irrigated environments) show differences in characteristics that influence crop development (Table 2). These soils were characterized by low levels of several components, including SOM (1.08, 1.19, and 1.27%), NO3− (18.60, 13.66, and 14.55 ppm), available P (5.53, 6.79, and 4.39 ppm), and Na (25.00, 29.89, and 65.09 ppm, respectively). All these values are considered “low” according to the published classification criteria (Table 2). In contrast, Ca, Cu, Mg, and S were found at medium levels, while K, Mn, and B were present at high levels. These classifications were defined according to the thresholds established in the Soil and Water Analysis Interpretation Manual [36].

Table 2.
Physical and chemical properties of the soils at the experimental sites (droughted, rainfed, and irrigated) in the semi-arid highlands of north-central Mexico.
Regarding soil moisture dynamics (Table 2), the field capacity (FC) values (19.10, 22.90, and 20.10%) show values typical of sandy loam soils, indicating a low water-holding capacity at the three environments, a fact that restricts water availability to plant roots. The Permanent wilting point (PWP) values were 11.40, 12.20, and 13.30%, which occur when soil moisture falls below the threshold at which plants can no longer absorb water. The difference between FC and PWP is small, indicating that the soils do not hold moisture for long periods of time, thereby exposing the bean crop to severe water stress under rainfed and drought environments.
Concerning the soil physical properties (Table 2), the pH ranged from 6.79 (irrigated) to 8.60 (rainfed), indicating conditions ranging from slightly acidic to alkaline. The latter condition is notable in the rainfed environment. The BD was higher in the rainfed and irrigated plots (1.55 g cm−3) compared to the droughted environment (1.27 g cm−3), values that are not the ideal for a loam soil but are lower than the value indicative of poor soil health (>1.8 g cm−3) [37].
3.2. Influence of Environment and Genotype on “Pinto” Bean Performance
3.2.1. Genotype × Environment Influence on Agronomic Traits of “Pinto” Bean Lines
Significant differences (p ≤ 0.01) were observed for environment, genotype, and their GEI across all evaluated agronomic traits, apart from SP, which showed no significant GEI (Table 3). The environment exerted the strongest influence on SY, ADB, TDB, and HI, a result seen in the considerably larger mean squares for the Environment (E) factor compared to the Genotype (G) and G × E factors. The relatively low residual error suggests that the evaluation was carried out with adequate precision.

Table 3.
Mean squares of agronomic variables of 25 “Pinto” beans genotypes evaluated in three environments (drought, rainfed, and irrigated).
3.2.2. Genotype × Environment Influence on “Pinto” Bean Lines Yield
In the comparison of means for SY across environments, the highest value was obtained under irrigated conditions, reaching 2279.1 kg ha−1 (Table 4), while the lowest SY was recorded under drought-stress conditions, at 592.7 kg ha−1. These SY were statistically different from the rainfed environment (1512.3 kg ha−1). Regarding yield components, the highest W100S was observed in the rainfed conditions (35.2 g), followed by the irrigated (32.5 g). In contrast, the lightest seeds were produced under drought-stress conditions (24.2 g). Total dry biomass (TDB) followed a similar pattern, reaching its highest value under irrigation (3927.3 kg ha−1) and its lowest under drought (1371.4 kg ha−1). The highest average HI was observed under rainfed conditions (0.61), while the drought-stressed environment exhibited the lowest HI (0.43). The significant GEI for HI (Table 3) explains these differences in biomass conversion efficiency across environments.

Table 4.
Mean comparison of yield-related traits of 25 “Pinto” bean genotypes grown under different environmental conditions.
3.3. Performance of “Pinto” Bean Genotypes Under Three Different Environments
Significant variability was observed in SY and other agronomic traits among the 25 “Pinto” bean genotypes evaluated in the three environments (drought stress, rainfed, and irrigated; Table 5). According to Tukey’s multiple range tests (p ≤ 0.05), line 13 ((D38/AZNA)/PS-30-2-1) achieved the highest averages SY (1874.3 kg ha−1), TDB (3184.0 kg ha−1), and PP (26), and for that reason it was included in the statistically superior group for these variables. In contrast, line 18 (PS/FME-M-30-4-1-M) showed the lowest values for SY (1137.4 kg ha−1) and TDB (1995.3 kg ha−1).

Table 5.
Comparison of means of 25 “Pinto” beans genotypes evaluated in three environments with different moisture availability during the growth cycle (drought, rainfed, and irrigated) in the semi-arid plateau of north-central Mexico.
Regarding W100S, among all genotypes, line 24 (Ll2-82-2-M-M) exhibited the highest value (32.2 g), which was significantly greater than that of the reference genotype 25 (“Pinto Saltillo”; 29.1 g). For HI, line 24 (Ll2-82-2-M-M) and line 4 (PS/Dalia-M-2-3-3-M) showed the highest values (0.57 and 0.56, respectively). Conversely, line 16 (SR/JANASA-M-86-M) presented the lowest HI (0.48).
