Bio-Efficiency of Foliar Herbicides Applied with Drift-Reducing Nozzles
Abstract
1. Introduction
2. Materials and Methods
2.1. Dose–Response Experiments
2.2. Measurements
2.2.1. Plant Response
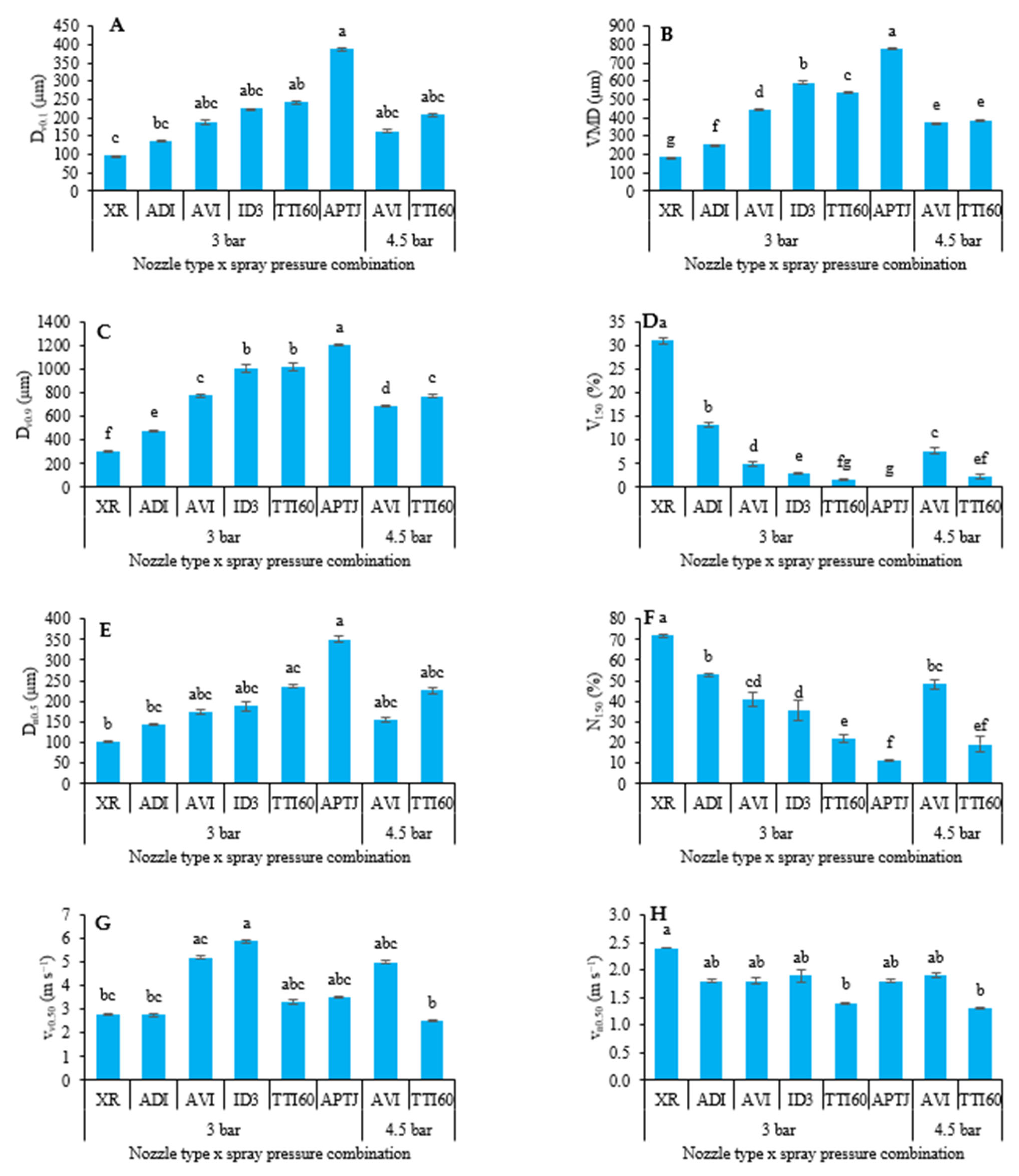
2.2.2. Droplet Size and Velocity Characteristics
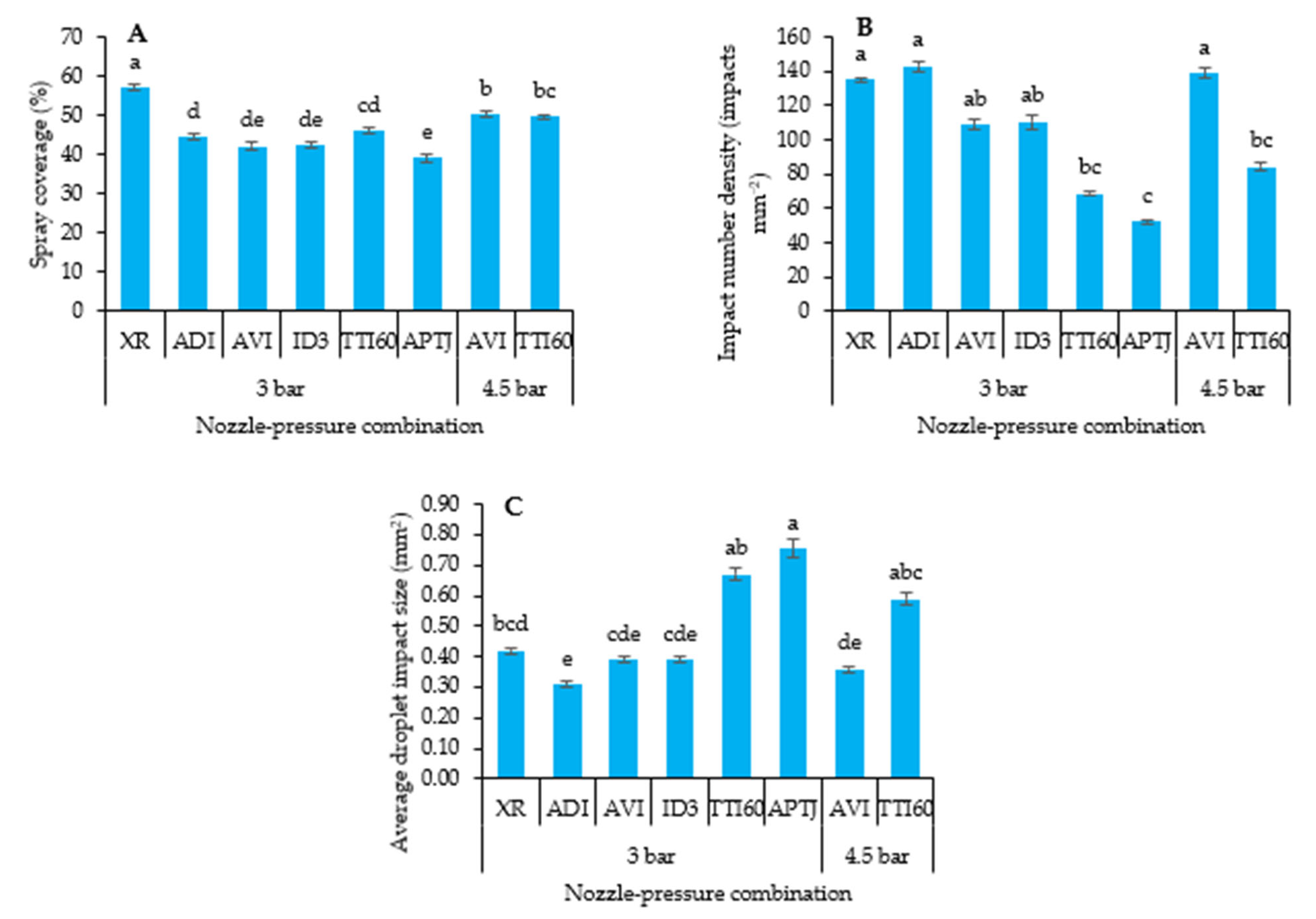
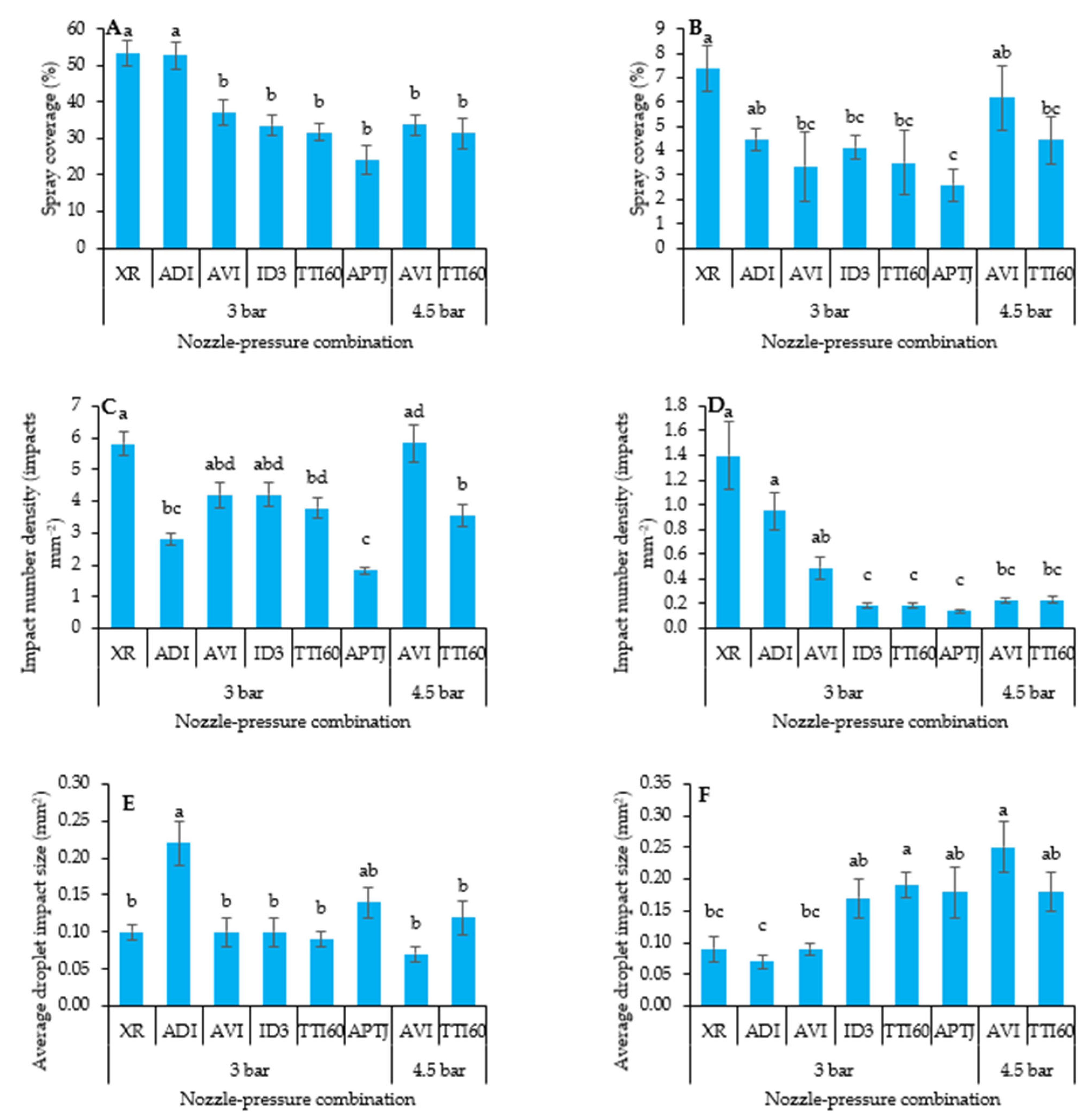
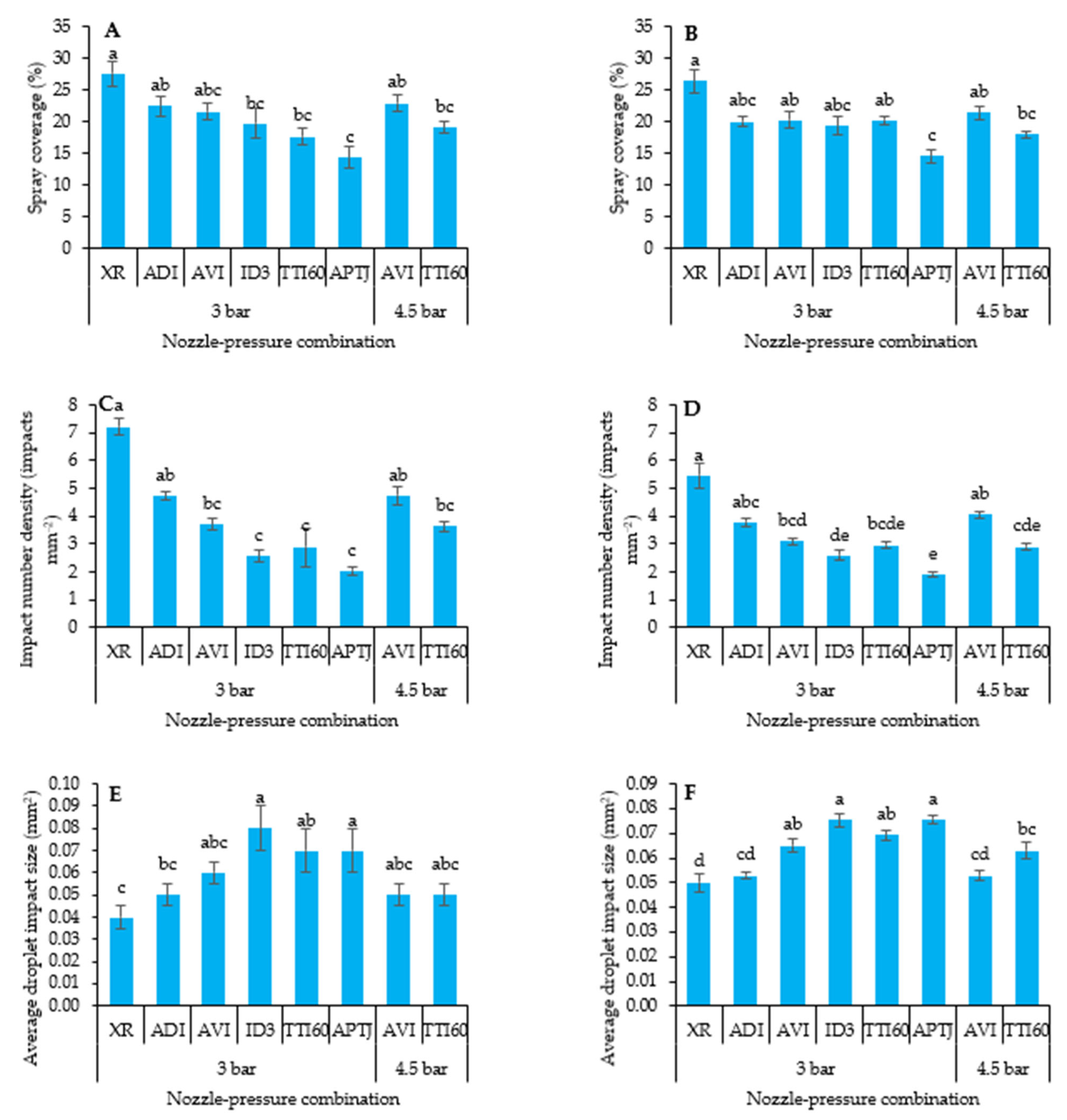
2.2.3. Spray Coverage Characteristics
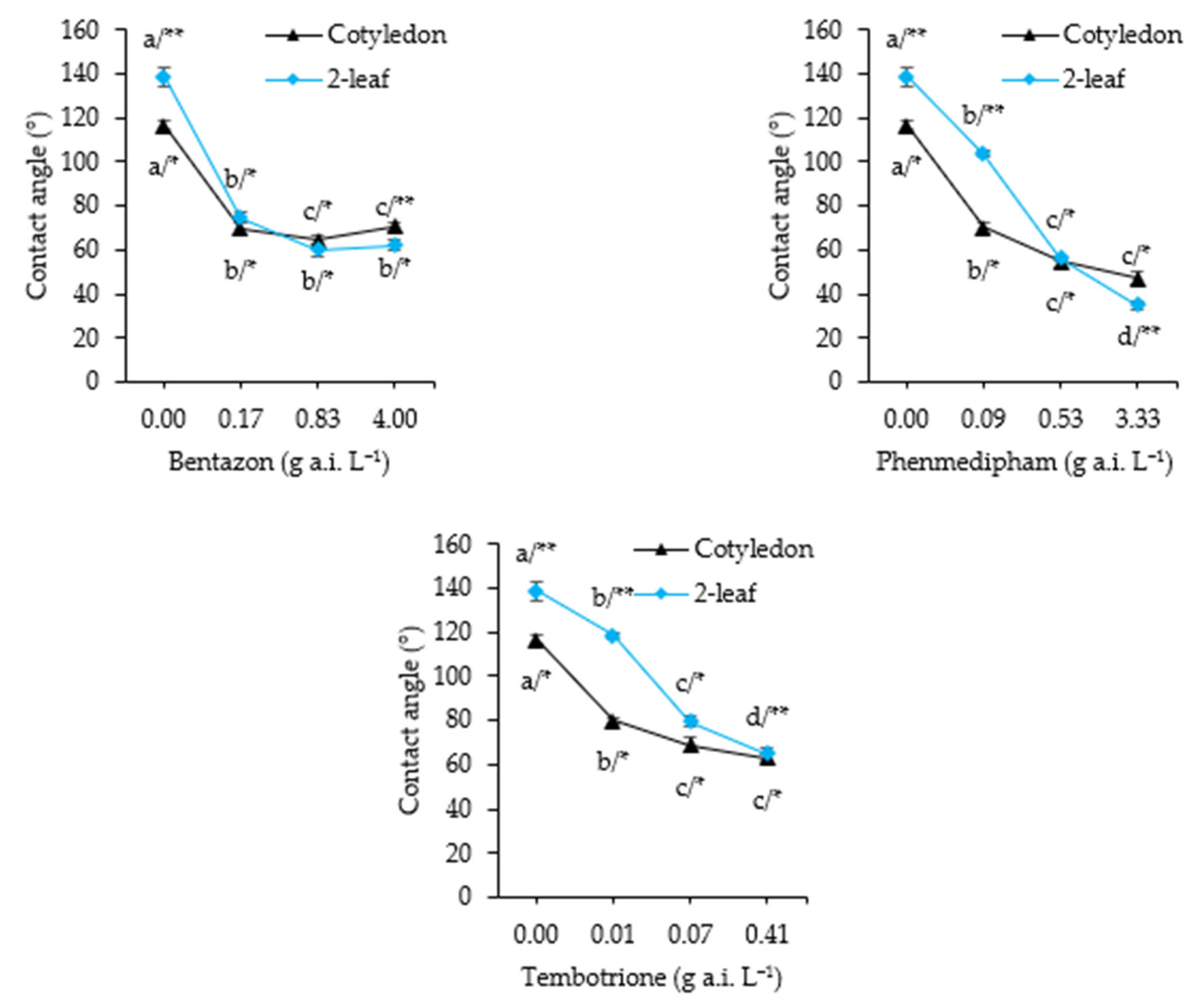
