Corn-Domesticated Bacteria Synergy Removes Pyrene and Enhances Crop Biomass: A Sustainable Farmland Remediation Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrichment and Domestication of Pyrene-Degrading Bacteria
2.2. DNA Isolation and Amplicon Sequencing of Bacterial 16S rRNA
2.3. Determination of Pyrene Removal Efficiency
2.4. Preparation of Soil and Pot Experiments
2.5. Determination of Pyrene Concentrations in Soil and Plants
2.6. Statistical Analysis
3. Results
3.1. Pyrene Removal Rates of Domesticated Bacteria
3.2. Removal Efficiency of Pyrene in Contaminated Soil by Different Bacterial Treatments
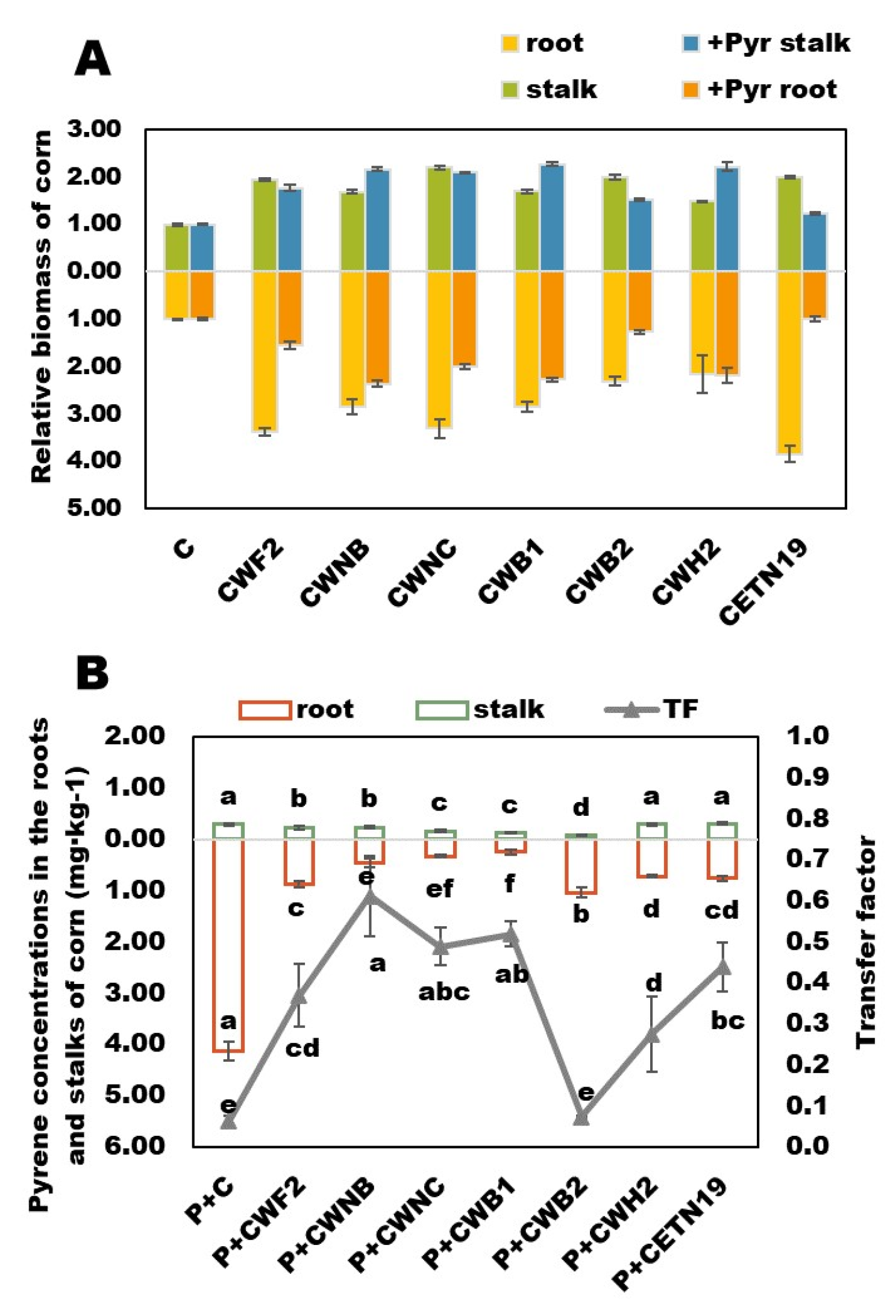
3.3. Effects of Different Bacteria Treatments Combined with Corn on Biomass and Pyrene Accumulation in Corn Plants
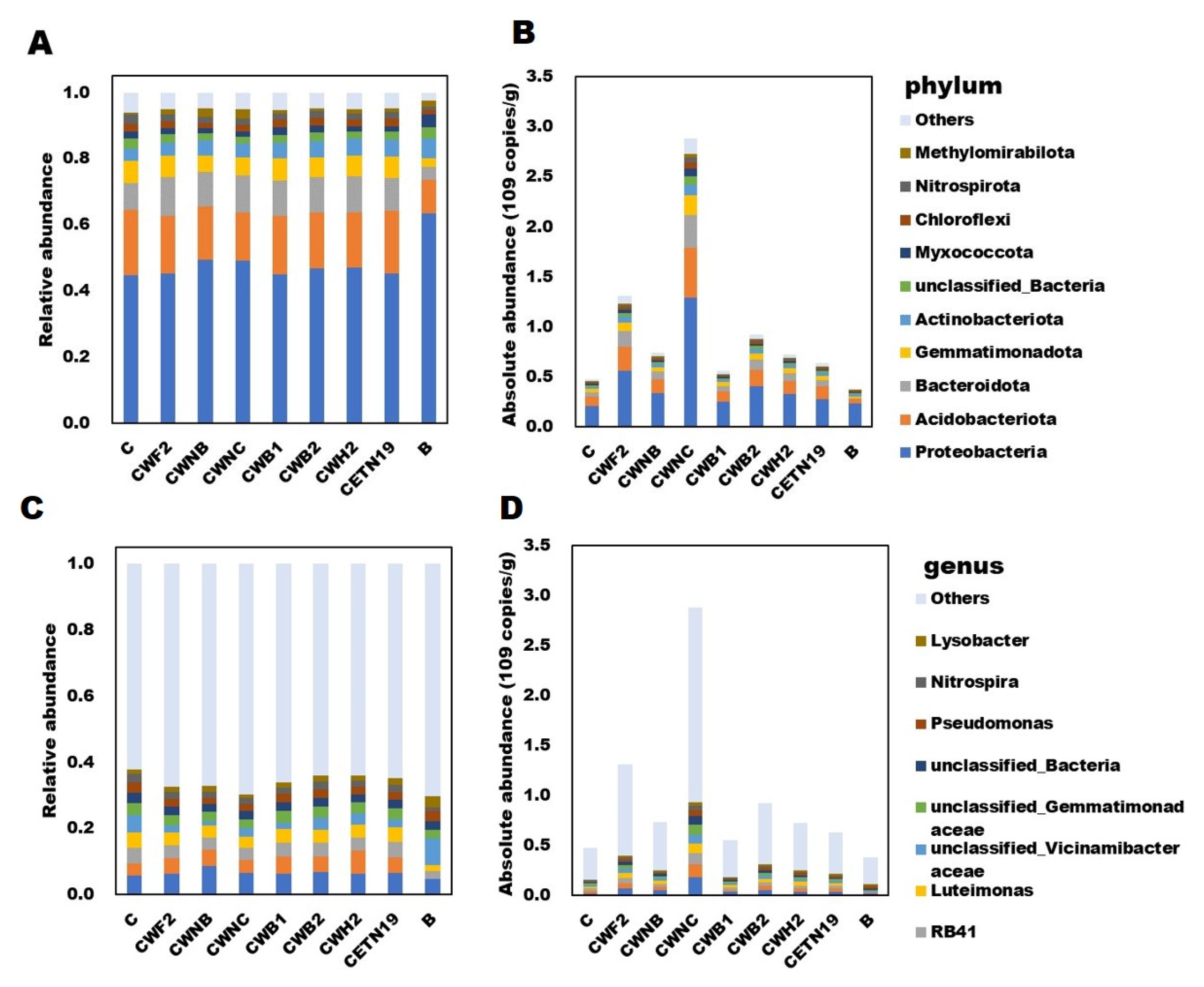
3.4. Bacterial Community Composition and Functional Characteristics in Domesticated Bacteria Treatments
3.5. Bacterial Community Dynamics in Corn–Bacteria Treatments for Pyrene Removal in Polluted Soils
3.6. Co-Correlation Network and Biomarkers Analysis Within Corn–Bacteria Combined Treatments
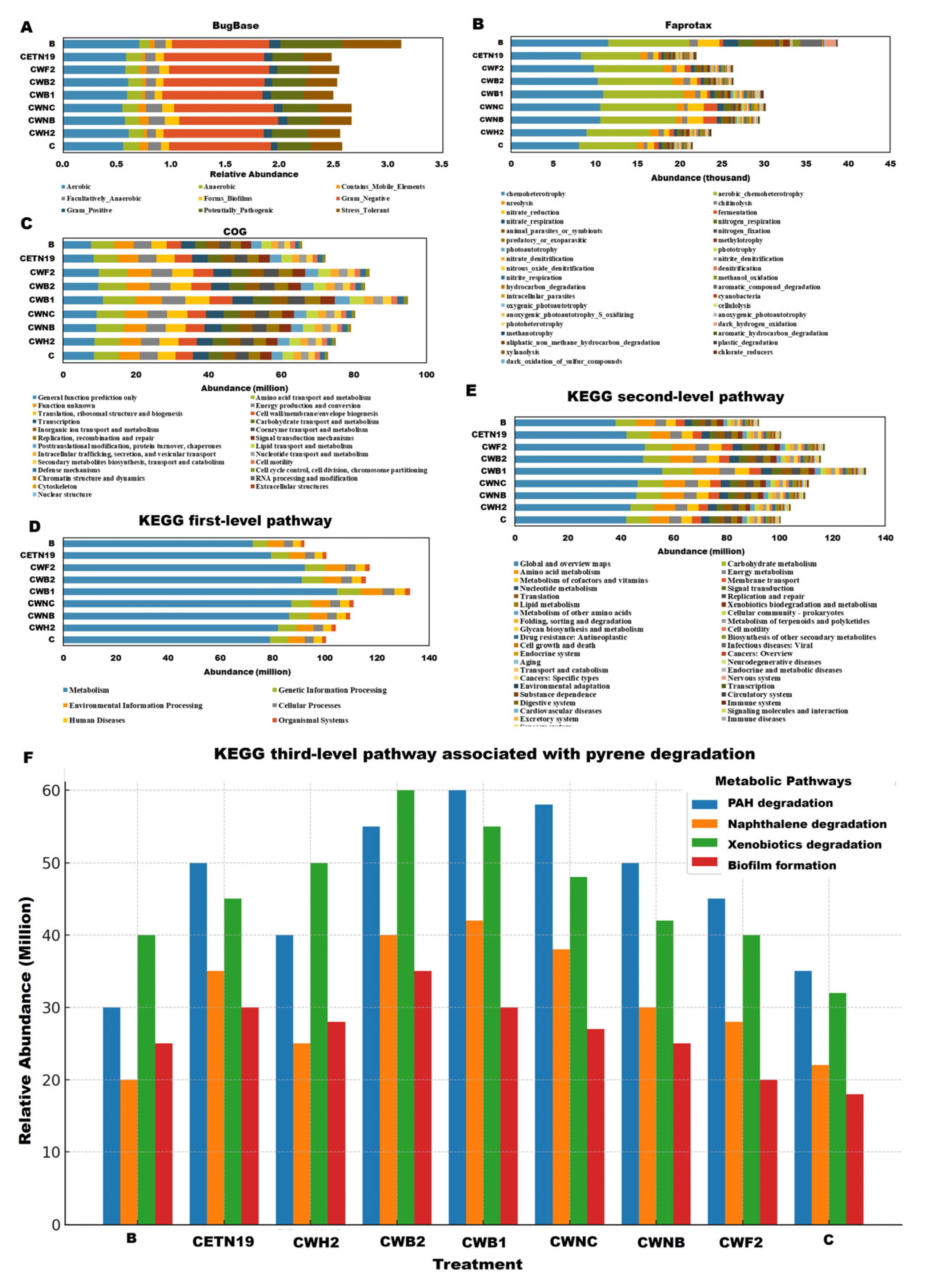
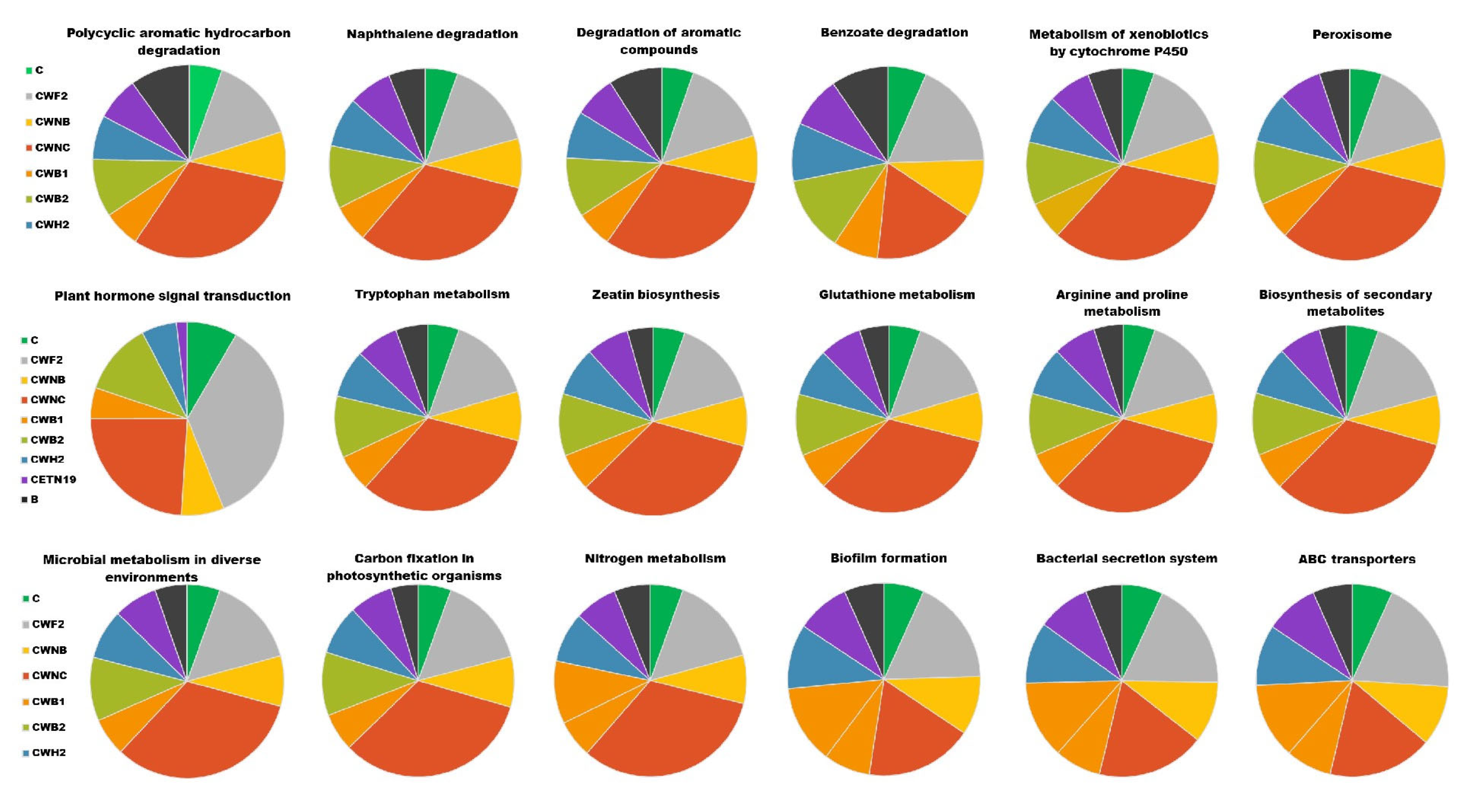
3.7. Predicted Functional Characteristics of Bacterial Communities and Enzymes Across Different Treatments Combined with Corn in Pyrene-Contaminated Soils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Addey, C.I.; Oderinde, O.; Okoro, J.O.; Uwamungu, J.Y.; Ikechukwu, C.K.; Okeke, E.S.; Ejeromedoghene, O.; Odii, E.C. Toxic Chemicals and Persistent Organic Pollutants Associated with Micro-and Nanoplastics Pollution. Chem. Eng. J. Adv. 2022, 11, 100310. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Y.; Du, L.; Yu, Y. Contamination level, sources, and health risk of polycyclic aromatic hydrocarbons in suburban vegetable field soils of Changchun, Northeast China. Sci. Rep. 2022, 12, 11301. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhu, Y.; Zhuo, S.; Liu, W.; Zeng, E.Y.; Wang, X.; Xing, B.; Tao, S. Occurrence of nitro- and oxy-PAHs in agricultural soils in eastern China and excess lifetime cancer risks from human exposure through soil ingestion. Environ. Int. 2017, 108, 261–270. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Kurek, M.; Hanula, M.; Wierzbicka, A.; Półtorak, A. Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review. Molecules 2022, 27, 4665. [Google Scholar] [CrossRef]
- Łyszczarz, S.; Lasota, J.; Szuszkiewicz, M.M.; Błońska, E. Soil texture as a key driver of polycyclic aromatic hydrocarbons (PAHs) distribution in forest topsoils. Sci. Rep. 2021, 11, 14708. [Google Scholar] [CrossRef]
- Gundlapalli, M.; Sivagami, K.; Gopalakrishnan, M.; Harshini, P.; Janjaroen, D.; Ganesan, S. Biodegradation of low molecular weight polycyclic aromatic hydrocarbons in soil: Insights into bacterial activities and bioremediation techniques. Sustain. Chem. Environ. 2024, 7, 100146. [Google Scholar] [CrossRef]