For PP, line 7 (PS/Dalia-M-2-3-6-M) produced the highest number of PP (26.0), whereas line 9 ((D38/AZNA)/PS-23-2-1) had the lowest (17.0). Regarding SP, line 11 (RAR/CAR-M-54-M-M) reached the highest value (5.4), while lines 12 ((D38/AZNA)/PS-29-2-1) and 21 (RAR/CAR-M-56-M-M) recorded the lowest values (4.7 and 4.9, respectively).
The data also revealed that ADB was highest in line 16 SR/JANASA-M-86-M, with 1316.2 kg ha−1, and lowest in line 18 (PS/FME-M-5-2-M), with 858.0 kg ha−1. PL ranged from 101.0 mm in line 23 to 115.2 mm in line 11 (RAR/CAR-M-54-M-M), indicating considerable variability among the evaluated genotypes for this trait, which is relevant for differentiating lines with superior reproductive structures.
3.4. Correlation of Agronomic Variables
Correlation analysis between agronomic traits and seed yield (Table 6) revealed important relationships among the attributes recorded in the Pinto bean lines. Seed yield (Y) shows a strong positive correlation with total dry biomass (TDB; r = 0.950, p ≤ 0.01); however, TDB includes seed yield; thus, they are not independent traits, along with aboveground dry biomass (ADB; r = 0.878, p ≤ 0.01), indicating that greater vegetative development favors seed yield.

Table 6.
Correlation of agronomic variables in 25 Pinto beans genotypes evaluated in three environments.
Seeds per pod (SP) was positively correlated with 100-seed weight (W100S; r = 0.487, p ≤ 0.05), indicating a moderate association between both variables. The correlations of pods per plant (PP) and seeds per pod (SP) with seed yield (SY) were moderate (r = 0.355 and 0.120, respectively), suggesting that these components contribute to yield but to a lesser extent than ADB.
3.5. Additive Main Effects and Multiplicative Interaction (AMMI 2)
The first two principal components of the AMMI 2 model explained 100% of the variation in the GEI for SY, ADB, TDB, HI, PP, and PL, indicating that the interaction patterns were fully captured by these two axes (Figure 2a–f). This result shows that the response of the 25 “Pinto” bean genotypes across the three environments can be completely interpreted using the first two principal components of the AMMI 2 biplot. The biplots also revealed that the three environments are in opposite quadrants, indicating different line responses across environments and highlighting the contrasting environmental conditions of the test sites.
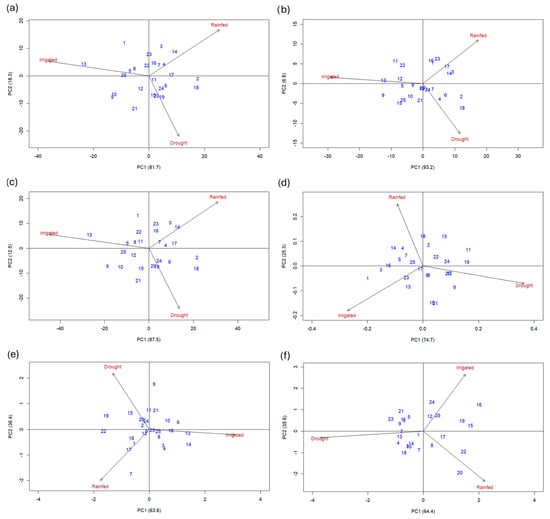
Figure 2.
AMMI 2 biplots showing the Genotype × Environment interaction (GEI) of 25 Pinto bean genotypes across three environments (irrigated, rainfed, and drought). The biplots represent the first two principal components (PC1 and PC2) for the additive main traits: (a) seed yield (SY), (b) aboveground dry biomass (ADB), (c) total dry biomass (TDB), (d) harvest index (HI), (e) pods per plant (PP), and (f) pod length (PL). Genotype codes are detailed in Table 1, and environmental descriptions are provided in Figure 1.
On the other hand, line 18 contributed the greatest variability to the GEI across five traits. Lines 9, 14, and 21 each contributed variability in four traits; lines 1, 13, 15, and 19 to three traits; and lines 2, 3, 10, 11, 22, and 23 to two traits. Additionally, lines 7, 17, 16, and 24 showed unique contributions to the interaction. Specific lines showed strong interactions for individual traits: line 7 for PP, line 17 for ADB, and lines 16 and 24 for PL.
The biplot also allowed for the identification of specific adaptation patterns. For SY, line 13 (3569 kg ha−1) was best adapted to irrigated conditions, line 14 (1995 kg ha−1) to rainfed conditions, and lines 19 (656 kg ha−1) and 20 (672 kg ha−1) to drought-stress conditions. For ADB, line 13 performed best under irrigation, lines 3, 14, and 17 under rainfed conditions, and lines 2 and 18 under drought stress.
Line 13 also performed best under irrigation for ADB and TDB, while lines 3 and 14 excelled under rainfed conditions for these traits. For TDB, lines 19, 20, and 24 showed specific adaptation to drought stress. Regarding HI, lines 1, 13, and 23 were well-suited for irrigated conditions, lines 4 and 18 for rainfed, and lines 12, 19, 20, and 24 for drought stress.