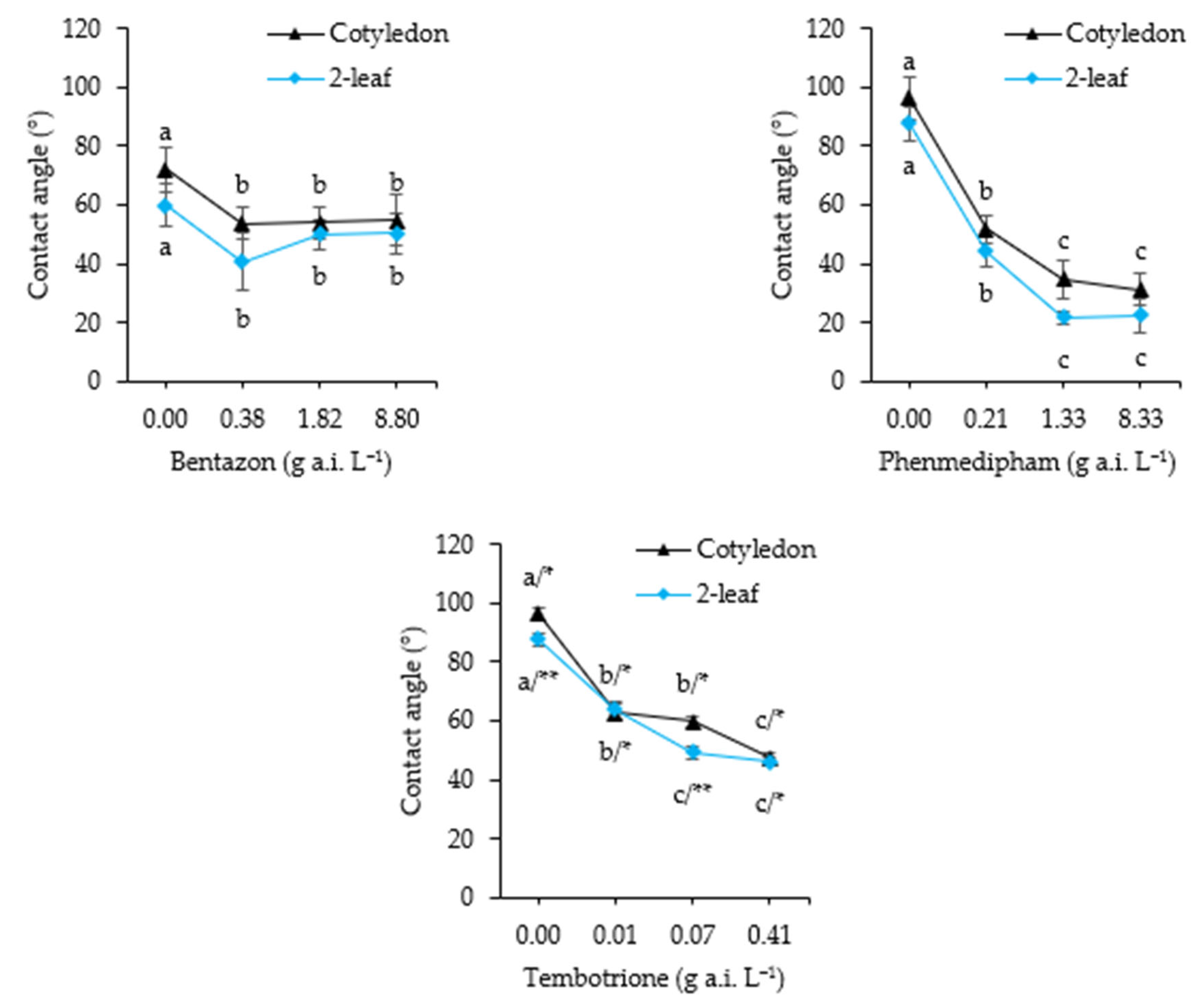
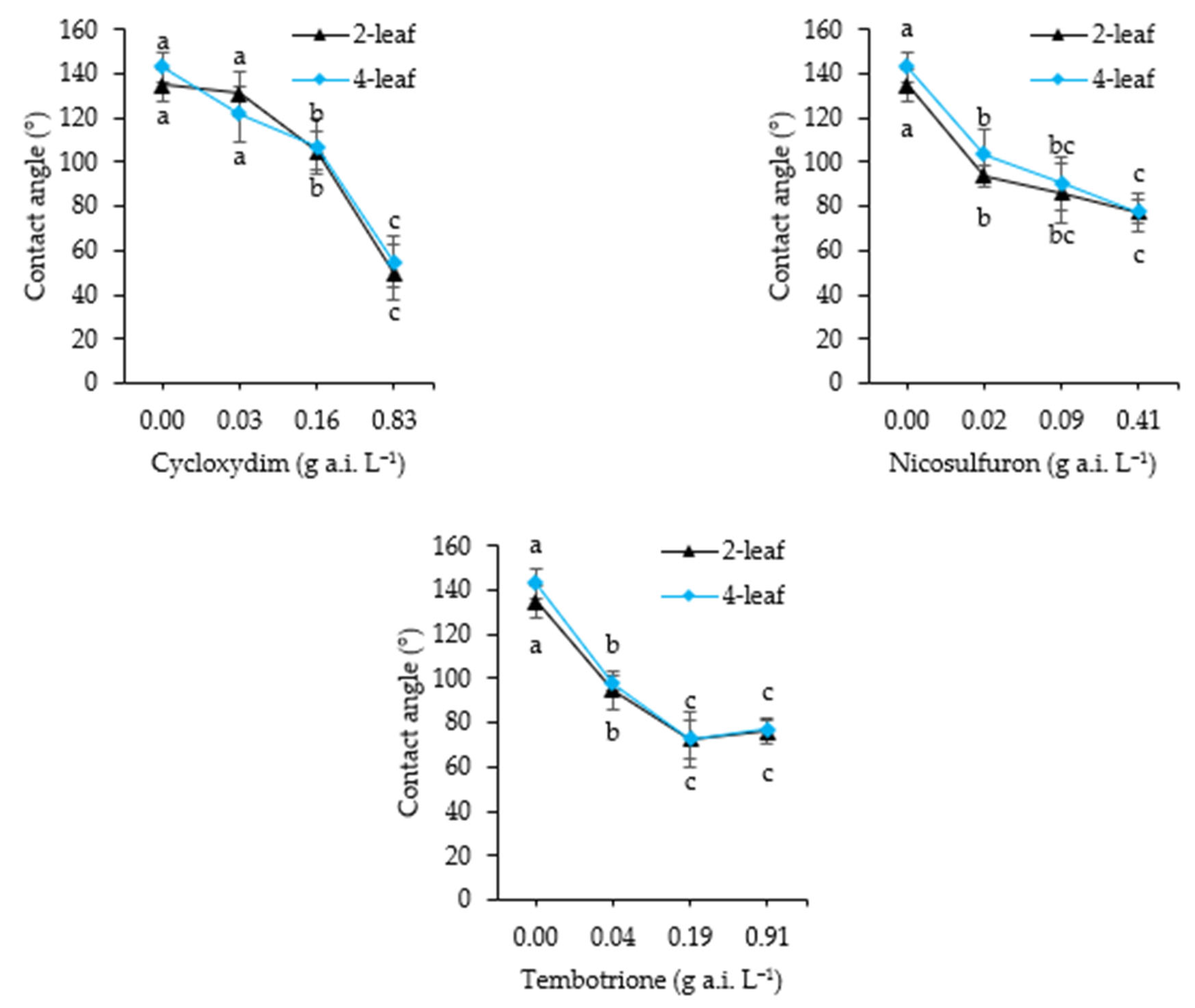
2.2.4. Contact Angle
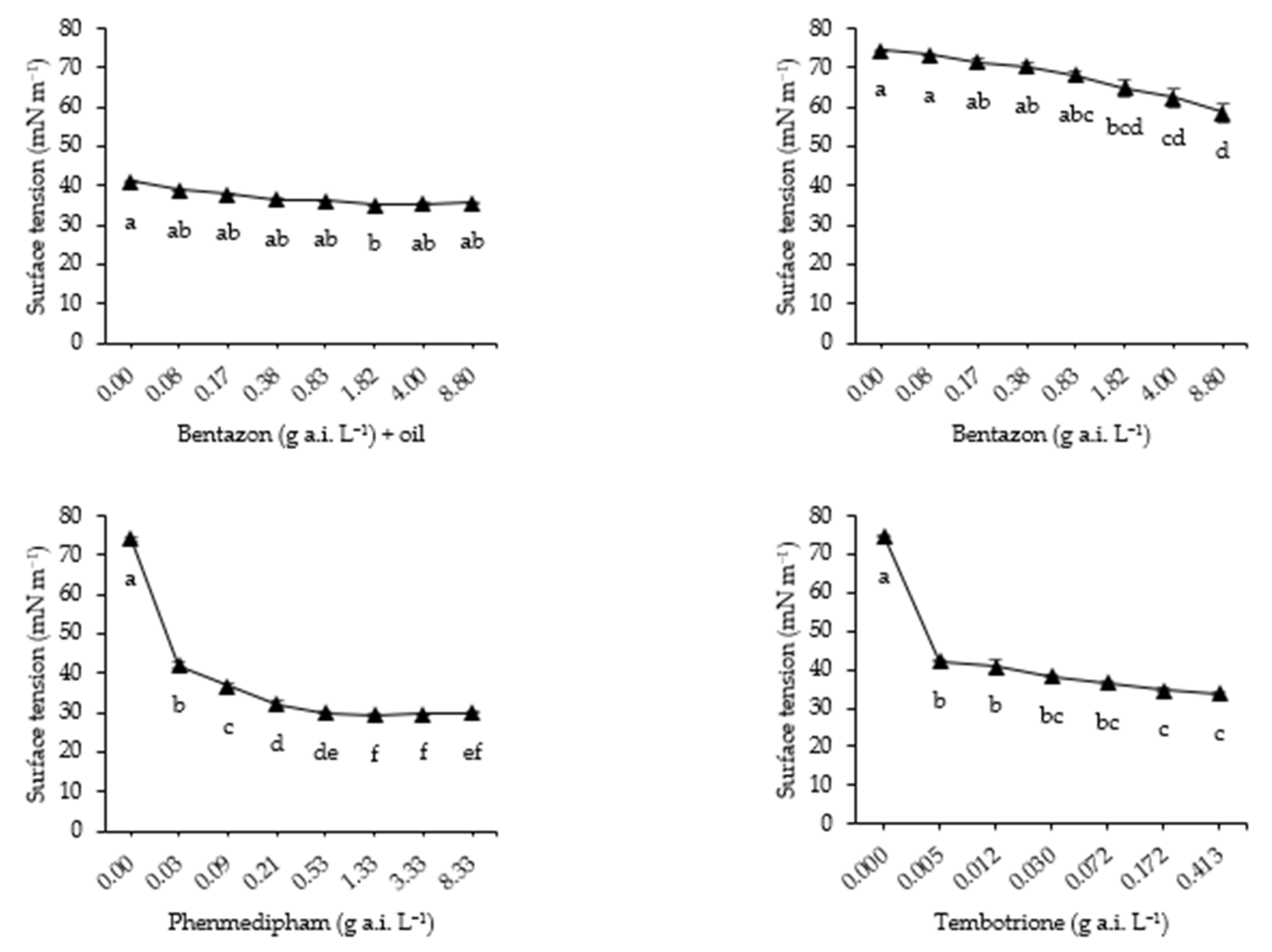
2.2.5. Surface Tension
2.3. Statistical Analysis
3. Results
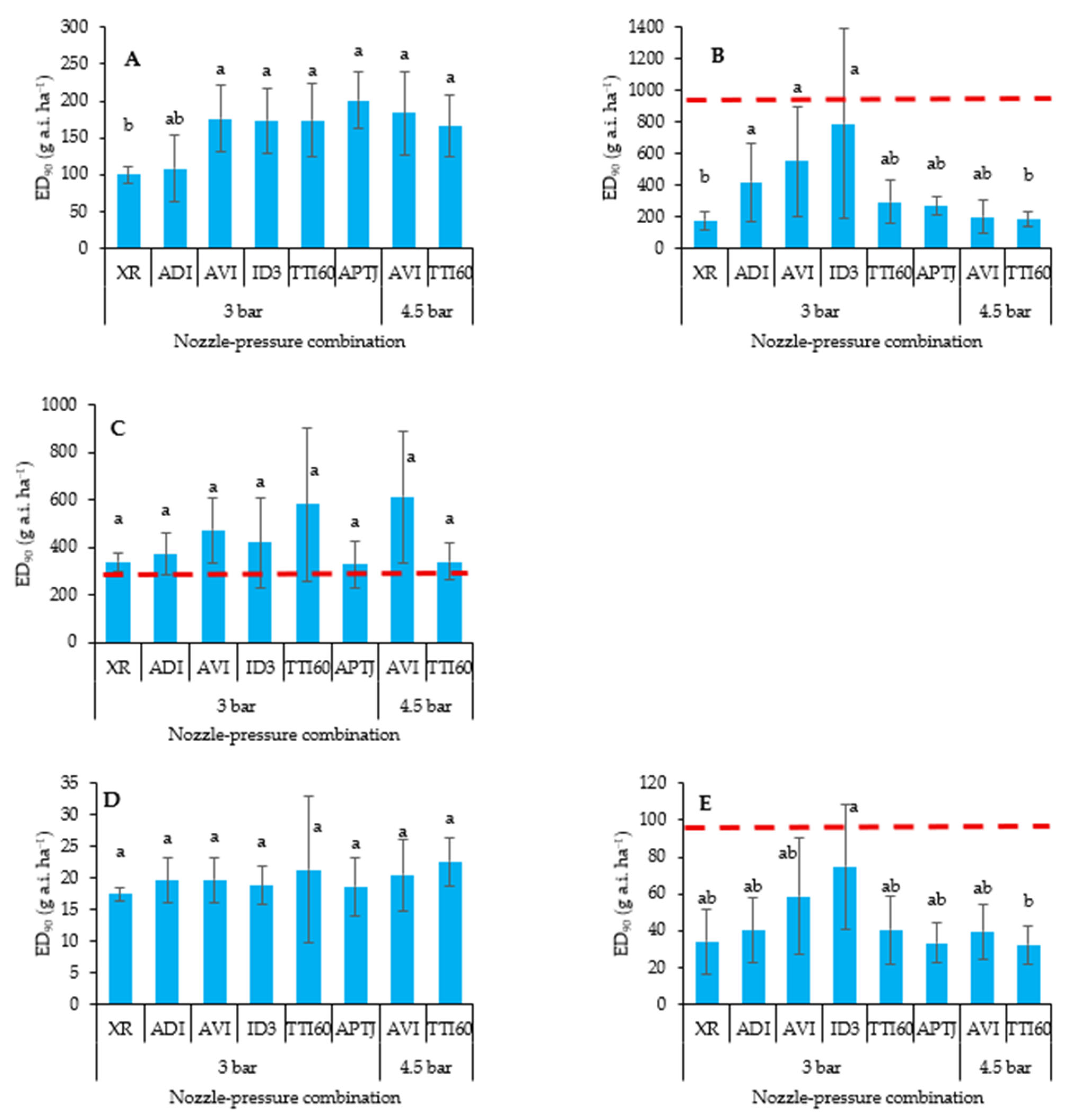
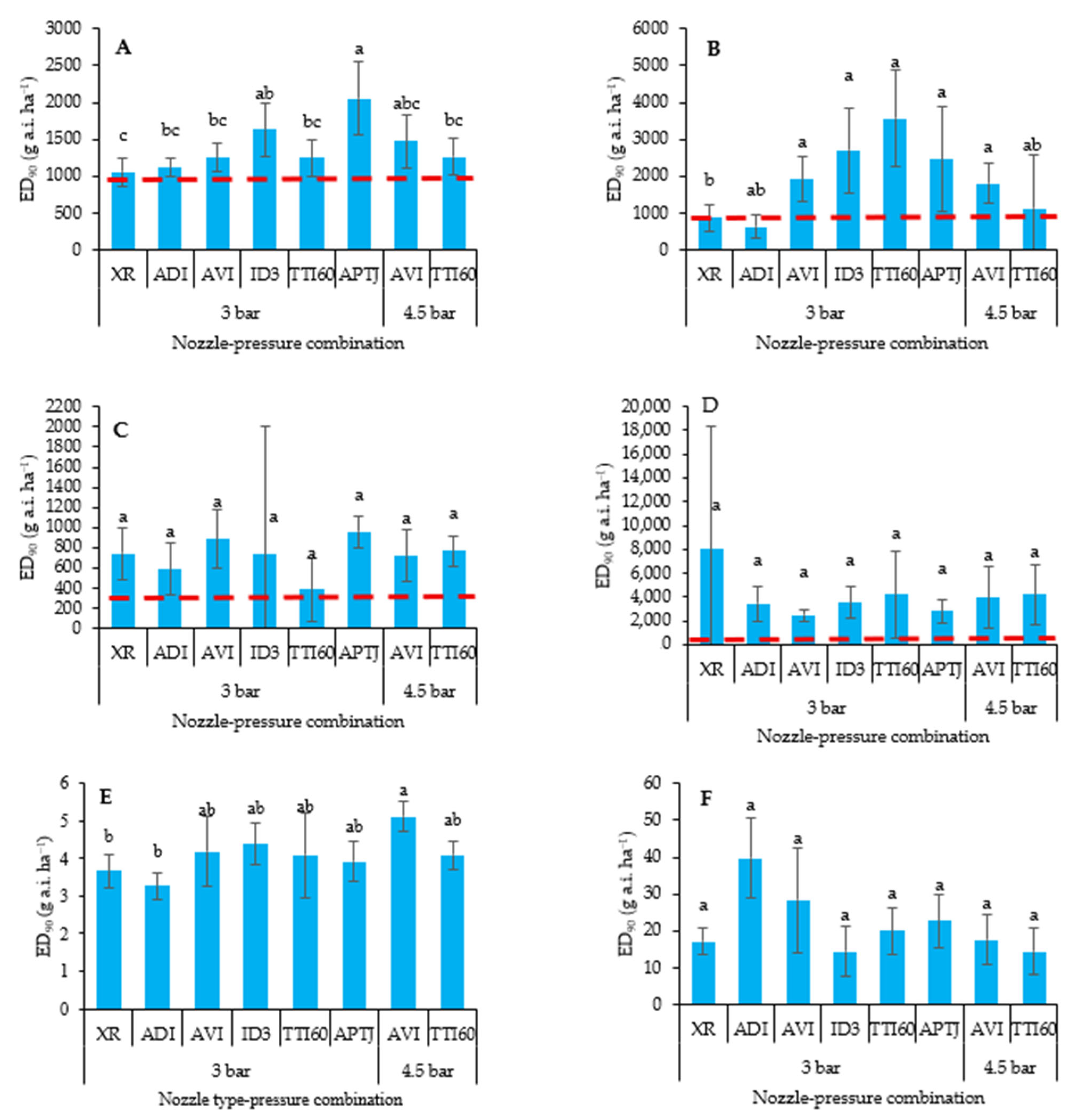
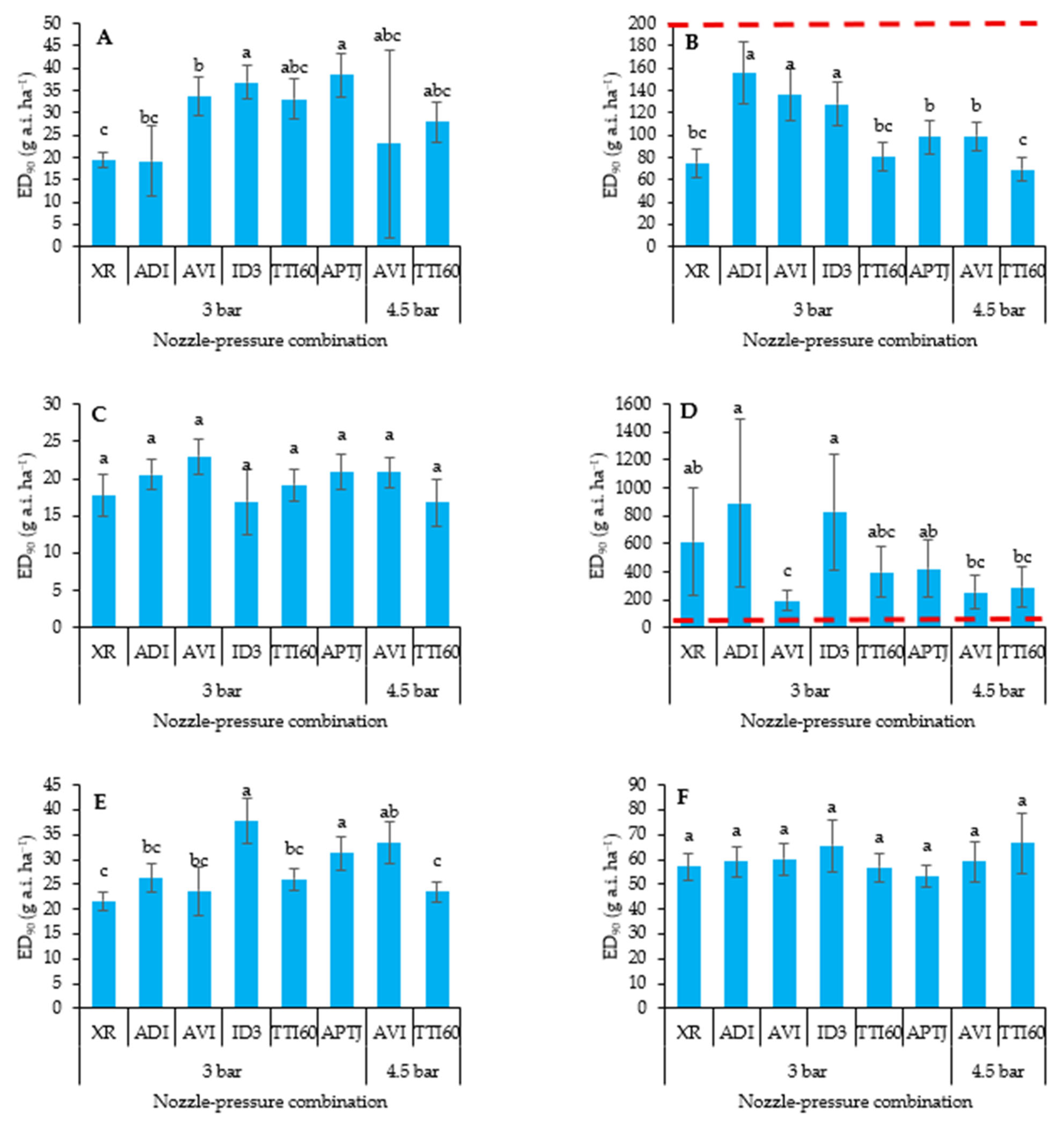
3.1. Dry Biomass Reduction
3.2. Droplet Size and Velocity Characteristics
3.3. Spray Coverage
3.3.1. Spray Coverage on WSP
3.3.2. Kaolinite Spray Coverage on Plant Leaves