- Cai, H.; Yao, S.; Huang, J.; Zheng, X.; Sun, J.; Tao, X.; Lu, G. Polycyclic Aromatic Hydrocarbons Pollution Characteristics in Agricultural Soils of the Pearl River Delta Region, China. Int. J. Environ. Res. Public Health 2022, 19, 16233. [Google Scholar] [CrossRef]
- Ashkanani, Z.; Mohtar, R.; Al-Enezi, S.; Smith, P.K.; Calabrese, S.; Ma, X.; Abdullah, M. AI-assisted systematic review on remediation of contaminated soils with PAHs and heavy metals. J. Hazard. Mater. 2024, 468, 133813. [Google Scholar] [CrossRef]
- Chen, X.; Chu, S.; Chi, Y.; Wang, J.; Wang, R.; You, Y.; Hayat, K.; Khalid, M.; Zhang, D.; Zhou, P.; et al. Unraveling the role of multi-walled carbon nanotubes in a corn-soil system: Plant growth, oxidative stress and heavy metal(loid)s behavior. Plant Physiol. Biochem. 2023, 200, 107802. [Google Scholar] [CrossRef]
- Lou, F.; Okoye, C.O.; Gao, L.; Jiang, H.; Wu, Y.; Wang, Y.; Li, X.; Jiang, J. Whole-genome sequence analysis reveals phenanthrene and pyrene degradation pathways in newly isolated bacteria Klebsiella michiganensis EF4 and Klebsiella oxytoca ETN19. Microbiol. Res. 2023, 273, 127410. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, N.; Dai, W.; Liu, Y.; Tian, K.; Wang, H.; Liu, Y. Degradation of benzo [a] pyrene in the soil enhanced by soapwort: The role of soapwort and functional microbial community. J. Hazard. Mater. 2023, 458, 131993. [Google Scholar] [CrossRef] [PubMed]
- Al-Thukair, A.A.; Malik, K. Pyrene metabolism by the novel bacterial strains Burkholderia fungorum (T3A13001) and Caulobacter sp (T2A12002) isolated from an oil-polluted site in the Arabian Gulf. Int. Biodeterior. Biodegrad. 2016, 110, 32–37. [Google Scholar] [CrossRef]
- Elenga-Wilson, P.S.; Kayath, C.A.; Mokemiabeka, N.S.; Nzaou, S.A.E.; Nguimbi, E.; Ahombo, G. Profiling of Indigenous Biosurfactant-Producing Bacillus Isolates in the Bioremediation of Soil Contaminated by Petroleum Products and Olive Oil. Int. J. Microbiol. 2021, 2021, 9565930. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Y.; Chen, Z.; Song, Y.; Sun, F.; Liu, J.; Waigi, M.G. Colonization and performance of a pyrene-degrading bacterium Mycolicibacterium sp. Pyr9 on root surfaces of white clover. Chemosphere 2021, 263, 127918. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Mawad, A.M.M.; Mostafa, Y.M.; Shoreit, A. Biodegradation ability and catabolic genes of petroleum-degrading Sphingomonas koreensis strain ASU-06 isolated from Egyptian oily soil. Biomed Res. Int. 2014, 2014, 127674. [Google Scholar] [CrossRef]
- Alaidaroos, B.A. Advancing Eco-Sustainable Bioremediation for Hydrocarbon Contaminants: Challenges and Solutions. Processes 2023, 11, 3036. [Google Scholar] [CrossRef]
