Green Manuring Reduces Agronomic Indicators of Fodder Winter Barley Regardless of Fertilization Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Description
2.2. Experimental Design and Soil Amendments
2.3. Soil and Plant Analyses
2.3.1. Soil Analysis
2.3.2. Grain Analysis
2.4. Photosynthesis and Transpiration
2.5. Soil Microorganisms
2.5.1. Microbial Respiration and Dehydrogenase Activity
2.5.2. Community-Level Physiological Profiling (CLPP)
2.6. Statistical Analysis
3. Results
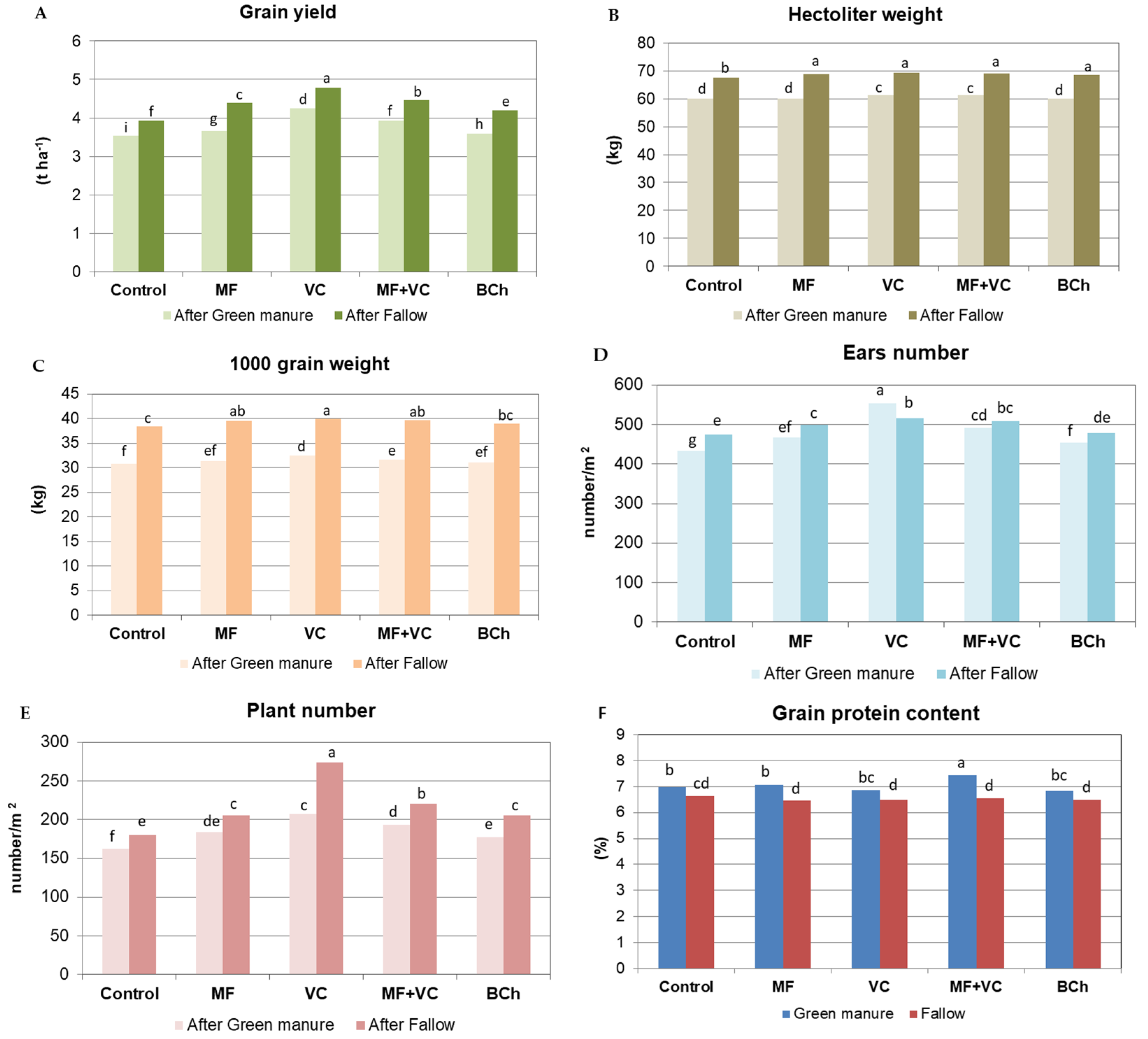
3.1. Agronomic Parameters
3.2. Changes in Soil Physicochemical Properties
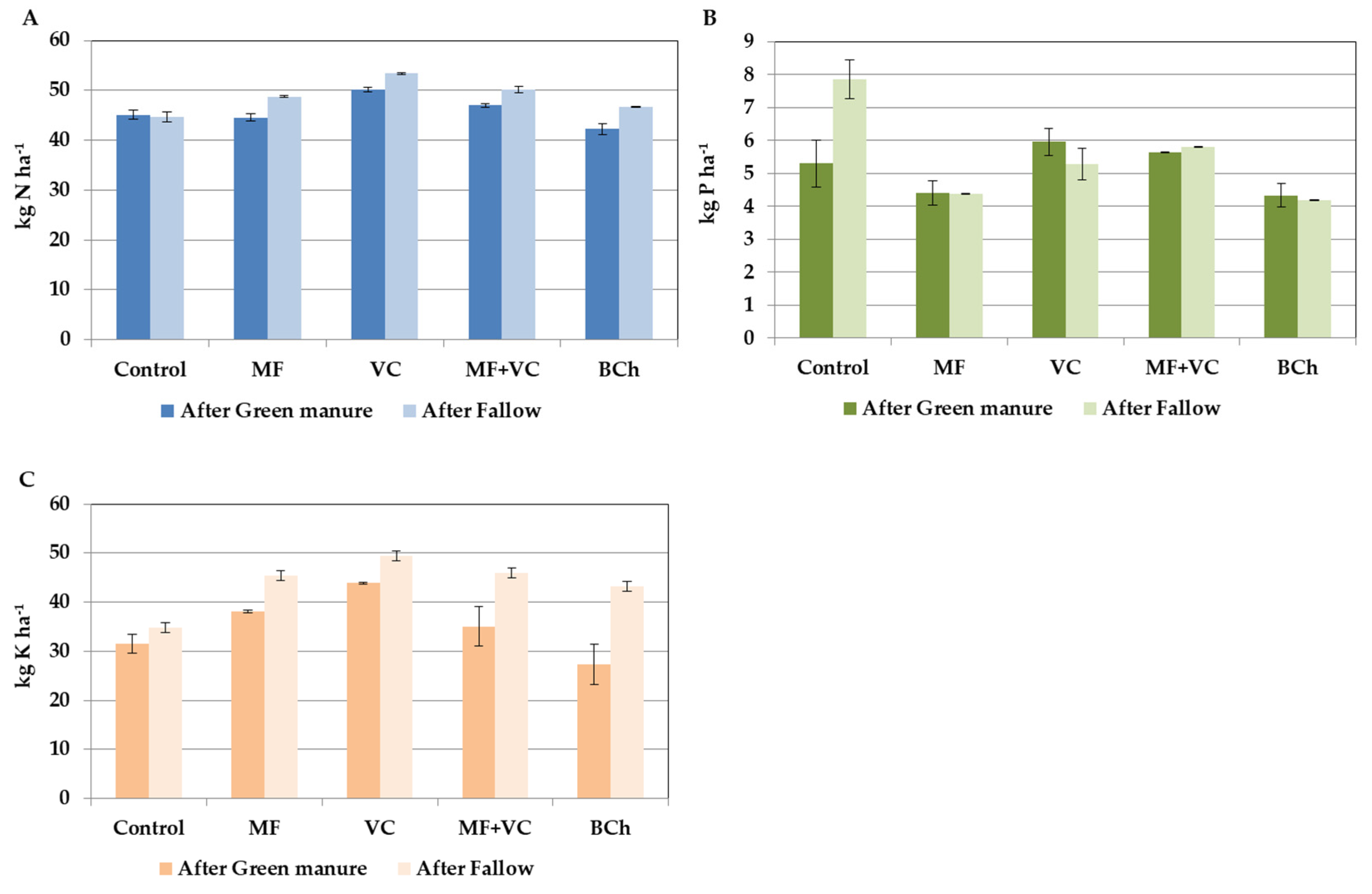
3.3. Export of Nutrients
