High Proportion of Blue Light Contributes to Product Quality and Resistance to Phytophthora Infestans in Tomato Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Site
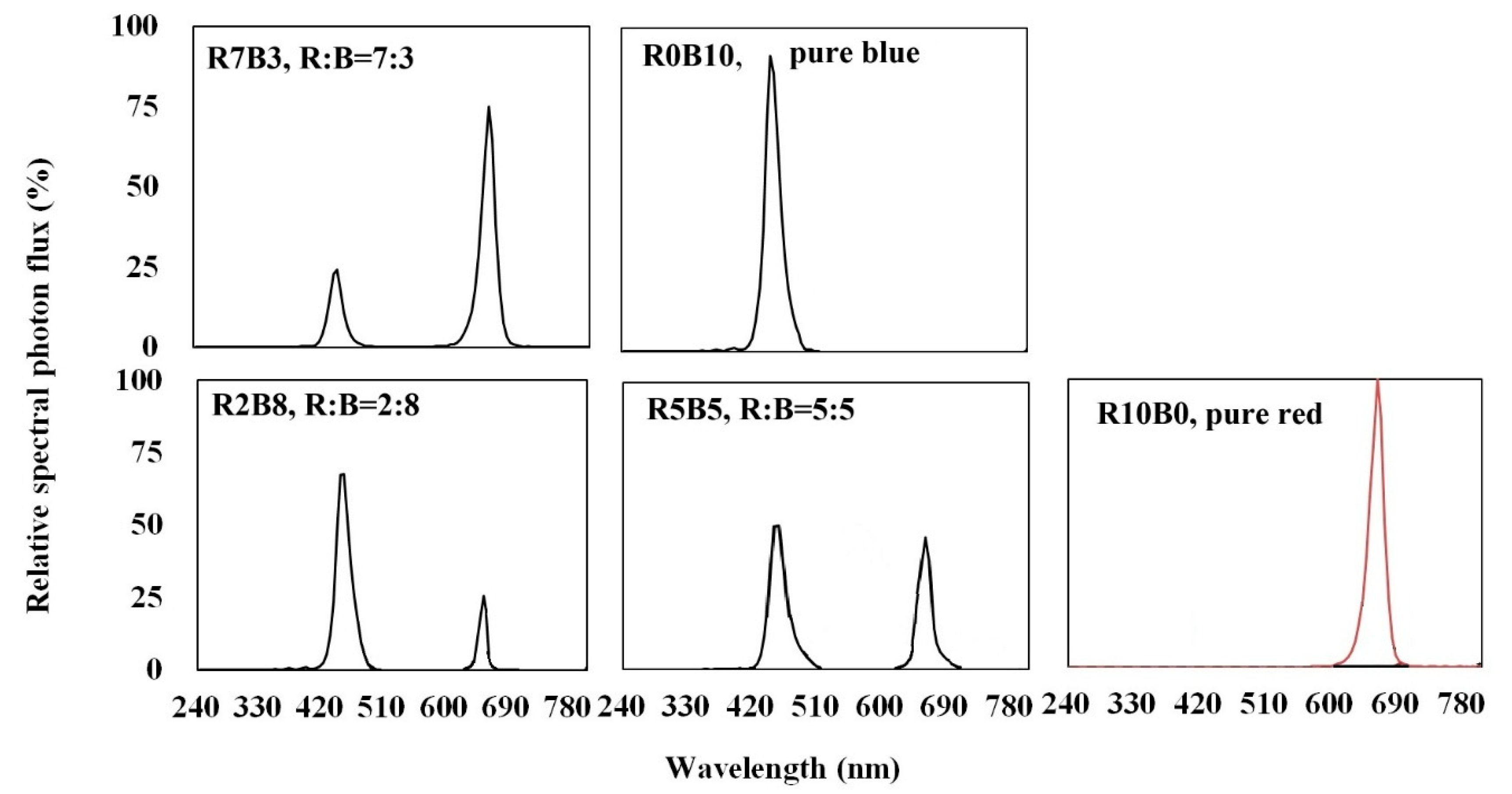
2.2. Experiment Design
2.3. Plant Morphology Analyses
2.4. Photosynthesis Parameter Measurements
2.5. Measurements of AsA-GSH Cycle Indicators
2.6. Plant Endogenous Hormones Measurements
2.7. Pathogen Inoculation and Glucose Measurements
2.8. Data Statistics and Analysis
3. Results
3.1. Plant Growth and Development
3.2. Leaf Photosynthesis Capacity
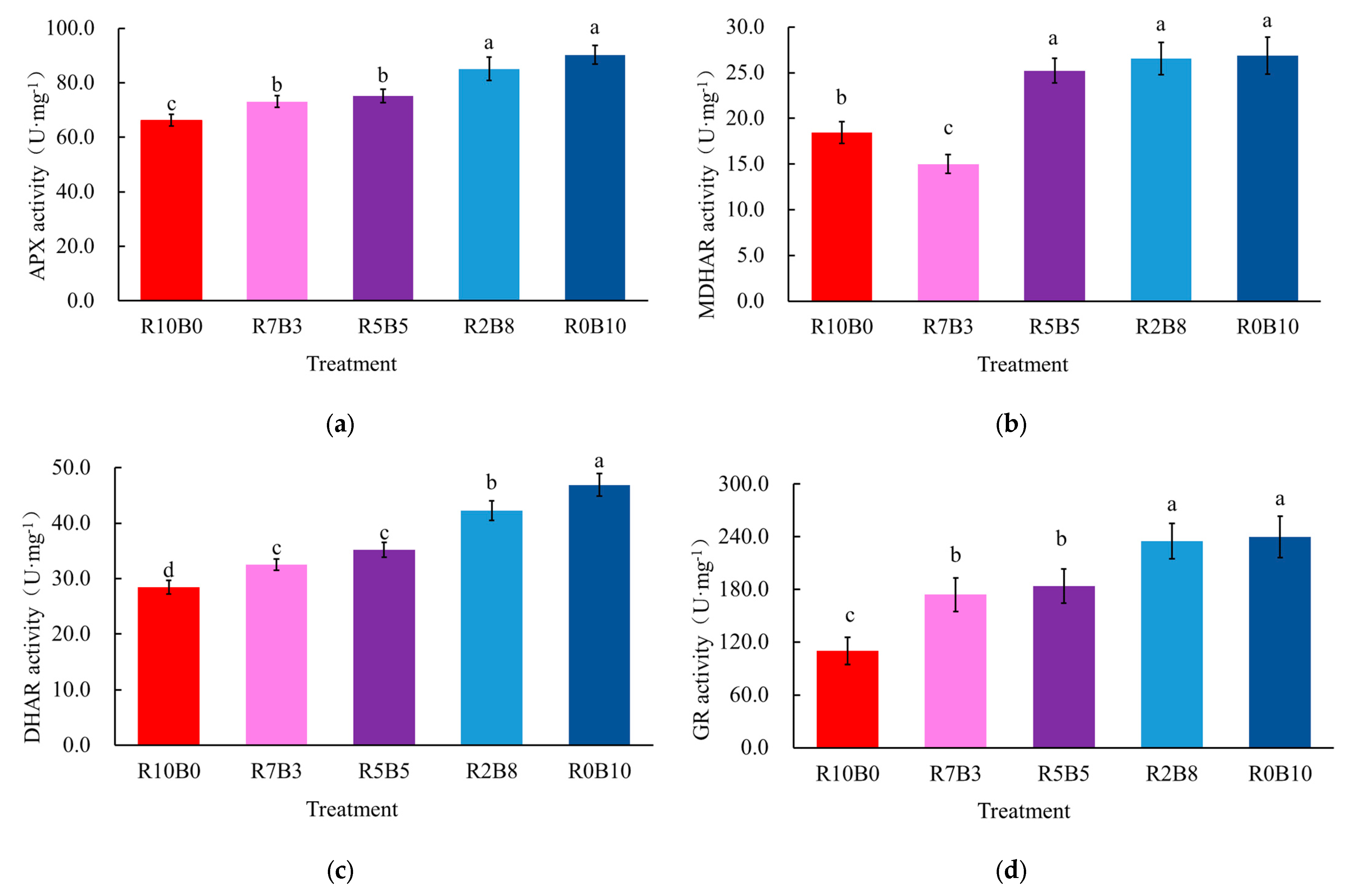
3.3. Enzyme Activities of Key Enzymes in the AsA-GSH Cycle
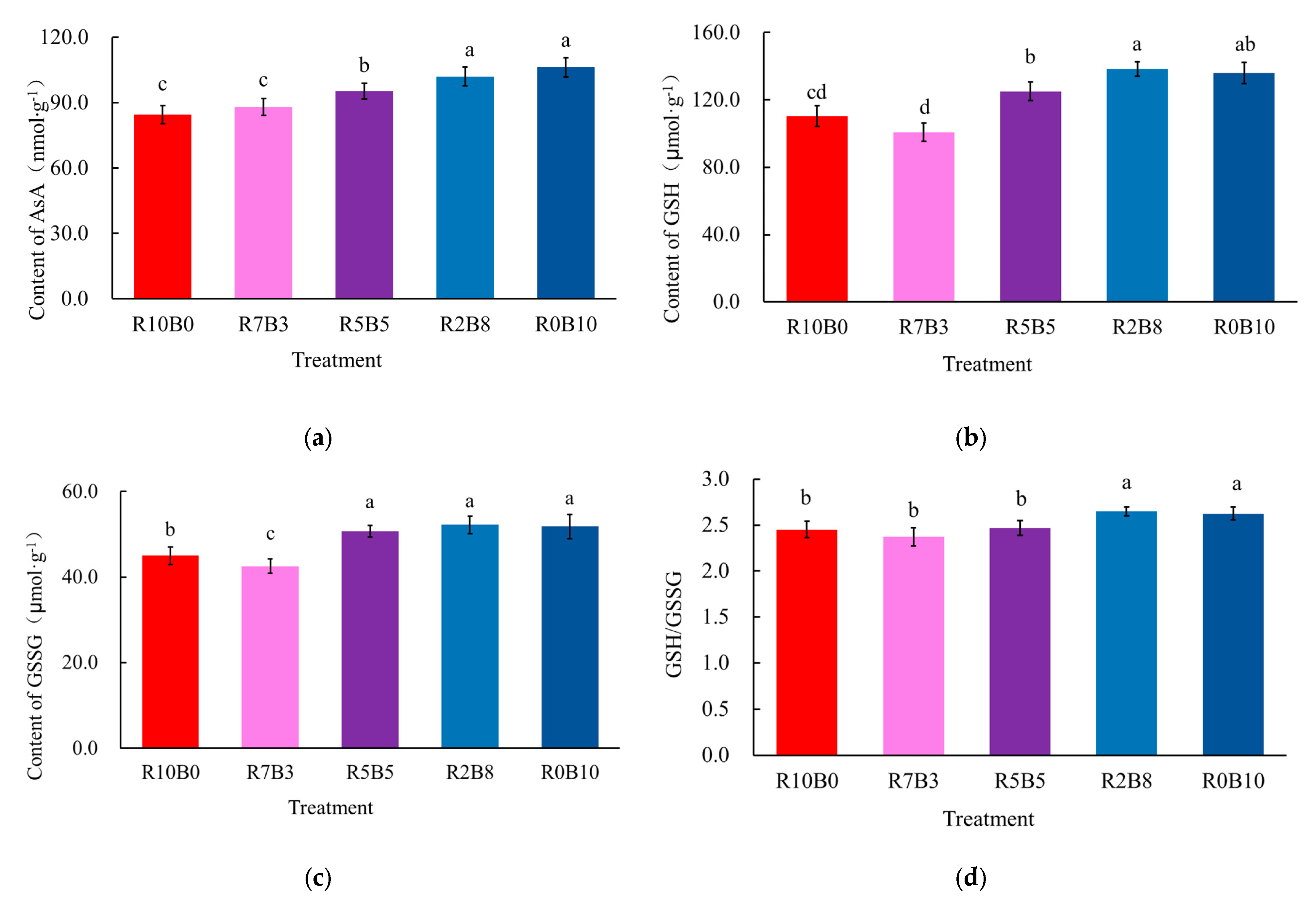
3.4. Contents of Key Substances in the AsA-GSH Cycle
3.5. Contents of Endogenous Hormones in Seedlings
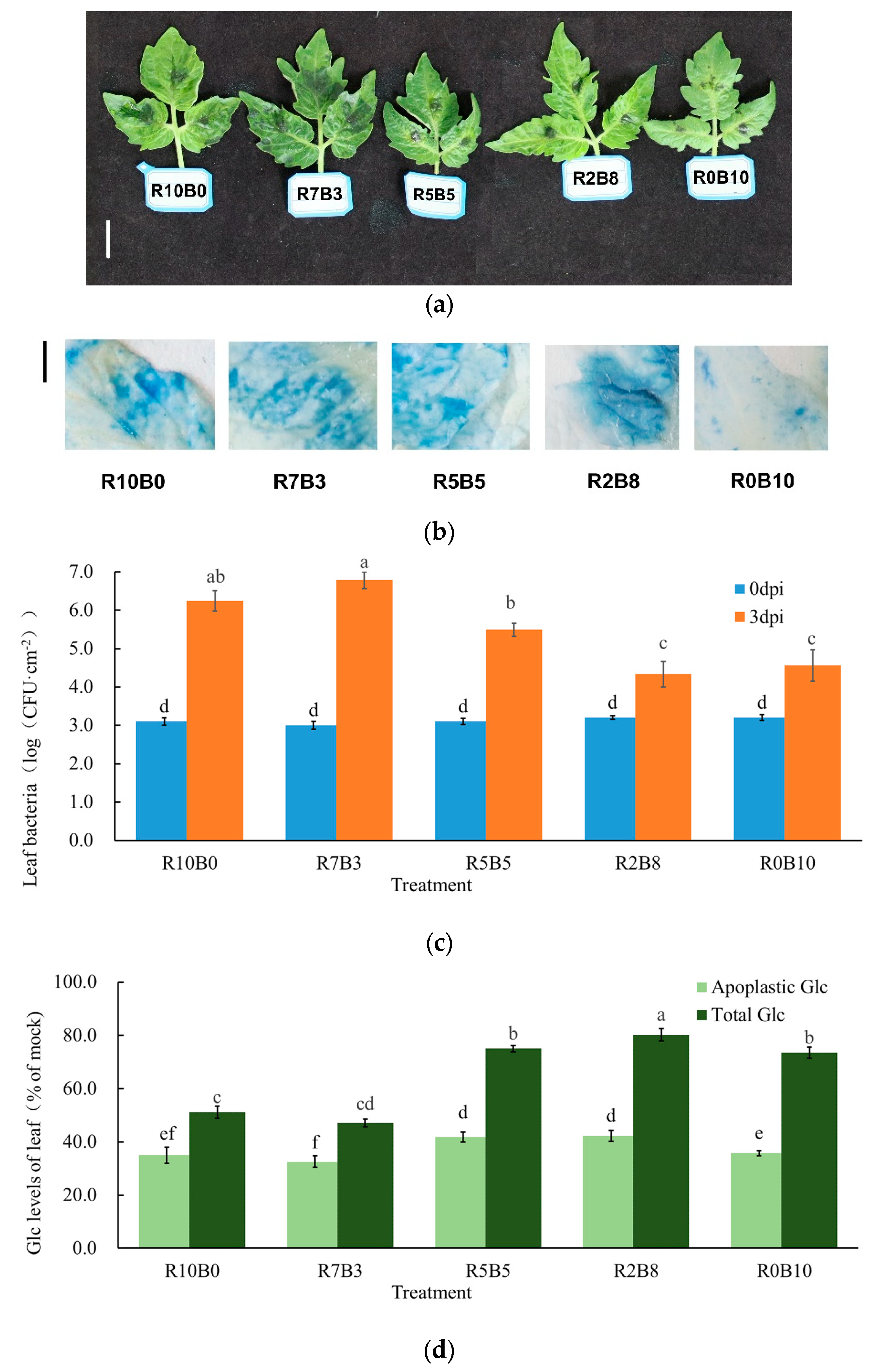
3.6. Disease Susceptibility and Glucose Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of extreme root restriction on the nutritional and flavor quality, and sucrose metabolism of tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Genoud, T.; Buchala, A.J.; Chua, N.H.; Métraux, J.P. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J. 2002, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, X.; Zhang, Z. The Magnaporthe grisea species complex and plant pathogenesis. Mol. Plant Pathol. 2016, 17, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Zhang, S.B.; Hu, J.X.; Sun, W.X.; Padilla, J.; He, Y.L.; Li, Y.; Yin, Z.Y.; Liu, X.Y.; Wang, W.H.; et al. Phosphorylation guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 17572–17577. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, C.; Gao, L. Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Front. Plant Sci. 2016, 7, 1832. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, T.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, M. Supplemental lighting applied within or underneath the canopy enhances leaf photosynthesis, stomatal regulation and plant development of tomato under limiting light conditions. Acta Hortic. 2018, 1227, 645–652. [Google Scholar] [CrossRef]