- Geng, S.; Qin, W.; Cao, W.; Wang, Y.; Ding, A.; Zhu, Y.; Fan, F.; Dou, J. Pilot-scale bioaugmentation of polycyclic aromatic hydrocarbon (PAH)-contaminated soil using an indigenous bacterial consortium in soil-slurry bioreactors. Chemosphere 2022, 287, 132183. [Google Scholar] [CrossRef]
- Vila, J.; López, Z.; Sabaté, J.; Minguillón, C.; Solanas, A.M.; Grifoll, M. Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: Actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2001, 67, 5497–5505. [Google Scholar] [CrossRef]
- Åkesson, A.; Curtsdotter, A.; Eklöf, A.; Ebenman, B.; Norberg, J.; Barabás, G. The importance of species interactions in eco-evolutionary community dynamics under climate change. Nat. Commun. 2021, 12, 4759. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, X.; Wang, Q.; Zou, L.; Peng, L.; Li, L.; Tu, W.; Li, Q. A novel method of domestication combined with ARTP to improve the reduction ability of Bacillus velezensis to Cr(VI). J. Environ. Chem. Eng. 2023, 11, 109091. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Ravi Kanth Reddy, P.; Obaisi, A.I.; Elghandour, M.M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Cipriano-Salazar, M.; Camacho-Díaz, L.M. Sustainable agriculture options for production, greenhouse gasses and pollution alleviation, and nutrient recycling in emerging and transitional nations—An overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wu, Y.; Wang, Y.; Gao, L.; Li, X.; Jiang, J. Fermentation profile, aerobic stability, and microbial community dynamics of corn straw ensiled with Lactobacillus buchneri PC-C1 and Lactobacillus plantarum PC1-1. Microbiol. Res. 2023, 270, 127329. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Y.; Okoye, C.O.; Gao, L.; Chen, X.; Wang, Y.; Jiang, J. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid bacteria: A promising feed additive for sustainable agriculture. Ind. Crops Prod. 2025, 224, 120315. [Google Scholar] [CrossRef]
- Gao, L.; Okoye, C.O.; Wang, C.; Lou, F.; Jiang, J. Enhanced Remediation of Phenanthrene and Naphthalene by Corn-Bacterial Consortium in Contaminated Soil. Plants 2024, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Chane, A.D.; Košnář, Z.; Hřebečková, T.; Jozífek, M.; Doležal, P.; Tlustoš, P. Persistent polycyclic aromatic hydrocarbons removal from sewage sludge-amended soil through phytoremediation combined with solid-state ligninolytic fungal cultures. Fungal Biol. 2024, 128, 1675–1683. [Google Scholar] [CrossRef]
- Grobelak, A.; Grosser, A.; Kacprzak, M.; Kamizela, T. Sewage sludge processing and management in small and medium-sized municipal wastewater treatment plant-new technical solution. J. Environ. Manag. 2019, 234, 90–96. [Google Scholar] [CrossRef]
- Ma, J.; Xu, L.; Jia, L. Characterization of pyrene degradation by Pseudomonas sp. strain Jpyr-1 isolated from active sewage sludge. Bioresour. Technol. 2013, 140, 15–21. [Google Scholar] [CrossRef]