In terms of PP, lines 13 and 14 stood out under irrigation, lines 7 and 17 under rainfed conditions, and lines 15, 20, and 24 under drought stress. For SP, the highest values were observed in lines 19 and 25 under irrigation, lines 8 and 10 under rainfed conditions, and lines 4 and 18 under drought stress. Line 13 excelled in five traits under irrigation, while line 14 excelled in three traits under rainfed conditions. Under drought stress, lines 19 and 20 outperformed the other genotypes in three and four traits, respectively. Regarding trait stability, lines located near the biplot’s origin showed consistent performance across all environments. Line 11 and 22 showed the highest stability for SY; lines 19, 20, and 24 for ADB; lines 16, 20, and 23 for TDB; lines 6, 17, and 18 for HI; lines 5, 12, 23, and 24 for PP; and line 1 for PL.
4. Discussion
The soil physicochemical properties and the accumulated rainfall during the growing season at the rainfed and drought environments reflect the natural conditions characteristic of the semi-arid region of north-central Mexico. Also, the historic information for climatic parameters shown in Figure 1 clearly indicates that rainfall was higher in previous decades. In addition, the soils at the test sites were characterized by low SOM and limited levels of available nitrogen and P, both essential nutrients for crop growth [38,39,40]. Their sandy loam texture further reduces water retention capacity, as indicated by low FC values of 19.1, 22.9, and 20.1%. This combination presents a considerable challenge for agricultural production, particularly the low available P content (4.39–6.79 ppm), since it is an essential nutrient for common beans, a crop with a high demand for P for root development and flowering. Furthermore, the bean crop is particularly vulnerable to water stress during critical growth stages, like flowering and grain filling [15]. Under these conditions, the combination of low soil water-holding capacity and the bean plant’s sensitivity to water deficit limit seed yields, underscoring the importance of strategies for improving water-use efficiency and soil moisture retention.
In response to these limitations, “Pinto” bean breeding projects focus on identifying lines with high efficiency in nutrient and soil moisture uptake, enabling more stable yields in resource-restricted environments. Under such conditions, both soil health and the plant’s root system capacity to explore the soil profile are critical factors for improving productivity and resilience.
In this region, the predominant growth habit is the prostrate indeterminate type III, which is typical of the “Durango” and “Jalisco” races [41]. This habit is associated with high yield potential and defensive traits, such as photoperiod sensitivity, staggered flowering, extensive ground cover [17,42], and a high harvest index (HI). However, over the last decades, the genetic diversity used in the commercial production of the “Durango” race has declined sharply, primarily due to the widespread adoption of the cultivar “Pinto Saltillo” [10]. In this study, some of the new lines showed higher yields (e.g., line 13, with 1874.0 kg ha−1) compared to the reference cultivar “Pinto Saltillo” (with 1480.7 kg ha−1), as well as line 17 with lower yield (1302.6 kg ha−1) but high stability, highlighting their potential value for commercial breeding.
Certain bean lines displayed remarkable performance across several important agronomic traits, such as line 13 evaluated in this study, which achieved the highest averages for SY (1874.3 kg ha−1), TDB (3184.0 kg ha−1), ADB (1309.7 g), and PP (26), placing it in the statistically superior group for these variables. Its SP (5.1) and PL (107 mm) were intermediate, suggesting that its high yield was primarily driven by greater biomass accumulation and a higher number of pods rather than by seed size or pod length. This indicates that line 13 may possess traits associated with drought escape or tolerance, such as efficient biomass partitioning and resource allocation to reproductive structures, which are critical under water-limited conditions [15,43]. Line 17 also demonstrated stability across environments, which may be related to its intermediate values for PP (24), SP (5), and PL (103 mm). This balance among reproductive traits suggests a conservative strategy that supports consistent performance under variable water regimes. Although root traits, canopy architecture, and phenology were not directly measured in this study, previous research indicates that drought tolerance in the common bean may be associated with early flowering, efficient resource allocation, deeper root systems, and canopy structures that improve water-use efficiency [15,43]. These findings suggest that such mechanisms could underlie the superior performance and stability observed in line 17 under drought stress. Future evaluations should include these physiological and morphological traits to better understand the mechanisms of drought tolerance in common beans. The cultivar Pinto Saltillo has become dominant not only in commercial production but also as a parental line in the development of new cultivars of this grain type [44,45].
Given the increasing climatic variability during the crop cycle, enhancing stability and resilience to environmental stress is a priority for the improvement of “Durango race Pinto” beans. Incorporating broader genetic diversity is essential [15,39] to address environmental variability and ensure crop sustainability in the semi-arid region.
As shown in Table 1, 19 of the 24 lines in this study originated from interracial crosses, primarily between the “Durango” and “Jalisco” races (lines 1, 2, 3, 4, 5, 6, 7, 14, 15, 17, 18, 19, 22, and 23), which are genetically closely related within the same gene pool [12] but with important differences in adaptation, the “Jalisco race” to more humid environments of Central-West Mexico, while the Durango race is adapted to the semi-arid Central-North. Additionally, four lines were derived from Durango × Mesoamerican crosses (lines 8, 11, 20, and 21), and one line represents an inter-gene pool cross between the “Durango” and “Nueva Granada” races (line 16). Recently a Pinto cultivar derived from an interspecific cross (Pinto Saltillo/Yellow Tepary) was registered for commercial production in the semi-arid highlands [46] and incorporated into our breeding projects as parental stock.