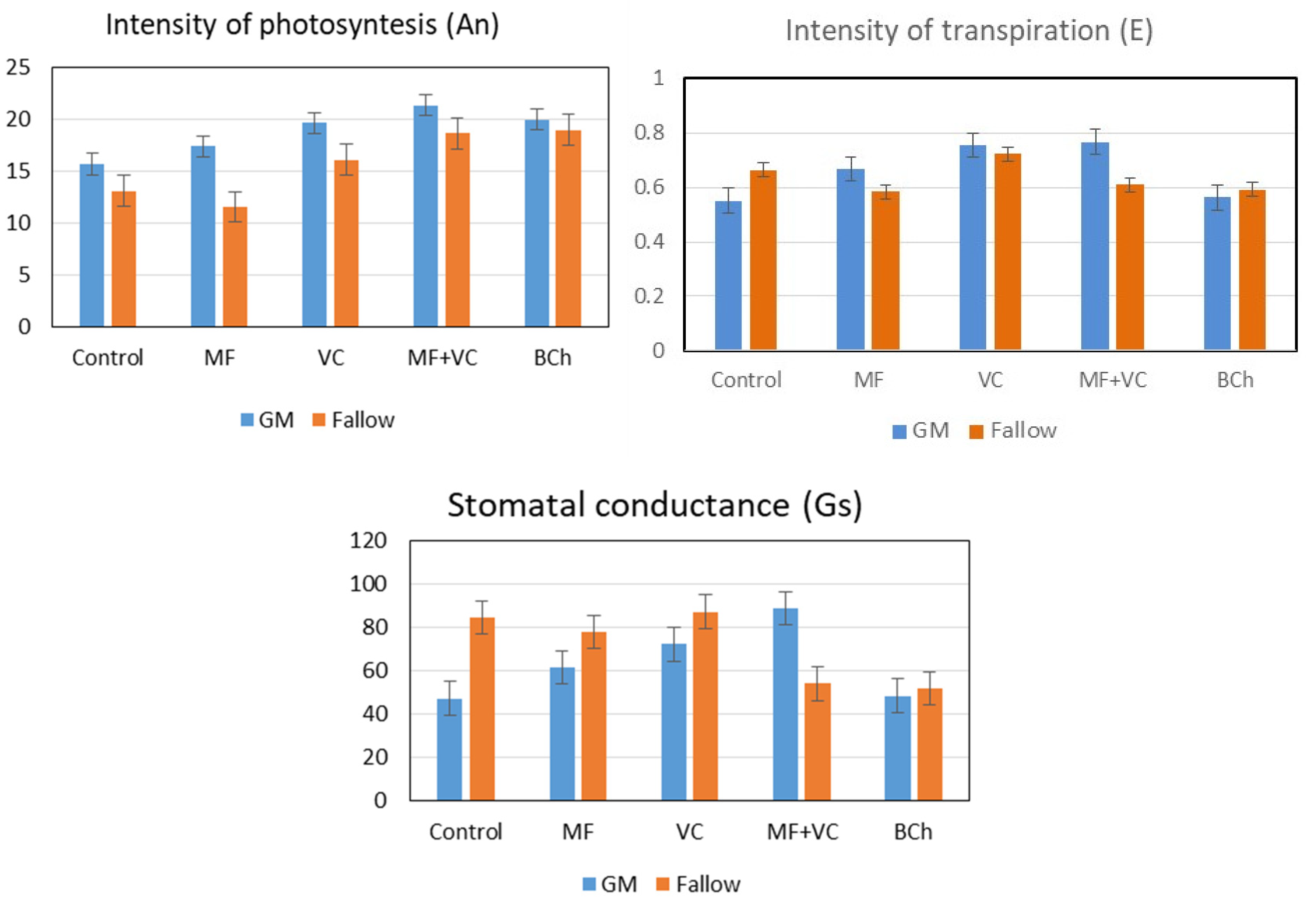
3.4. Photosynthesis and Transpiration
3.5. Soil Microorganisms
3.5.1. Microbial Respiration and Dehydrogenase Activity
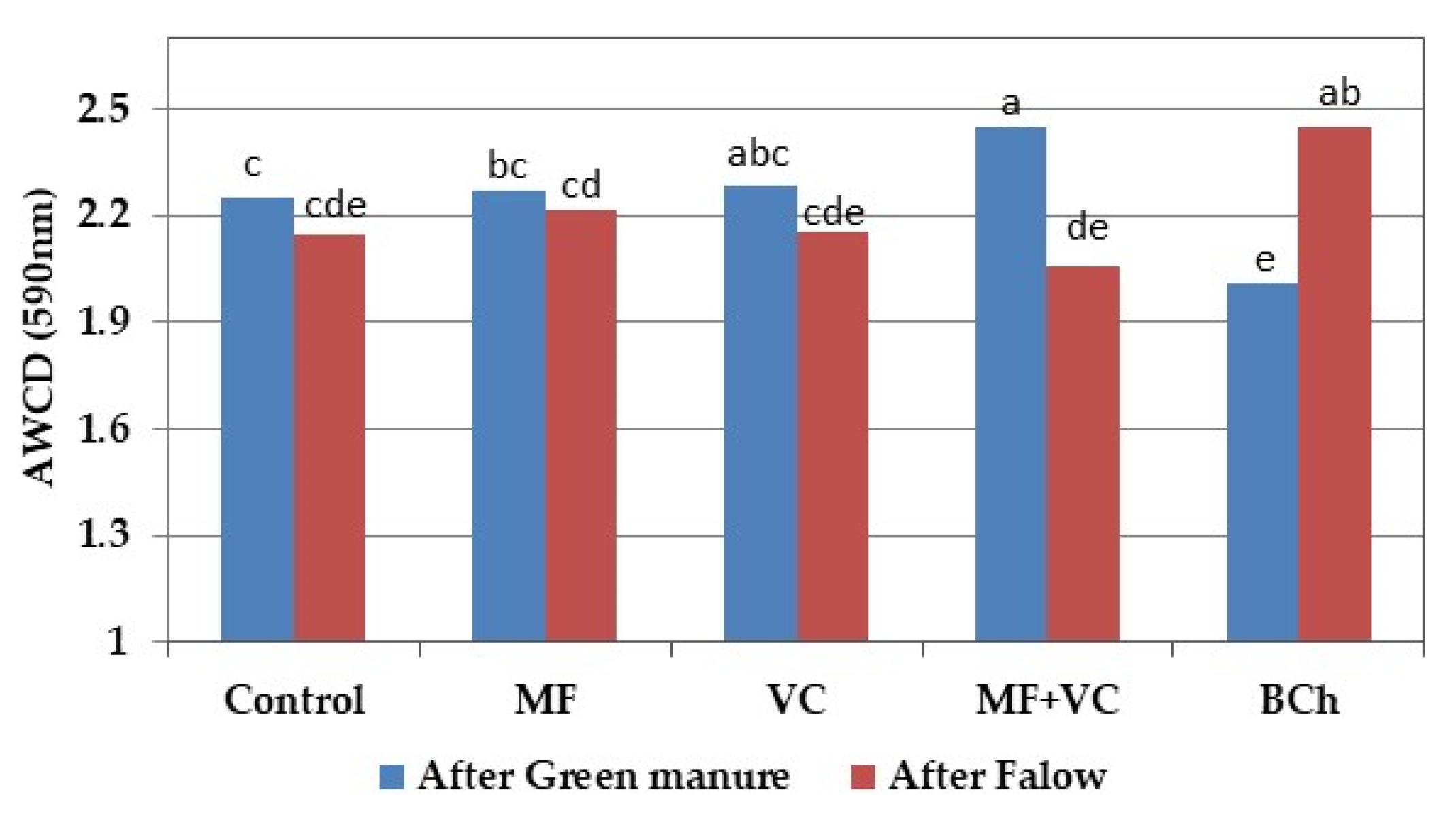
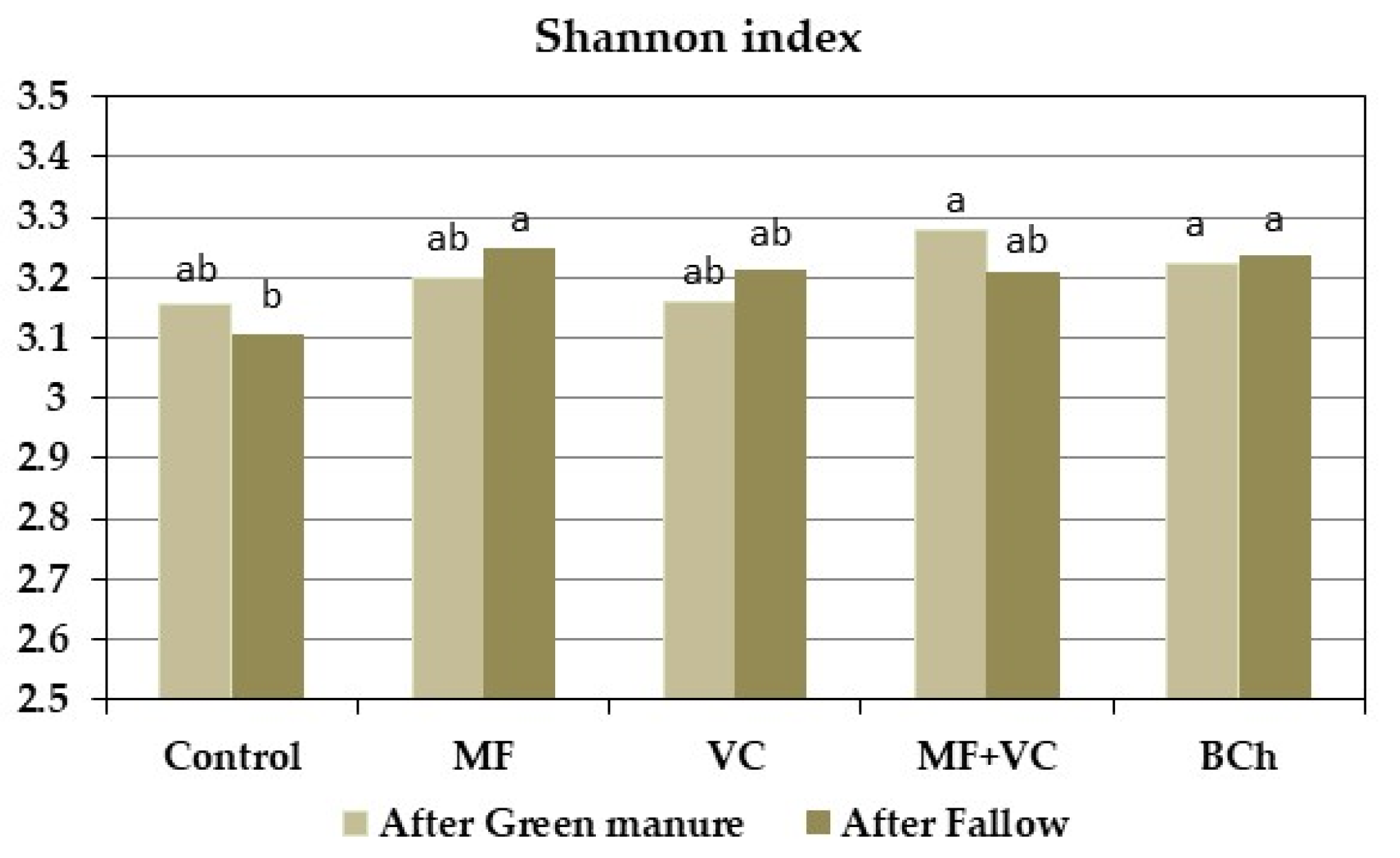
3.5.2. Community-Level Physiological Profiling
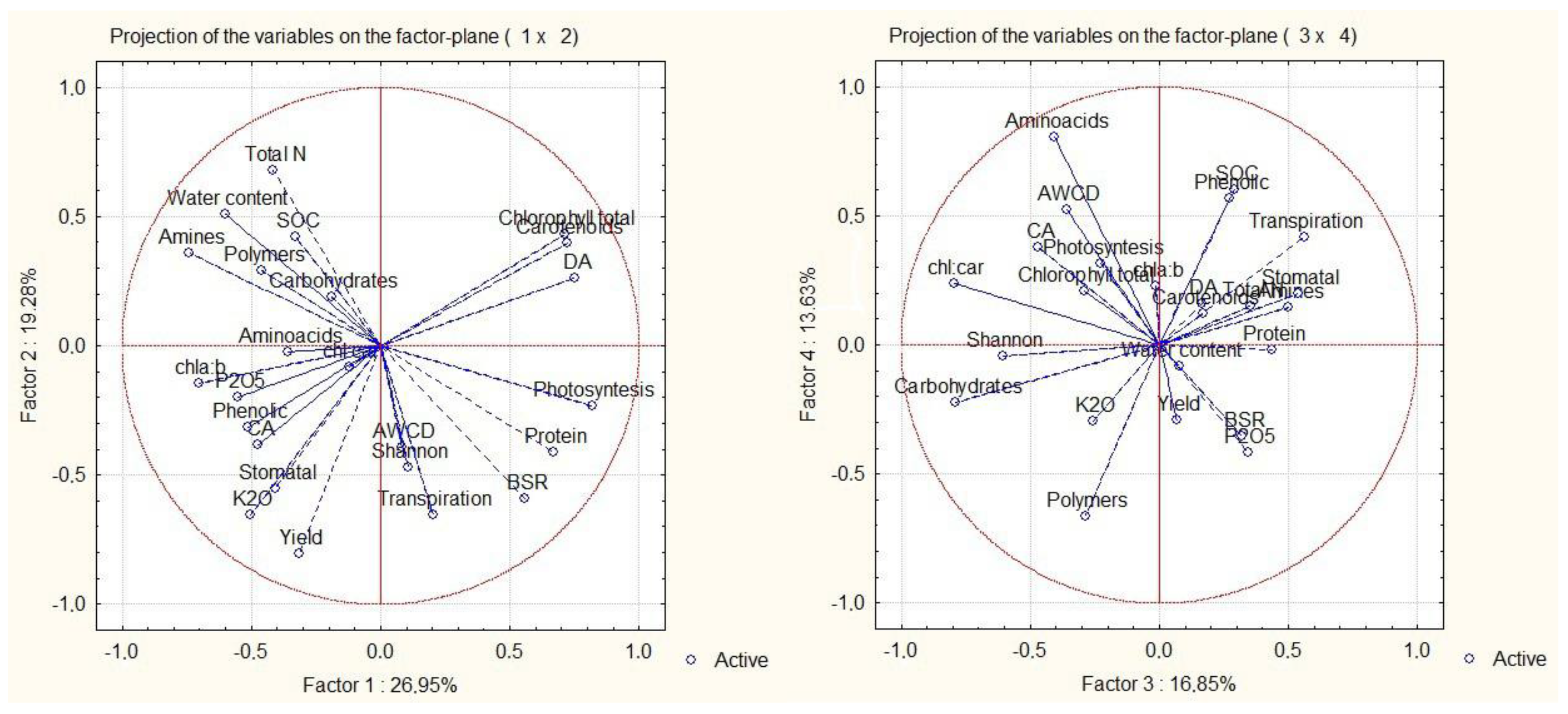
3.6. PCA Analisys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kehinde, B.A.; Sharma, P. Recently isolated antidiabetic hydrolysates and peptides from multiple food sources: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 322–340. [Google Scholar] [CrossRef]
- FAO. FAO STAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 September 2025).