- Panwar, R.; Mathur, J. Microbial-assisted phytodegradation for the amelioration of pyrene-contaminated soil using Pseudomonas aeruginosa and Aspergillus oryzae with alfalfa and sunflower. 3 Biotech 2023, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Wang, J.; Zhang, H.; Li, J.; Wu, F. Enhanced rhizodegradation of polycyclic aromatic hydrocarbons in corn straw-amended soil related to changing of bacterial community and functional gene expression. Bull Environ Contam Toxicol. 2022, 108, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-C.; Dan, A.; Chao, Y.-Q.; Li, H.-Y.; Li, C.; Lin, Q.-Q.; Li, Y.-Y.; Qiu, R.-L. Mechanism of polycyclic aromatic hydrocarbons degradation in the rhizosphere of Phragmites australis: Organic acid co-metabolism, iron-driven, and microbial response. Environ. Pollut. 2023, 327, 121608. [Google Scholar] [CrossRef] [PubMed]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef]
- Alotaibi, F.; Lee, S.-J.; St-Arnaud, M.; Hijri, M. Salix purpurea and Eleocharis obtusa Rhizospheres Harbor a Diverse Rhizospheric Bacterial Community Characterized by Hydrocarbons Degradation Potentials and Plant Growth-Promoting Properties. Plants 2021, 10, 1987. [Google Scholar] [CrossRef]
- Karaś, M.A.; Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Sokołowski, W.; Melianchuk, K.; Komaniecka, I. Assessment of Phenanthrene Degradation Potential by Plant-Growth-Promoting Endophytic Strain Pseudomonas chlororaphis 23aP Isolated from Chamaecytisus albus (Hacq.) Rothm. Molecules 2023, 28, 7581. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Z.; Liu, Y.; Xie, X.; Huang, L.; Zhang, R.; Dong, B. Effect of pyrene-induced changes in root activity on growth of Chinese cabbage (Brassica campestris L.), and the health risks caused by pyrene in Chinese cabbage at different growth stages. Chem. Biol. Technol. Agric. 2022, 9, 7. [Google Scholar] [CrossRef]
- Cao, X.; Wang, L.; Lin, J.; Wu, G.; Tang, K.; Tang, J.; Yan, Z.; An, M.; Liu, Z.; Zhou, Z. Differential bioaccumulation and tolerances of massive and branching scleractinian corals to polycyclic aromatic hydrocarbons in situ. Sci. Total Environ. 2024, 931, 172920. [Google Scholar] [CrossRef]
- Tarigholizadeh, S.; Sushkova, S.; Rajput, V.D.; Ranjan, A.; Arora, J.; Dudnikova, T.; Barbashev, A.; Mandzhieva, S.; Minkina, T.; Wong, M.H. Transfer and Degradation of PAHs in the Soil-Plant System: A Review. J. Agric. Food Chem. 2024, 72, 46–64. [Google Scholar] [CrossRef]
- Nascimento, A.L.; Souza, A.J.; Andrade, P.A.M.; Andreote, F.D.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Sewage Sludge Microbial Structures and Relations to Their Sources, Treatments, and Chemical Attributes. Front. Microbiol. 2018, 9, 1462. [Google Scholar] [CrossRef]
- Serwecińska, L.; Font-Nájera, A.; Strapagiel, D.; Lach, J.; Tołoczko, W.; Bołdak, M.; Urbaniak, M. Sewage sludge fertilization affects microbial community structure and its resistome in agricultural soils. Sci. Rep. 2024, 14, 21034. [Google Scholar] [CrossRef]