The analysis of variance for the 25 “Pinto” bean genotypes revealed a strong environmental influence on key traits, including SY, ADB, TDB, and HI. As shown in Table 3, the environmental effect was the dominant source of variation, which highlights the determining role of environmental conditions in the phenotypic expression of the tested materials. The significant differences among environments (p ≤ 0.01) indicate that agronomic performance is strongly conditioned by site-specific factors, which emphasizes the need for selection strategies tailored to specific environments to optimize adaptation and productivity. The significant GEI across most traits indicates that genotypes responded differently to the environmental conditions of the test sites. This finding reinforces the importance of evaluating stability and adaptability to identify lines with consistent performance across environments. Conversely, the absence of GEI for SP suggests that this yield component is relatively stable and less influenced by environmental fluctuations, facilitating its selection in breeding projects.
Finally, although the impact of the genotypes on the results was smaller than that from the environments, the variability observed among the lines tested underscores the diversity present in the evaluated material and its impact on yield and agronomic traits under rainfed and irrigated environments. This variability is crucial for further breeding efforts, as it enables the identification of lines with high yield stability and adaptation to stress factors such as drought or low soil fertility, conditions typical of the semi-arid highlands of north-central Mexico.
Correlation analysis between agronomic traits and seed yield in “Pinto” bean lines revealed key relationships influencing crop productivity. SY exhibited a strong positive correlation with TDB (r = 0.950, p ≤ 0.01) and ADB (r = 0.685, p ≤ 0.01), indicating that greater biomass accumulation favors seed production. In contrast, SY showed weak and no significant correlations with other components such as W100S (r = 0.089) and SP (r = 0.120), suggesting that, under the conditions faced by the genotypes in our trials, biomass production was the primary driver of yield. These findings emphasize the importance of total biomass in yield determination and suggest that selecting genotypes with high dry matter production could enhance productivity across contrasting environments. For instance, several lines produced more biomass than the check in the drought-stressed environment (Figure 2c). Overall, these results highlight the genetic diversity among the evaluated “Pinto” bean lines and emphasize the role of genotype in yield potential and biomass allocation efficiency.
Conversely, the HI shows a small positive correlation with SY (r = 0.257) but a negative correlation with aboveground dry biomass (ADB; r = –0.422), indicating that increased vegetative growth does not necessarily improve the efficiency of biomass conversion to grain. This underscores the need to balance biomass accumulation with harvest efficiency in breeding projects, as excessive vegetative growth may not proportionally increase yield.
Moreover, the HI is considered positively associated with drought tolerance, reflecting a genotype’s ability to remobilize photosynthates to seeds under water-limited conditions [15,47,48,49,50]. This makes HI a key component of drought-adaptation mechanisms and an important criterion for selecting resilient “Pinto” bean genotypes.
As for utilizing the AMMI 2 model, it has proven to be an effective tool for evaluating GEIs, enabling the identification of genotypes with superior stability and adaptability across diverse production environments. In this study, the first two principal components (PC1 and PC2) explained 100% of the GEI variability, confirming the model’s effectiveness in capturing the response patterns of the 24 “Pinto” bean lines and reference cultivar Pinto Saltillo. This observation aligns with the findings of Gauch and Zobel [51], who reported that the first two principal components are generally sufficient to represent environmental interactions in AMMI analyses. However, other authors have suggested using up to four components for more complex scenarios [52,53].
The AMMI 2 biplot (Figure 2) is centered at the origin (0,0), which is divided into four sectors by vertical and horizontal axes. Genotypes located farthest from the origin along a sector are considered winning genotypes for the environments within that sector, as their positions reflect strong positive interactions with those conditions. Conversely, the magnitude of the interaction between a genotype and an environment is represented by the distance of their vectors from the origin, while genotypes located near the origin exhibit greater stability across environments [54,55].
Furthermore, the ideal genotype can be statistically identified as one with a high average yield and a short vector length, indicating high stability across environments [56]. By correctly interpreting this model, breeders can efficiently identify lines with desirable patterns of interaction, adaptation, and stability, while eliminating materials with low stability or poor performance across environments [57,58]. For example, Figure 2a visually confirms that line 13, a top performer under irrigation, is positioned in the irrigated sector, while lines 19 and 20, which performed best under drought, are aligned with the drought vector, while lines 11 and 17 seem to be more stable.
The biplot analysis revealed that environments with short vectors, such as drought for seed yield and rainfed for HI (Figure 2), are less discriminating for the GEI. According to Murphy et al. [59], these environments are considered more suitable for identifying genotypes with average yield and broad adaptability. For the evaluated traits, most lines clustered near the biplot origin (Figure 2a–f), indicating a uniform response across environments. In contrast, genotypes positioned farther from the origin displayed greater environmental sensitivity and stronger GEI, reflecting performance variability across sites [60].
The AMMI model is a valuable tool for selecting stable and adaptable genotypes while discarding those with inconsistent performance [49]. Consistent with the findings of Khan et al. [54] and Stansluos et al. [55], genotypes located near the origin in the biplot demonstrated greater stability, whereas those positioned farther away exhibited an environment-dependent response.