- Fan, C.Y.; Manivannan, A.; Wei, H. Light quality-mediated influence of morphogenesis in micropropagated horticultural crops: A comprehensive overview. BioMed Res. Int. 2022, 2022, 4615079. [Google Scholar] [CrossRef]
- Fang, S.; Lang, T.; Cai, M.; Han, T. Light keys open locks of plant photoresponses: A review of phosphors for plant cultivation LEDs. J. Alloys Compd. 2022, 902, 163825. [Google Scholar] [CrossRef]
- Bhattarai, T.; Ebong, A.; Raja, M.Y.A. A review of Light-Emitting Diodes and Ultraviolet Light-Emitting Diodes and their applications. Photonics 2024, 11, 491. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, J.; Meng, Y.; Wang, L.; Wang, X.; Wu, Z. Effect of different light ratios in plant factories on the growth and quality of water dropwort. North. Hortic. 2022, 6, 8–14, (In Chinese with English Abstract). [Google Scholar]
- Wang, Z.; Ma, J.; He, S.; He, D.; Shang, W.; Wang, H. Effects of different proportions of light qualities from LED light sources on the growth of tissue-cultured seedlings of Photinia × fraseri. Jiangsu Agri. Sci. 2019, 47, 152–155. [Google Scholar]
- Campos, M.D.; Félix, M.R.; Patanita, M.; Materatski, P.; Varanda, C. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic. Res. 2021, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Huang, R. Regulation of ascorbic acid synthesis in plants. Plant Signal Behav. 2013, 8, e24536. [Google Scholar] [CrossRef]
- Fenech, M.; Amorim-Silva, V.; del Valle, A.E.; Arnaud, D.; Ruiz-Lopez, N.; Castillo, A.G.; Smirnoff, N.; Botella, M.A. The role of GDP-l-galactose phosphorylase in the control of ascorbate biosynthesis. Plant Physiol. 2021, 185, 1574–1594. [Google Scholar] [CrossRef]