- Tang, L.; Bao, Z.; Zhao, X.; Wang, X.; Gao, Y.; Lu, C.; Ling, W. Variations of different PAH fractions and bacterial communities during the biological self-purification in the soil vertical profile. J. Hazard. Mater. 2023, 458, 131903. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Inoue, M.; Omae, K.; Yoshida, T.; Sako, Y. Chapter Three—Anaerobic and hydrogenogenic carbon monoxide-oxidizing prokaryotes: Versatile microbial conversion of a toxic gas into an available energy. Adv. Appl. Microbiol. 2020, 110, 99–148. [Google Scholar] [PubMed]
- Obayori, O.S.; Salam, L.B.; Oyetibo, G.O.; Idowu, M.; Amund, O.O. Biodegradation potentials of polyaromatic hydrocarbon (pyrene and phenanthrene) by Proteus mirabilis isolated from an animal charcoal polluted site. Biocatal. Agric. Biotechnol. 2017, 12, 78–84. [Google Scholar] [CrossRef]
- Sun, S.; Su, Y.; Chen, S.; Cui, W.; Zhao, C.; Liu, Q. Bioremediation of oil-contaminated soil: Exploring the potential of endogenous hydrocarbon degrader Enterobacter sp. SAVR S-1. Appl. Soil Ecol. 2022, 173, 104387. [Google Scholar] [CrossRef]
- Bridges, C.M.; Gage, D.J. Development and application of aerobic, chemically defined media for Dysgonomonas. Anaerobe 2021, 67, 102302. [Google Scholar] [CrossRef]
- Pang, L.; Huang, Z.; Yang, P.; Wu, M.; Zhang, Y.; Pang, R.; Jin, B.; Zhang, R. Effects of biochar on the degradation of organophosphate esters in sewage sludge aerobic composting. J. Hazard. Mater. 2023, 442, 130047. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Biofilms formation in plant growth-promoting bacteria for alleviating agro-environmental stress. Sci. Total Environ. 2024, 907, 167774. [Google Scholar] [CrossRef]
- Ma, B.; Gavzy, S.J.; Saxena, V.; Song, Y.; Piao, W.; Lwin, H.W.; Lakhan, R.; Iyyathurai, J.; Li, L.; France, M.; et al. Strain-specific alterations in gut microbiome and host immune responses elicited by tolerogenic Bifidobacterium pseudolongum. Sci. Rep. 2023, 13, 1023. [Google Scholar] [CrossRef]
- Wang, W.; Shen, P.; Lu, Z.; Mo, F.; Liao, Y.; Wen, X. Metagenomics reveals the abundance and accumulation trend of antibiotic resistance gene profile under long-term no tillage in a rainfed agroecosystem. Front. Microbiol. 2023, 14, 1238708. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Do, P.T.; Pham, Y.B.; Doan, T.O.; Nguyen, X.C.; Lee, W.K.; Nguyen, D.D.; Vadiveloo, A.; Um, M.-J.; Ngo, H.H. Roles, mechanism of action, and potential applications of sulfur-oxidizing bacteria for environmental bioremediation. Sci. Total Environ. 2022, 852, 158203. [Google Scholar] [CrossRef]
- Lu, C.; Hong, Y.; Liu, J.; Gao, Y.; Ma, Z.; Yang, B.; Ling, W.; Waigi, M.G. A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut. 2019, 251, 773–782. [Google Scholar] [CrossRef]