In this study, “Pinto” bean lines 11, 22, and 17 emerged as the most stable for seed yield, positioning them as promising candidates for breeding programs targeting resilience and yield stability under semi-arid conditions. The application of AMMI-based selection strategies facilitates the identification of lines with high water- and nutrient-use efficiency, thereby contributing to the sustainability of “Pinto” bean production in regions characterized by low and erratic rainfall patterns and marginal soils.
In bean breeding programs, yield has traditionally been the main characteristic considered in genotype selection. However, this approach presents limitations when there are conditions of high environmental variability. Several studies have reported that high-yielding genotypes often exhibit a strong GEI interaction, which compromises their phenotypic stability and limits their applicability across contrasting environments, as their performance may be outstanding under optimal conditions but highly variable or even deficient under suboptimal scenarios [53,54].
In contrast, prioritizing yield stability in the selection process allows the identification of genotypes with consistent performance across diverse environments, facilitating their adoption in regions with climatic and soil heterogeneity [61]. In our study, line 11 demonstrated stable performance under rainfed, drought, and irrigated conditions, making it a strong candidate for use as a parental line in local breeding programs. Considering its stability, this bean line represents a valuable genetic resource for developing resilient cultivars adapted to semi-arid regions.
On the other hand, line 13 showed the highest yield but lower stability across environments. Because these two lines originated from different parental backgrounds (Table 1), crossing them represents an ideal complementary strategy to combine high yield potential with stability. This approach will facilitate the development of new genotypes capable of both high productivity and resilience under variable environmental conditions. While the GEI interactions were relatively small, they were sufficient to cause a reclassification of cultivars among environments. Yield stability contributed to the consistent performance of lines that ranked just below the top-yielding ones, as cultivars with both high and low yields exhibited variable performance across environments. This reinforces the importance of incorporating stability into selection criteria, ensuring that new cultivars can maintain reliable yields under the unpredictable conditions typical of semi-arid production systems. For breeders, balancing maximum yield with stability is essential to develop cultivars that not only achieve high productivity under favorable conditions but also provide consistent performance and resilience across diverse environments.
Although the drought-stress data were obtained during a single cropping season (2023), the results of this research are valid because the statistical analyses ensure accurate comparisons among genotypes and the experimental approach reflects typical environmental conditions of the region, making the findings relevant to local production systems. Nevertheless, this single-year dataset may not fully capture the variability of drought conditions across different years, which could limit the broader generalization of the results. Therefore, future research should be carried out under variable climate scenarios within a single cropping cycle to strengthen and enhance these findings of this research.
5. Conclusions
Despite the strong environmental influence on the seed yield and agronomic traits of the evaluated “Pinto” bean lines under irrigated, rainfed, and drought-stress conditions, the observed variability in seed yield, dry biomass, harvest index (HI), and seeds per pod across environments highlights the importance of identifying resilient genotypes. Both genetic and environmental factors critically determine “Pinto” bean productivity, underscoring the need for breeding strategies tailored to specific environmental conditions to enhance adaptation and yield stability.
Identifying lines with consistent performance under contrasting water regimes is essential for sustainable production in semi-arid regions, particularly under increasing climate variability. Among the tested lines, line 11 demonstrated superior seed yield combined with stability across environments, making it a strong candidate for use as a parental line in local breeding programs or for registration as a new cultivar for the semi-arid highlands of north-central Mexico.
The application of the AMMI 2 model proved effective for detecting stable genotypes in terms of seed yield and agronomic traits by characterizing Genotype × Environment interactions. These findings provide valuable guidance for both genetic improvement programs and the development of agronomic management strategies aimed at maximizing Pinto bean productivity under resource-limited conditions.