- Castro, J.C.; Castro, C.G.; Cobos, M. Genetic and biochemical strategies for regulation of L-ascorbic acid biosynthesis in plants through the L-galactose pathway. Front. Plant Sci. 2023, 14, 1099829. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Liao, K.L.; Jones, R.D.; McCarter, P.; Tunc-Ozdemir, M.; Draper, J.A.; Elston, T.C.; Kramer, D.; Jones, A.M. A shadow detector for photosynthesis efficiency. J. Theor. Biol. 2017, 414, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sugars and plant innate immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Häusler, R.E.; Heinrichs, L.; Schmitz, J.; Flugge, U.I. How sugars might coordinate chloroplast and nuclear gene expression during acclimation to high light intensities. Mol. Plant 2014, 7, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Heloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trda, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; Luo, Q.; Hu, Z.; Ma, Q.; Li, Y.; Lin, T.; Liang, X.; Yu, J.; Foyer, C.H.; et al. Glucose sensing by regulator of G protein signaling 1 (RGS1) plays a crucial role in coordinating defense in response to environmental variation in tomato. New Phytol. 2022, 236, 561–575. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and theopportunities of modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Sun, J.; Mu, J. Recent advances on fluorescence-based enzyme linked immunosorbent assay. Chin. J. Anal. Chem. 2023, 51, 331–339. [Google Scholar]
- Thiha, A.; Ibrahim, F. A colorimetric enzyme-linked immunosorbent assay (ELISA) detection platform for a point-of-care dengue detection system on a lab-on-compact-disc. Sensors 2015, 15, 11431–11441. [Google Scholar] [CrossRef]