- Thomas, F.; Corre, E.; Cébron, A. Stable isotope probing and metagenomics highlight the effect of plants on uncultured phenanthrene-degrading bacterial consortium in polluted soil. ISME J. 2019, 13, 1814–1830. [Google Scholar] [CrossRef]
- Naloka, K.; Kuntaveesuk, A.; Muangchinda, C.; Chavanich, S.; Viyakarn, V.; Chen, B.; Pinyakong, O. Pseudomonas and Pseudarthrobacter are the key players in synergistic phenanthrene biodegradation at low temperatures. Sci. Rep. 2024, 14, 11976. [Google Scholar] [CrossRef]
- Wald, J.; Hroudova, M.; Jansa, J.; Vrchotova, B.; Macek, T.; Uhlik, O. Pseudomonads Rule Degradation of Polyaromatic Hydrocarbons in Aerated Sediment. Front. Microbiol. 2015, 6, 1268. [Google Scholar] [CrossRef]
- Feng, F.; Yang, Y.; Liu, Q.; Wu, S.; Yun, Z.; Xu, X.; Jiang, Y. Insights into the characteristics of changes in dissolved organic matter fluorescence components on the natural attenuation process of toluene. J. Hazard. Mater. 2024, 476, 134952. [Google Scholar] [CrossRef]
- Xu, G.; Geng, S.; Cao, W.; Zuo, R.; Teng, Y.; Ding, A.; Fan, F.; Dou, J. Vertical distribution characteristics and interactions of polycyclic aromatic compounds and bacterial communities in contaminated soil in oil storage tank areas. Chemosphere 2022, 301, 134695. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Du, X.; Li, M.; Zhang, Z.; Liang, H.; Gao, D. Bioremediation of PAHs-contaminated site in a full-scale biopiling system with immobilized enzymes: Removal efficiency and microbial communities. Environ. Res. 2024, 262, 119763. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z.; Wang, Y.; Xu, J. Efficient removal of heavily oil-contaminated soil using a combination of fenton pre-oxidation with biostimulated iron and bioremediation. J. Environ. Manag. 2022, 308, 114590. [Google Scholar] [CrossRef] [PubMed]
- Acer, Ö.; Johnston, G.; Lineman, D.; Johnston, C. Evaluating degradation of polycyclic aromatic hydrocarbon (PAH) potential by indigenous bacteria isolated from highly contaminated riverbank sediments. Environ. Earth Sci. 2021, 80, 773. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Meng, Q.; Li, L. Effects of Graphene Oxide on Endophytic Bacteria Population Characteristics in Plants from Soils Contaminated by Polycyclic Aromatic Hydrocarbons. Molecules 2024, 29, 2342. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Qin, L.-J.; Long, T.; Wu, R.-T.; Niu, S.-H.; Liu, S.; Deng, W.-K.; Liao, X.-D.; Xing, S.-C. Effortless rule: Effects of oversized microplastic management on lettuce growth and the dynamics of antibiotic resistance genes from fertilization to harvest. J. Hazard. Mater. 2025, 492, 138046. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, R.; Zydlik, Z.; Wolna-Maruwka, A.; Niewiadomska, A.; Kayzer, D. The Effect of Biofumigation on the Microbiome Composition in Replanted Soil in a Fruit Tree Nursery. Agronomy 2023, 13, 2507. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Landry, N.B.J.; Duan, Y.; Li, X.; Zhou, X. The impact of Ricinus straw on tomato growth and soil microbial community. Front. Microbiol. 2024, 15, 1499302. [Google Scholar] [CrossRef]