Author Contributions
Conceptualization, O.G.B. and J.A.A.G.; methodology, O.G.B., Y.J.H. and J.A.A.G.; software, J.S.G.J.A.R. and G.C.A.; validation, O.G.B., J.S.G.J.A.R. and J.A.A.G.; formal analysis, J.S.G.J.A.R., J.A.A.G. and O.G.B.; investigation, O.G.B. and J.A.A.G.; resources, J.A.A.G.; data curation, O.G.B. and J.S.G.J.A.R.; writing—original draft preparation, G.C.A., O.G.B. I.F.C.D. and U.A.L.; writing—review and editing, G.C.A., J.A.A.G., Y.J.H., I.F.C.D. and U.A.L.; visualization, J.A.A.G. and Y.J.H.; supervision, J.A.A.G.; project administration, J.A.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Forestry, Agricultural and Livestock Research (INIFAP), grant number 16215236227.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The row data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SOM | Soil organic matter |
| N | Nitrogen |
| P | Phosphorus |
| C | Calcium |
| Mg | Magnesium |
| K | Potassium |
| Na | Sodium |
| Mn | Manganese |
| CaCO3 | Calcium carbonate |
| S | Sulfur |
| B | Boron |
| Cu | Copper |
| Fe | Iron |
| BD | Soil bulk density |
| pH | Hydrogen potential |
| FC | Soil field capacity |
| CEC | Cation exchange capacity |
| PWP | Permanent wilting point |
| PP | Number of Pods per Plant |
| PL | Pod Length (PL |
| SP | Number of seeds per pod |
| ADB | Aboveground Dry Biomass |
| SY | Seed Yield |
| W100S | 100-seed Weight |
| TDB | Total Dry Biomass |
| HI | Harvest index |
| DGB | Dry Grain Biomass |
References
- Graham, P.H.; Ranalli, P. Common bean (Phaseolus vulgaris L.). Field Crops Res. 1997, 53, 131–146. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legum. Sci. 2023, 5, e155. [Google Scholar] [CrossRef]
- Pujolà, M.; Farreras, A.; Casañas, F. Protein and starch content of raw, soaked and cooked beans (Phaseolus vulgaris L.). Food Chem. 2007, 102, 1034–1041. [Google Scholar] [CrossRef]
- Kotue, T.C.; Marlyne, J.M.; Wirba, L.Y.; Amalene, S.R.H.; Nkenmeni, D.C.; Kwuimgoin, I.; Djote, W.N.B.; Kansci, G.; Fokou, E.; Fokam, D.P. Nutritional properties and nutrients chemical analysis of common beans seed. MOJ Biol. Med. 2018, 3, 41–47. [Google Scholar] [CrossRef]
- Cid-Ríos, J.A.; Acosta-Gallegos, J.A.; Echavarría-Cháirez, F.G.; Bañuelos-Valenzuela, R.; Prado-García, A.A. Identification of bean lines (Phaseolus vulgaris) with low genotype–environment interactions under rainfed in two semiarid sites of North-Central Mexico. Agronomy 2025, 15, 1160. [Google Scholar] [CrossRef]
- SIAP. Secretaria de Agricultura y Desarrollo Rural. Servicio de Información Agroalimentaria y Pesquera. Anuario Estadístico de la Producción Agrícola. 2022. Available online: https://nube.agricultura.gob.mx/cierre_agricola/ (accessed on 10 March 2025).
- Dominguez, S.A.; Darias, R.R.; Yordanys, M.D.Y.; Negrin, E.A. Tolerance of common bean varieties (Phaseolus vulgaris) to field drought conditions. Cent. Agricola 2019, 46, 22–29. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-57852019000300022&lng=en&tlng= (accessed on 20 July 2025).
- FIRA. Fideicomisos Instituidos en Relación con la Agricultura. Panorama Agroalimentario, Frijol. 2012. Available online: https://www.fira.gob.mx/Nd/index.jsp?q=produccion+de+frijol+pinto+ (accessed on 10 June 2025).
- Miklas, P.N.; Soler-Garzon, A.; Pastor Corrales, M.A.; Cichy, K.A. Registration of ‘USDA Diamondback’ slow-darkening pinto bean. J. Plant Regist. 2024, 18, 52–60. [Google Scholar] [CrossRef]
- Sánchez Valdez, I.; Ibarra Pérez, F.J.; Rosales Serna, R.; Singh, S.P.; Acosta Gallegos, J.A. Pinto Saltillo: Nueva variedad de frijol para el Altiplano de México. Agric. Técnica En México 2001, 27, 73–75. Available online: https://www.redalyc.org/pdf/608/60827108.pdf (accessed on 7 August 2025).
- Rosales-Serna, R.; Flores-Gallardo, H.; López González, G.C.; Rubiños-Panta, J.E.; Ortiz-Sánchez, I.A.; Flores Magdaleno, H.; Santana-Espinoza, S.; Domínguez-Martínez, P.A. Phenology and water productivity in improved pinto bean varieties grown in Durango, Mexico. Rev. Fitotec. Mex. 2021, 44, 511–519. [Google Scholar] [CrossRef]
- Rosales-Serna, R.; Acosta-Gallegos, J.A.; Ibarra-Pérez, F.J.; Cuéllar-Robles, E.I. Pinto bravo: A new dry bean variety for the semiarid Mexican highlands. Rev. Mex. De Cienc. Agrícolas 2011, 2, 985–991. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-09342011000600015&lng=es&nrm=iso (accessed on 28 June 2025).
- Singh, S.P. Drought resistance in the race Durango dry bean landraces and cultivars. Agron. J. 2007, 99, 1219–1225. [Google Scholar] [CrossRef]
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; García, R.; Rivera, M. Evidence for genotypic differences among elite lines of common bean in the ability to remobilize photosynthate to increase yield under drought. J. Agric. Sci. 2017, 155, 857–875. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- White, J.W.; Laing, D.R. Photoperiod response of flowering in diverse genotypes of common bean (Phaseolus vulgaris). Field Crops Res. 1989, 22, 113–128. [Google Scholar] [CrossRef]
- Acosta-Gallegos, J.A.; White, J. Phenological plasticity as an adaptation by common bean to rainfed environments. Crop Sci. 1995, 35, 199–204. [Google Scholar] [CrossRef]
- Comstock, R.E.; Moll, R.H. Genotype-Environment Interactions. In Statistical Genetics and Plant Breeding; Hanson, W.D., Robinson, H.F., Eds.; NAS-NRC: Washington, DC, USA, 1963; pp. 164–196. [Google Scholar]
- Naghavi, A.; Sofalian, O.; Asghari, A.; Sedghi, M. Relation between freezing tolerance and seed storage proteins in winter bread wheat. Turk. J. Field Crops 2010, 15, 154–158. Available online: https://dergipark.org.tr/en/download/article-file/158758 (accessed on 23 August 2025).