- Farrokhzad, Y.; Babaei, A.; Yadollahi, A.; Kashkooli, A.B.; Mokhtassi-Bidgoli, A.; Hesami, S. In vitro photomorphogenesis, plant growth regulators, melatonin content, and DNA methylation under various wavelengths of light in Phalaenopsis amabilis. Plant Cell Tiss. Organ. Cult. 2022, 149, 535–548. [Google Scholar] [CrossRef]
- Ding, S.; Shao, X.; Li, J.; Ahammed, G.J.; Yao, Y.; Ding, J.; Hu, Z.; Yu, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Function and Mechanisms of Apoplastic Glucose Signaling in Tomato Disease Resistance Under Low Light Condition. Ph.D. Thesis, Zhejiang University, Hangzhou, China,, 2021. [Google Scholar]
- Zhang, N.; Pombo, M.A.; Rosli, H.G.; Martin, G.B. Tomato wall-associated kinase SlWak1 depends on Fls2/Fls3 to promote apoplastic immune responses to Pseudomonas syringae. Plant Phys. 2020, 183, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of daily light integral on tomato (Solanum lycopersicon L.) grafting and quality in a controlled environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Si, C.; Lin, Y.; Luo, S.; Yu, Y.; Liu, R.; Naz, M.; Dai, Z. Effects of LED light quality combinations on growth and leaf colour of tissue culture generated plantlets in Sedum rubrotinctum. Hortic. Sci. Technol. 2024, 42, 53–67. [Google Scholar] [CrossRef]
- Gu, L.; Han, J.; Wood, J.D.; Chang, C.Y.; Sun, Y. Sun-induced Chl fluorescence and its importance for biophysical modeling of photosynthesis based on light reactions. New Phytol. 2019, 233, 1179–1191. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED supplemental lighting improves quality of tomato seedlings by increasing biomass accumulation in a controlled environment. Agronomy 2024, 14, 1888. [Google Scholar] [CrossRef]
- An, R.; Liu, X.; Luo, S.; Li, G.; Hu, H.; Li, P. Taxifolin delays the degradation of chlorophyll in pakchoi (Brassica rapa L. subsp. chinensis) by regulating the ascorbate-glutathione cycle. Postharvest Biol. Technol. 2022, 191, 111982. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, C.; Jing, J.; Zhuang, M.; Zhang, J.; Wang, K.; Zhang, H. Metabolomics analysis of cucumber fruit in response to foliar fertilizer and pesticides using UHPLC-Q-Orbitrap-HRMS. Food Chem. 2021, 369, 130960. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Liu, Y.; Zhang, L.; Li, D.; Zhao, X.; Zhang, J.; Sui, Y. Promoting anthocyanin biosynthesis in purple lettuce through sucrose supplementation under nitrogen limitation. Horticulturae 2024, 10, 838. [Google Scholar] [CrossRef]
- Ahmad, N.; Naeem, M.; Ali, H.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Siddiqui, S.A.; Khan, A.A.; Iqbal, B. From challenges to solutions: The impact of melatonin on abiotic stress synergies in horticultural plants via redox regulation and epigenetic signaling. Sci. Hortic. 2023, 321, 112369. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Gong, X.; Xue, X.; Hu, Y. Effects of different LED light spectra on growth and alterations of endogenous hormone of potato plantlets grown in vitro. Chin. Potato J. 2020, 34, 257–267, (In Chinese with English Abstract). [Google Scholar]
- Li, X.; Zhao, S.; Bao, X.; Yang, Y.; Wu, Y.; Yang, Z. Effect of increasing the percentage of green light on morphology, photosynthetic traits and carbohydrates of cucumber seedlings. J. China Agric. Univ. 2024, 29, 58–65, (In Chinese with English Abstract). [Google Scholar]
- Farrokhzad, Y.; Babaei, A.; Yadollahi, A.; Kashkooli, A.B.; Mokhtassi-Bidgoli, A.; Hessami, S. Informative title: Development of lighting intensity approach for shoot proliferation in Phalaenopsis amabilis through combination with silver nanoparticles. Sci. Hortic. 2022, 292, 110582. [Google Scholar] [CrossRef]
- Wan, L.; Li, H.; Li, C.; Wang, A.; Yang, Y.; Wang, P. Hyperspectral sensing of plant diseases: Principle and methods. Agronomy 2022, 12, 1451. [Google Scholar] [CrossRef]