- Jiménez-Vázquez, K.R.; López-Bucio, J.S.; Ruíz-Herrera, L.F.; López-Bucio, J. Root cap transcription factors control directional root growth in Arabidopsis seedlings in response to the plant growth-promoting rhizobacterium Achromobacter sp. 5B1. Plant J. 2025, 122, e70226. [Google Scholar] [CrossRef]
- Rebelo Romão, I.; Rodrigues Dos Santos, A.S.; Velasco, L.; Martínez-Ferri, E.; Vilchez, J.I.; Manzanera, M. Seed-Encapsulation of Desiccation-Tolerant Microorganisms for the Protection of Maize from Drought: Phenotyping Effects of a New Dry Bioformulation. Plants 2022, 11, 1024. [Google Scholar] [CrossRef]
- Ren, H.; Chen, S.; Shang, J.; Gao, Y.; Deng, Y.; Wang, Z.; Hu, G.; Wang, B. Insights into the Response and Evolution of Microbial Communities During Long-Term Natural Remediation of Contaminated Abandoned Shale Gas Wells. Water Air Soil Pollut. 2024, 235, 729. [Google Scholar] [CrossRef]
- Jiménez-Volkerink, S.N.; Jordán, M.; Smidt, H.; Minguillón, C.; Vila, J.; Grifoll, M. Metagenomic insights into the microbial cooperative networks of a benz(a)anthracene-7,12-dione degrading community from a creosote-contaminated soil. Sci. Total Environ. 2024, 907, 167832. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q. Plant–Bacteria Partnerships for the Remediation of Hydrocarbon Contaminated Soils. Chemosphere 2012, 90, 1317–1332. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Shen, Y.; Chen, X.; He, G.; He, X.; Wang, G.; He, H.; Lv, Z. The response of nutrient cycle, microbial community abundance and metabolic function to nitrogen fertilizer in rhizosphere soil of Phellodendron chinense Schneid seedlings. Front. Microbiol. 2023, 14, 1302775. [Google Scholar] [CrossRef]
- Meng, S.; Peng, T.; Liu, Y.; Zhang, S.; Qian, Z.; Huang, T.; Xie, Q.; Gu, J.-D.; Hu, Z. Novel insights into the synergetic degradation of pyrene by microbial communities from mangroves in China. J. Hazard. Mater. 2024, 469, 133907. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hou, J.; Huang, Y.; Liu, W.; Christie, P. Metagenomics reveals mechanism of pyrene degradation by an enriched bacterial consortium from a coking site contaminated with PAHs. Sci. Total Environ. 2023, 904, 166759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Zhang, T.; Yin, B.; Li, R.; Sheng, Z.; Li, S. Variations in soil microbial communities in different saline soils under typical Populus spp. vegetation in alpine region of the Qaidam Basin, NW China. Ecotoxicol. Environ. Saf. 2024, 282, 116747. [Google Scholar] [CrossRef]
- Zada, S.; Zhou, H.; Xie, J.; Hu, Z.; Ali, S.; Sajjad, W.; Wang, H. Bacterial degradation of pyrene: Biochemical reactions and mechanisms. Int. Biodeterior. Biodegrad. 2021, 162, 105233. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Jeon, C.O. Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci. Rep. 2019, 9, 860. [Google Scholar] [CrossRef]