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Yadav, M.R.; Kumar, S.; Lal, M.K.; Kumar, D.; Kumar, R.; Yadav, R.K.; Kumar, S.; Nanda, G.; Singh, J.; Udawat, P. Mechanistic understanding of leakage and consequences and recent technological advances in improving nitrogen use efficiency in cereals. Agronomy 2023, 13, 527. [Google Scholar] [CrossRef]
- Lyu, H.; Li, Y.; Wang, Y.; Wang, P.; Shang, Y.; Yang, X.; Wang, F.; Yu, A. Drive soil nitrogen transformation and improve crop nitrogen absorption and utilization—A review of green manure applications. Front. Plant Sci. 2024, 14, 1305600. [Google Scholar] [CrossRef]
- Gao, S.J.; Zhang, R.G.; Cao, W.D.; Fan, Y.Y.; Gao, J.S.; Huang, J. Long-term rice-rice-green manure rotation changing the microbial communities in typical red paddy soil in south China. J. Integr. Agric. 2015, 14, 2512–2520. [Google Scholar] [CrossRef]
- Becker, M.; Ladha, J.K.; Ali, M. Green manure technology: Potential, usage, and limitations. A case study for lowland rice. Plant Soil 1995, 174, 181–194. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.X.; Wang, J.S.; Wang, B.; Chen, H.Y.H.; Xu, X.F.; Wang, C.G.; He, N.P.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Change Biol. 2019, 25, 14557. [Google Scholar] [CrossRef]
- Carter, M.S.; Sørensen, P.; Petersen, S.O.; Ma, X.; Ambus, P. Effects of green manure storage and incorporation methods on nitrogen release and N2O emissions after soil application. Biol. Fertil. Soils 2014, 50, 1233–1246. [Google Scholar] [CrossRef]
- Portugal, J.R.; Arf, O.; Buzetti, S.; Portugal, A.R.P.; Garcia, N.F.S.; Meirelles, F.C.; Garé, L.M.; Abrantes, F.L.; Rodrigues, R.A.F. Do cover crops improve the productivity and industrial quality of upland rice? Agron. J. 2020, 112, 327–343. [Google Scholar] [CrossRef]
- Astier, M.; Maass, J.M.; Etchevers-Barra, J.D.; PeA, J.J.; González, F.D.L. Short-term green manure and tillage management effects on maize yield and soil quality in an andisol. Soil Till. Res. 2006, 88, 153–159. [Google Scholar] [CrossRef]
- Rashmi, I.; Shirale, A.; Kartikha, K.S.; Shinogi, K.C.; Meena, B.P.; Kala, S. Leaching of plant nutrients from agricultural lands. In Essential Plant Nutrients; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Bergström, L.; Kirchmann, H.; Aronsson, H.; Torstensson, G.; Mattsson, L. Use efficiency and leaching of nutrients in organic and conventional cropping systems in Sweden. In Organic Crop Production—Ambitions and Limitations; Kirchmann, H., Bergström, L., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Zuo, W.; Xu, L.; Qiu, M.; Yi, S.; Wang, Y.; Shen, C.; Zhao, Y.; Li, Y.; Gu, C.; Shan, Y.; et al. Effects of Different Exogenous Organic Materials on Improving Soil Fertility in Coastal Saline-Alkali Soil. Agronomy 2023, 13, 61. [Google Scholar] [CrossRef]
- Schröder, P.; Beckers, B.; Daniels, S.; Gnädinger, F.; Maestri, E.; Marmiroli, N.; Mench, M.; Millán, R.; Obermeier, M.M.; Oustriere, N.; et al. Intensify production, transform biomass to energy and novel goods and protect soils in Europe—A vision how to mobilize marginal lands. Sci. Total Environ. 2018, 616–617, 1101–1123. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of Vermicompost on Soil and Plant Health and Promoting Sustainable Agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, Z.; Xu, L.; Zhao, Y.; Yi, S.; Shen, C.; Wang, Y.; Li, Y.; Zuo, W.; Gu, C.; et al. Effects of Vermicompost Application on Growth and Heavy Metal Uptake of Barley Grown in Mudflat Salt-Affected Soils. Agronomy 2022, 12, 1007. [Google Scholar] [CrossRef]
- Nasiri, S.; Andalibi, B.; Tavakoli, A.; Delavar, M.A.; El-Keblawy, A.; Zwieten, L.V.; Mastinu, A. The mineral biochar alters the biochemical and microbial properties of the soil and the grain yield of Hordeum vulgare L. under drought stress. Land 2023, 12, 559. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Houborg, R.; McCabe, M.F.; Cescatti, A.; Gitelson, A.A. Leaf chlorophyll constraint on model simulated gross primary productivity in agricultural systems International. J. Appl. Earth Obs. Geoinform. 2015, 43, 160–176. [Google Scholar] [CrossRef]
- Zaytseva, I.Y.; Noskova, E.N.; Lisitsyn, E.M.; Schennikova, I.N. Relationship of barley leaf’s pigment content with development of yield structure elements. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 042051. [Google Scholar] [CrossRef]