- Sun, J.; Tan, X.; Liu, B.; Battino, M.; Meng, X.; Zhang, F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biol. Technol. 2024, 215, 113006. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Lin, J.; Lu, X.; Ye, B.; Lv, H.; Du, X.; Chen, T. Hormone metabolism and substance accumulation in cucumber plants: Downy mildew infection and potassium stress. Agriculture 2025, 15, 994. [Google Scholar] [CrossRef]
- Kangasjärvi, S.; Neukermans, J.; Li, S.; Aro, E.M.; Noctor, G. Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 2012, 63, 1619–1636. [Google Scholar] [CrossRef]
- Heinrichs, L.; Schmitz, J.; Flügge, U.I.; Häusler, R.E. The mysterious rescue of adg1-1/tpt-2-an Arabidopsis thaliana double mutant impaired in acclimation to high light-by exogenously supplied sugars. Front. Plant Sci. 2012, 3, 265. [Google Scholar] [CrossRef]
- Schmitz, J.; Schöttler, M.A.; Krueger, S.; Geimer, S.; Schneider, A.; Kleine, T.; Leister, D.; Bell, K.; Flügge, U.I.; Häusler, R.E. Defects in leaf carbohydrate metabolism compromise acclimation to high light and lead to a high chlorophyll fluorescence phenotype in Arabidopsis thaliana. BMC Plant Biol. 2012, 12, 8. [Google Scholar] [CrossRef]
- Schmitz, J.; Heinrichs, L.; Scossa, F.; Fernie, A.R.; Oelze, M.L.; Dietz, K.J.; Rothbart, M.; Grimm, B.; Flügge, U.I.; Häusler, R.E. The essential role of sugar metabolism in the acclimation response of Arabidopsis thaliana to high light intensities. J. Exp. Bot. 2014, 65, 1619–1636. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Neale, H.C.; Geilfus, C.M.; Jackson, R.W.; Arnold, D.L.; Preston, G.M. Early changes in apoplast composition associated with defence and disease in interactions between phaseolus vulgaris and the halo blight pathogen pseudomonas syringae pv. phaseolicola. Plant Cell Environ. 2016, 39, 2172–2184. [Google Scholar] [CrossRef]