3.4. Contact Angle
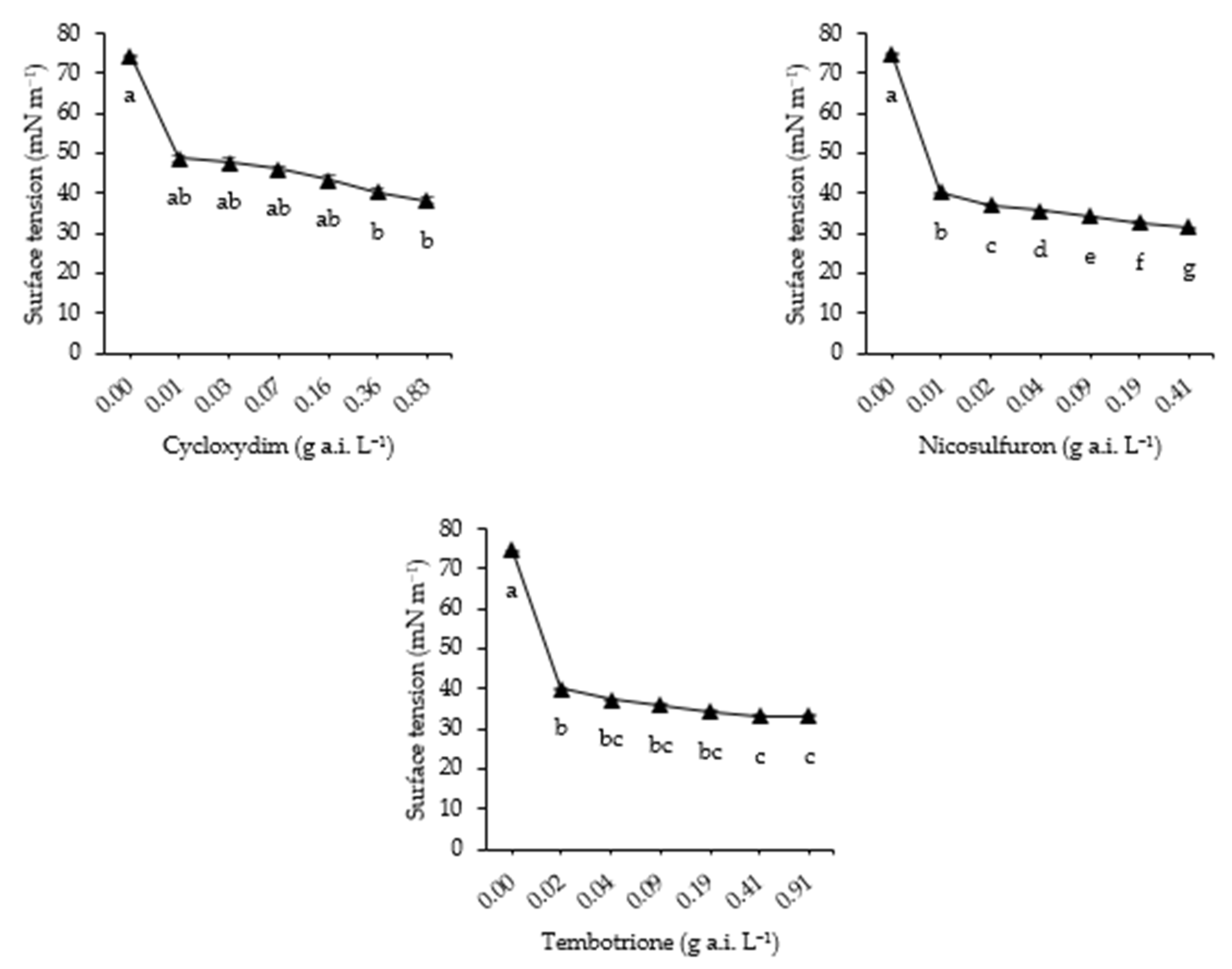
3.5. Surface Tension
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudsk, P. Optimising Herbicide Performance. In Weed Management Handbook, 9th ed.; Blackwell Science: West Sussex, UK, 2002; pp. 323–344. [Google Scholar]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Nuyttens, D.; De Schampheleire, M.; Baetens, K.; Sonck, B. The Influence of Operator-Controlled Variables on Spray Drift