- Yu, K.; Lenz-Wiedemann, V.; Chen, X.; Bareth, G. Estimating leaf chlorophyll of barley at different growth stages using spectral indices to reduce soil background and canopy structure effects. ISPRS J. Photogramm. Remote Sens. 2014, 97, 58–77. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Liang, R.; Hou, R.; Li, J.; Lyu, Y.; Hang, S.; Gong, H.; Ouyang, Z. Effects of different fertilization on rhizosphere bacterial communities of winter wheat in the North China Plain. Agronomy 2020, 10, 93. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Popova, V.; Petkova, M.; Shilev, S. Metagenomic approach unravelling bacterial diversity in combined composting and vermicomposting technology of agricultural wastes. Ecol. Balk. 2023, 15, 135–150. [Google Scholar]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis; Sparks, D.L., Ed.; Part 3. SSSA Book Ser. 5; SSSA: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity, electrical conductivity, and total dissolved solids. In Methods of Soil Analysis; Sparks, D.L., Ed.; Part 3. SSSA Book Ser. 5; SSSA: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Keeny, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis; Part 2, Chemical and microbiological methods; Page, A.L., Miller, D.R., Keeny, D.R., Eds.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Foster, J.C. Soil sampling and storage. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 49–121. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3. Chemical Methods; Black, C.A., Ed.; Soil Science of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute (IRRI): Manila, Philippines, 1976; pp. 27–34. Available online: http://books.irri.org/9711040352_content.pdf (accessed on 10 September 2025).
- FAO. Food Energy—Methods of Analysis and Conversion Factors; Report of a Technical Workshop Rome, 3–6 December 2002; FAO: Rome, Italy, 2003; ISSN 0254-4725. [Google Scholar]
- Shlyk, A.A. Biosynthesis of chlorophyll b. Annu. Rev. Plant Physiol. 1971, 22, 169–184. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Bushmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Petrova, S.; Velcheva, I.; Nikolov, B.; Vasileva, T.; Bivolarski, V. Antioxidant Responses and Adaptation Mechanisms of Tilia tomentosa Moench, Fraxinus excelsior L. and Pinus nigra J. F. Arnold towards Urban Air Pollution. Forests 2022, 13, 1689. [Google Scholar] [CrossRef]
- Hidayati, N.; Triadiati; Anas, I. Photosynthesis and transpiration rates of rice cultivated under the system of rice intensification and the effects on growth and yield. Hayati J. Biosci. 2016, 23, 67–72. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Morley, P.G.; Jump, A.S.; West, M.D.; Donoghue, D.N.M. Spectral response of chlorophyll content during leaf senescence in European beech trees. Environ. Res. Commun. 2020, 2, 071002. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, Y.; Berlyn, G.P. Photosynthetic parameters of urban greening trees growing on paved land. For.-Biogeosci. For. 2019, 12, 403–410. [Google Scholar] [CrossRef]
- Alef, K. Soil respiration. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 214–219. [Google Scholar]
- Thalmann, A. Zur metodik der bestimmung der dehydrogenaseactivitat im boden mittels thriphenyltetrazolium chloride (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Alef, K. Dehydrogenase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 228–231. [Google Scholar]
- Insam, H.; Goberna, M. Use of Biolog for the community level physiological profiling (CLPP) of environmental samples. Mol. Microb. Ecol. Manag. 2008, 452, 853–860. [Google Scholar]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and compost application either alone or in combination affects vegetable yield in a volcanic mediterranean soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Buscot, F. What Are Soils? In Microorganisms in Soils: Roles in Genesis and Functions; Varma, A., Buscot, F., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3. [Google Scholar] [CrossRef]