- Dodds, P.N.; Lagudah, E.S. Starving the enemy. Science 2016, 354, 1377–1378. [Google Scholar] [CrossRef]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 354, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kunz, M.; Dandekar, T. Plant-pathogen maneuvering over apoplastic sugars. Trends Plant Sci. 2017, 22, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mccormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 2013, 496, 181. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Kopyra, A.; Dobrowolski, J.W.; Czech, T.; Neugschwandtner, R.W.; Gambuś, F.; Kot, D. Chapter One—The Use of Laser Biotechnology in Agri-Environment as a Significant Agronomical Advance Increasing Crop Yield and Quality. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 170, pp. 1–33. [Google Scholar]
- Dłużniewska, J.; Klimek-Kopyra, A.; Czech, T.; Dobrowolski, J.W.; Dacewicz, E. The Use of Coherent Laser Stimulation of Seeds and a Fungal Inoculum to Increase the Productivity and Health of Soybean Plants. Agronomy 2021, 11, 1923. [Google Scholar] [CrossRef]

| Treatment | Fresh Weight Aboveground (g) | Dry Weight Aboveground (mg) | Stem Height (cm) | Leaf Area (cm2) | SPAD | Root Length (cm) | Health Index |
|---|---|---|---|---|---|---|---|
| R10B0 | 2.15 ± 0.19 d 1 | 27.34 ± 1.84 d | 6.82 ± 0.27 d | 14.09 ± 0.72 c | 34.76 ± 3.06 d | 28.00 ± 1.01 c | 0.52 ± 0.07 c |
| R7B3 | 3.15 ± 0.12 bc | 37.12 ± 4.13 c | 10.78 ± 0.69 c | 17.89 ± 0.79 b | 47.13 ± 2.23 c | 33.02 ± 3.02 b | 0.52 ± 0.09 c |
| R5B5 | 3.56 ± 0.37 bc | 38.21 ± 3.33 c | 10.47 ± 0.71 c | 20.31 ± 0.78 a | 50.98 ± 2.13 bc | 38.17 ± 3.95 abc | 0.58 ± 0.01 b |
| R2B8 | 4.13 ± 0.17 ab | 54.26 ± 3.04 a | 13.18 ± 0.81 a | 22.12 ± 1.31 a | 55.39 ± 2.36 ab | 45.16 ± 3.96 a | 0.67 ± 0.08 a |
| R0B10 | 4.36 ± 0.41 a | 50.07 ± 3.39 ab | 13.32 ± 0.33 a | 21.31 ± 0.98 a | 59.85 ± 2.77 a | 40.32 ± 2.09 ab | 0.62 ± 0.03 a |
| Treatment | MEL (ng·g−1) | ABA (ng·g−1) | KIN (ng·g−1) | ZE (ng·g−1) | IAA (ng·g−1) | IBA (ng·g−1) |