- Tosquy-Valle, O.H.; Ibarra-Perez, F.J.; Rodriguez-Rodriguez, J.R.; Esqueda-Esquivel, V.A.; Andrés-Meza, P. Adaptation and disease resistance of elite tropical black bean lines. Legum. Res. 2023, 46, 1126–1133. [Google Scholar] [CrossRef]
- Yoseph, T.; Mekbib, F.; Amsalu, B.; Tadele, Z. Genotype by environment interaction and yield stability of drought tolerant mung bean [Vigna radiata (L.) Wilczek] genotypes in Ethiopia. J. Agric. Environ. Sci. 2022, 7, 43–62. Available online: https://boris-portal.unibe.ch/server/api/core/bitstreams/ac361021-fc6c-4ae7-9d93-5b3ebd34bd61/content (accessed on 6 August 2025).
- Rao, P.J.M.; Sandhyakishore, N.; Srinivasan, S.; Sandeep, S.; Praveen, G.; Neelima, G.; Anil Kumar, G. AMMI and GGE stability analysis of drought tolerant chickpea (Cicer arietinum L.) genotypes for target environments. Legum. Res. 2023, 46, 1105–1116. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- IIEG (Instituto de Información Estadística y Geográfica de Jalisco). Diagnóstico del Municipio. 2023. Available online: https://iieg.gob.mx/ns/wp-content/uploads/2023/08/Ojuelos-1.pdf (accessed on 20 June 2025).
- Secretaría de Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-021-RECNAT-2000, que Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis; Diario Oficial de la Federación: México, 2002. Available online: https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2280.pdf (accessed on 21 July 2025).
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Kjeldahl, J.A. A new method for the determination of nitrogen in organic compounds. Analyst 1883, 8, 22–24. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1322199 (accessed on 1 July 2025).
- Bray, R.H.; Kurtz, L.T. Determination of total, soluble, and reactive phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Khorshidi, M.; Lu, N. Determination of cation exchange capacity from soil water retention curve. J. Eng. Mech. 2017, 143, 04017023. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for Zinc, Iron, Manganee, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Lindsay, J.I.; Gumbs, F.A. Erodibility Indices Compared ti Measured Values of Selected Trinidad Soils. Soil Sci. Soc. Am. J. 1982, 46, 393–396. [Google Scholar] [CrossRef]
- Kah, M.; Haderlein, S.R. Determination of total sulfur in soils and sediments using a modified version of the azomethine-H method. Environ. Sci. Technol. 2005, 39, 6673–6680. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Soil reaction and cation exchange capacity. In Soil Sampling and Methods of Analysis; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 143–145. Available online: https://eclass.uoa.gr/modules/document/file.php/GEOL105/%CE%91%CE%A3%CE%9A%CE%97%CE%A3%CE%97_%CE%95%CE%94%CE%91%CE%A6%CE%9F%CE%A3%20%CE%9B%CE%91%CE%A5%CE%A1%CE%99%CE%9F%CE%A5_2018%20%28%CE%A7%20%CE%A3%CF%84%CE%BF%CF%85%CF%81%CE%B1%CF%8A%CF%84%CE%B7%29/Soil%20Sampling%20and%20analysis_Canadian%20Society%20of%20soil%20science.pdf (accessed on 1 July 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 10 March 2025).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.3-8; National Engineering University (UNI): Lima, Peru, 2023; Available online: https://CRAN.R-project.org/package=agricolae (accessed on 14 March 2025).
- Castellanos, J.Z.; Uvalle-Bueno, J.K.; Aguilar-Santelises, A. Manual de Interpretación de Análisis de Suelos y Aguas Agrícolas, Plantas y ECP.; Instituto de Capacitación para la Productividad Agrícola: Ciudad de México, México, 2000. [Google Scholar]
- Klopp, H.; Vly, S. Bulk Density Is an Indicator of Soil Health. South Dakota State University Extension. 2023. Available online: https://extension.sdstate.edu/bulk-density-indicator-soil-health (accessed on 17 September 2025).
- Bot, A.; Benites, J. The Importance of Soil Organic Matter; FAO Soils Bulletin 80; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Available online: https://www.fao.org/4/a0100e/a0100e00.htm (accessed on 25 June 2025).
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. Soil organic carbon and mineral nitrogen contents in soils as affected by their pH, texture and fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- Flynn, F. Interpreting Soil Tests—Unlock the Secrets of Your Soil; New Mexico State University: Las Cruces, NM, USA, 2010; Available online: https://pubs.nmsu.edu/_circulars/CR6761.pdf (accessed on 21 July 2025).