- Zarei, T.; Moradi, A.; Kazemeini, S.A.; Akhgar, A.; Rahi, A.A. The role of ACC deaminase producing bacteria in improving sweet corn (Zea mays L. var saccharata) productivity under limited availability of irrigation water. Sci. Rep. 2020, 10, 20361. [Google Scholar] [CrossRef]


| Treatment | Removal Rate (%) | |||
|---|---|---|---|---|
| 7 d | 15 d | 30 d | ||
| Control | Blank control | 1.02 ± 0.01 Cd | 5.81 ± 0.75 Ce | 10.15 ± 1.91 Df |
| ASS * | WNB | 33.95 ± 1.86 Ab | 53.56 ± 0.58 Ab | 75.30 ± 0.77 Ac |
| WNC | 36.63 ± 6.31 Ab | 55.44 ± 2.81 Ab | 87.69 ± 1.21 Aa | |
| WH2 | 44.43 ± 3.60 Aa | 65.06 ± 0.61 Aa | 82.30 ± 1.03 Ab | |
| PFS | WB1 | 27.73 ± 0.96 Bc | 38.03 ± 7.65 Be | 70.90 ± 0.62 Bd |
| WB2 | 33.50 ± 4.19 Bb | 59.50 ± 0.70 Bb | 76.58 ± 0.84 Bc | |
| WF2 | 32.69 ± 0.73 Bb | 52.16 ± 0.72 Bc | 62.61 ± 5.49 Be | |
| DS | ETN19 | 38.91 ± 3.25 Bb | 47.70 ± 1.93 Bd | 61.99 ± 8.93 Ce |
| Treatment | Removal Rate (%) | Treatment | Removal Rate (%) | ||
|---|---|---|---|---|---|
| Control | B | 16.53 ± 0.72 Ce | Control + corn | C | 43.12 ± 3.17 Bb |
| ASS * | WNB | 73.93 ± 1.55 Aa | ASS + corn | CWNB | 97.68 ± 0.48 Aa |
| WNC | 64.46 ± 1.67 Abcd | CWNC | 97.70 ± 0.62 Aa | ||
| WH2 | 67.02 ± 4.67 Ab | CWH2 | 96.95 ± 0.37 Aa | ||
| PFS | WB1 | 61.55 ± 0.91 Bd | PFS + corn | CWB1 | 98.45 ± 0.73 Aa |
| WB2 | 63.85 ± 1.90 Bbcd | CWB2 | 96.85 ± 0.65 Aa | ||
| WF2 | 66.37 ± 0.86 Bbc | CWF2 | 96.95 ± 1.48 Aa | ||
| DS | ETN19 | 62.81 ± 0.46 Bcd | DS + corn | CETN19 | 96.40 ± 0.62 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Okoye, C.O.; Lou, F.; Ezenwanne, B.C.; Wu, Y.; Chen, X.; Wang, Y.; Li, X.; Jiang, J. Corn-Domesticated Bacteria Synergy Removes Pyrene and Enhances Crop Biomass: A Sustainable Farmland Remediation Strategy. Agriculture 2025, 15, 2083. https://doi.org/10.3390/agriculture15192083
Gao L, Okoye CO, Lou F, Ezenwanne BC, Wu Y, Chen X, Wang Y, Li X, Jiang J. Corn-Domesticated Bacteria Synergy Removes Pyrene and Enhances Crop Biomass: A Sustainable Farmland Remediation Strategy. Agriculture. 2025; 15(19):2083. https://doi.org/10.3390/agriculture15192083
Chicago/Turabian StyleGao, Lu, Charles Obinwanne Okoye, Feiyue Lou, Bonaventure Chidi Ezenwanne, Yanfang Wu, Xunfeng Chen, Yongli Wang, Xia Li, and Jianxiong Jiang. 2025. "Corn-Domesticated Bacteria Synergy Removes Pyrene and Enhances Crop Biomass: A Sustainable Farmland Remediation Strategy" Agriculture 15, no. 19: 2083. https://doi.org/10.3390/agriculture15192083
APA StyleGao, L., Okoye, C. O., Lou, F., Ezenwanne, B. C., Wu, Y., Chen, X., Wang, Y., Li, X., & Jiang, J. (2025). Corn-Domesticated Bacteria Synergy Removes Pyrene and Enhances Crop Biomass: A Sustainable Farmland Remediation Strategy. Agriculture, 15(19), 2083. https://doi.org/10.3390/agriculture15192083