|---|---|---|---|---|---|---|
| R10B0 | 2.16 ± 0.13 d | 2.43 ± 0.18 a | 1.83 ± 0.05 c | 0.81 ± 0.01 b | 13.17 ± 0.15 d | 3.16 ± 0.75 d |
| R7B3 | 2.52 ± 0.17 c | 2.22 ± 0.12 ab | 2.16 ± 0.06 b | 0.75 ± 0.05 c | 14.44 ± 0.18 bc | 3.02 ± 1.28 d |
| R5B5 | 3.49 ± 0.12 b | 2.17 ± 0.17 ab | 1.91 ± 0.09 c | 0.77 ± 0.04 cb | 14.08 ± 0.23 cd | 3.91 ± 1.23 b |
| R2B8 | 4.01 ± 0.20 ab | 2.12 ± 0.17 b | 2.22 ± 0.04 a | 0.93 ± 0.05 a | 16.22 ± 0.12 a | 4.05 ± 1.01 a |
| R0B10 | 4.92 ± 0.12 a | 2.69 ± 0.14 a | 2.15 ± 0.03 b | 0.90 ± 0.03 a | 15.49 ± 0.21 ab | 3.54 ± 1.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Ma, Y.; Zhang, K.; Song, Y.; Liu, Z.; Li, M.; Zheng, Y.; Ge, S.; Pan, T.; Xie, J.; et al. High Proportion of Blue Light Contributes to Product Quality and Resistance to Phytophthora Infestans in Tomato Seedlings. Agriculture 2025, 15, 2082. https://doi.org/10.3390/agriculture15192082
Jiang C, Ma Y, Zhang K, Song Y, Liu Z, Li M, Zheng Y, Ge S, Pan T, Xie J, et al. High Proportion of Blue Light Contributes to Product Quality and Resistance to Phytophthora Infestans in Tomato Seedlings. Agriculture. 2025; 15(19):2082. https://doi.org/10.3390/agriculture15192082
Chicago/Turabian StyleJiang, Chengyao, Yue Ma, Kexin Zhang, Yu Song, Zixi Liu, Mengyao Li, Yangxia Zheng, Sang Ge, Tonghua Pan, Junhua Xie, and et al. 2025. "High Proportion of Blue Light Contributes to Product Quality and Resistance to Phytophthora Infestans in Tomato Seedlings" Agriculture 15, no. 19: 2082. https://doi.org/10.3390/agriculture15192082
APA StyleJiang, C., Ma, Y., Zhang, K., Song, Y., Liu, Z., Li, M., Zheng, Y., Ge, S., Pan, T., Xie, J., & Lu, W. (2025). High Proportion of Blue Light Contributes to Product Quality and Resistance to Phytophthora Infestans in Tomato Seedlings. Agriculture, 15(19), 2082. https://doi.org/10.3390/agriculture15192082