from Field Crop Sprayers. Trans. ASABE 2007, 50, 1129–1140. [Google Scholar] [CrossRef]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide Impact on Non-Target Plant Reproduction: What Are the Toxicological and Ecological Implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Dexter, A.G. Herbicide Spray Drift. In North Dakota State University Extension Service; (Revised 1995); NDSU Extension Service EXT A-657: Fargo, ND, USA, 1993; p. 11. [Google Scholar]
- Vieira, B.C.; Luck, J.D.; Amundsen, K.L.; Werle, R.; Gaines, T.A.; Kruger, G.R. Herbicide Drift Exposure Leads to Reduced Herbicide Sensitivity in Amaranthus spp. Sci. Rep. 2020, 10, 2146. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.J.; Priest, C.D.; Brayden, B.H.; Hanzas, J.P.; Arpino, M.R.; Richardson, L.; Stryker, J.; Banman, C.; Rodney, S.I.; Chapple, A.; et al. A Field Spray Drift Study to Determine the Downwind Effects of Isoxaflutole Herbicide to Nontarget Plants. Integr. Environ. Assess. Manag. 2021, 18, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Agentschap Landbouw & Zeevisserij IPM Richtlijnen-Checklist 2024. Belgisch Staatsblad 2024; Ministrieel Besluit 2 Februari 2024 tot Wijziging van het Ministrieel Besluit van 1 April 2021 tot Vaststelling van de Driftreducerende Middelen of Maatregelen., 48. Available online: https://lv.vlaanderen.be/sites/lv/files/2024-07/IPM_richtlijnen_checklist_2024_juli.pdf (accessed on 9 October 2025).