- Sinclair, T.; Rufty, T.W. Photosynthesis and Yield. In Bringing Skepticism to Crop Science; Springer Briefs in Agriculture; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Kirschbaum, M.U. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Van der Boogard, R. Vertical and horizontal development of the root system of carrots following green manure. Plant Soil 1999, 212, 145–153. [Google Scholar] [CrossRef]
- Debele, G.G.; Kassie, K.; Getachew, T. Improving carbon sequestration and yield of barley (Hordeum vulgare) through combined application of green manure and mineral fertilizers under cambisols in ethiopian highlands. In Proceedings of the EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024; EGU24-802. [Google Scholar] [CrossRef]
- Liang, K.; Wang, X.; Du, Y.; Li, G.; Wei, Y.; Liu, Y.; Li, Z.; Wei, X. Effect of Legume Green Manure on Yield Increases of Three Major Crops in China: A Meta-Analysis. Agronomy 2022, 12, 1753. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Condron, L.M.; Simpson, Z.P.; McDowell, R.W. Inclusion of Leguminous Green Manures Enhances Crop Biomass, Nutrient Uptake, Soil Phosphorus Dynamics and Bioavailability. J. Sustain. Agric. Environ. 2024, 3, e70035. [Google Scholar] [CrossRef]
- Frøseth, R.L.; Bakken, A.K.; Bleken, M.A.; Riley, H.; Pommeresche, R.; Thorup-Kristensen, K.; Hansen, S. Effects of green manure herbage management and its digestate from biogas production on barley yield, N recovery, soil structure and earthworm populations. Eur. J. Agron. 2014, 52, 90–102. [Google Scholar] [CrossRef]
- Talgre, L.; Lauringson, E.; Roostalu, K.; Astover, A.; Makke, A. Green manure as a nutrient source for succeeding crops. Plant Soil Environ. 2012, 58, 275–281. [Google Scholar] [CrossRef]
- Talgre, L. BIOMASS Production of Different Green Manure Crops and Their Effect on the Succeeding Crops Yield. Doctoral Thesis, University of Thartu, Tartu, Estonia, 2013. [Google Scholar]
- Kirchmann, H.; Bergström, L.; Kätterer, T.; Andrén, O.; Andersson, R. Can Organic Crop Production Feed the World? In Organic Crop Production—Ambitions and Limitations; Kirchmann, H., Bergström, L., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 37–72. [Google Scholar]
- Khalifa, T.H.; Mariey, S.A.; Ghareeb, Z.E.; Khatab, I.A.; Alyamani, A. Effect of organic amendments and nano-zinc foliar application on alleviation of water stress in some soil properties and water productivity of barley yield. Agronomy 2022, 12, 585. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C.; Castellini, M.; Garofalo, P.; Samarelli, I.; Lacolla, G.; Montesano, F.F.; Spagnuolo, M.; Mastrangelo, M.; Stellacci, A.M. Residual effect of compost and biochar amendment on soil chemical, biological, and physical properties and durum wheat response. Agronomy 2024, 14, 749. [Google Scholar] [CrossRef]

| Treatments | Concentration (t ha−1) | N | P | K |
|---|---|---|---|---|
| (g m−2) | ||||
| 1. Control | 0 | 0 | 0 | 0 |
| 2. Mineral fertilizer (MF, 15:15:15) | 0.1 | 6.50 | 1.50 | 1.50 |
| 3. Vermicompost (VC) | 12 | 15.20 | 13.05 | 19.54 |
| 4. Mineral fertilizer + vermicompost (MF+VC) | 0.05 + 6 | 10.85 | 7.28 | 10.50 |
| 5. Biochar (BCh) | 10 | 13.1 | 6.4 | 79.5 |
| Unit | Vermicompost | Biochar | |
|---|---|---|---|
| TOC | (%) | 17.71 ± 1.3 | 65.00 ± 4.23 |
| pH | 7.78 ± 0.01 | - | |
| EC | (µS cm−1) | 1510 ± 12.4 | - |
| Total N | (%) | 1.31 ± 0.16 | 1.31 ± 0.11 |
| P | (%) | 1.08 ± 0.08 | 0.64 ± 0.03 |
| K | (%) | 1.59 ± 0.20 | 7.95 ± 0.67 |
| Ca | (%) | 3.11 ± 0.33 | 4.28 ± 0.27 |
| Mg | (%) | 0.73 ± 0.08 | 3.06 ± 0.27 |
| Fe | (%) | 0.51 ± 0.05 | 0.13 ± 0.01 |
| pH | EC (µS cm−1) | TOC (g kg−1) | N-NH4 (mg kg−1) | N-NO3 (mg kg−1) | ||||||
| GM | F | GM | F | GM | F | GM | F | GM | F | |
| Before sowing | 8.10 ± 0.05 | 122.2 ± 2.9 | 1.04 ± 0.12 | 6.48 ± 0.53 | 7.05 ± 0.17 | |||||
| Control | 7.97 c | 8.21 a | 130.13 cd | 123.80 ef | 1.10 bc | 1.30 b | 10.13 a | 10.13 a | 13.82 ab | 16.58 a |
| MF | 7.97 c | 7.99 bc | 133.37 bc | 134.63 b | 0.82 bc | 0.63 c | 11.16 a | 11.90 a | 10.92 ab | 10.19 b |
| VC | 8.02 bc | 8.18 a | 145.77 a | 145.97 a | 2.29 a | 2.39 a | 9.19 a | 7.37 a | 10.07 b | 9.31 b |
| MF+VC | 8.01 bc | 8.17 a | 125.30 e | 130.37 bcd | 0.88 bc | 1.03 bc | 8.29 a | 9.70 a | 10.03 b | 10.49 b |
| BCh | 8.07 abc | 8.12 ab | 120.97 f | 127.47 de | 1.14 bc | 1.22 bc | 7.37 a | 8.29 a | 11.46 ab | 9.67 b |
| S.E. for comp. | 0.067 | 2.06 | 0.36 | 2.34 | 2.76 | |||||
| Crit. value comp. | 0.14 | 4.30 | 0.74 | 4.88 | 5.76 | |||||