- Singh, S.P. Broadening the genetic base of common bean cultivars. Crop Sci. 2001, 41, 1659–1675. [Google Scholar] [CrossRef]
- Acosta-Gallegos, J.A.; Kohashi-Shibata, V. Effect of water stress on growth and yield of indeterminate dry bean (Phaesolus vulgaris) cultivars. Field Crops Res. 1989, 20, 81–90. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S. Physiological traits associated with drought resistance in Andean and Mesoamerican genotypes of common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Mier, J.C.; Konzen, E.R.; Palkovic, A.; Rao, I.M.; Beebe, S.; Gepts, P. Effect of drought stress on the genetic architecture of photosynthate allocation and remobilization in pods of common bean (Phaseolus vulgaris L.), a key species for food security. BMC Plant Biol. 2019, 19, 171. [Google Scholar] [CrossRef]
- Assefa, T.; Beebe, S.E.; Rao, I.M.; Cuasquer, J.B.; Duque, M.C.; Rivera, M.; Battisti, A.; Lucchin, M. Pod harvest index as a selection criterion to improve drought resistance in white pea bean. Field Crops Res. 2013, 148, 24–33. [Google Scholar] [CrossRef]
- Jiménez-Galindo, J.C.; Padilla-Chacón, D.; Anaya-López, J.L.; Acosta-Gallegos, J.A.; Ramírez-Cabral, N.; Sánchez-Gutiérrez, R.A.; Ortega-Ortega, A.; Figueroa-Gonzalez, J.J. ‘Tepehuán-RS’ a new drought tolerant, high grain yield in low plant densities and slow darkening pinto bean cultivar. J. Plant Regist. 2025, 19, e220420. [Google Scholar] [CrossRef]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Return to agrobiodiversity: Participatory plant breeding. Diversity 2022, 14, 126. [Google Scholar] [CrossRef]
- Díaz, L.M.; Blair, M.W. Race structure within the Mesoamerican gene pool of common bean (Phaseolus vulgaris L.) as determined by microsatellite markers. Theor. Appl. Genet. 2006, 114, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Hageman, A.; Van Volkenburgh, E. Sink strength maintenance underlies drought tolerance in common bean. Plants 2021, 10, 489. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. AMMI analysis of yield trials. In Genotype by Environment Interaction; Kang, M.S., Gauch, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 85–122. [Google Scholar]
- Sivapalan, S.; Brien, L.O.; Ortiz-Ferrara, G.; Hollamby, G.J.; Barclay, I.; Martin, P.J. An adaptation analysis of Australian and CIMMYT/ICARDA wheat germplasm in Australian production environments. Aust. J. Agric. Res. 2000, 51, 903–915. [Google Scholar] [CrossRef]
- Tariku, S.; Lakew, T.; Bitew, M.; Asfaw, M. Genotype by environment interaction and grain yield stability analysis of rice (Oryza sativa L.) genotypes evaluated in north western Ethiopia. Net J. Agric. Sci. 2013, 1, 10–16. Available online: https://www.netjournals.org/pdf/NJAS/2013/1/13-015.pdf (accessed on 26 July 2025).
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Mamun, M.A. AMMI and GGE biplot analysis for yield performance and stability assessment of selected Bambara groundnut (Vigna subterranea L. Verdc.) genotypes under the multi-environmental trials (METs). Sci. Rep. 2021, 11, 22791. [Google Scholar] [CrossRef]
- Stansluos, A.A.L.; Öztürk, A.; Niedbała, G.; Türkoğlu, A.; Haliloğlu, K.; Szulc, P.; Omrani, A.; Wojciechowski, T.; Piekutowska, M. Genotype–trait (GT) biplot analysis for yield and quality stability in some sweet corn (Zea mays L. saccharata Sturt.) genotypes. Agronomy 2023, 13, 1538. [Google Scholar] [CrossRef]
- White, J.W.; Castillo, J.A.; Ehleringer, J.R.; Garcia, C.J.A.; Singh, S.P. Relations of carbon isotope discrimination and other physiological traits to yield in common bean (Phaseolus vulgaris) under rainfed conditions. J. Agric. Sci. 1994, 122, 275–284. [Google Scholar] [CrossRef]
- Yan, W. Singular-value partitioning in biplot analysis of multi-environment trial data. Agron. J. 2002, 94, 990–996. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop. Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Murphy, S.E.; Lee, E.A.; Woodrow, L.; Seguin, P.; Kumar, J.; Rajcan, I.; Ablett, G.R. Genotype × Environment interaction and stability for isoflavone content in soybean. Crop. Sci. 2009, 49, 1313–1321. [Google Scholar] [CrossRef]
- Akter, A.; Hassan, M.; Umma, K.M.; Islam, M.; Hossain, K.; Rahman, M. AMMI biplot analysis for stability of grain yield in hybrid rice (Oryza sativa L.). J. Rice Res. 2014, 2, 126. [Google Scholar] [CrossRef]
- Sokolovic, D.; Babic, S.; Petrovic, M.; Solís, I.; Cougnon, M.; Gutierrez, N.; Pärssinen, P.; Reheul, D.; Radovic, J.; Torres, A.M. Genotype by environment interactions and phenotypic traits stability of the EUCLEG faba bean collection. Front. Plant Sci. 2025, 15, 1480110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).