- FOD VVVL. Dienst Gewasbeschermingsmiddelen en Meststoffen Bescherming van het Oppervlaktewater bij het Gebruik van Gewasbeschermingsmiddelen. Available online: https://fytoweb.be/sites/default/files/guide/attachments/bescherming_van_het_oppervlaktewater_20240701.pdf (accessed on 31 August 2025).
- Nuyttens, D.; Taylor, W.A.; De Schampheleire, M.; Verboven, P.; Dekeyser, D. Influence of Nozzle Type and Size on Drift Potential by Means of Different Wind Tunnel Evaluation Methods. Biosyst. Eng. 2009, 103, 271–280. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Recent Developments in Our Understanding of the Plant Cuticle as a Barrier to the Foliar Uptake of Pesticides†. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Taylor, F.E.; Davies, L.G.; Cobb, A.H. An Analysis of the Epicuticular Wax of Chenopodium album Leaves in Relation to Environmental Change, Leaf Wettability and the Penetration of the Herbicide Bentazone. Ann. Appl. Biol. 1981, 98, 471–478. [Google Scholar] [CrossRef]
- Sanyal, D.; Bhowmik, P.C.; Reddy, K.N. Influence of Leaf Surface Micromorphology, Wax Content, and Surfactant on Primisulfuron Droplet Spread on Barnyardgrass (Echinochloa crus-galli) and Green Foxtail (Setaria viridis). Weed Sci. 2006, 54, 627–633. [Google Scholar] [CrossRef]
- Kudsk, P.; Kristensen, J.L. Effect of Environmental Factors on Herbicide Performance. Proc. First Int. Weed Control Congr. 1992, 1, 173–186. [Google Scholar]
- Ramsdale, B.K.; Messersmith, C.G. Drift-Reducing Nozzle Effects on Herbicide Performance 1. Weed Technol. 2001, 15, 453–460. [Google Scholar] [CrossRef]
- Creech, C.F. Herbicide Application Technology Impacts on Herbicide Spray Characteristics and Performance; University of Nebraska: Lincoln, NE, USA, 2015. [Google Scholar]
- De Cauwer, B.; De Meuter, I.; De Ryck, S.; Dekeyser, D.; Zwertvaegher, I.; Nuyttens, D. Performance of Drift-Reducing Nozzles in Controlling Small Weed Seedlings with Contact Herbicides. Agronomy 2023, 13, 1342. [Google Scholar] [CrossRef]
- ISO 5682-1:2017; Equipment for Crop Protection—Spraying Equipment—Part 1: Test Methods for Sprayer Nozzles. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/60053.html (accessed on 24 August 2025).
- Kashdan, J.T.; Shrimpton, J.S.; Whybrew, A. Two-Phase Flow Characterization by Automated Digital Image Analysis. Part 2: Application of PDIA for Sizing Sprays. Part Part Syst. Charact. 2004, 21, 15–23. [Google Scholar] [CrossRef]
- Nuyttens, D.; Baetens, K.; De Schampheleire, M.; Sonck, B. Effect of Nozzle Type, Size and Pressure on Spray Droplet Characteristics. Biosyst. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- ISO 25358:2018(En); Crop Protection Equipment—Droplet-Size Spectra from Atomizers—Measurement and Classification. ISO: Geneva, Switzerland, 2018.
- Laurén, S. Agrochemicals—How Do Surface Tension and Wettability Affect the Efficiency? Available online: https://www.biolinscientific.com/blog/agrochemicals-how-do-surface-tension-and-wettability-affect-the-efficiency (accessed on 11 February 2025).
- Ebnesajjad, S. Surface Tension and Its Measurement. In Handbook of Adhesives and Surface Preparation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–30. ISBN 978-1-4377-4461-3. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; ISBN 3-900051-07-0. [Google Scholar]
- Ritz, C.; Streibig, J.C. Bioassay Analysis Using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudemo, M.; Jensen, J.E. Dose-Response Curves and Statistical Models. In Herbicide Bioassays; CRC Press: Boca Raton, FL, USA, 1993; pp. 29–55. [Google Scholar]
- Butler Ellis, M.C.; Bradley, A. The Influence of Formulation on Spray Drift. In International Advances in Pesticide Application; Aspects of Applied Biology: Warwick, UK, 2002; pp. 251–258. [Google Scholar]
- Massinon, M.; De Cock, N.; Forster, W.A.; Nairn, J.J.; McCue, S.W.; Zabkiewicz, J.A.; Lebeau, F. Spray Droplet Impaction Outcomes for Different Plant Species and Spray Formulations. Crop Prot. 2017, 99, 65–75. [Google Scholar] [CrossRef]
- Zwertvaegher, I.K.; Verhaeghe, M.; Brusselman, E.; Verboven, P.; Lebeau, F.; Massinon, M.; Nicolaï, B.M.; Nuyttens, D. The Impact and Retention of Spray Droplets on a Horizontal Hydrophobic Surface. Biosyst. Eng. 2014, 126, 82–91. [Google Scholar] [CrossRef]
- Gao, Z.-M.; Hu, W.; Dong, X.-Y.; Zhao, X.-Y.; Wang, S.; Chen, J.; Qiu, B.-J. Motion Behavior of Droplets on Curved Leaf Surfaces Driven by Airflow. Front. Plant Sci. 2024, 15, 1450831. [Google Scholar] [CrossRef] [PubMed]
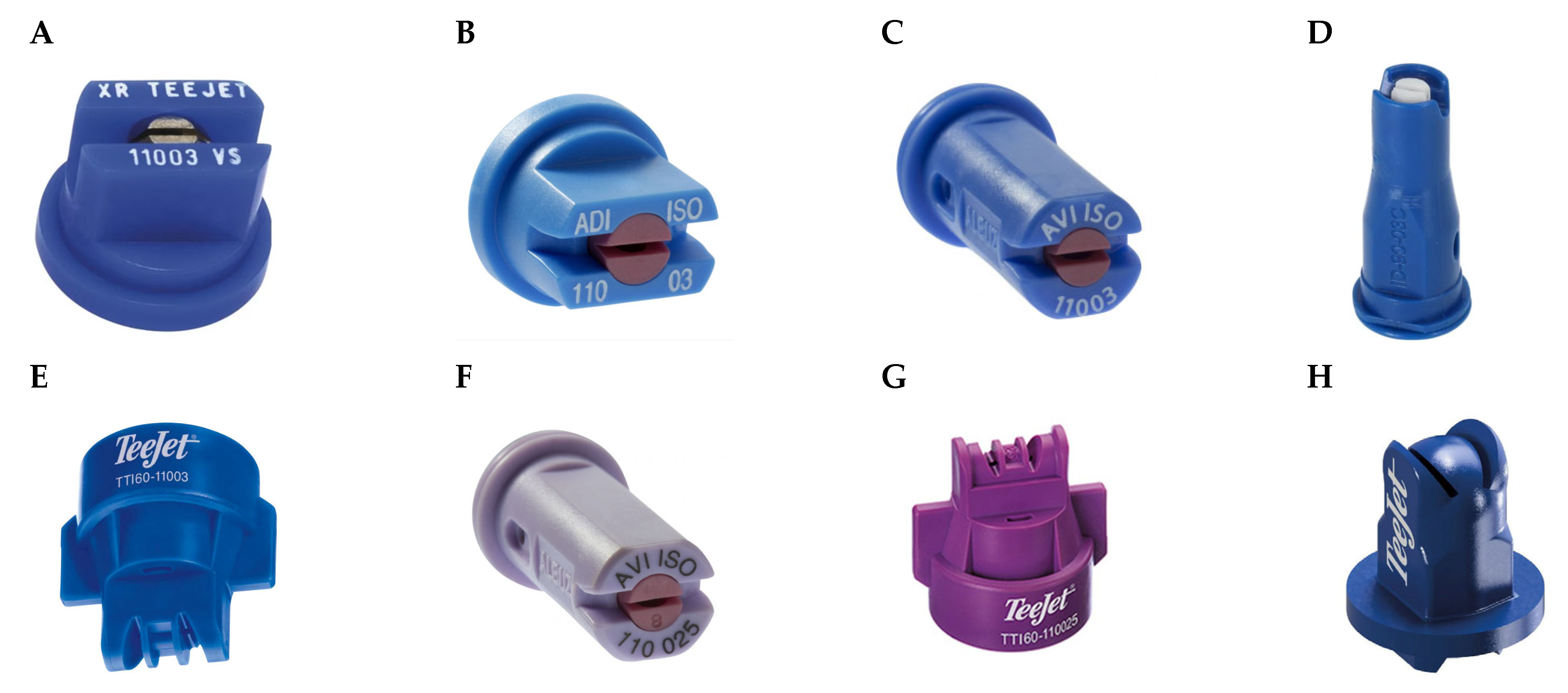
| Herbicide (Product Name, Concentration, Formulation, Supplier) | Herbicide Mode of Action (HRAC/WSSA-Group/ Legacy HRAC) | Herbicide Doses (g a.i. L−1) |
|---|---|---|
| Bentazon 1 (Basagran, 87% a.i., SG, BASF Agricultural Solutions) | Photosystem II-Inhibitor (6/C3) | 0.000–0.078–0.171–0.376–0.826–1.818–4.000 2 0.000–0.171–0.376–0.826–1.818–4.000–8.800 3 |