| TN (mg kg−1) | TP (mg kg−1) | TK (mg kg−1) | Soil humidity (%) | |||||||
| GM | F | GM | F | GM | F | GM | F | |||
| Before sowing | 13.52 ± 0.83 | 177.62 ± 12.05 | 298.31 ± 25.74 | 28.4 ± 1.1 | ||||||
| Control | 23.96 ab | 26.71 a | 197.59 de | 272.69 bc | 292.75 e | 355.43 cd | 30.2 bc | 28.4 bc | ||
| MF | 22.08 abc | 22.08 abc | 269.10 bcd | 357.88 a | 343.48 d | 457.60 a | 28.2 bc | 23.4 c | ||
| VC | 19.26 bc | 16.69 c | 275.50 bc | 307.75 ab | 365.48 bcd | 395.4 bc | 38.8 a | 33.4 ab | ||
| MF+VC | 18.32 bc | 20.20 abc | 155.06 e | 165.38 e | 367.88 bcd | 400.35 bc | 35.0 ab | 32.3 ab | ||
| BCh | 18.82 bc | 17.96 bc | 222.81 cde | 210.13 cde | 362.8 cd | 410.65 b | 31.7 abc | 29.3 bc | ||
| S.E. for comp. | 3.55 | 36.51 | 22.54 | 1.05 | ||||||
| Crit. value comp. | 7.24 | 74.56 | 46.03 | 2.13 | ||||||
| chl a | chl b | chl a+b | car | chl a/b | chl a+b/car | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | F | GM | F | GM | F | GM | F | GM | F | GM | F | |
| Control | 1.92 c | 1.44 c | 1.92 b | 0.84 c | 3.84 b | 2.29 c | 1.72 b | 0.95 b | 1.00 b | 1.71 a | 2.24 b | 2.41 b |
| MF | 3.09 a | 1.50 c | 2.01 a | 0.95 c | 5.10±a | 2.45 c | 2.28 a | 1.05 b | 1.54 a | 1.59 b | 2.23 b | 2.33 b |
| VC | 1.74 c | 1.32 c | 1.73 b | 0.77 d | 3.47 c | 2.09 d | 1.60 c | 1.03 b | 0.99 b | 1.72 a | 2.17 c | 2.03 c |
| MF+VC | 2.36 b | 2.66 a | 1.6 c | 1.84 a | 3.96 b | 4.51 a | 1.63 c | 1.90 a | 1.47 a | 1.45 c | 2.44 b | 2.37 b |
| BCh | 3.07 a | 2.13 b | 2.09 a | 1.44 b | 5.16 a | 3.57 b | 1.81 b | 0.77 c | 1.47 a | 1.48 c | 2.85 a | 4.64 a |
| Soil Respiration (μg C-CO2 g−1 h−1) | Dehydrogenase Activity (μg TPF g−1 h−1) | |||
|---|---|---|---|---|
| GM | F | GM | F | |
| Control | 2.82 bcd | 2.25 e | 0.28 c | 0.32 bc |
| MF | 2.67 d | 2.70 cd | 0.34 b | 0.32 bc |
| VC | 4.19 a | 2.89 bcd | 0.41 a | 0.46 a |
| MF+VC | 3.15 b | 2.90 bcd | 0.36 b | 0.42 a |
| BCh | 3.05 bc | 2.82 bcd | 0.28 c | 0.31 bc |
| S.E. for comp. | 0.17 | 0.025 | ||
| Crit. value for comp. | 0.35 | 0.052 | ||
| Fields | Treatm. | Aminoacids | Amines & Amides | Charbohydrates | Carboxylic Acids | Phenolic Compounds | Polymers |
|---|---|---|---|---|---|---|---|
| After GM | Control | 2.34 cd | 1.53 b | 2.42 a | 2.21 cd | 1.13 bc | 2.68 ab |
| MF | 2.58 ab | 1.60 b | 2.17 ab | 2.47 abc | 1.82 a | 2.35 bc | |
| VC | 2.23 d | 1.15 b | 1.76 d | 2.29 bcd | 1.59 ab | 2.46 bc | |
| MF+VC | 2.63 a | 1.44 b | 2.06 bc | 2.46 abc | 1.53 ab | 2.28 c | |
| BCh | 2.32 d | 1.43 b | 2.06 bc | 2.05 d | 0.82 c | 2.58 abc | |
| After Fallow | Control | 2.62 a | 2.96 a | 1.81 cd | 2.37 bcd | 2.00 a | 2.53 bc |
| MF | 2.39 bcd | 1.96 b | 2.45 a | 2.60 ab | 1.63 ab | 2.91 a | |
| VC | 2.37 bcd | 1.60 b | 2.03 bcd | 2.30 bcd | 1.51 ab | 2.40 bc | |
| MF+VC | 2.55 abc | 1.17 b | 2.10 b | 2.14 cd | 1.64 ab | 2.43 bc | |
| BCh | 2.72 a | 1.07 b | 2.42 a | 2.80 a | 1.65 ab | 2.57 abc | |
| S.E. for comparison | 0.1 | 0.45 | 0.14 | 0.17 | 0.29 | 0.18 | |
| Critical value for comp. | 0.22 | 0.93 | 0.29 | 0.37 | 0.6 | 0.37 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilev, S.; Yanev, M.; Petrova, S.; Minev, N.; Popova, V.; Neykova, I.; Mitkov, A.; Szulc, W.; Yordanov, Y. Green Manuring Reduces Agronomic Indicators of Fodder Winter Barley Regardless of Fertilization Type. Agriculture 2025, 15, 2145. https://doi.org/10.3390/agriculture15202145
Shilev S, Yanev M, Petrova S, Minev N, Popova V, Neykova I, Mitkov A, Szulc W, Yordanov Y. Green Manuring Reduces Agronomic Indicators of Fodder Winter Barley Regardless of Fertilization Type. Agriculture. 2025; 15(20):2145. https://doi.org/10.3390/agriculture15202145
Chicago/Turabian StyleShilev, Stefan, Mariyan Yanev, Slaveya Petrova, Nikolay Minev, Vanya Popova, Ivelina Neykova, Anyo Mitkov, Wiesław Szulc, and Yordan Yordanov. 2025. "Green Manuring Reduces Agronomic Indicators of Fodder Winter Barley Regardless of Fertilization Type" Agriculture 15, no. 20: 2145. https://doi.org/10.3390/agriculture15202145
APA StyleShilev, S., Yanev, M., Petrova, S., Minev, N., Popova, V., Neykova, I., Mitkov, A., Szulc, W., & Yordanov, Y. (2025). Green Manuring Reduces Agronomic Indicators of Fodder Winter Barley Regardless of Fertilization Type. Agriculture, 15(20), 2145. https://doi.org/10.3390/agriculture15202145