| Phenmedipham (Astrix, 160 g a.i. L−1, EC, UPL Europe Ltd.) | Photosystem II-Inhibitor (5/C1) | 0.000–0.034–0.085–0.213–0.533–1.333–3.333 2 0.000–0.085–0.213–0.533–1.333–3.333–8.333 3 |
| Tembotrione (Laudis, 44 g a.i. L−1, OD, Bayer Crop Science) | HPPD-inhibitor (27/F2) | 0.000–0.005–0.012–0.030–0.072–0.172–0.413 2, 3 0.000–0.018–0.039–0.085–0.188–0.413–0.908 4 |
| Cycloxydim (Focus plus, 100 g a.i. L−1, EC, BASF Agricultural Solutions) | ACCase-inhibitor (1/A) | 0.000–0.013–0.030–0.068–0.158–0.362–0.830 4 |
| Nicosulfuron (Samson Extra 60, 60 g a.i. L−1, OD, ISK Biosciences Europe) | ALS-inhibitor (2/B) | 0.000–0.008–0.018–0.039–0.085–0.188–0.413 4 |
| Nozzle Type | Manufacturer | Description | Drift Reduction Class (%) 1 | Spray Pressure (Bar) | Belt Speed (km h−1) |
|---|---|---|---|---|---|
| XR 110 03 | TeeJet | Standard single 110° flat-fan | 0 | 3 | 3.35 |
| ADI 110 03 | Albuz | Pre-orifice single 110° flat-fan | 50 | 3 | 5.35 |
| AVI 110 03 | Albuz | Air induction single 110° flat-fan | 75 | 3 | 3.98 |
| ID3 120 03 | Lechler | Air induction single 120° flat-fan | 90 | 3 | 4.60 |
| TTI60 110 03 | TeeJet | Air induction dual 110° flat-fan with 30° forward and backward angle | 90 | 3 | 2.81 |
| AVI 110 025 | Albuz | Air induction single 110° flat-fan | 75 | 4.5 | 4.00 |
| TTI60 110 025 | TeeJet | Air induction dual 110° flat-fan with 30° forward and backward angle | 75 | 4.5 | 2.97 |
| APTJ 110 03 | TeeJet | Non-air induction dual 110° flat-fan with 30° forward and backward angle | ? 2 | 3 | 2.76 |
| Weed Species (Experiment) | Growth Stage 1 | Date | ||
|---|---|---|---|---|
| Sowing | Spraying | Harvesting | ||
| C. album (Exp. 1) | BBCH10 | 26 May 2023 | 6 June 2023 | 22 June 2023 (bentazon, phenmedipham) 26 June 2023 (tembotrione) |
| BBCH12 | 17 May 2023 | |||
| S. nigrum (Exp. 2) | BBCH10 | 29 June 2023 | 13 July 2023 | 4 August 2023 (bentazon, phenmedipham) 7 August 2023 (tembotrione) |
| BBCH12 | 21 June 2023 | |||
| E. crus-galli (Exp. 3) | BBCH12 | 4 August 2023 | 16 August 2023 | 7 September 2023 |
| BBCH14 | 27 July 2023 | |||
| Weed Species | Timing | Experimental Period 1 | Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Avg. | Min. | Max. | Avg. | |||
| C. album | Pre-application | BBCH12: 17 May–5 June BBCH10: 26 May–5 June | 8.3 9.6 | 20.2 21.8 | 14.4 15.6 | 52.3 46.6 | 91.0 88.0 | 71.9 68.1 |
| Day of application | 06 June | 10.8 | 16.8 | 24.1 | 56.4 | 91.0 | 75.3 | |
| Post-application | 6 June–22 June (bentazon; phenmedipham) 6 June–26 June (tembotrione) | 14.3 14.3 | 27.3 27.2 | 20.9 20.9 | 46.3 46.3 | 88.1 88.9 | 66.8 67.4 | |
| S. nigrum | Pre-application | BBCH12: 21 June–12 July BBCH10: 29 June–12 July | 13.9 13.5 | 25.0 24.8 | 19.4 19.0 | 52.8 51.4 | 92.3 92.3 | 73.0 72.3 |
| Day of application | 13 July | 13.6 | 23.5 | 18.2 | 50.8 | 91.0 | 72.1 | |
| Post-application | 13 July–4 August (bentazon; phenmedipham) 13 July–7 August (tembotrione) | 14.0 13.6 | 22.0 21.5 | 17.6 17.3 | 60.9 61.7 | 93.3 93.4 | 79.2 79.8 | |
| E. crus-galli | Pre-application | BBCH14: 27 July–15 August BBCH12: 04 August–15 August | 13.0 20.7 | 21.7 28.9 | 17.5 17.5 | 65.1 61.9 | 95.5 | 83.0 |
| Day of application | 16 August | 13.2 | 18.9 | 23.9 | 64.0 | 97.6 | 81.1 | |
| Post-application | 16 August–7 September | 14.1 | 25.1 | 19.5 | 66.2 | 100.0 | 86.7 | |
| ImageJ Function | Parameters | Spray Coverage on WSP | Spray Coverage on Plant Leaves | |
|---|---|---|---|---|
| Blue Area | Total Leaf Area | White Area | ||
| Color threshold | Hue | 0–255 | 0–255 | 0–221 |
| Saturation | 0–155 | 0–183 | 0–32 or 0–51 | |
| Brightness | 0–255 | 64–255 | 125–255 | |
| Analyse particles | Pixel size | 0–infinity | 350–infinity | [10–250]-infinity |
| Circularity | 0.01–1 | 0.1–1 | 0.01–1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Ryck, S.; Van Hecke, E.; Zwertvaegher, I.; Nuyttens, D.; Vanwijnsberghe, J.; Zewdie, T.A.; Verboven, P.; De Meester, M.; De Cauwer, B. Bio-Efficiency of Foliar Herbicides Applied with Drift-Reducing Nozzles. Agriculture 2025, 15, 2115. https://doi.org/10.3390/agriculture15202115
De Ryck S, Van Hecke E, Zwertvaegher I, Nuyttens D, Vanwijnsberghe J, Zewdie TA, Verboven P, De Meester M, De Cauwer B. Bio-Efficiency of Foliar Herbicides Applied with Drift-Reducing Nozzles. Agriculture. 2025; 15(20):2115. https://doi.org/10.3390/agriculture15202115
Chicago/Turabian StyleDe Ryck, Sander, Eline Van Hecke, Ingrid Zwertvaegher, David Nuyttens, Jan Vanwijnsberghe, Tewodros Andargie Zewdie, Pieter Verboven, Mattie De Meester, and Benny De Cauwer. 2025. "Bio-Efficiency of Foliar Herbicides Applied with Drift-Reducing Nozzles" Agriculture 15, no. 20: 2115. https://doi.org/10.3390/agriculture15202115
APA StyleDe Ryck, S., Van Hecke, E., Zwertvaegher, I., Nuyttens, D., Vanwijnsberghe, J., Zewdie, T. A., Verboven, P., De Meester, M., & De Cauwer, B. (2025). Bio-Efficiency of Foliar Herbicides Applied with Drift-Reducing Nozzles. Agriculture, 15(20), 2115. https://doi.org/10.3390/agriculture15202115







